Abstract
Hepatitis B virus (HBV)-related liver disease is the leading indication for liver transplantation (LT) in Asia, especially in China. With the introduction of hepatitis B immunoglobulin (HBIG) and oral antiviral drugs, the recurrent HBV infection rate after LT has been evidently reduced. However, complete eradication of recurrent HBV infection after LT is almost impossible. Recurrent graft infection may lead to rapid disease progression and is a frequent cause of death within the first year after LT. At present, the availability of new oral medications, especially nucleoside or nucleotide analogues such as adefovir dipivoxil, entecavir and tenofovir disoproxil fumarate, further strengthens our ability to treat recurrent HBV infection after LT. Moreover, since combined treatment with HBIG and antiviral agents after liver re-transplantation may play an important role in improving the prognosis of recurrent HBV infection, irreversible graft dysfunction secondary to recurrent HBV infection in spite of oral medications should no longer be considered an absolute contraindication for liver re-transplantation. Published reviews focusing on the therapeutic strategies for recurrent HBV infection after LT are very limited. In this article, the current therapeutic strategies for recurrent HBV infection after LT and evolving new trends are reviewed to guide clinical doctors to choose an optimal treatment plan in different clinical settings.
Keywords: Therapy, Hepatitis B virus, Recurrent hepatitis B virus infection, Antiviral drugs, Liver transplantation
INTRODUCTION
Hepatitis B virus (HBV)-related liver disease accounts for approximately 5%-10% of all liver diseases after liver transplantations (LT) performed each year in the United States and is the leading indication for LT in Asia[1,2]. It was reported that the recurrent HBV infection rate after LT is as high as 80%-100% without any prophylaxis[3,4]. The recurrent HBV infection rate has been decreased from 90% to approximately 30%-40% since the introduction of hepatitis B immunoglobulin (HBIG)[5,6]. With the advances in oral antiviral drugs, combined treatment with HBIG and lamivudine (LAM), recommended by many centers, has achieved encouraging outcomes, with the recurrent HBV infection rate reduced to less than 10% during a follow-up period of 1-2 years[7-11].
However, combined treatment with HBIG and LAM cannot control recurrent HBV infection. Recurrent graft infection may lead to rapid disease progression and is a frequent cause of death within the first year after LT[12]. Furthermore, the incidence of acquiring de novo HBV infection after LT in patients who are negative for hepatitis B surface antigen (HBsAg) is 1.7%-3.5%, and patients with de novo HBV infection are also at a risk for severe progressive liver injury[13-15]. The aggressive clinical course is probably due to stimulation of viral replication and direct cytotoxicity of HBV under immunosuppressive therapy. Therefore, suppression of HBV replication is paramount to prevent disease progression in the transplanted liver.
Unfortunately, almost all published reviews focusing on the prophylactic strategies against recurrent HBV infection after LT, have drawn less prominence to the treatment of recurrent HBV infection in recipients after LT. Published reviews, focusing on the therapeutic strategies against recurrent HBV infection after LT, are very limited, and almost all of them are already nearly obsolete. In the following, the current therapeutic strategies for recurrent HBV infection after LT and evolving new trends are reviewed.
INTERFERON
In the pre-LAM era, interferon α is a common therapeutic option for patients with recurrent HBV infection after LT. However, with the advent of LAM, it has not been used as a first-line treatment drug. Patients using interferon α have a lower efficacy and a higher risk of precipitating allograft rejection than those using LAM[16,17]. Furthermore, treatment of recurrent HBV infection after LT with interferon α can lead to side effects such as neutropenia.
LAM
LAM can potentially inhibit HBV replication by competitively suppressing the reverse transcriptase and termination of proviral DNA chain extension, and has been used in treatment of recurrent HBV infection, with an excellent safety profile in both compensated and decompensated cirrhotic patients. The use of LAM in treatment of recurrent HBV infection after LT has shown promising results as is shown in a multicenter North American study on 52 patients with chronic hepatitis B after LT, demonstrating that use of LAM for 52 wk can result in loss of serum HBV DNA in 60%, undetectable hepatitis B e antigen (HBeAg) in 31%, undetectable HBsAg in 6%, normalization of serum alanine transaminase (ALT) levels in 71% of patients, respectively[18]. The results from other studies[19-27] are summarized in Table 1, showing that LAM can suppress HBV DNA to undetectable levels in 32.5%-100%, anti-HBeAg seroconversion in 4.2%-100%, and anti-HBsAg seroconversion in 0%-83.3% of patients, respectively, after 4.6-36 mo of treatment. Notably, use of LAM in treatment of de novo HBV infection or acute recurrent HBV infection of the graft after LT tends to effectively suppress HBV DNA, and converse serum anti-HBeAg and anti-HBsAg.
Table 1.
Use of LAM in treatment of recurrent HBV graft infection after LT
| Author (Ref.) | n |
Pre-treatment |
Treatment duration (mo) mean (range) | LAM dosage (mg/d) |
Post-treatment |
||||
| HBV DNA+ (%) | HBeAg+ (%) | HBV DNA negative following treatment (%) | HBeAg seroconversion (%) | HBsAg seroconversion (%) | Development of LAM resistant mutants (%) | ||||
| Ben-Ari et al[20] | 8 | 100 | 62.5 | 36 (24-50) | 100 | 32.5 | 20 | 0 | 62.5 |
| Umeda et al[19] | 61 | 100 | NA | 4.6 (0.7-11) | 100 | NA | NA | 83.3 | 0 |
| Castells et al[21] | 71 | 100 | 85.7 | 24.5 (12-49) | 100 | 71.4 | 50 | 14.3 | 14.3 |
| Fontana et al[22] | 33 | 94 | 75 | 21 (4-36) | NA | 72 | 4.2 | 0 | 29.4 |
| Andreone et al[23] | 112 | 100 | 18.2 | 17 (8-27) | 100 | 100 | 100 | 9.1 | 27.3 |
| Perrillo et al[18] | 52 | 90.4 | 86.5 | 12 | 100 | 68.1 | 11.1 | 3.8 | 26.9 |
| Nery et al[24] | 11 | 90.9 | NA | 15 (13-21) | NA | 90 | NA | NA | 27.3 |
| Fischer et al[25] | 12 | 100 | NA | 10.5 (5-43) | 100-150 | 83.3 | NA | NA | NA |
| Rayes et al[26] | 41 | 100 | NA | 12-36 | 150 | 75.6 | NA | NA | 24.4 |
| Malkan et al[27] | 41 | 100 | 75 | 11 (4-28) | 100-150 | 100 | NA | 25 | 0 |
All patients had de novo HBV infection after LT;
This study reported treatment of acute recurrent graft HBV infection. NA: Not available; HBV: Hepatitis B virus; LT: Liver transplantation; HBeAg: Hepatitis B e antigen; HBsAg: Hepatitis B surface antigen; LAM: Lamivudine.
However, the major factor limiting the use of LAM in treatment of graft HBV infection after LT is the development of mutations in thyrosine-methionine-aspartate-aspartate (YMDD) motif of the HBV DNA polymerase gene, which confers resistance to LAM. In non-immunosuppressed patients, the LAM resistance rate is 15%-20%[28]. LAM resistance can be detected in 45% of immunosuppressed patients within the first year of treatment[29,30]. It has been reported that YMDD mutation occurs in 26.9%, 27.3%, 29.4% and 62.5% of patients with recurrent HBV infection[18,20,22,24] after 12, 15, 21 and 36 mo of treatment with LAM, respectively. It has also been reported that YMDD mutation occurs in patients with de novo HBV infection after LT, in 0%, 0% and 14.3% of patients with recurrent HBV infection[19,21,27] after 4.6, 11 and 24.5 mo of treatment with LAM, respectively. One possible explanation for it is the short-term use of LAM in patients with de novo HBV infection and low HBV-DNA levels at the acute phase of de novo HBV reactivation.
Available data from the literature regarding the outcomes secondary to YMDD mutation are controversial. Perrillo et al[18] reported that the YMDD mutant virus is not consistently associated with hepatic disease progression, whereas elevated serum ALT can be frequently observed at the time when serum HBV DNA becomes detectable again, and the mean ALT values after breakthrough often remain below the pretreatment values. However, McCaughan et al[31] reported that patients infected with procure mutant strains of HBV develop drug resistance or liver failure after 11 mo of rescue therapy with LAM. Mutimer et al[32] also demonstrated that LAM-resistant phenotype can cause severe graft damage. Hence, long-term, randomized, blinded, and controlled clinical trials are needed to further observe the clinical outcomes following LAM resistance.
In summary, LAM therapy results in not only a loss of viral replication markers in serum and an improved hepatic biochemical profile, but also improvement or stabilization in liver histology. However, LAM resistance and its possible accompanying clinical deterioration have limited its long-term use in treatment of recurrent HBV infection after LT.
Adefovir dipivoxil
Adefovir dipivoxil (ADV), a nucleotide analog that selectively inhibits viral polymerases and reverse transcriptase, is effective against both negative and positive HBeAg[33-36]. Furthermore, it has been shown that ADV has an excellent activity against wild-type as well as LAM-resistant HBV strains[37-41], suggesting that ADV contributes to the development of LAM-resistant mutants and de novo LAM-resistant HBV infection after LT. Schiff et al[42] in a landmark multicenter study showed that PCR DNA levels are decreased with a 1-year survival rate of 93%, undetectable serum HBV DNA, normal serum ALT, albumin, bilirubin and prothrombin time in 34%, 49%, 76%, 75% and 20% of patients, respectively, after 24 and 48 wk of treatment with ADV. Four years later, Schiff et al[43] further showed that the serum HBV DNA levels are undetectable in 40%, 65% and 78% of patients with recurrent HBV infection and LAM resistance 48, 96 and 114 wk after ADV treatment (10 mg, once a day), respectively, as well as loss or seroconversion of HBeAg in 31% and 11% of patients by week 48, and in 58% and 34% of patients by week 96. Liver function and other parameters after treatment with ADV are shown in Figure 1. Other studies[44-48] are summarized in Table 2. Toniutto et al[38] reported that ADV plus LAM can achieve favorable outcomes of HBsAg seroconversion and undetectable HBV DNA in patients with de novo graft HBV infection and LAM resistance.
Figure 1.
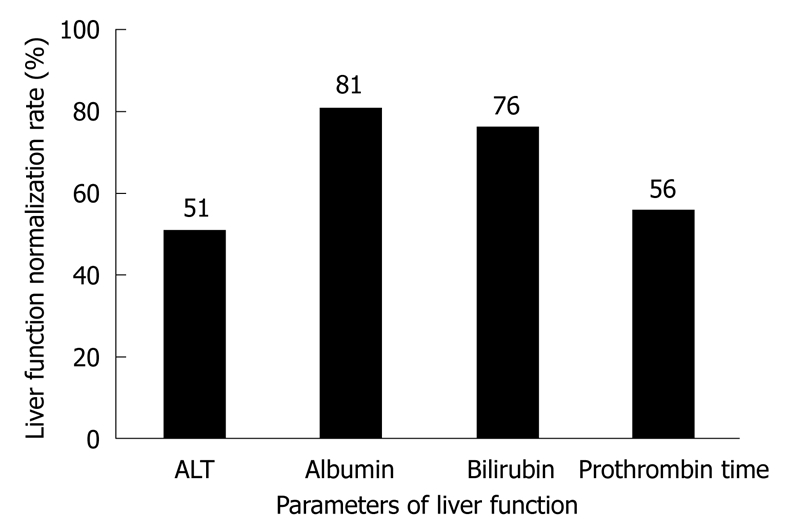
Alanine transaminase (ALT), albumin and bilirubin levels and prothrombin time 48 wk after adefovir dipivoxil treatment[43].
Table 2.
Use of ADV in treatment of recurrent graft infection after LT
| Author (Ref.) | n |
Pre-treatment |
Treatment duration (mo), mean (range) | ADV dosage (mg/d) | ConcurrentLAM use |
Post-treatment |
||||
| HBV DNA+ (%) | HBeAg+ (%) | HBV DNA negative following treatment (%) | HBsAg seroconversion (%) | ALT normalization (%) | Development of ADV mutants | |||||
| Akyildiz et al[44] | 11 | 81.8 | 9.1 | 18 (6-48) | 10 | Yes | 77.8 | 11.1 | 81.8 | None |
| Limquiaco et al[45] | 7 | 100 | 71.4 | 35 (22-48) | 10 | Yes | 28.6 | 20 | 86 | None |
| Bárcena et al[46] | 42 | 100 | 71.4 | 21.5 (12-31) | 10 | No | 64 | 20 | 70.5 | None |
| Herreros de Tejada Echanojáuregui et al[47] | 7 | 100 | 71.4 | 11 | 10 | Yes1 | 42.9 | 20 | NA | None |
| Neff et al[48] | 9 | 100 | 77.8 | 30 (6-48) | 10 | No | 0 | 57.1 | NA | None |
LAM was changed to ADV in 5 patients and ADV was added to LAM in the other 2 patients. ADV: Adefovir dipivoxil.
Mildly elevated serum creatinine level may occur after treatment with ADV, especially with calcineurin inhibitors, but only a small number of patients require dose adjustment, and even discontinuance. However, renal function should be regularly monitored, with dose adjustments based on renal function, as necessary.
In short, ADV is a safe and effective treatment option for recurrent HBV infection, especially as a salvage treatment for recurrent HBV infection due to LAM resistance.
Entecavir
Entecavir (ETV), a very potent anti-HBV selective guanosine analogue, approved by the US FDA in 2005, can be used in treatment of chronic HBV infection. Unfortunately, few reports are available on ETV in treatment of recurrent HBV infection. Most data concerning its efficacy and safety are obtained from patients with no LT. It was reported that 0.5 mg of ETV or 100 mg of LAM, once daily for 48 wk, can improve the histology in 72% and 62% of patients, respectively, with serum HBV DNA undetectable in 67% and 36% of patients, respectively, and normal ALT in 68% and 60% of patients, respectively, and no evidence of viral resistance to ETV[49]. It has been shown that 0.5 mg of ETV or 100 mg of LAM, once daily for 48 wk, can also achieve favorable outcomes in patients with nucleoside-naive HBeAg-negative chronic hepatitis B[50], suggesting that the histologic, virologic and biochemical improvement rates are significantly higher after ETV treatment than after LAM treatment, in patients with nucleoside-naive HBeAg-positive or -negative chronic hepatitis B, with no evidence of viral resistance to ETV, which is consistent with the findings in other studies[51-53]. The mean decrease in serum HBV DNA after 2, 4, 8, 12, 24 and 48 wk of ETV and ADV treatment in patients is shown in Figure 2. A recent study demonstrated that ETV therapy is safe and efficient for recipients with ADV-resistant HBV infection[54].
Figure 2.
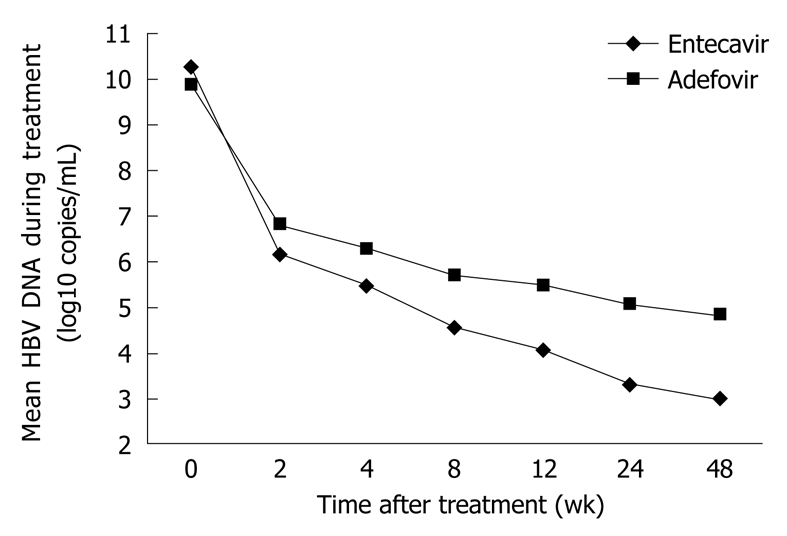
Mean serum hepatitis B virus (HBV) DNA level in patients after entecavir (ETV) and adefovir (ADV) treatment[53].
ETV resistance is associated with the LAM-resistance substitutions M204V/I and L180M in combination with an additional substitution at residues T184, S202 or M250 in the reverse-transcriptase region of HBV polymerase[55]. In other words, ETV is associated with a high genetic barrier to resistance requiring multiple mutations for resistance development. In nucleoside-naïve patients, the probability of developing resistance to ETV remains consistently low (< 1.2%) even after 96 wk of treatment[56]. By contrast, ETV gives rise to ETV-resistant mutants in patients with LAM resistance. The rate of ETV resistance in LAM-resistant patients 4 years after treatment of may reach 35%[57], which is due to a particular selection mode of ETV strains that follows a 2-step process, with the selection of primary resistance mutations at position M204V/I (which are also resistant to LAM) followed by the addition of secondary resistance mutations on the same viral genomes[58]. Once these secondary substitutions occur, high-level resistance to ETV occurs. Therefore, the high probability of resistance to long-term ETV in LAM resistant patients with no LT suggests that ETV is not a good choice for LAM-resistant patients after LT, although ETV has been tried in some LAM resistant patients after LT[54]. However, ETV can be used in non-LAM resistant patients, due to its great potency, high genetic barrier and absence of nephrotoxicity. More data are urgently needed to confirm the safety and efficacy of ETV after LT.
Tenofovir disoproxil fumarate
Tenofovir disoproxil fumarate (TDF), a nucleotide analogue, inhibits viral polymerases by direct binding to DNA or by terminating the DNA chain due to the absence of a requisite 3’ hydroxyl on the tenofovir molecule[59]. In 2001, TDF was licensed for HIV therapy as a potent inhibitor of HIV replication. Subsequently, it has been proved to be a potent and selective anti-HBV agent in vitro[60]. Several clinical studies have currently confirmed the efficacy of TDF in suppressing HBV replication. Marcellin et al[61] showed that the antiviral efficacy of TDF is higher than that of ADV with no resistance mutation in patients with HBeAg-negative or HBeAg-positive chronic HBV infection. Detail data regarding viral suppression, liver function improvement, and serologic response are shown in Figure 3. It has been shown that TDF has an excellent antiviral activity against both wild-type and LAM-resistant HBV both in vitro and in vivo[62,63]. Furthermore, TDF shows a stronger antiviral effect than ADV on LAM-resistant HBV[64,65]. Hann et al[66] reported that the mean level of HBV DNA is 1.5 ± 1.0 log10 copies/mL and 4.3 ± 2.2 log10 copies/mL, respectively, in patients with LAM resistance after treatment with TDF and ADV. A recent study demonstrated that TDF plus LAM can safely and markedly suppress HBV replication in patients with or without resistance to ADV[67].
Figure 3.
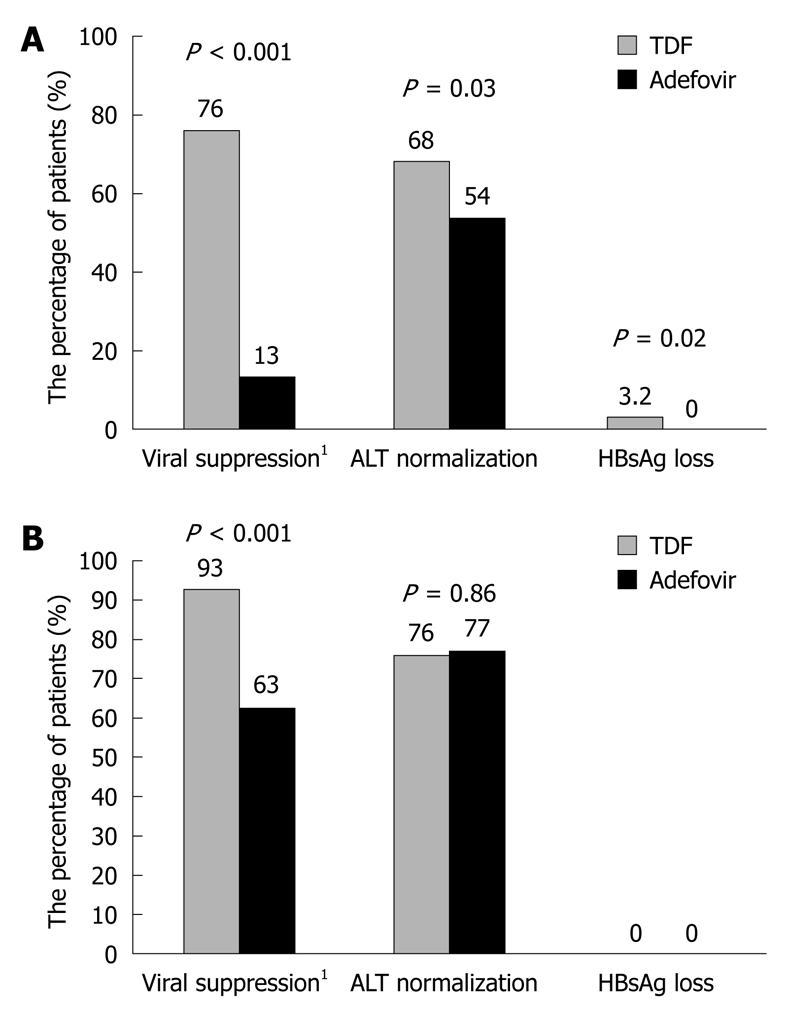
Viral suppression, ALT normalization and hepatitis B surface antigen (HBsAg) loss in hepatitis B e antigen (HBeAg)-positive (A) and HBeAg-negative (B) patients after tenofovir disoproxil fumarate (TDF) and adefovir treatment[61]. 1HBV DNA level of less than 400 copies/mL.
The daily TDF dose (300 mg) has been approved as a standard dose for controlling HBV infection. To reduce the cost of therapy, a low TDF dose (75 mg) can be used to control HBV viremia in patients with chronic HBeAg-negative hepatitis B, which can more effectively inhibit HBV replication than ADV at the standard dose[68]. However, further studies are needed to determine its efficacy and safety in a larger number of HBeAg-positive subjects.
Only 2 reports are available on TDF-related mutations conferring resistance to TDF[69,70]. Transfection experiments showed that rtA194T mutation alone can result in a 7.6-fold decrease in susceptibility to TDF, but rtA194T mutation in combination with ADV-resistant mutations can lead to a more than 10-fold decrease in susceptibility to TDF[70]. However, TDF susceptibility to rtA194T mutation is not consistent with its clinical significance. Since 2005, ADV-resistance mutations (rtN236T, rtA194T, rtA181V) have not caused a significant change in TDF susceptibility[71,72]. Only one study is available on the application of TDF in treatment of recurrent HBV infection after LT[73]. Although TDF can significantly decrease LAM-resistant HBV variant replication after LT, further studies are needed to determine its efficacy and safety profile with a long follow-up time and a large cohort of patients.
RETRANSPLANTATION
With the advent of new antiviral drugs such as ADV, ETV and TDF, satisfactory outcomes can be achieved in patients with recurrent HBV infection by suppressing viral replication. However, some patients with irreversible graft dysfunction secondary to recurrent HBV infection still need a second LT to replace the primary graft[74,75]. To date, second LT accounts for 5%-22% of all LTs in adults[76]. Samuel et al[77] reported that recurrent graft HBV infection is the direct cause of death in patients with hepatitis B after a second LT. Crippin et al[78] showed that the mortality rate of patients with recurrent HBV infection is 95% after a second LT, which is consistent with the findings in other studies[12,79-81].
Ishitani et al[82] showed that long-term HBIG therapy can continuously maintain a serum anti-HBs level > 500 IU/L and prevent recurrent HBV infection in 86% of patients after a second LT. Roche et al[80] reported that prophylactic intravenous ganciclovir can maintain an anti-HBs titre greater than 500 IU/L, with a satisfactory prognosis of all patients after a second LT. Lo et al[83] demonstrated that combined injection of ADV, LAM and HBIG can maintain an anti-HBs titre greater than 100 IU/L in patients with LAM-resistant HBV mutants after a second LT.
In summary, recurrent HBV infection after a primary LT should no longer be considered an absolute contraindication for a second LT. Combined HBIG and antiviral agents may play an important role in improving the prognosis of patients with recurrent HBV infection after a second LT. Further randomized study is needed to definitely confirm it.
CONCLUSION
The emergence of new oral medications, especially nucleoside or nucleotide analogues such as ADV, ETV and TDF, further increases our ability to treat recurrent HBV infection after LT. By recognizing the efficacies and features of such drugs, various therapeutic strategies, according to different clinical settings, have been recommended to control the recurrent graft infection after LT (Table 3). For the de novo or wild-type HBV infection after LT, long-term use of LAM is limited by the high risk of developing mutations in YMDD motif. Hence, ADV is the first choice in this setting, if needing long-term administration. For the LAM-resistant HBV infection after LT, ADV, administered in combination with LAM or as a monotherapy, appears to be safe and effective in this setting. Theoretically, “add therapy” may minimize the risk of resistance, but needs long-term, randomized, blinded, controlled clinical trials to further confirm it. In addition, ETV is not a good choice for LAM-resistant patients after LT because of its high probability of resistance in patients with LAM resistance without LT. ETV therapy for ADV-resistant HBV infection after LT is safe and efficient in this setting. Additionally, combined TDF and LAM therapy may be an alternative approach based on its favorable therapeutic effects in patients with out LT. However, no or few data are available on the efficacy of ETV and TDF in patients after LT. Further clinical trials are needed to evaluate their efficacy and safety in this special setting. Second LT is the only rescue procedure for patients with irreversible graft dysfunction secondary to recurrent HBV infection. Since combined HBIG and antiviral agents may play an important role in improving the prognosis of patients with recurrent HBV infection after LT, recurrent HBV infection after a primary LT should no longer be considered an absolute contraindication for a second LT.
Table 3.
Summary of recommended therapeutic strategies for recurrent HBV infection after LT
| Clinical setting | Recommended first line therapies | Recommended second line therapies |
| De novo HBV infection, wild-type HBV infection | LAM for short-term therapy; ADV for long-term therapy | ETV, TDF |
| LAM resistance | ADV, ADV + LAM | TDF |
| ADV resistance | ETV | TDF + LAM |
| Irreversible graft dysfunction | SLT |
ETV: Entecavir; TDF: Tenofovir disoproxil fumarate; SLT: Second liver transplantation.
Footnotes
Peer reviewer: Dr. Thamara Perera, Senior Transplant Fellow, The Liver Transplant Unit, Queen Elizabeth Hospital, Edgbaston, Birmingham, B15 2TH, United Kingdom
S- Editor Wang YR L- Editor Wang XL E- Editor Zheng XM
References
- 1.Seaberg EC, Belle SH, Beringer KC, Schivins JL, Detre KM. Liver transplantation in the United States from 1987-1998: updated results from the Pitt-UNOS Liver Transplant Registry. Clin Transpl. 1998:17–37. [PubMed] [Google Scholar]
- 2.Lo CM, Fan ST, Liu CL, Lai CL, Wong J. Prophylaxis and treatment of recurrent hepatitis B after liver transplantation. Transplantation. 2003;75:S41–S44. doi: 10.1097/01.TP.0000047027.68167.07. [DOI] [PubMed] [Google Scholar]
- 3.Shouval D, Samuel D. Hepatitis B immune globulin to prevent hepatitis B virus graft reinfection following liver transplantation: a concise review. Hepatology. 2000;32:1189–1195. doi: 10.1053/jhep.2000.19789. [DOI] [PubMed] [Google Scholar]
- 4.Rosenau J, Bahr MJ, Tillmann HL, Trautwein C, Klempnauer J, Manns MP, Böker KHW. Lamivudine and low-dose hepatitis B immune globulin for prophylaxis of hepatitis B reinfection after liver transplantation possible role of mutations in the YMDD motif prior to transplantation as a risk factor for reinfection. J Hepatol. 2001;34:895–902. doi: 10.1016/s0168-8278(01)00089-7. [DOI] [PubMed] [Google Scholar]
- 5.McGory RW, Ishitani MB, Oliveira WM, Stevenson WC, McCullough CS, Dickson RC, Caldwell SH, Pruett TL. Improved outcome of orthotopic liver transplantation for chronic hepatitis B cirrhosis with aggressive passive immunization. Transplantation. 1996;61:1358–1364. doi: 10.1097/00007890-199605150-00013. [DOI] [PubMed] [Google Scholar]
- 6.Terrault NA, Zhou S, Combs C, Hahn JA, Lake JR, Roberts JP, Ascher NL, Wright TL. Prophylaxis in liver transplant recipients using a fixed dosing schedule of hepatitis B immunoglobulin. Hepatology. 1996;24:1327–1333. doi: 10.1002/hep.510240601. [DOI] [PubMed] [Google Scholar]
- 7.Marzano A, Salizzoni M, Debernardi-Venon W, Smedile A, Franchello A, Ciancio A, Gentilcore E, Piantino P, Barbui AM, David E, et al. Prevention of hepatitis B virus recurrence after liver transplantation in cirrhotic patients treated with lamivudine and passive immunoprophylaxis. J Hepatol. 2001;34:903–910. doi: 10.1016/s0168-8278(01)00080-0. [DOI] [PubMed] [Google Scholar]
- 8.Markowitz JS, Martin P, Conrad AJ, Markmann JF, Seu P, Yersiz H, Goss JA, Schmidt P, Pakrasi A, Artinian L, et al. Prophylaxis against hepatitis B recurrence following liver transplantation using combination lamivudine and hepatitis B immune globulin. Hepatology. 1998;28:585–589. doi: 10.1002/hep.510280241. [DOI] [PubMed] [Google Scholar]
- 9.Han SH, Ofman J, Holt C, King K, Kunder G, Chen P, Dawson S, Goldstein L, Yersiz H, Farmer DG, et al. An efficacy and cost-effectiveness analysis of combination hepatitis B immune globulin and lamivudine to prevent recurrent hepatitis B after orthotopic liver transplantation compared with hepatitis B immune globulin monotherapy. Liver Transpl. 2000;6:741–748. doi: 10.1053/jlts.2000.18702. [DOI] [PubMed] [Google Scholar]
- 10.Steinmüller T, Seehofer D, Rayes N, Müller AR, Settmacher U, Jonas S, Neuhaus R, Berg T, Hopf U, Neuhaus P. Increasing applicability of liver transplantation for patients with hepatitis B-related liver disease. Hepatology. 2002;35:1528–1535. doi: 10.1053/jhep.2002.33681. [DOI] [PubMed] [Google Scholar]
- 11.Rosenau J, Tillmann HL, Bahr MJ, Trautwein C, Boeker KH, Nashan B, Klempnauer J, Manns MP. Successful hepatitis B reinfection prophylaxis with lamivudine and hepatitis B immune globulin in patients with positive HBV-DNA at time of liver transplantation. Transplant Proc. 2001;33:3637–3638. doi: 10.1016/s0041-1345(01)02564-7. [DOI] [PubMed] [Google Scholar]
- 12.Todo S, Demetris AJ, Van Thiel D, Teperman L, Fung JJ, Starzl TE. Orthotopic liver transplantation for patients with hepatitis B virus-related liver disease. Hepatology. 1991;13:619–626. [PMC free article] [PubMed] [Google Scholar]
- 13.Roche B, Samuel D, Gigou M, Feray C, Virot V, Schmets L, David MF, Arulnaden JL, Bismuth A, Reynes M, et al. De novo and apparent de novo hepatitis B virus infection after liver transplantation. J Hepatol. 1997;26:517–526. doi: 10.1016/s0168-8278(97)80416-3. [DOI] [PubMed] [Google Scholar]
- 14.Fabia R, Levy MF, Crippin J, Tillery W, Netto GJ, Aguanno J, Dysert P, Goldstein RM, Husberg BS, Gonwa TA, et al. De novo hepatitis B infection after liver transplantation: source of disease, incidence, and impact. Liver Transpl Surg. 1998;4:119–127. doi: 10.1002/lt.500040210. [DOI] [PubMed] [Google Scholar]
- 15.Chazouillères O, Mamish D, Kim M, Carey K, Ferrell L, Roberts JP, Ascher NL, Wright TL. "Occult" hepatitis B virus as source of infection in liver transplant recipients. Lancet. 1994;343:142–146. doi: 10.1016/s0140-6736(94)90934-2. [DOI] [PubMed] [Google Scholar]
- 16.Terrault NA, Holland CC, Ferrell L, Hahn JA, Lake JR, Roberts JP, Ascher NL, Wright TL. Interferon alfa for recurrent hepatitis B infection after liver transplantation. Liver Transpl Surg. 1996;2:132–138. doi: 10.1002/lt.500020209. [DOI] [PubMed] [Google Scholar]
- 17.Wright HI, Gavaler JS, Van Theil DH. Preliminary experience with alpha-2b-interferon therapy of viral hepatitis in liver allograft recipients. Transplantation. 1992;53:121–124. doi: 10.1097/00007890-199201000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Perrillo R, Rakela J, Dienstag J, Levy G, Martin P, Wright T, Caldwell S, Schiff E, Gish R, Villeneuve JP, et al. Multicenter study of lamivudine therapy for hepatitis B after liver transplantation. Lamivudine Transplant Group. Hepatology. 1999;29:1581–1586. doi: 10.1002/hep.510290507. [DOI] [PubMed] [Google Scholar]
- 19.Umeda M, Marusawa H, Ueda M, Takada Y, Egawa H, Uemoto S, Chiba T. Beneficial effects of short-term lamivudine treatment for de novo hepatitis B virus reactivation after liver transplantation. Am J Transplant. 2006;6:2680–2685. doi: 10.1111/j.1600-6143.2006.01542.x. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Ari Z, Mor E, Shapira Z, Tur-Kaspa R. Long-term experience with lamivudine therapy for hepatitis B virus infection after liver transplantation. Liver Transpl. 2001;7:113–117. doi: 10.1053/jlts.2001.21308. [DOI] [PubMed] [Google Scholar]
- 21.Castells L, Vargas V, Rodríguez F, Allende H, Buti M, Sánchez-Avila JF, Jardí R, Margarit C, Pumarola T, Esteban R, et al. Clinical impact and efficacy of lamivudine therapy in de novo hepatitis B infection after liver transplantation. Liver Transpl. 2002;8:892–900. doi: 10.1053/jlts.2002.35555. [DOI] [PubMed] [Google Scholar]
- 22.Fontana RJ, Hann HW, Wright T, Everson G, Baker A, Schiff ER, Riely C, Anschuetz G, Riker-Hopkins M, Brown N. A multicenter study of lamivudine treatment in 33 patients with hepatitis B after liver transplantation. Liver Transpl. 2001;7:504–510. doi: 10.1053/jlts.2001.24896. [DOI] [PubMed] [Google Scholar]
- 23.Andreone P, Caraceni P, Grazi GL, Belli L, Milandri GL, Ercolani G, Jovine E, D'Errico A, Dal Monte PR, Ideo G, et al. Lamivudine treatment for acute hepatitis B after liver transplantation. J Hepatol. 1998;29:985–989. doi: 10.1016/s0168-8278(98)80127-x. [DOI] [PubMed] [Google Scholar]
- 24.Nery JR, Weppler D, Rodriguez M, Ruiz P, Schiff ER, Tzakis AG. Efficacy of lamivudine in controlling hepatitis B virus recurrence after liver transplantation. Transplantation. 1998;65:1615–1621. doi: 10.1097/00007890-199806270-00013. [DOI] [PubMed] [Google Scholar]
- 25.Fischer L, Sterneck M, Zöllner B, Rogiers X. Lamivudine improves the prognosis of patients with hepatitis B after liver transplantation. Transplant Proc. 2000;32:2128–2130. doi: 10.1016/s0041-1345(00)01600-6. [DOI] [PubMed] [Google Scholar]
- 26.Rayes N, Seehofer D, Hopf U, Neuhaus R, Naumann U, Bechstein WO, Neuhaus P. Comparison of famciclovir and lamivudine in the long-term treatment of hepatitis B infection after liver transplantation. Transplantation. 2001;71:96–101. doi: 10.1097/00007890-200101150-00016. [DOI] [PubMed] [Google Scholar]
- 27.Malkan G, Cattral MS, Humar A, Al Asghar H, Greig PD, Hemming AW, Levy GA, Lilly LB. Lamivudine for hepatitis B in liver transplantation: a single-center experience. Transplantation. 2000;69:1403–1407. doi: 10.1097/00007890-200004150-00033. [DOI] [PubMed] [Google Scholar]
- 28.Lai CL, Dienstag J, Schiff E, Leung NW, Atkins M, Hunt C, Brown N, Woessner M, Boehme R, Condreay L. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36:687–696. doi: 10.1086/368083. [DOI] [PubMed] [Google Scholar]
- 29.Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000--summary of a workshop. Gastroenterology. 2001;120:1828–1853. doi: 10.1053/gast.2001.24839. [DOI] [PubMed] [Google Scholar]
- 30.Seehofer D, Rayes N, Berg T, Neuhaus R, Müller AR, Hopf U, Bechstein WO, Neuhaus P. Lamivudine as first- and second-line treatment of hepatitis B infection after liver transplantation. Transpl Int. 2000;13:290–296. doi: 10.1007/s001470050704. [DOI] [PubMed] [Google Scholar]
- 31.McCaughan GW, Spencer J, Koorey D, Bowden S, Bartholomeusz A, Littlejohn M, Verran D, Chui AK, Sheil AG, Jones RM, et al. Lamivudine therapy in patients undergoing liver transplantation for hepatitis B virus precore mutant-associated infection: high resistance rates in treatment of recurrence but universal prevention if used as prophylaxis with very low dose hepatitis B immune globulin. Liver Transpl Surg. 1999;5:512–519. doi: 10.1002/lt.500050601. [DOI] [PubMed] [Google Scholar]
- 32.Mutimer D, Pillay D, Shields P, Cane P, Ratcliffe D, Martin B, Buchan S, Boxall L, O'Donnell K, Shaw J, et al. Outcome of lamivudine resistant hepatitis B virus infection in the liver transplant recipient. Gut. 2000;46:107–113. doi: 10.1136/gut.46.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Wulfsohn MS, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800–807. doi: 10.1056/NEJMoa021812. [DOI] [PubMed] [Google Scholar]
- 34.Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808–816. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- 35.Xiong X, Flores C, Yang H, Toole JJ, Gibbs CS. Mutations in hepatitis B DNA polymerase associated with resistance to lamivudine do not confer resistance to adefovir in vitro. Hepatology. 1998;28:1669–1673. doi: 10.1002/hep.510280629. [DOI] [PubMed] [Google Scholar]
- 36.Perrillo R, Schiff E, Yoshida E, Statler A, Hirsch K, Wright T, Gutfreund K, Lamy P, Murray A. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology. 2000;32:129–134. doi: 10.1053/jhep.2000.8626. [DOI] [PubMed] [Google Scholar]
- 37.Zeng M, Mao Y, Yao G, Wang H, Hou J, Wang Y, Ji BN, Chang CN, Barker KF. A double-blind randomized trial of adefovir dipivoxil in Chinese subjects with HBeAg-positive chronic hepatitis B. Hepatology. 2006;44:108–116. doi: 10.1002/hep.21225. [DOI] [PubMed] [Google Scholar]
- 38.Toniutto P, Fumo E, Caldato M, Apollonio L, Perin A, Pirisi M. Favourable outcome of adefovir-dipivoxil treatment in acute de novo hepatitis B after liver transplantation. Transplantation. 2004;77:472–473. doi: 10.1097/01.TP.0000113466.53834.8A. [DOI] [PubMed] [Google Scholar]
- 39.Peters MG, Hann Hw H, Martin P, Heathcote EJ, Buggisch P, Rubin R, Bourliere M, Kowdley K, Trepo C, Gray Df D, et al. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2004;126:91–101. doi: 10.1053/j.gastro.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 40.Perrillo R, Hann HW, Mutimer D, Willems B, Leung N, Lee WM, Moorat A, Gardner S, Woessner M, Bourne E, et al. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology. 2004;126:81–90. doi: 10.1053/j.gastro.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 41.Benhamou Y, Bochet M, Thibault V, Calvez V, Fievet MH, Vig P, Gibbs CS, Brosgart C, Fry J, Namini H, et al. Safety and efficacy of adefovir dipivoxil in patients co-infected with HIV-1 and lamivudine-resistant hepatitis B virus: an open-label pilot study. Lancet. 2001;358:718–723. doi: 10.1016/s0140-6736(01)05840-8. [DOI] [PubMed] [Google Scholar]
- 42.Schiff ER, Lai CL, Hadziyannis S, Neuhaus P, Terrault N, Colombo M, Tillmann HL, Samuel D, Zeuzem S, Lilly L, et al. Adefovir dipivoxil therapy for lamivudine-resistant hepatitis B in pre- and post-liver transplantation patients. Hepatology. 2003;38:1419–1427. doi: 10.1016/j.hep.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 43.Schiff E, Lai CL, Hadziyannis S, Neuhaus P, Terrault N, Colombo M, Tillmann H, Samuel D, Zeuzem S, Villeneuve JP, et al. Adefovir dipivoxil for wait-listed and post-liver transplantation patients with lamivudine-resistant hepatitis B: final long-term results. Liver Transpl. 2007;13:349–360. doi: 10.1002/lt.20981. [DOI] [PubMed] [Google Scholar]
- 44.Akyildiz M, Karasu Z, Zeytunlu M, Aydin U, Ozacar T, Kilic M. Adefovir dipivoxil therapy in liver transplant recipients for recurrence of hepatitis B virus infection despite lamivudine plus hepatitis B immunoglobulin prophylaxis. J Gastroenterol Hepatol. 2007;22:2130–2134. doi: 10.1111/j.1440-1746.2006.04609.x. [DOI] [PubMed] [Google Scholar]
- 45.Limquiaco JL, Wong J, Wong VW, Wong GL, Tse CH, Chan HY, Kwan KY, Lai PB, Chan HL. Lamivudine monoprophylaxis and adefovir salvage for liver transplantation in chronic hepatitis B: a seven-year follow-up study. J Med Virol. 2009;81:224–229. doi: 10.1002/jmv.21369. [DOI] [PubMed] [Google Scholar]
- 46.Bárcena R, Del Campo S, Moraleda G, Casanovas T, Prieto M, Buti M, Moreno JM, Cuervas V, Fraga E, De la Mata M, et al. Study on the efficacy and safety of adefovir dipivoxil treatment in post-liver transplant patients with hepatitis B virus infection and lamivudine-resistant hepatitis B virus. Transplant Proc. 2005;37:3960–3962. doi: 10.1016/j.transproceed.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 47.Herreros de Tejada Echanojáuregui A, Moreno Planas JM, Rubio González E, Portero Azorin F, López Monclús J, Revilla Negro J, Lucena de la Poza JL, Sánchez Turrión V, Barrios Peinado C, Cuervas-Mons Martínez V. Adefovir dipivoxil therapy in liver transplant recipients with lamivudine-resistant hepatitis B virus. Transplant Proc. 2005;37:1507–1508. doi: 10.1016/j.transproceed.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 48.Neff GW, O'brien CB, Nery J, Shire N, Montalbano M, Ruiz P, Nery C, Safdar K, De Medina M, Tzakis AG, et al. Outcomes in liver transplant recipients with hepatitis B virus: resistance and recurrence patterns from a large transplant center over the last decade. Liver Transpl. 2004;10:1372–1378. doi: 10.1002/lt.20277. [DOI] [PubMed] [Google Scholar]
- 49.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 50.Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, DeHertogh D, Wilber R, Zink RC, Cross A, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2006;354:1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 51.Schiff E, Simsek H, Lee WM, Chao YC, Sette H Jr, Janssen HL, Han SH, Goodman Z, Yang J, Brett-Smith H, et al. Efficacy and safety of entecavir in patients with chronic hepatitis B and advanced hepatic fibrosis or cirrhosis. Am J Gastroenterol. 2008;103:2776–2783. doi: 10.1111/j.1572-0241.2008.02086.x. [DOI] [PubMed] [Google Scholar]
- 52.Gish RG, Lok AS, Chang TT, de Man RA, Gadano A, Sollano J, Han KH, Chao YC, Lee SD, Harris M, et al. Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2007;133:1437–1444. doi: 10.1053/j.gastro.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 53.Leung N, Peng CY, Hann HW, Sollano J, Lao-Tan J, Hsu CW, Lesmana L, Yuen MF, Jeffers L, Sherman M, et al. Early hepatitis B virus DNA reduction in hepatitis B e antigen-positive patients with chronic hepatitis B: A randomized international study of entecavir versus adefovir. Hepatology. 2009;49:72–79. doi: 10.1002/hep.22658. [DOI] [PubMed] [Google Scholar]
- 54.Kamar N, Milioto O, Alric L, El Kahwaji L, Cointault O, Lavayssière L, Sauné K, Izopet J, Rostaing L. Entecavir therapy for adefovir-resistant hepatitis B virus infection in kidney and liver allograft recipients. Transplantation. 2008;86:611–614. doi: 10.1097/TP.0b013e3181806c8c. [DOI] [PubMed] [Google Scholar]
- 55.Baldick CJ, Tenney DJ, Mazzucco CE, Eggers BJ, Rose RE, Pokornowski KA, Yu CF, Colonno RJ. Comprehensive evaluation of hepatitis B virus reverse transcriptase substitutions associated with entecavir resistance. Hepatology. 2008;47:1473–1482. doi: 10.1002/hep.22211. [DOI] [PubMed] [Google Scholar]
- 56.Colonno RJ, Rose R, Baldick CJ, Levine S, Pokornowski K, Yu CF, Walsh A, Fang J, Hsu M, Mazzucco C, et al. Entecavir resistance is rare in nucleoside naïve patients with hepatitis B. Hepatology. 2006;44:1656–1665. doi: 10.1002/hep.21422. [DOI] [PubMed] [Google Scholar]
- 57.Sherman M, Yurdaydin C, Sollano J, Silva M, Liaw YF, Cianciara J, Boron-Kaczmarska A, Martin P, Goodman Z, Colonno R, et al. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology. 2006;130:2039–2049. doi: 10.1053/j.gastro.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 58.Villet S, Ollivet A, Pichoud C, Barraud L, Villeneuve JP, Trépo C, Zoulim F. Stepwise process for the development of entecavir resistance in a chronic hepatitis B virus infected patient. J Hepatol. 2007;46:531–538. doi: 10.1016/j.jhep.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 59.De Clercq E. Clinical potential of the acyclic nucleoside phosphonates cidofovir, adefovir, and tenofovir in treatment of DNA virus and retrovirus infections. Clin Microbiol Rev. 2003;16:569–596. doi: 10.1128/CMR.16.4.569-596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ying C, De Clercq E, Nicholson W, Furman P, Neyts J. Inhibition of the replication of the DNA polymerase M550V mutation variant of human hepatitis B virus by adefovir, tenofovir, L-FMAU, DAPD, penciclovir and lobucavir. J Viral Hepat. 2000;7:161–165. doi: 10.1046/j.1365-2893.2000.00210.x. [DOI] [PubMed] [Google Scholar]
- 61.Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–2455. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 62.Lada O, Benhamou Y, Cahour A, Katlama C, Poynard T, Thibault V. In vitro susceptibility of lamivudine-resistant hepatitis B virus to adefovir and tenofovir. Antivir Ther. 2004;9:353–363. [PubMed] [Google Scholar]
- 63.Kuo A, Dienstag JL, Chung RT. Tenofovir disoproxil fumarate for the treatment of lamivudine-resistant hepatitis B. Clin Gastroenterol Hepatol. 2004;2:266–272. doi: 10.1016/s1542-3565(04)00017-5. [DOI] [PubMed] [Google Scholar]
- 64.van Bömmel F, Zöllner B, Sarrazin C, Spengler U, Hüppe D, Möller B, Feucht HH, Wiedenmann B, Berg T. Tenofovir for patients with lamivudine-resistant hepatitis B virus (HBV) infection and high HBV DNA level during adefovir therapy. Hepatology. 2006;44:318–325. doi: 10.1002/hep.21253. [DOI] [PubMed] [Google Scholar]
- 65.van Bömmel F, Wünsche T, Mauss S, Reinke P, Bergk A, Schürmann D, Wiedenmann B, Berg T. Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infection. Hepatology. 2004;40:1421–1425. doi: 10.1002/hep.20464. [DOI] [PubMed] [Google Scholar]
- 66.Hann HW, Chae HB, Dunn SR. Tenofovir (TDF) has stronger antiviral effect than adefovir (ADV) against lamivudine (LAM)-resistant hepatitis B virus (HBV) Hepatol Int. 2008;2:244–249. doi: 10.1007/s12072-008-9045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choe WH, Kwon SY, Kim BK, Ko SY, Yeon JE, Byun KS, Kim GH, Lee CH. Tenofovir plus lamivudine as rescue therapy for adefovir-resistant chronic hepatitis B in hepatitis B e antigen-positive patients with liver cirrhosis. Liver Int. 2008;28:814–820. doi: 10.1111/j.1478-3231.2008.01685.x. [DOI] [PubMed] [Google Scholar]
- 68.Del Poggio P, Zaccanelli M, Oggionni M, Colombo S, Jamoletti C, Puhalo V. Low-dose tenofovir is more potent than adefovir and is effective in controlling HBV viremia in chronic HBeAg-negative hepatitis B. World J Gastroenterol. 2007;13:4096–4099. doi: 10.3748/wjg.v13.i30.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brunelle MN, Jacquard AC, Pichoud C, Durantel D, Carrouée-Durantel S, Villeneuve JP, Trépo C, Zoulim F. Susceptibility to antivirals of a human HBV strain with mutations conferring resistance to both lamivudine and adefovir. Hepatology. 2005;41:1391–1398. doi: 10.1002/hep.20723. [DOI] [PubMed] [Google Scholar]
- 70.Sheldon J, Camino N, Rodés B, Bartholomeusz A, Kuiper M, Tacke F, Núñez M, Mauss S, Lutz T, Klausen G, et al. Selection of hepatitis B virus polymerase mutations in HIV-coinfected patients treated with tenofovir. Antivir Ther. 2005;10:727–734. [PubMed] [Google Scholar]
- 71.Delaney WE 4th, Ray AS, Yang H, Qi X, Xiong S, Zhu Y, Miller MD. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob Agents Chemother. 2006;50:2471–2477. doi: 10.1128/AAC.00138-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qi X, Xiong S, Yang H, Miller M, Delaney WE 4th. In vitro susceptibility of adefovir-associated hepatitis B virus polymerase mutations to other antiviral agents. Antivir Ther. 2007;12:355–362. [PubMed] [Google Scholar]
- 73.Neff GW, Nery J, Lau DT, O'Brien CB, Duncan R, Shire NJ, Ruiz P, Nery C, Montalbano M, Muslu H, et al. Tenofovir therapy for lamivudine resistance following liver transplantation. Ann Pharmacother. 2004;38:1999–2004. doi: 10.1345/aph.1E280. [DOI] [PubMed] [Google Scholar]
- 74.Lerut J, Laterre PF, Roggen F, Mauel E, Gheerardyn R, Ciccarelli O, Donataccio M, de Ville de Goyet J, Reding R, Goffette P, et al. Adult hepatic retransplantation. UCL experience. Acta Gastroenterol Belg. 1999;62:261–266. [PubMed] [Google Scholar]
- 75.Chen GH, Fu BS, Cai CJ, Lu MQ, Yang Y, Yi SH, Xu C, Li H, Wang GS, Zhang T. A single-center experience of retransplantation for liver transplant recipients with a failing graft. Transplant Proc. 2008;40:1485–1487. doi: 10.1016/j.transproceed.2008.01.076. [DOI] [PubMed] [Google Scholar]
- 76.Pfitzmann R, Benscheidt B, Langrehr JM, Schumacher G, Neuhaus R, Neuhaus P. Trends and experiences in liver retransplantation over 15 years. Liver Transpl. 2007;13:248–257. doi: 10.1002/lt.20904. [DOI] [PubMed] [Google Scholar]
- 77.Samuel D, Muller R, Alexander G, Fassati L, Ducot B, Benhamou JP, Bismuth H. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med. 1993;329:1842–1847. doi: 10.1056/NEJM199312163292503. [DOI] [PubMed] [Google Scholar]
- 78.Crippin J, Foster B, Carlen S, Borcich A, Bodenheimer H Jr. Retransplantation in hepatitis B--a multicenter experience. Transplantation. 1994;57:823–826. doi: 10.1097/00007890-199403270-00009. [DOI] [PubMed] [Google Scholar]
- 79.König V, Hopf U, Neuhaus P, Bauditz J, Schmidt CA, Blumhardt G, Bechstein WO, Neuhaus R, Lobeck H. Long-term follow-up of hepatitis B virus-infected recipients after orthotopic liver transplantation. Transplantation. 1994;58:553–559. doi: 10.1097/00007890-199409150-00005. [DOI] [PubMed] [Google Scholar]
- 80.Roche B, Samuel D, Feray C, Majno P, Gigou M, Reynes M, Bismuth H. Retransplantation of the liver for recurrent hepatitis B virus infection: the Paul Brousse experience. Liver Transpl Surg. 1999;5:166–174. doi: 10.1002/lt.500050304. [DOI] [PubMed] [Google Scholar]
- 81.O'Grady JG, Smith HM, Davies SE, Daniels HM, Donaldson PT, Tan KC, Portmann B, Alexander GJ, Williams R. Hepatitis B virus reinfection after orthotopic liver transplantation. Serological and clinical implications. J Hepatol. 1992;14:104–111. doi: 10.1016/0168-8278(92)90138-f. [DOI] [PubMed] [Google Scholar]
- 82.Ishitani M, McGory R, Dickson R, Caldwell S, Bickston S, McCullough C, Pruett T, Terrault N, Roberts J, Ascher N, et al. Retransplantation of patients with severe posttransplant hepatitis B in the first allograft. Transplantation. 1997;64:410–414. doi: 10.1097/00007890-199708150-00006. [DOI] [PubMed] [Google Scholar]
- 83.Lo CM, Cheung ST, Ng IO, Liu CL, Lai CL, Fan ST. Fibrosing cholestatic hepatitis secondary to precore/core promoter hepatitis B variant with lamivudine resistance: successful retransplantation with combination adefovir dipivoxil and hepatitis B immunoglobulin. Liver Transpl. 2004;10:557–563. doi: 10.1002/lt.20133. [DOI] [PubMed] [Google Scholar]


