Abstract
Staphylococcal skin infections are quite common in human patients. These infections often clear spontaneously, but may also progress locally and/or disseminate to cause serious and sometimes fatal deep infections. The present studies were undertaken to examine the clearance phase of experimental cutaneous Staphylococcus aureus infections in a mouse model system. Previous work in this system has shown that staphylococci applied to the skin rapidly disseminate to the spleen and kidney. In the present experiments the bacteria were found to persist at the skin infection site at a time (8 days after inoculation) when they had disappeared from the spleen and kidney. Examination of the infected skin at earlier times revealed rapid (within 6 h) invasion into the stratum corneum, stratum Malpighii, and dermis, but subsequent redistribution of bacteria (at 1–2 days) to more superficial sites, particularly crusts located just above the skin surface. The crusts seen in these infections were of two distinct types, which were termed type 1 and type 2. Type 1 crusts appeared first, consisted of bacteria, inflammatory cells, and debris, and developed over an intact epidermis. Type 2 crusts arose from the process of dermal necrosis previously reported to take place at 2 days in this model system. In the latter situation the bacteria were not really cleared from the epidermis and dermis; rather those layers were transformed into a superficial crust that contained the bacteria. Deep hair follicle infections in the dermis were found in these infections, but they did not persist and did not seem to be a reservoir for organisms in the dermis. Resolution of these experimental infections appeared to involve redistribution of invading bacteria to more superficial locations in crusts above the skin surface, marked proliferation of the epidermis, loss of the bacteria-laden crusts from the skin, and eventual healing of the cutaneous damage.
Keywords: Staphylococcus aureus, Epidermis, Dermis, Cutaneous bacterial infection, Neutrophil, Stratum corneum
Introduction
Staphylococcus aureus is a well-recognized human commensal as well as frequent cause of both cutaneous and deep or disseminated infections. Increased interest in this pathogen has been driven recently by the upsurge in infections caused by community-associated methicillin-resistant S. aureus. Surveys have shown that approximately 32% of the US population is colonized by S. aureus and 0.8% by the methicillin-resistant strains [14, 18]. Skin and soft tissue infections account for a majority of the disease produced by either community or hospital associated strains of this organism [15]. S. aureus can be found to be colonizing the anterior nares, pharynx, and skin of healthy humans, with the nares being the predominant niche [37, 39]. Determinants of S. aureus colonization states are poorly understood; however, colonization is a recognized risk factor for cutaneous and systemic infection with this organism [9, 14, 28, 37, 39].
We have recently used experimental cutaneous S. aureus infections in mice to study invasion into the skin and dissemination of this organism from that site; these experiments involved epicutaneous inoculation of S. aureus onto minimally disrupted skin [11]. In this model system the bacteria disseminated to the spleen and kidneys within 6 h after inoculation in almost all of the animals. Although both the cutaneous and deep infection sites produced by the epicutaneous inoculations did eventually resolve spontaneously, the cutaneous ones appeared to persist for longer periods. Therefore, the mechanisms involved in clearance of the infections from the skin could be different from those that function at deeper sites.
Studies of other cutaneous infections, particularly superficial mycoses and bacterial skin infections, have implicated several clearance processes that may function somewhat differently in the skin; these include neutrophil accumulation into the superficial layers of the epidermis [10, 29, 33, 40], epidermal proliferation [2, 20, 21, 33, 35], and shedding of viable organisms from the skin [32, 34]. The goal of the present study was to carefully examine the S. aureus-infected skin at later time points in this model system to elucidate the mechanisms by which the organisms are cleared from the skin.
Materials and methods
Organisms
Staphylococcus aureus strain 25923 from the American Type Culture Collection was used for these experiments. The organisms were cultured overnight in trypticase soy broth, washed three times in sterile water, and then used as an inoculum of 107 CFU. This inoculum was chosen from a review of previous similar cutaneous infection models [1, 19, 25]. S. aureus strain ATCC 25923 has been shown to be virulent in other animal models of staphylococcal infection [5, 6]. Virulence factors and enzymes of this staphylococcal strain include fibronectin binding protein A, the Panton–Valentine leukocidin, coagulase, catalase, DNase, heat shock protein 60, thermostable nuclease, α-toxin, and staphylococcal enterotoxins G, I, M, N, and O; this strain is negative for β-lactamase, exfoliative toxins, and toxic shock syndrome toxin 1 (TSST) [4, 5, 27]. In the previous study using this model system, we used a second S. aureus strain (Newman) with approximately similar results to those obtained with strain ATCC 25923 [11].
Animals
C57BL/6 mice were used for all the studies and were obtained from Charles Rivers Laboratories (Wilmington, MA). The animals were both male and female, from 8 to 14 weeks of age, and were described by the supplier as being free of specific pathogens (including S. aureus). The mice for these studies were housed in a separate BSL 2 enhanced section of the Veterinary Medical Unit at the Milwaukee VA Medical Center. The experimental procedures were approved by the appropriate committees at the Milwaukee Veterans Affairs Medical Center and the Medical College of Wisconsin. It should be noted that mouse skin structure is generally similar to that of humans except for a thinner epidermis and grouping of hair follicles together in areas of those that are actively growing (anagen) or those that are resting (telogen) [16].
Epicutaneous inoculations
The skin over the animal's flanks was shaved with an electric razor, swabbed with iodine, washed with alcohol followed by saline, and then dried with gauze. The skin surface was prepared by gentle tape stripping 7× with Transpore tape (approximately 27 mm in width, from 3M, Minneapolis, MN). This technique has previously been found to cause only minimal damage to the epidermis or dermis [11]. An inoculum of 107 S. aureus CFU in 0.025 ml of saline was added to 4 mm filter paper discs placed onto the prepared skin of the animal's left flank, with saline added to the disc on the opposite side. Both sites were covered with 1.0 cm2 pieces of plastic sheet, which were then dressed with Transpore tape and Nexcare waterproof tape (3M).
Monitoring of infections
The dressings were removed after 1–24 h and the sites washed four times with saline-soaked gauze pads. At various times from 1 h to 14 days after inoculation the animals were killed and skin removed from the inoculated sites for histology. Paraffin sections were prepared, stained with tissue gram stains, and analyzed as discussed below. In some cases when the mice were killed, skin scrapings or homogenates of spleen or kidneys in 1 ml of saline were cultured on trypticase soy agar, with results recorded as log10 CFU per ml. For skin scrapings the entire inoculation site was abraded with a scalpel blade until a glistening surface could be seen; the material removed was vortexed in 1 ml of saline and then cultured for CFU determinations. In that a 0.1 ml sample from these 1.0 ml specimens was cultured, the lower limit of log10 CFU values was recorded as 1.0 when no CFU were obtained on the plates.
Staphylococcus aureus or saline inoculated skin sections were examined in a blinded fashion for bacteria and cutaneous changes at a 400× magnification under light microscopy. For the purposes of the present study the epidermis was defined as the stratum Malpighii or the epidermal keratinocyte layers above the dermal–epidermal junction, but excluding the stratum corneum or crusts. In ten random fields in each section the site of bacteria location was determined as being in the stratum corneum or crusts, the stratum Malpighii, the dermis, or combination thereof. A section was considered to be positive for bacteria if it contained at least five readily identifiable organisms (although most sections contained many more). In preliminary studies the crusts were found to be of two distinct types, as discussed below; location of the organisms in each type was also determined. Each hair follicle infundibular outlet across the sections was also examined for the presence of bacteria, with the data being recorded as a percent of outlets infected. Hair follicles located in the dermis were also examined for bacteria located >100 μm below the skin surface, with the data being recorded as the percent of hair follicles with deep infections; in this case the denominator was taken as the total number of outlets, in that each outlet will have a corresponding dermal section at this depth. Thickness of the stratum Malpighii was measured in microns using an ocular micrometer at ten random sites across the section as a measure of epidermal proliferation; locations with either neutrophil infiltration or visible epidermal damage were excluded from this analysis.
Statistics
Data were expressed as mean ± SE, with statistical comparisons carried out using the GraphPad Prism 4.0a statistical package. For multiple comparisons ANOVA with Tukey's tests were used to determine statistical significance. Generally 4–11 mice per point were studied in 3–6 experiments (each consisting of animals inoculated in a similar manner on a particular day). Statistical significance was considered to be P < 0.05 in this study.
Results
Clearance of S. aureus CFU from skin, spleen, and kidney
After epicutaneous inoculation onto flank skin, the organisms were found thereafter not only in cultures of skin, but also those of spleen and kidney, as previously reported from this model system [11]. The number of S. aureus CFU were highest at 2–4 days and rapidly declined thereafter in both skin and the deep organs (Fig. 1). At 8 days after inoculation, bacteria were still found in the skin (mean ± SE log10 of S. aureus CFU of 4.3 ± 0.7) although cultures of spleen and kidney had become negative by this time.
Fig. 1.
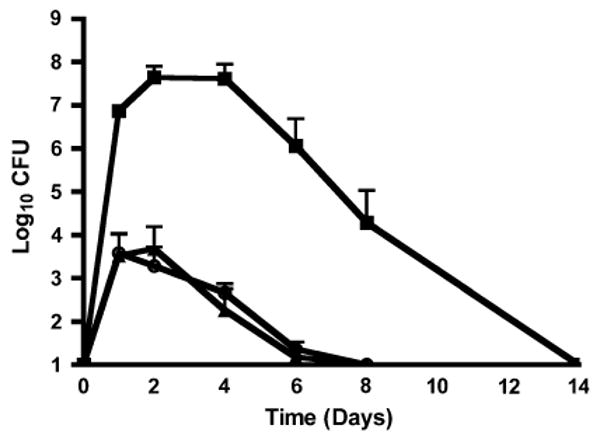
S. aureus CFU (log10 on the ordinate) cultured from skin (filled squares), spleen (filled triangles), and kidney (open circles) at various times (in days on the abscissa) after epicutaneous inoculation on flank skin of C57BL/6 mice. Bars represent SE. Note that CFU in skin from 1 to 8 days were significantly greater than those in either spleen or kidney at these times (P < 0.001 in all cases by ANOVA and Tukey's tests). Data come from 5 to 8 animals per point studied in 3–5 experiments
Formation of crusts
Crusts at the skin surface formed on the S. aureus-infected skin as early as 6 h after epicutaneous inoculation. There appeared to be two distinct types of crusts that developed in these infections, and they were arbitrarily designated type 1 (Fig. 2a) and type 2 (Fig. 2b) for the purposes of this study. Type 1 crusts appeared over an intact epidermis and consisted of organisms, neutrophils, and cellular debris. Type 2 crusts formed from degenerated dermis and epidermis; they contained this material plus organisms and some inflammatory cells, and developed over remaining subcutaneous tissue (muscle and adipose cells). The organisms in both types of crusts were generally grouped into round masses of bacteria (instead of being dispersed in the crust), and were not usually directly associated with host inflammatory cells (Fig. 2a, b).
Fig. 2.
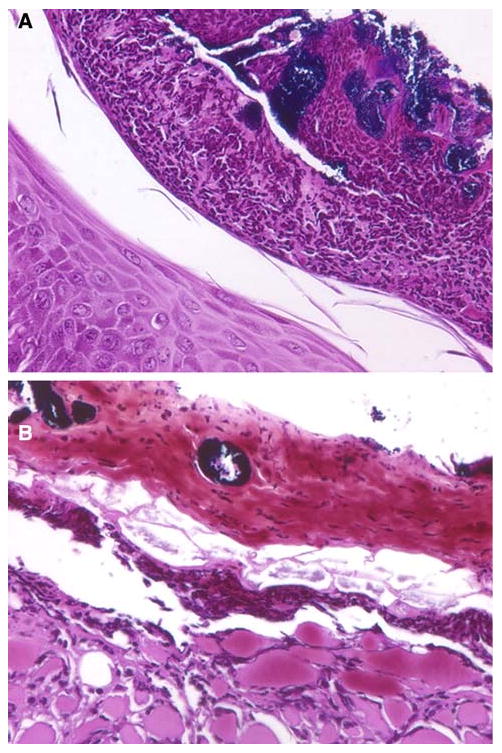
Photomicrographs of crusts in S. aureus-infected skin of C57BL/6 mice. a Type 1 crust at 4 days after inoculation with organisms and inflammatory cells located above an intact epidermis; b type 2 crust at 2 days after inoculation with organisms, degenerated dermis and some inflammatory cells located above remaining subcutaneous tissue (tissue gram stains with original magnification of ×1,000 under oil immersion)
Redistribution of organisms in skin during the experimental infections
At 6 h after inoculation organisms were found in the stratum corneum and crusts, the stratum Malpighii, and the dermis (Fig. 3a); at later time points (2–4 days) fewer fields with organisms were found for stratum Malpighii and dermis, suggesting that the bacteria had left or been cleared from these deeper sites. However, since the stratum Malpighii and dermis were converted into type 2 crusts by the dermal necrosis process, it would appear that the organisms contained therein had become displaced to these crusts rather than actually being cleared from the dermis and epidermis. Although at 6 h more fields containing organisms were found for the stratum corneum than crusts, this situation reversed between 1 and 2 days after inoculation when there appeared to be a shift in location from the stratum corneum to the crusts (Fig. 3b). Type 1 crusts containing organisms appeared to develop earlier than did the type 2 crusts, which were not seen until at least 2 days after inoculation (Fig. 3c). In some animals both types of crusts could be found in different areas within the same section of infected skin. Crusts of both types and the bacteria they contained were seen to decrease rapidly after 2 days and had disappeared by 8 days. It should be noted that total numbers of CFU in the skin were highest at 2 days after inoculation and that at this time most of the bacteria were localized to crusts at the skin surface.
Fig. 3.
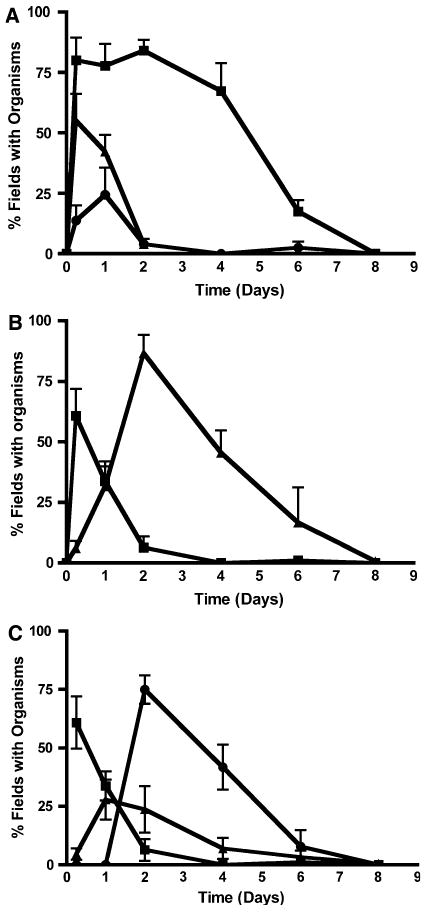
Location of S. aureus bacteria in the skin (as percent of fields with organisms in particular sites on the ordinate) at various times (in days on the abscissa) after epicutaneous inoculation in C57BL/6 mice. a Organisms in stratum corneum or crusts (filled squares), stratum Malpighii (filled triangles), or dermis (filled circles); b organisms in stratum corneum (filled squares) or crusts (filled triangles); c organisms in stratum corneum (filled squares), type 1 crusts (filled triangles), or type 2 crusts (filled circles). Note that at 6 h there were significantly more organisms in the stratum corneum than crusts (P < 0.01 by ANOVA and Tukey's tests), whereas by 2 or 4 days there were more organisms in the crusts than stratum corneum (P < 0.001 in each case). Data come from 4 to 11 animals studied in 3–5 experiments
Hair follicle infections
Infections of the hair follicles were noted for both the follicle infundibular outlets and deeper follicle sites within the dermis (Fig. 4a, b). The deeper infections (defined as being located >100 μm below the skin surface) were much less common than those at the surface; both types of infection declined rapidly over time, with very few being found at 4 days and none by 8 days (Fig. 5).
Fig. 4.
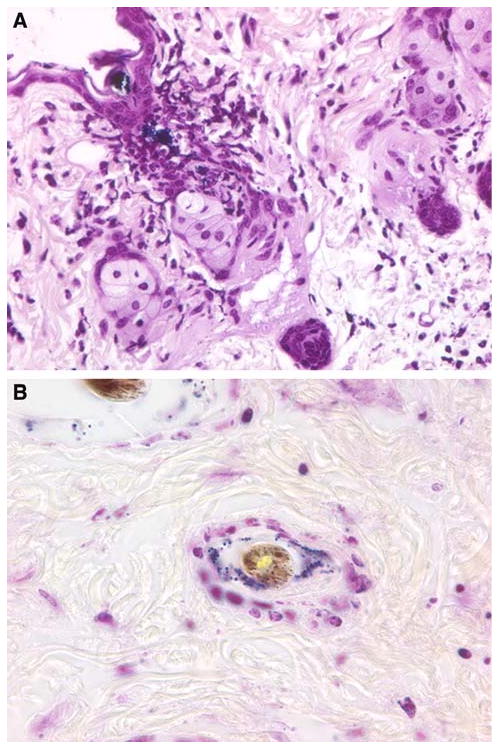
Photomicrographs of infected hair follicles 1 day after epicutaneous inoculation with S. aureus in C57BL/6 mice. a Infection of the infundibular outlet; b infection of a deeper follicular site in the dermis (tissue gram stains with original magnification of ×1,000 under oil immersion)
Fig. 5.
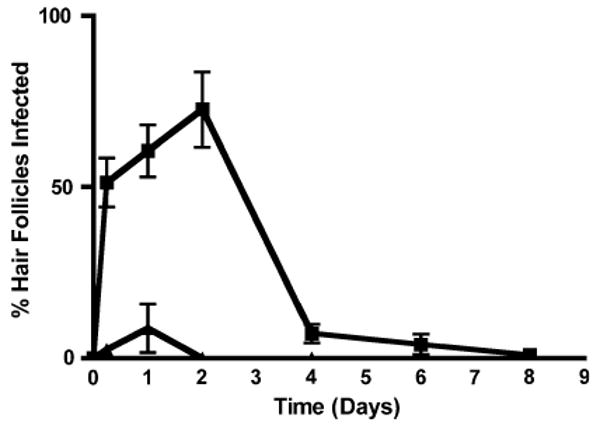
Hair follicles infected (as percent of total on the ordinate) versus time after epicutaenous inoculation with S. aureus (in days on the abscissa). Note that the number of follicles infected at the infundibular outlets (filled squares) was greater than those infected at follicle locations deeper than 100 μm below the skin surface (filled triangles), but that both types of infection appeared to resolve by 8 days. Data come from 5 to 9 animals per point studied in 3–6 experiments
Epidermal proliferation
Whereas the stratum Malpighii of uninfected skin from C57BL/6 mice is generally quite thin, usually one or two cells thick (Fig. 6a), that from skin inoculated 8 days previously in this model system was much thicker (Fig. 6b). During these infections the stratum Malpighii thickness increased from 17.2 ± 0.6 μm in uninfected skin to 147.9 ± 50.6 in skin 8 days after infection (Fig. 7). There was some irregularity in the data for this value at the later time points, perhaps because in certain areas the dermis and epidermis had undergone necrosis and the skin was actively repairing itself. It should be noted that by 8 days and later the crusts had disappeared from the skin surface and visible damage had resolved.
Fig. 6.
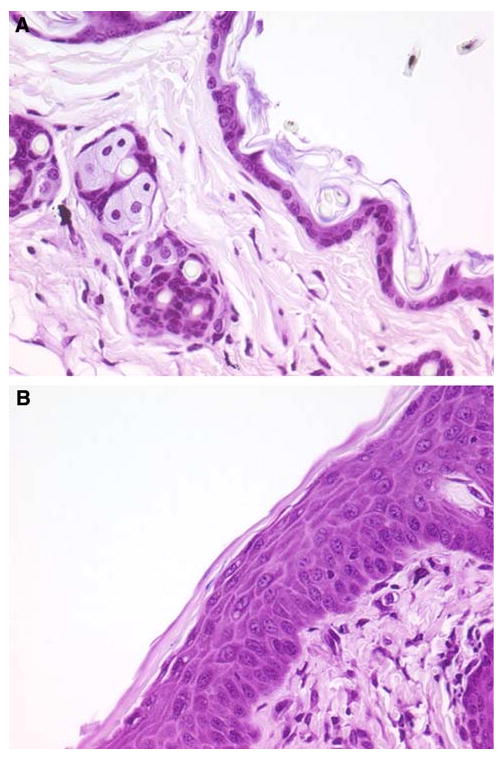
Photomicrographs showing epidermal proliferation in S. aureus-infected skin of C57BL/6 mice. a Normal uninfected mouse skin showing a thin stratum Malpighii; b mouse skin taken at 8 days after inoculation with S. aureus showing increased thickness of the stratum Malpighii (tissue gram stains with original magnification of ×1,000 under oil immersion)
Fig. 7.
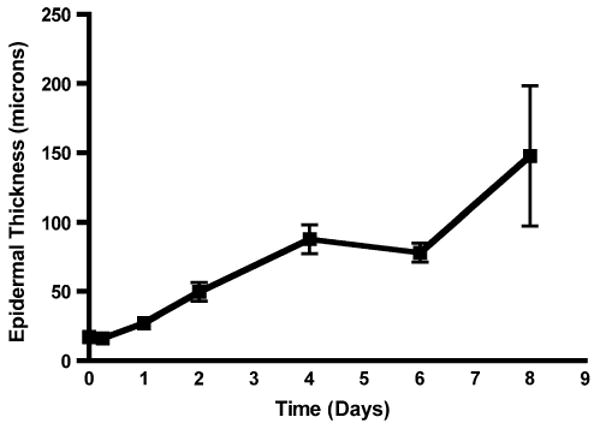
Epidermal proliferation as measured by increased thickness of the stratum Malpighii (in microns on the ordinate) at various times after epicutaneous inoculation with S. aureus (in days on the abscissa). The value for 0 days represents uninfected skin. Note that there was significantly increased stratum Malpighii thickness at day 8 compared to uninfected C57BL/6 mouse skin or that inoculated 6 h or 1 day beforehand (P < 0.001 by ANOVA and Tukey's tests in all cases). Data come from 6 to 8 animals per point studied in 3–6 experiments
Discussion
These studies were designed to examine the clearance phase of cutaneous S. aureus infections after epicutaneous inoculation of the organisms onto relatively intact (tape stripped) skin on the flanks of C57BL/6 mice. Previous work in this model system has shown that the organisms disseminate rapidly from the skin to the spleen and kidney. The present experiments demonstrate that bacteria persist at the skin infection site at a time (8 days after inoculation) by which they have disappeared from the spleen and kidney. Although the numbers of CFU at this time are too low to allow for microscopic visualization in microscopic sections, observations at earlier time points revealed rapid redistribution of the bacteria from deeper to more superficial sites in the skin soon after inoculation. In fact, the organisms appeared to rapidly (within 6 h) invade into the epidermis and dermis, only to disappear from these layers soon afterwards and then to be found populating crusts at the skin surface at later times. Similarly, bacteria in the stratum corneum also rapidly disappeared from this site. The crusts seen in these infections were clearly of two different types, which we termed type 1 and type 2. The former developed first and consisted of bacteria, inflammatory cells, and debris that overlay an intact epidermis. The latter developed from the process of dermal necrosis previously reported to take place at 2 days in this model system [11]. In that the dermis and epidermis were lost during dermal necrosis and generation of the type 2 crusts, it would appear that the organisms did not really disappear from the skin itself; rather the dermis and epidermis were merely converted into a crust that then contained the same bacteria. In any event, the result was relocation of the organisms into crusts of either or both types at the skin surface by 2 days after inoculation. Infections of deep hair follicle sites in the dermis could be a way for the bacteria to persist at deep sites in the infected skin; however, although this process did occur, it appeared to be fairly infrequent and short-lived. Resolution of these experimental infections seemed to involve redistribution of invading bacteria to more superficial locations in crusts above the skin surface, eventual loss of these crusts from the skin, and healing of cutaneous damage in conjunction with proliferation of the epidermis.
The mouse model system used in these studies does produce infections that are similar to those seen in human patients. As has been found in other animal models of cutaneous staphylococcal infections, the resulting damage to the skin involved production of folliculitis, microabscesses, epidermal and dermal necrosis, and cutaneous ulcers [1, 13, 17, 19, 25, 26]; these cutaneous changes are similar to those previously described for human staphylococcal skin infections [13, 31]. Whereas it is not common to find staphylococcal bacteremia in human patients with minor infections of the skin, it should be noted that our method to demonstrate dissemination by culturing whole spleen and kidneys is likely much more sensitive. When blood cultures have been performed in experimental staphylococcal skin infections in mice, the results were generally negative [25, 26]. One potential limitation of this model system would seem to be that the experimental infections uniformly appear to resolve spontaneously, without the formation of more chronic abscesses that are sometimes seen in human patients.
In these experimental infections the invading staphylococci rapidly became localized to both superficial and deep locations; the former included stratum corneum and crusts, and the latter the epidermal keratinocytes, dermis, spleen, kidney, and possibly other deep sties that were not sampled. The physical conditions are quite different at the skin surface than at deep sites within the body and it is likely that different mechanisms are required to clear bacteria from the superficial sites. The stratum corneum is known to have reduced water content, acidic pH, antimicrobial lipids, and tightly arrayed corneocytes that appear to provide significant defense against both water loss and microbial invasion [3, 7, 36, 38]. However, these characteristics would presumably make this site less favorable for phagocytic cell function, a process that likely plays an important role at deeper locations. If one considers the time period from 2 to 4 days after inoculation in this model system, a majority of cultured bacteria appear to be residing in the stratum corneum and crusts in the infected animals, with smaller numbers persisting in the deep organs or similar sites. These superficial bacteria are likely made up of those remaining from the inoculum, their progeny resulting from bacterial cell division at the skin surface, and bacteria that have been relocated from the dermis and epidermis to crusts by the dermal necrosis process described above. Since killing by phagocytic cells is likely inefficient at this site, other mechanisms are probably involved in the clearance process. Some of the organisms may be killed by antimicrobial lipids generated in skin secretions [3] or antimicrobial proteins produced by differentiating keratinocytes [8, 22, 23]. On the other hand, loss of viable bacteria through sloughing of the superficial crusts containing them is another likely possibility, as has been shown in studies of cutaneous candidiasis [32, 34]. At 4 days after inoculation, a time when skin-scraping cultures still show near maximal values, all of the organisms visible microscopically have become relocated to crusts located above the skin surface. Microscopic examination at 6–8 days demonstrates loss of the crusts and organisms they contain suggesting that sloughing of bacteria-laden crusts may be a significant mechanism of bacterial clearance in this model system.
Epidermal proliferation has long been implicated in the clearance of cutaneous infections, particularly superficial fungal infections. This process was a uniform finding in our staphylococcal model system and may well be involved in clearance of these infections as well. The process of shedding organisms located in the stratum corneum or crusts is likely enhanced by increased production, differentiation, and upward movement of keratinocytes [2, 20, 21, 35]. Stimuli that serve to promote keratinocyte differentiation also upregulate the production of keratinocyte antimicrobial proteins such as β-defensins, cathelicidins, and calprotectin [8, 22, 23]. Both the shedding and antimicrobial protein mechanisms could be involved in clearance of these infections. Epidermal proliferation likely also plays a role in healing the damaged skin caused by the dermal necrosis process. By 6–8 days after inoculation, microscopic examination shows that the ulcers caused by dermal necrosis have completely healed, that the skin is visibly intact, and that the stratum Malpighii has become markedly thickened.
The mechanisms involved in eliminating superficial bacteria in these experimental infections would seem to be quite different from those that kill organisms that have invaded into the deeper tissues. Neutrophils likely predominate in mediating the latter function, especially in the skin where these cells make up a majority of infiltrating inflammatory cells in this model system. Neutrophils readily kill S. aureus and can do so by a variety of oxidative and non-oxidative mechanisms [12, 24, 30]. It appears likely that the aspect of host defense most important to survival of the animals in these experimental infections is indeed killing of bacteria that have invaded into the dermis and deep organs. Neutrophils and other inflammatory cells probably undertake this process. Since these cells would be unlikely to function effectively at the conditions extant in the stratum corneum or crusts at the skin surface, the other mechanisms described above may be required for bacterial clearance from this location.
Sequestration of organisms into crusts located just above the skin surface was a consistent finding in this model system. Although two types of crusts were noted in these infections, the characteristics of the bacteria found in each were quite similar. The organisms were generally aggregated into round masses and most of the bacteria were not in direct contact with host inflammatory cells. In that the organization of bacteria in the crusts of this model system has these particular characteristics, we propose the following acronym to describe them: sequestered, cell-unassociated, aggregated, bacteria, or SCABs. These superficial organisms would seem to represent a short period of skin colonization after the acute invasive infections have cleared; they could also serve as a reservoir for repeated cutaneous infections and thus necessitate clearance by whatever effective means are available to the host.
In summary, since phagocytic cell killing of microorganisms is probably ineffective at the skin surface due to unfavorable conditions there, alternative mechanisms to clear bacteria from this site would seem to be required for resolution of the experimental staphylococcal skin infections in our system. Our findings indicate that for S. aureus organisms located at the skin surface these mechanisms include sequestration of the organisms into crusts, epidermal proliferation, and subsequent sloughing of the bacteria-laden crusts.
Acknowledgments
This work was supported by the United States Department of Veterans Affairs and by the NIH/NIAID Regional Center of Excellence for Bio-defense and Emerging Infectious Diseases Research (RCE) Program. The authors wish to acknowledge membership within and support from the Region V ‘Great Lakes’ RCE (NIH award 1-U54-AI-057153).
Contributor Information
Charles C. Onunkwo, Division of Infectious Diseases, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI 53226, USA; Consultant Care Division and Research Service/151, VA Medical Center, Milwaukee, WI 53295, USA
Beth L. Hahn, Division of Infectious Diseases, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI 53226, USA; Consultant Care Division and Research Service/151, VA Medical Center, Milwaukee, WI 53295, USA
Peter G. Sohnle, Email: psohnle@mcw.edu, Division of Infectious Diseases, Department of Medicine, Medical College of Wisconsin, Milwaukee, WI 53226, USA; Consultant Care Division and Research Service/151, VA Medical Center, Milwaukee, WI 53295, USA
References
- 1.Abe Y, Akiyama H, Arata J. Furuncle-like lesions in mouse experimental skin infections with Staphylococcus aureus. J Dermatol. 1993;20:198–202. doi: 10.1111/j.1346-8138.1993.tb03861.x. [DOI] [PubMed] [Google Scholar]
- 2.Berk SH, Penneys NS, Weinstein GD. Epidermal activity in annular dermatophytosis. Arch Dermatol. 1976;112:485–488. [PubMed] [Google Scholar]
- 3.Bibel DJ, Aly R, Shah S, Shinefeld HR. Sphingosines: antimicrobial barriers of the skin. Acta Derm Venereol. 1993;73:407–411. doi: 10.2340/0001555573407411. [DOI] [PubMed] [Google Scholar]
- 4.Blaiotta G, Ercolini D, Pennacchia C, Fusco V, Casaburi A, Pepe O, Villani F. PCR detection of staphylococcal enterotoxin genes in Staphylococcus spp. strains isolated from meat and dairy products. Evidence for new variants of seG and seI in S. aureus AB-8802. J Appl Microbiol. 2004;97:719–730. doi: 10.1111/j.1365-2672.2004.02349.x. [DOI] [PubMed] [Google Scholar]
- 5.Callegan MC, Hobden JA, Hill JM, Insler MS, O'Callaghan RJ. Topical antibiotic therapy for the treatment of experimental Staphylococcus aureus keratitis. Investig Ophthalmol Vis Sci. 1992;33:3017–3023. [PubMed] [Google Scholar]
- 6.Cirioni O, Giacometti A, Ghiselli R, Kamysz W, Orlando F, Mocchegiani F, Silvestri C, Licci A, Lukasiak J, Saba V, Scalise G. Temporin A alone and in combination with imipenem reduces lethality in a mouse model of staphylococcal sepsis. J Infect Dis. 2005;192:1613–1620. doi: 10.1086/496888. [DOI] [PubMed] [Google Scholar]
- 7.Cartlidge P. The epidermal barrier. Semin Neonatol. 2000;5:273–280. doi: 10.1053/siny.2000.0013. [DOI] [PubMed] [Google Scholar]
- 8.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, Gudmundsson GH. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 9.Gorwitz RJ. A review of community-associated methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J. 2008;27:1–7. doi: 10.1097/INF.0b013e31815819bb. [DOI] [PubMed] [Google Scholar]
- 10.Hahn BL, Bischof TS, Sohnle PG. Superficial exudates of neutrophils prevent invasion of Bacillus anthracis bacilli into abraded skin of resistant mice. Int J Exp Pathol. 2008;89:180–187. doi: 10.1111/j.1365-2613.2008.00584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn BL, Onunkwo CC, Watts CJ, Sohnle PG. Systemic dissemination and cutaneous damage in a mouse model of staphylococcal skin infections. Microb Pathog. 2009;47:16–23. doi: 10.1016/j.micpath.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome—oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- 13.Iwatsuki K, Yamasaki O, Morizane S, Oono T. Staphylococcal cutaneous infections—invasion, evasion and aggression. J Dermatol Sci. 2006;42:203–214. doi: 10.1016/j.jdermsci.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Kil EH, Heymann WR, Weinberg JM. Methicillin resistant Staphylococcus aureus : an update for the dermatologist. Part 1—epidemiology. Cutis. 2008;81:227–233. [PubMed] [Google Scholar]
- 15.Kil EH, Heymann WR, Weinberg JM. Methicillin resistant staphylococcus aureus: an update for the dermatologist. Part 2—pathogenesis and cutaneous. Cutis. 2008;81:247–254. [PubMed] [Google Scholar]
- 16.Kligman AM. Pathophysiology of ringworm infections in animals with skin cycles. J Invest Dermatol. 1956;27:171–185. doi: 10.1038/jid.1956.90. [DOI] [PubMed] [Google Scholar]
- 17.Kraft WG, Johnson PT, David BC, Morgan D. Cutaneous infection in normal and immunocompromised mice. Infect Immun. 1986;52:707–713. doi: 10.1128/iai.52.3.707-713.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuehnert MJ, Kruszan-Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, McDougal LK, Chaitran J, Jensen B, Fridkin SK, Killgore G, Tenover FC. Prevalence of Staphylococcus aureus nasal colonization in the US, 2001–2006. J Infect Dis. 2006;193:172–179. doi: 10.1086/499632. [DOI] [PubMed] [Google Scholar]
- 19.Kugelberg E, Norstrom T, Petersen TK, Duvold T, Andersson DI, Hughes D. Establishment of a superficial skin infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes. Antimicrob Agents Chemother. 2005;49:3435–3441. doi: 10.1128/AAC.49.8.3435-3441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lepper AWD. Experimental bovine Trichophyton verrucosum infection. The cellular responses in primary lesions of the skin resulting from surface or intradermal inoculation. Res Vet Sci. 1974;16:287–298. [PubMed] [Google Scholar]
- 21.Lepper AWD, Anger HS. Experimental bovine Trichophyton verrucosum infection. Comparison of the rate of epidermal cell proliferation and keratinization in non-infected and reinoculated cattle. Res Vet Sci. 1976;20:117–121. [PubMed] [Google Scholar]
- 22.Liu L, Wang L, Jia HP, Zhao C, Heng HHQ, Schutte BC, McCray PB, Jr, Ganz T. Structure and mapping of the human β-defensin HBD-2 gene and its expression at sites of inflammation. Gene. 1998;222:237–244. doi: 10.1016/s0378-1119(98)00480-6. [DOI] [PubMed] [Google Scholar]
- 23.Madsen P, Rasmussen HH, Leffers H, Honore B, Celis JE. Molecular cloning and expression of a novel keratinocyte protein (psoriasis-associated fatty acid-binding protein) that is highly upregulated in psoriatic skin and that shares similarity to fatty acid-binding proteins. J Invest Dermatol. 1992;99:299–305. doi: 10.1111/1523-1747.ep12616641. [DOI] [PubMed] [Google Scholar]
- 24.Mayer-Scholl A, Averhoff P, Zychlinsky A. How do neutrophils and pathogens interact? Curr Opin Microbiol. 2004;7:62–66. doi: 10.1016/j.mib.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Molne L, Tarkowski A. An experimental model of cutaneous infection induced by superantigen-producing Staphylococcus aureus. J Invest Dermatol. 2000;114:1120–1125. doi: 10.1046/j.1523-1747.2000.00973.x. [DOI] [PubMed] [Google Scholar]
- 26.Molne L, Verdrengh M, Tarkowski A. Role of neutrophil leukocytes in cutaneous infection caused by Staphylococcus aureus. Infect Immun. 2000;68:6162–6167. doi: 10.1128/iai.68.11.6162-6167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monecke S, Berger-Bachi B, Coombs G, Holmes A, Kay I, Kearns A, Linde HJ, O'Brien F, Slickers P, Ehricht R. Comparative genomics and DNA array-based genotyping of pandemic Staphylococcus aureus strains encoding Panton-Valentine leukocidin. Clin Microbiol Infect. 2007;13:236–249. doi: 10.1111/j.1469-0691.2006.01635.x. [DOI] [PubMed] [Google Scholar]
- 28.Mylotte JM, McDermott C, Spooner JA. Prospective study of 114 consecutive episodes of Staphylococcus aureus bacteremia. Rev Infect Dis. 1987;9:891–907. doi: 10.1093/clinids/9.5.891. [DOI] [PubMed] [Google Scholar]
- 29.Ray TL, Wuepper KD. Experimental cutaneous candidiasis in rodents. J Invest Dermatol. 1976;66:29–33. doi: 10.1111/1523-1747.ep12478053. [DOI] [PubMed] [Google Scholar]
- 30.Segal AW. How do neutrophils kill microbes? Annu Rev Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh G, Marples RR, Kligman AM. Experimental Staphylococcus aureus infections in humans. J Invest Dermatol. 1971;57:149–162. doi: 10.1111/1523-1747.ep12261498. [DOI] [PubMed] [Google Scholar]
- 32.Sohnle PG, Frank MM, Kirkpatrick CH. Mechanisms involved in elimination of organisms from experimental cutaneous Candida albicans infections in guinea pigs. J Immunol. 1976;117:523–530. [PubMed] [Google Scholar]
- 33.Sohnle PG, Hahn BL. Epidermal proliferation and the neutrophilic infiltrates of experimental cutaneous candidiasis in mice. Arch Dermatol Res. 1989;281:279–283. doi: 10.1007/BF00431063. [DOI] [PubMed] [Google Scholar]
- 34.Sohnle PG, Hahn BL. The fate of individual organisms during clearance of experimental cutaneous Candida albicans infections in mice. Acta Derm Venereol. 1992;72:241–244. [PubMed] [Google Scholar]
- 35.Sohnle PG, Kirkpatrick CH. Epidermal proliferation in the defense against cutaneous candidiasis. J Invest Dermatol. 1978;70:130–133. doi: 10.1111/1523-1747.ep12258536. [DOI] [PubMed] [Google Scholar]
- 36.Tsuruta D, Green KJ, Getsios S, Jones JCR. The barrier function of skin—how to keep a tight lid on water loss. Trends Cell Biol. 2002;12:355–357. doi: 10.1016/s0962-8924(02)02316-4. [DOI] [PubMed] [Google Scholar]
- 37.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med. 2001;344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 38.Watts CJ, Wagner DK, Sohnle PG. Fungal infections, cutaneous. In: Schaechter M, editor. Encyclopedia of microbiology. Elsevier Science; Oxford: 2009. pp. 382–388. [Google Scholar]
- 39.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, Nouwen JL. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 40.Yee J, Giannias B, Kapadia B, Chartrand L, Christou NV. Exudative neutrophils—modulation of microbicidal function in the inflammatory microenvironment. Arch Surg. 1994;129:99–105. doi: 10.1001/archsurg.1994.01420250111014. [DOI] [PubMed] [Google Scholar]


