Abstract
AIM: To analyze α-methylacyl CoA racemase (AMACR) expression in relation to various dysplasia phenotypes and clinicopathological parameters of sporadic colorectal adenomas.
METHODS: Fifty-five cases of sporadic colorectal adenomas were categorized according to the Vienna classification for Gastrointestinal Neoplasia. These corresponded to a total of 98 different intra-lesion microscopic fields that were further independently assigned a histological grade based on the old nomenclature (mild, moderate, severe dyplasia and carcinoma in situ). AMACR expression was evaluated by immunohistochemistry and statistical analysis was performed to investigate possible associations with various clinicopathologic parameters of adenomas i.e. gender, age, localization, grade of dysplasia, size and configuration.
RESULTS: Patient age ranged from 41 to 84 years (mean 65 ± 13.2 years); 37 patients were males and 18 were females. Adenomas ranged in size between 0.5 and 30 cm (mean 2 ± 1.3 cm), including 18 tubular, 16 villous, 20 mixed or tubulovillous, and 1 giant sessile villous adenoma. AMACR expression was observed in 3 out of 16 (18.8%) of low-grade vs 23 out of 35 (62.8%) of high-grade lesions (P = 0.002). Most adenomas exhibiting high grade dysplasia with in situ carcinoma-like areas stained positive for AMACR (15/17 or 88.2%) as compared to adenomas with high grade dysplasia which contained severe dysplasia-like foci (6/15 or 40%), (P = 0.005). In AMACR positive adenomas featuring severe dysplasia-like or in situ carcinoma-like areas, AMACR staining was not necessarily observed in the in situ component. Positivity in intra-lesion of mild, moderate or severe dysplasia-like foci was more often encountered in adenomas harboring in situ, intramucosal or infiltrative carcinoma [21/33 (63.6%) vs 9/40 (22.5%), P < 0.001]. Strong AMACR expression was found in 11 out of 17 villous adenomas, but in only 1 out of 18 tubular lesions (P = 0.005). Larger lesions, i.e. > 1 cm stained more frequently for AMACR than smaller ones [27/45 (60%) vs 2/10 (20%), P = 0.02]. Overall, AMACR expression was associated with the grade of dysplasia, as well as with the size and configuration of adenomas, i.e. the consensus risk factors applied to colorectal adenoma patient surveillance.
CONCLUSION: It may be worthy to further evaluate the possible use of AMACR as an additional risk factor for the assessment of colorectal adenoma patients.
Keywords: α-methylacyl CoA racemase, Adenoma, Colorectal, Dysplasia, Carcinoma in situ, Immunohistochemistry
INTRODUCTION
α-methylacyl CoA racemase (AMACR) is a mitochondrial and peroxisomal enzyme implicated in the degradation of branched chained fatty acids and fatty acid derivatives. Conversion of several (2R)-methyl-branched-chain fatty acyl-coenzyme-A esters to their (S)-stereoisomeres, which is catalyzed by AMACR, is necessary for the completion of the β-oxidation pathway[1-3]. AMACR is expressed in a variety of tumors and precancerous conditions[4-6], with prostate cancer being the one most extensively investigated[7,8]. Various studies have analyzed AMACR expression in adenocarcinoma of the colon, where it is thought to be associated with tumor differentiation and localization[9-14]. Recently, much attention has been given to the expression of this biomarker in preneoplastic conditions arising in the gastrointestinal tract. It has been suggested that AMACR could be used as an adjunct in the characterization of dysplasia in Crohn’s disease, ulcerative colitis and Barrett’s esophagous[15-17]. In spite of all this promising data, there is still limited information regarding AMACR expression in relation to clinicopathologic parameters as well as to various dysplasia phenotypes in sporadic colorectal adenomas[5,6,10,11,17]. In the paper by Went et al[5], no significant differences were found between mild, moderate and severe dysplasia. On the contrary, Strater et al[17] found moderate to strong AMACR expression in 73% of high-grade cases but only in 37% of low-grade lesions. However, the results of these studies appear contradictory; in addition, given the degree of interobserver variability in regard to dysplasia classification, interpretation of these findings is hindered by the absence of criteria upon which dysplasia of adenomas was categorized[5,17]. Current guidelines on the classification of dysplasia in sporadic adenomas of the colorectum, namely those published by the American College of Gastroenterology and included in the Vienna classification of Gastrointestinal Neoplasia, strongly discourage the use of terms such as carcinoma in situ/intramucosal carcinoma due to, among other reasons, concerns for misinterpretation of their clinical significance that might lead to overtreatment[18-23]. Nonetheless, albeit prone to interobserver variability, specific cytoarchitectural features, including mild, moderate, severe and carcinoma in situ changes, do correspond to the biologic progression of dysplasia[20,23]. The goal of our study was to analyze the immunohistochemical expression of AMACR in various histologic patterns of dysplasia in sporadic colorectal adenomas in the frame of clinically oriented current classification schemes.
MATERIALS AND METHODS
Case selection
Fifty-five cases of colorectal adenomas including 4 adenomatous polyps having undergone malignant transformation with submucosal invasion were retrieved from the surgical pathology archives at “Georgios Gennimatas” General Community Hospital from 2005 to 2009. All 55 cases came from individual patients. Out of these, 51 were endoscopically or surgically removed adenomatous polyps and 4 were incidental findings in patients who had undergone colectomy for adenocarcinoma of the colorectum. Clinical data were reviewed to ensure that all cases were indeed sporadic adenomas. Hyperplastic polyps and serrated adenomas were not included in this study based on evidence of their distinguishing biology[24-26]. Hematoxylin-eosin (HE) stained slides were reviewed by two different pathologists in a blinded manner. For diagnostic purposes, each case was characterized by the highest grade of dysplasia present, according to the modified Vienna classification of Gastrointestinal Neoplasia using both the main categories (classical Western classification) as well as the subdivisions of group 4 (high-grade dysplasia)[19,21]. In addition, when more than one dysplastic pattern was histologically recognized on the same slide, these were given a separate grade of dysplasia corresponding to the presumably different biologic potentials and based upon previously reported pathologic criteria[20,27]. In this context terminology was based on the old nomenclature (mild, moderate, severe dysplasia, carcinoma in situ and intramucosal carcinoma). Interobserver disagreement on the interpretation of histology was present in 5 cases and a consensus diagnosis was reached at a multi-headed microscope. Size, architectural configuration and localization of lesions were also recorded. Normal appearing non-neoplastic intestinal mucosa was present in 29 cases.
Immunohistochemistry
One 4 micron formalin-fixed, paraffin-embedded tissue section including the highest grade of dysplasia was selected for each case. Prostate cancer sections were used as a positive control. Immunohistochemistry was carried out on a Bond Automated Immunostainer using the Bond Polymer Define Detection Kit (Cat. No. DS9713). Briefly, sections were deparaffinized, rehydrated and treated with 3% H2O2 to block endogenous peroxidase activity. Antigen retrieval was performed at 100°C/pH 6 for 30 min. After incubation with the primary polyclonal antibody to AMACR (P504S) (Biocare Medical, Concord, USA) at 1:30 dilution for 30 min, sections were further incubated with a secondary Polymer Poly-HRP anti-mouse/rabbit IgG. 3,3’-diaminobenzidine was used as a substrate for visualization. Finally, slides were counterstained with hematoxylin.
Interpretation
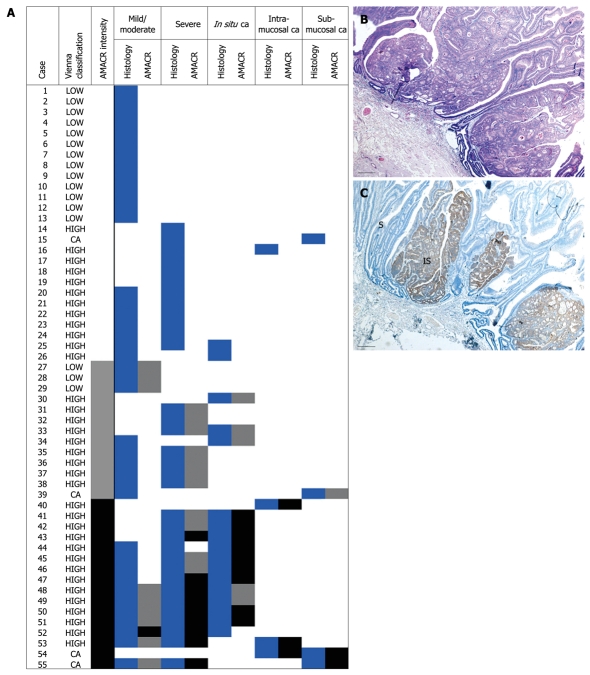
A lesion was considered negative when less than 5% of cells showed immunoreactivity. Positivity was divided into weak (mild, cytoplasmic granular staining) and strong (diffuse, intense cytoplasmic staining). Intra-lesion areas with different grades of dysplasia were evaluated separately, but case-specific intensity was based on the interpretation of immunostaining in the most intense focus, independently of the corresponding dysplastic grade. For example, adenomas containing strongly positive severe dysplasia and negative carcinoma in situ foci were considered positive (case 52 in Figure 1A).
Figure 1.
α-methylacyl CoA racemase (AMACR) expression in colorectal adenomas is related to the grade of dysplasia. A: Cumulative results from all dysplastic areas within each one of the 55 cases examined. Histology columns: white = absence, blue = presence of the indicated histological pattern. AMACR column: white = negative, grey = weak, black = strong staining; B and C: Case 44, as listed in A. In this adenoma with high grade dysplasia (HE staining), AMACR staining is restricted to the in situ component (IS) with severely dysplastic (S) adjacent glands showing negative or focal weak immunoreactivity. Bars: 100 microns.
Statistical analysis
Statistical analysis was performed using the SPSS software v.17. A P value of < 0.05 was considered statistically significant as determined by the Mann-Whitney test for two independent samples.
RESULTS
Clinicopathological features of colorectal adenomas
Patient age ranged from 41 to 84 years (mean 65 ± 13.2 years); 37 patients were males and 18 were females. Adenomas ranged in size between 0.5 and 30 cm (mean 2 ± 1.3 cm, whereby the largest lesion corresponded to a giant sessile, villous adenoma), including 18 tubular, 17 villous (containing a villous component > 75%), 20 mixed or tubulovillous (containing a villous component > 25%, but < 75%). The majority were retrieved from the left colon with only 7 cases being located in the proximal 2/3 of the transverse colon. The incidence of dysplastic lesions based on the Vienna classification is summarized in Table 1. The well established interrelations between size, histological configuration of colorectal adenomas and grade of dysplasia[28] were reproduced by our data. High grade dysplasia was assigned to 33 out of 41 adenomas greater than 1 cm and 30 out of 34 containing a villous component. In contrast the majority of smaller (7/10) or tubular lesions (12/17) were low grade adenomas. (P = 0.004 and P < 0.001, respectively). Similarly, lesions ≥ 1 cm were more likely to contain villous features [31/41 (75.6%) vs 3/10 (30%), P = 0.007]. Not surprisingly the 3 out of 4 high grade polyps showing evidence of submucosal invasion were villous adenomas and all 4 measured more than 1 cm.
Table 1.
Incidence of various dysplasia histological phenotypes n (%)
| Clinically oriented | Biologic potential | Total cases | Subgroups |
| Low grade dysplasia | Mild | 16 (29.1) | 6 (10.9) |
| Moderate | 10 (18.2) | ||
| High grade dysplasia | Severe | 35 (63.6) | 15 (27.3) |
| In situ carcinoma | 17 (30.9) | ||
| Intramucosal carcinoma | 3 (5.5) | ||
| Infiltrative carcinoma | Submucosal carcinoma | 4 (7.3) |
Immunohistochemical findings
The results of immunohistochemistry are summarized in Table 2 and Figure 1A. AMACR was positive in 29 adenomas (52.7%), and in all 4 adenocarcinomas, but was consistently negative in nonneoplastic intestinal mucosa in all 29 cases where normal appearing colonic epithelium was included in the section. Immunopositivity was strongly related to the grade of dysplasia independent of staining intensity (Figure 1A-C). Twenty-three out of 35 high grade but only 3/16 low grade lesions showed immunopositivity for AMACR (P = 0.002). Moreover, when staining was compared among adenomas containing only severe dysplasia-like areas and those with carcinoma in situ-like morphology, the latter group was more often positive [6/15 (40%) vs 15/17 (88.2%), P = 0.005] (Figure 1B and C).
Table 2.
AMACR expression in sporadic colorectal adenomas n (%)
| Vienna classification of GI neoplasia | Biologic potential |
Staining |
||||
| Negative | Weak | Strong | Subtotal positive | Total | ||
| Low grade (n = 16) | Mild | 13 (81.3) | 3 (18.8) | 0 (0) | 3 (18.8) | 16 |
| moderate | ||||||
| High grade (n = 35) | Severe | 9 (60) | 6 (40) | 0 (0) | 6 (40) | 15 |
| In situ carcinoma | 2 (11.8) | 3 (17.6) | 12 (70.6)1 | 15 (88.2) | 17 | |
| Intramucosal carcinoma | 1 | 0 | 2 | 2 | 3 | |
| Infiltrative carcinoma (n = 4) | Submucosal carcinoma | 1 | 1 | 2 | 3 | 4 |
| 26 (47.3) | 13 (23.7) | 16 (29) | 29 (52.7) | 55 | ||
In 3 cases (#48, 49, 52 in Figure 1A) higher grade focus and focus of most intense staining did not match, as described in Materials and Methods, Interpretation. AMACR: α-methylacyl CoA racemase; GI: Gastrointestinal.
As shown in Figure 1A, with the exception of 3 cases (#48, 49, 52), the most intense AMACR staining was observed in areas with the highest degree of dysplasia. On the contrary, no significant difference in staining intensity was observed between adenomas with mild to moderate dysplasia vs adenomas with severe dysplasia (3/16 vs 6/15, P = 0.2). AMACR immunostaining was also found to be associated with size and configuration of adenomas. Adenomas with a villous component (villous or tubulovillous) were more often positive for AMACR [22/37 (59.5%) vs 7/18 (37.5%), P = 0.03] and the same applied to lesions measuring ≥ 1 cm in greatest diameter [27/45 (60%) vs 2/10 (20%), P = 0.02]. Moreover, as shown in Table 3, strong expression was found in more than half of villous adenomas (11/17), but in only 1 tubular lesion (P = 0.005).
Table 3.
AMACR expression in relation to pathological features of adenomas n (%)
| n |
Staining |
||||
| Negative | Weak | Strong | Total positivity | ||
| Configuration | 55 | ||||
| Tubular | 18 | 11 (61) | 6 (33) | 1 (6) | 7 (39) |
| Villous | 17 | 5 (29.4) | 1 (5.9) | 11 (64.7) | 12 (76.5) |
| Tubulovillous | 20 | 10 (50) | 6 (30) | 4 (20) | 10 (50) |
| Size | 55 | ||||
| < 1 cm | 10 | 8 (80) | 1 (10) | 1 (10) | 2 (20) |
| ≥ 1 cm | 45 | 18 (40) | 12 (26.7) | 15 (33.3) | 27 (60) |
When areas with different degrees of dysplasia (and, hence, putative different biologic potential) within the same lesion were separately evaluated for AMACR expression (n = 98), positive staining in intra-lesion mild, moderate or severe dysplasia-like foci was more frequently encountered in adenomas also harboring a higher grade focus, i.e. in situ, intramucosal or infiltrative carcinoma, [21/33 (63.6%) vs 9/40 (22.5%), P < 0.001], (Table 4 and Figure 1A). Interestingly strong AMACR expression in 9 foci with severe dysplasia and in 1 focus with mild/moderate dysplasia was restricted in adenomas exhibiting intra-lesion cytological and architectural features of a higher grade component in another field (Figure 1A, cases # 43, 47-53, 55). This intra-lesion heterogeneity of AMACR expression is exemplified in Figure 2.
Table 4.
AMACR staining in areas with mild/moderate or severe dysplasia n (%)
| Higher grade component |
Staining |
|||
| Negative | Weak | Strong | Total positivity | |
| Present (n = 33) | 12 (36.4) | 11 (33.3) | 10 (30.3) | 21 (63.6) |
| Absent (n = 40) | 31 (77.5) | 9 (22.5) | 0 (0) | 9 (22.5) |
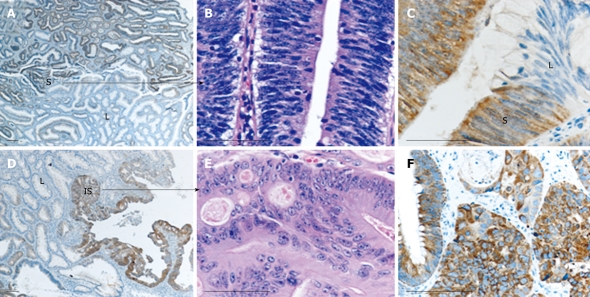
Figure 2.
Heterogeneity of AMACR staining may be observed in different dysplastic areas within the same lesion. Case 47, as listed in Figure 1A, is shown. This case contained areas of mild/moderate [low-grade (L)] and severe (S) dysplasia, as well as carcinoma in situ (IS) elements. Severely dysplastic (A-C) and carcinoma in situ areas (D-F) are strongly positive for AMACR, while mild/moderate dysplastic areas are negative. Histological distinction between severe dysplasia and carcinoma in situ is apparent in B and E, respectively (HE staining). Bars: 100 microns.
However, when considering severe dysplasia-like and in situ carcinoma-like foci as individual cases (that is, not taking into account their occurrence within the same or in different lesions), we did not observe significant differences in AMACR expression (P = 0.06). Similarly, no association was found between AMACR expression and either age, gender or localization of adenomas (data not shown).
DISCUSSION
Colonic adenomas are considered adenocarcinoma precursors[29]. Even though this might not be entirely endorsed[30], the predictive value of high grade dysplasia for the subsequent development of advanced adenomas and cancer is well documented and indisputable[31-34]. In this setting, it is widely accepted that severe dysplasia and carcinoma in situ bear a similar clinical significance and are therefore merged into a single category in most classification/reporting protocols. Molecular studies have shown an increased frequency of genetic alterations and DNA aneuploidy with increasing grade of dysplasia as assigned by the Vienna classification[35]. Following a detailed evaluation of the examined dysplastic lesions and taking into account intra-lesion heterogeneity, our study is the first one to report a positive association between AMACR and increasing grade of dysplasia in sporadic colorectal adenomas at the immunohistochemical level.
The observed overall prevalence of AMACR expression in colorectal adenomas in our study (52.7%) appears to be somewhat lower than in previous publications (64%-91%)[5,6,10,11,17]. It was difficult to compare our findings with the ones presented in these studies, in which the applied criteria for the classification of dysplasia were not provided. In the two studies that, to our knowledge, evaluated AMACR expression in relation to dysplasia, results did not significantly differ between low and high grade adenomas[17] or among adenomas with mild, moderate or severe dysplasia[5]. The above discrepancies can be attributed to the different antibodies used in each case and also to the different evaluation criteria set for immunopositivity, such as lower thresholds. These differences are further reflected in the reported positivity of normal colonic epithelia[5,11,14,17], which was not observed in our series.
It was previously suggested that AMACR expression might be closely related to the early stages of neoplastic progression in the colon[10]. The estimated prevalence of AMACR in adenomas bearing in situ carcinoma cytoarchitectural features in our study (88.2%) is similar to that reported for colorectal carcinoma in the majority of publications (62%-83%)[5,9-12]. In comparison, we observed AMACR positivity in only 40% of severely dysplastic and 18.8% of low grade lesions. These findings might suggest a more advanced state towards malignancy of in situ carcinoma as compared to severe dysplasia, although both lesions are currently incorporated into the high grade category for management reasons.
The differences observed between lesions exhibiting morphological changes corresponding to the previously used terms of severe dysplasia and in situ carcinoma at the molecular level prompt for morphologically identifying these lesions at least for research purposes[20,21]. Of note, however, co-existing severely dysplastic and in situ carcinoma areas in the same lesion did not significantly differ in terms of AMACR expression. AMACR expression seemed to be related to the co-existence of in situ carcinoma rather than to in situ carcinoma itself, since it was present in severely dysplastic areas in the vicinity of negative in situ carcinomas. Severe dysplasia-like morphology does not differ in the simultaneous presence or absence of adjacent in situ carcinoma. However, the same morphologic features might bear significant differences at the molecular level that seem to be more prominent in later stages of the dysplasia-carcinoma sequence.
Another implication of the above described intra-lesion AMACR heterogeneity is that, when examining the expression of AMACR and possibly other markers in adenomas on tissue microarrays, multiple areas should be sampled for adequate evaluation of the lesion.
Taken together, our data indicate that AMACR may play some role in the process of malignant transformation in the colon. What this role might be cannot be established by this study. As has also been suggested for AMACR in the prostate, it is uncertain whether its expression is directly involved in the process of malignant transformation or whether it is just an epiphenomenon triggered by the increased metabolic requirements of malignant cells[36].
Studies like the present one are performed to approach a basic question: How can molecular or genetic information help to stratify risk in patients with adenomatous polyps[33]. Currently, size ≥ 1 cm, villous configuration and high grade dysplasia constitute the consensus criteria based on which an adenoma is characterized as “advanced” and patient surveillance is adjusted accordingly[18,31,33,34]. Based on these parameters, patients can be stratified at the time of colonoscopy into lower or higher risk groups for subsequent advanced neoplasia (adenomas with high grade dysplasia or cancer). However, debate on the clinical relevance of these factors continues to exist and many authors still address the need for more objective and standardized pathologic criteria[37-40]. As we show here, except for its association with the grade of dysplasia, AMACR expression in colonic adenomas is also related to two additional pathologic risk factors, i.e. size and villous configuration, a finding not previously reported. Hence, AMACR could be a candidate parameter for further evaluation as an additional risk factor for the development of subsequent advanced neoplasia. Large scale studies with long term patient follow-up would be required for the evaluation of any marker in this setting, since patients with adenoma seldom develop carcinoma within a period of 3 years[32].
Another observation that needs to be further evaluated is the significant difference in AMACR expression between severe dysplasia-like changes and carcinoma in situ-like changes, when these appear as independent lesions. Taking into account the drawbacks of our study (small sample size and lack of patient follow up), none of our findings directly raises any issues concerning the current guidelines for the reporting of dysplasia in colorectal adenomas that have been imposed by the need for simplicity and standardization. However as shown here a more detailed approach to the cytoarchitectural features of dysplasia in the context of observational studies might unravel meaningful and potentially useful associations.
COMMENTS
Background
α-methylacyl CoA racemase (AMACR) has been widely used in prostate pathology as an adjunct in the differential diagnosis of prostate cancer. Recently many papers have addressed the possible application of this biomarker in distinguishing low-grade from high-grade dysplasia in premalignant lesions of the gastrointestinal tract like Barrett’s esophagus, Crohn’s disease and ulcerative colitis.
Research frontiers
Although immunohistochemistry with AMACR in colorectal carcinoma has been extensively investigated, there are not yet any clear-cut conclusions regarding the expression of AMACR in sporadic colorectal adenomas in relation to histology. This is the first study to report associations between AMACR expression and cytoarchitectural features of colorectal polyps.
Innovations and breakthroughs
Recent studies have shown a positive association between AMACR expression and grade of dysplasia in inflammatory bowel disease and Barrett’s esophagus with higher grade lesions showing strong staining at the immunohistochemical level. In this study, the authors report for the first time a significant association between AMACR expression and grade of dysplasia in sporadic colorectal adenomas, which may also be extended to other pathologic parameters like villousness and size.
Applications
AMACR may be considered as a candidate adjunct marker for further evaluation regarding risk stratification of patients undergoing colonoscopic surveillance.
Terminology
AMACR is a mitochondrial and peroxisomal enzyme implicated in the degradation of branched chained fatty acids and fatty acid derivatives. There are no conclusive results about the role of AMACR in carcinogenesis. However, multiple studies have addressed its potential use as a biomarker in cancer diagnostics.
Peer review
The study evaluate the expression of AMACR with polyps characteristics and suggested that it could be use as an additional risk factor for the assessment of colorectal adenoma.
Footnotes
Supported by Private Funding
Peer reviewers: Gerrit A Meijer, Professor, Department of Pathology, VU University Medical Center, Postbus 7057, Amsterdam, 1007 MB, The Netherlands; Damian Casadesus Rodriguez, MD, PhD, Calixto Garcia University Hospital, J and University, Vedado, Havana City, Cuba
S- Editor Wang YR L- Editor O’Neill M E- Editor Ma WH
References
- 1.Ferdinandusse S, Denis S, IJlst L, Dacremont G, Waterham HR, Wanders RJ. Subcellular localization and physiological role of alpha-methylacyl-CoA racemase. J Lipid Res. 2000;41:1890–1896. [PubMed] [Google Scholar]
- 2.Schmitz W, Albers C, Fingerhut R, Conzelmann E. Purification and characterization of an alpha-methylacyl-CoA racemase from human liver. Eur J Biochem. 1995;231:815–822. doi: 10.1111/j.1432-1033.1995.tb20766.x. [DOI] [PubMed] [Google Scholar]
- 3.Wanders RJ, Vreken P, Ferdinandusse S, Jansen GA, Waterham HR, van Roermund CW, Van Grunsven EG. Peroxisomal fatty acid alpha- and beta-oxidation in humans: enzymology, peroxisomal metabolite transporters and peroxisomal diseases. Biochem Soc Trans. 2001;29:250–267. doi: 10.1042/0300-5127:0290250. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Z, Fanger GR, Woda BA, Banner BF, Algate P, Dresser K, Xu J, Chu PG. Expression of alpha-methylacyl-CoA racemase (P504s) in various malignant neoplasms and normal tissues: astudy of 761 cases. Hum Pathol. 2003;34:792–796. doi: 10.1016/s0046-8177(03)00268-5. [DOI] [PubMed] [Google Scholar]
- 5.Went PT, Sauter G, Oberholzer M, Bubendorf L. Abundant expression of AMACR in many distinct tumour types. Pathology. 2006;38:426–432. doi: 10.1080/00313020600922470. [DOI] [PubMed] [Google Scholar]
- 6.Zhou M, Chinnaiyan AM, Kleer CG, Lucas PC, Rubin MA. Alpha-Methylacyl-CoA racemase: a novel tumor marker over-expressed in several human cancers and their precursor lesions. Am J Surg Pathol. 2002;26:926–931. doi: 10.1097/00000478-200207000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Jiang Z, Woda BA, Rock KL, Xu Y, Savas L, Khan A, Pihan G, Cai F, Babcook JS, Rathanaswami P, et al. P504S: a new molecular marker for the detection of prostate carcinoma. Am J Surg Pathol. 2001;25:1397–1404. doi: 10.1097/00000478-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Z, Wu CL, Woda BA, Dresser K, Xu J, Fanger GR, Yang XJ. P504S/alpha-methylacyl-CoA racemase: a useful marker for diagnosis of small foci of prostatic carcinoma on needle biopsy. Am J Surg Pathol. 2002;26:1169–1174. doi: 10.1097/00000478-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Chen ZM, Ritter JH, Wang HL. Differential expression of alpha-methylacyl coenzyme A racemase in adenocarcinomas of the small and large intestines. Am J Surg Pathol. 2005;29:890–896. doi: 10.1097/01.pas.0000167364.90899.59. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Z, Fanger GR, Banner BF, Woda BA, Algate P, Dresser K, Xu J, Reed SG, Rock KL, Chu PG. A dietary enzyme: alpha-methylacyl-CoA racemase/P504S is overexpressed in colon carcinoma. Cancer Detect Prev. 2003;27:422–426. doi: 10.1016/j.cdp.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Lin A, Weiser MR, Klimstra DS, Paty PB, Tang LH, Al-Ahmadie H, Hoo Park S, Guillem JG, Temple L, Wong WD, et al. Differential expression of alpha-methylacyl-coenzyme A racemase in colorectal carcinoma bears clinical and pathologic significance. Hum Pathol. 2007;38:850–856. doi: 10.1016/j.humpath.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Marx A, Simon P, Simon R, Mirlacher M, Izbicki JR, Yekebas E, Kaifi JT, Terracciano L, Sauter G. AMACR expression in colorectal cancer is associated with left-sided tumor localization. Virchows Arch. 2008;453:243–248. doi: 10.1007/s00428-008-0646-1. [DOI] [PubMed] [Google Scholar]
- 13.Nassar A, Amin MB, Sexton DG, Cohen C. Utility of alpha-methylacyl coenzyme A racemase (p504s antibody) as a diagnostic immunohistochemical marker for cancer. Appl Immunohistochem Mol Morphol. 2005;13:252–255. doi: 10.1097/00129039-200509000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Shi X, Gong E, Wu X. Alpha-methylacyl-CoA racemase/P504S overexpression in colorectal carcinoma is correlated with tumor differentiation. Appl Immunohistochem Mol Morphol. 2007;15:175–180. doi: 10.1097/01.pai.0000213107.20355.d8. [DOI] [PubMed] [Google Scholar]
- 15.Dorer R, Odze RD. AMACR immunostaining is useful in detecting dysplastic epithelium in Barrett’s esophagus, ulcerative colitis, and Crohn’s disease. Am J Surg Pathol. 2006;30:871–877. doi: 10.1097/01.pas.0000213268.30468.b4. [DOI] [PubMed] [Google Scholar]
- 16.Lisovsky M, Falkowski O, Bhuiya T. Expression of alpha-methylacyl-coenzyme A racemase in dysplastic Barrett’s epithelium. Hum Pathol. 2006;37:1601–1606. doi: 10.1016/j.humpath.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Strater J, Wiesmüller C, Perner S, Kuefer R, Möller P. Alpha-methylacyl-CoA racemase (AMACR) immunohistochemistry in Barrett’s and colorectal mucosa: only significant overexpression favours a diagnosis of intraepithelial neoplasia. Histopathology. 2008;52:399–402. doi: 10.1111/j.1365-2559.2007.02923.x. [DOI] [PubMed] [Google Scholar]
- 18.Bond JH. Polyp guideline: diagnosis, treatment, and surveillance for patients with colorectal polyps. Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 2000;95:3053–3063. doi: 10.1111/j.1572-0241.2000.03434.x. [DOI] [PubMed] [Google Scholar]
- 19.Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130–131. doi: 10.1136/gut.51.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubio CA, Nesi G, Messerini L, Zampi GC, Mandai K, Itabashi M, Takubo K. The Vienna classification applied to colorectal adenomas. J Gastroenterol Hepatol. 2006;21:1697–1703. doi: 10.1111/j.1440-1746.2006.04258.x. [DOI] [PubMed] [Google Scholar]
- 21.Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stolte M. The new Vienna classification of epithelial neoplasia of the gastrointestinal tract: advantages and disadvantages. Virchows Arch. 2003;442:99–106. doi: 10.1007/s00428-002-0680-3. [DOI] [PubMed] [Google Scholar]
- 23.West AB, Mitsuhashi T. Cancer or high-grade dysplasia? The present status of the application of the terms in colonic polyps. J Clin Gastroenterol. 2005;39:4–6. [PubMed] [Google Scholar]
- 24.Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol. 1990;14:524–537. doi: 10.1097/00000478-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Rubio CA. Colorectal adenomas: time for reappraisal. Pathol Res Pract. 2002;198:615–620. doi: 10.1078/0344-0338-00310. [DOI] [PubMed] [Google Scholar]
- 26.Yashiro M, Laghi L, Saito K, Carethers JM, Slezak P, Rubio C, Hirakawa K, Boland CR. Serrated adenomas have a pattern of genetic alterations that distinguishes them from other colorectal polyps. Cancer Epidemiol Biomarkers Prev. 2005;14:2253–2256. doi: 10.1158/1055-9965.EPI-04-0790. [DOI] [PubMed] [Google Scholar]
- 27.Morson BC, Sobin LH, Grundmann E, Johansen A, Nagayo T, Serck-Hanssen A. Precancerous conditions and epithelial dysplasia in the stomach. J Clin Pathol. 1980;33:711–721. doi: 10.1136/jcp.33.8.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Brien MJ, Winawer SJ, Zauber AG, Gottlieb LS, Sternberg SS, Diaz B, Dickersin GR, Ewing S, Geller S, Kasimian D. The National Polyp Study. Patient and polyp characteristics associated with high-grade dysplasia in colorectal adenomas. Gastroenterology. 1990;98:371–379. [PubMed] [Google Scholar]
- 29.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 30.Appelman HD. What is dysplasia in the gastrointestinal tract? Arch Pathol Lab Med. 2005;129:170–173. doi: 10.5858/2005-129-170-WIDITG. [DOI] [PubMed] [Google Scholar]
- 31.Atkin WS, Saunders BP. Surveillance guidelines after removal of colorectal adenomatous polyps. Gut. 2002;51 Suppl 5:V6–V9. doi: 10.1136/gut.51.suppl_5.v6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieberman DA, Weiss DG, Harford WV, Ahnen DJ, Provenzale D, Sontag SJ, Schnell TG, Chejfec G, Campbell DR, Kidao J, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077–1085. doi: 10.1053/j.gastro.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 33.Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O’Brien MJ, Levin B, Smith RA, Lieberman DA, Burt RW, Levin TR, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology. 2006;130:1872–1885. doi: 10.1053/j.gastro.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Yang G, Zheng W, Sun QR, Shu XO, Li WD, Yu H, Shen GF, Shen YZ, Potter JD, Zheng S. Pathologic features of initial adenomas as predictors for metachronous adenomas of the rectum. J Natl Cancer Inst. 1998;90:1661–1665. doi: 10.1093/jnci/90.21.1661. [DOI] [PubMed] [Google Scholar]
- 35.Sugai T, Habano W, Uesugi N, Jiao YF, Nakamura S, Sato K, Chiba T, Ishii M. Molecular validation of the modified Vienna classification of colorectal tumors. J Mol Diagn. 2002;4:191–200. doi: 10.1016/s1525-1578(10)60703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuefer R, Varambally S, Zhou M, Lucas PC, Loeffler M, Wolter H, Mattfeldt T, Hautmann RE, Gschwend JE, Barrette TR, et al. alpha-Methylacyl-CoA racemase: expression levels of this novel cancer biomarker depend on tumor differentiation. Am J Pathol. 2002;161:841–848. doi: 10.1016/s0002-9440(10)64244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Appelman HD. Con: High-grade dysplasia and villous features should not be part of the routine diagnosis of colorectal adenomas. Am J Gastroenterol. 2008;103:1329–1331. doi: 10.1111/j.1572-0241.2008.02005_3.x. [DOI] [PubMed] [Google Scholar]
- 38.Bosman FT. Dysplasia classification: pathology in disgrace? J Pathol. 2001;194:143–144. doi: 10.1002/1096-9896(200106)194:2<143::AID-PATH883>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 39.Odze R. A Balancing view: Pathologist-clinician interaction is essential. Am J Gastroenterol. 2008;103:1331–1333. doi: 10.1111/j.1572-0241.2008.02005_4.x. [DOI] [PubMed] [Google Scholar]
- 40.Rex DK, Goldblum JR. Pro: Villous elements and high-grade dysplasia help guide post-polypectomy colonoscopic surveillance. Am J Gastroenterol. 2008;103:1327–1329. doi: 10.1111/j.1572-0241.2008.02005_2.x. [DOI] [PubMed] [Google Scholar]