Abstract
AIM: To establish a model for prognosis assessment of extranodal follicular dendritic cell (FDC) sarcoma.
METHODS: Nine lesions were examined by routine and molecular approaches. Clinicopathological factors from the new cases and 97 reported cases were analyzed for their prognostic values.
RESULTS: The current lesions were found in five male and four female patients, located mainly in the head and neck area and averaging 7.2 cm in size. Six patients had recurrence or metastasis and three remained free of disease. The 106 patients (male/female ratio, 1.1:1) were aged from 9 to 82 years (median, 44 years). The tumor sizes ranged from 1.5 to 21 cm (mean, 7.4 cm). Abdominal/pelvic region was affected most frequently (43%). Surgical resection was performed in 100 patients, followed by radiation and/or chemotherapy in 35 of them. Follow-up data were available in 91 cases, covering a period of 3-324 mo (mean, 27 mo; median, 19 mo). Of the informative cases, 38 (42%) had recurrence or metastasis, and 12 (13%) died of the disease. These tumors were classified histologically into low- and high-grade lesions. A size ≥ 5 cm (P = 0.003), high-grade histology (P = 0.046) and a mitotic count ≥ 5/10 HPF (P = 0.013) were associated with tumor recurrence. The lesions were defined as low-, intermediate- and high-risk tumors, and their recurrence rates were 16%, 46% and 73%, and their mortality rates 0%, 4% and 45%, respectively.
CONCLUSION: Extranodal FDC tumors behave like soft tissue sarcomas. Their clinical outcomes are variable and can be evaluated according to their sizes and grades.
Keywords: Extranodal follicular dendritic cell sarcoma, Prognosis assessment, Histologic grade, Immunohistochemistry, In situ hybridization, Mutation detection
INTRODUCTION
Follicular dendritic cell (FDC) sarcoma was described by Monda et al[1] in 1986. It is considered to be derived from FDC, whose cell type normally forms a tight meshwork in the primary and secondary lymphoid follicles and participates in the immune system by interacting with B or T lymphocytes[2]. Epstein-Barr virus (EBV) infection was demonstrated in majority of the hepatic and splenic lesions and its causative effect has been proposed for the pathogenesis of the lesion[3-9], but the association is not evident in most of the tumors from other sites[10-13]. Majority of the tumors were found in lymph nodes. However, about one-third of the lesions were identified in extranodal sites. It was considered that extranodal FDC sarcoma occurred preferentially in the head and neck area[14].
FDC sarcoma is usually regarded as an indolent tumor with a tendency of local recurrence but a low risk of metastasis, behaving like a low-grade soft tissue sarcoma[2,15]. However, Perez-Ordonez et al[16] considered this tumor more aggressive through their observation of 13 cases. Because of the rarity of the tumor, assessment of its prognosis remains difficult, though intra-abdominal location, a high mitotic count (≥ 5/10 HPF), coagulative necrosis and marked cellular atypia were proposed to be predictors of an unfavorable outcome[3]. With more cases encountered in our pathological practice and reported from literatures, various morphologic and clinical representations were noticed. Clearly, histological grade of this malignancy remains to be defined and a model is needed to evaluate the recurrence risk of individual tumors within this category.
In this study, we presented our experience in nine cases of FDC sarcoma from northern China. In addition, clinicopathological features and corresponding data extracted from 97 reported cases were analyzed, with special respect to prognosis assessment of this disease.
MATERIALS AND METHODS
Samples and histological examination
As listed in Table 1, the tissue specimens were resected from nine patients in three medical centers, including the Fourth Military Medical University Tangdu Hospital in Xi’an (Cases 1 and 2), Chinese Academy of Medical Sciences Cancer Hospital in Beijing (Cases 3-6, 8 and 9) and the First Affiliated Hospital of Inner Mongolia Medical College in Huhehot (Case 7). These patients were admitted for a solid occupation in head and neck areas (n = 6), and thoracic and abdominal cavities (n = 3) from 2002 to 2009. Cases 1 and 2 had been described in a brief report[17], and follow-up data were complemented in the current study.
Table 1.
Clinicopathological and therapeutic data of nine new cases of extranodal FDC sarcoma (Cases 1-9)
| Case No. | Age (yr) | Gender | Sites | Size (cm) | HG | MC (/10 HPF) | Year of onset | Initial diagnoses | Initial treatment | Recurrence, DFT (mo) | Disease status | Follow-up (mo) |
| 1 | 60 | M | Tonsil | 5.0 | Low | < 1 | 2002 | Granuloma | Surg + RT | NED | 86 | |
| 2 | 35 | F | Parapharyn-geal space | 5.0 | High | 10 | 2003 | NPC | Surg | LR, 2 | DOD | 12 |
| 3 | 63 | M | Infratem-poral fossa | 4.0 | Low | 1 | 2003 | PNET | Surg + RT + ChT | NED | 72 | |
| 4 | 30 | F | Pyform sinus | 5.0 | High | 9 | 2004 | FDC sarcoma | Surg + RT | DM (lung), 25 | DOD | 25 |
| 5 | 23 | M | Mediastinum | 8.0 | Low | 3 | 2006 | MPNST | Surg + RT + ChT | DM (bone), 45 | AWD | 45 |
| 6 | 45 | M | Liver | 14.5 | Low | < 1 | 2007 | FDC sarcoma | Surg | NED | 27 | |
| 7 | 36 | F | Mesentery | 15.0 | High | 7 | 2007 | Malignant GIST | Surg | DM (liver, ovary), 4 | AWD | 27 |
| 8 | 28 | F | Parapharyn-geal space | 6.0 | High | 3 | 2008 | FDC sarcoma | Surg + RT + ChT | DM (lung), 14 | AWD | 22 |
| 9 | 55 | M | Tonsil | 2.0 | High | 9 | 2008 | FDC sarcoma | Surg + RT | LR, 18 | AWD | 21 |
AWD: Alive with disease; ChT: Chemotherapy; DFT: Disease-free time following operation; DM: Distant metastasis; DOD: Died of disease; GIST: Gastrointestinal stromal tumor; HG: Histological grades; LR: Local recurrence; MC: Mitotic counts; MPNST: Malignant nerve sheath tumor; NED: No evidence of disease; NPC: Nasopharyngeal carcinoma; PNET: Primitive neuroepidermal tumor; RT: Radiotherapy; Surg: Surgery; FDC: Follicular dendritic cell.
The formalin-fixed, paraffin-embedded tissue blocks were retrieved from the files. Sections of 4 μm in thickness were prepared and stained with hematoxylin and eosin (HE). Three pathologists (Su Q, Yang HY and Li L) reviewed the slides independently, and the diagnosis was confirmed by their histological and immunohistochemical phenotypes. As described previously[2], whorl, storiform and fascicular arrangements of spindle tumor cells were regarded as architectural features for typical lesions, while epithelioid and pleomorphic patterns were considered anaplastic phenotypes in this study. Other morphologic parameters, including mitotic activity, coagulative necrosis, nuclear atypia, distribution of infiltrating small lymphocytes and tumor sites, were also assessed. In addition, we also evaluated proportions of the typical tumor components, as described above, in each lesion.
Immunohistochemistry
Immunohistochemical reactions were performed on paraffin sections following deparaffinization, rehydration and antigen retrieval. The antigen retrieval was performed by heating in citrate (pH 6.0) or ethylene-diamine-tetraacetic acid (EDTA) buffer (pH 9.0; Table 2). In addition to routine markers for tumor diagnosis, such as pan-cytokeratins (CKs; AE1/AE3), epithelial membranous antigen (EMA), vimentin, desmin and smooth muscle-type actin (SMA), a panel of antibodies were applied to demonstrate histiocytic and dendritic cell linage differentiation, including those against CD68, S100 protein, CD21, CD23, CD35, CD1a and podoplanin (D2-40). Ki-67 antigen was detected to show proliferative activity of each lesion, and p53 protein accumulation was demonstrated by reaction with the antibody DO-7. After incubation with primary antibodies at room temperature for 1 h, the antigen-antibody reaction was demonstrated by a horseradish peroxidase (HRP)-labeled secondary antibody (EnVision™ Detection System, Dako A/S, Glostrup, Denmark) at room temperature for 15 min and visualized in a solution containing 0.5 mg/mL 3,3’-diaminobenzidine (DAB) and 0.01% hydrogen peroxide. Finally, the sections were counterstained slightly with hematoxylin and mounted in resin.
Table 2.
Primary antibodies and antigen retrieving procedures
| Antibodies | Origins and clones | Dilutions | Antigen retrieval | Sources |
| Pan-cytokeratin | MAb, AE1/AE3 | 1:80 | MW-citrate | Invitrogen |
| EMA | MAb, Mc5 | 1:100 | MW-citrate | Invitrogen |
| Vimentin | MAb, V9 | 1:200 | MW-citrate | NeoMarker |
| Desmin | MAb, ZC18 | 1:80 | MW-citrate | Invitrogen |
| SMA | MAb, IA4 | 1:100 | Not treated | Invitrogen |
| CD45 | MAb, RP2/18 | 1:120 | MW-citrate | Novacastro |
| CD68 | MAb, KP1 | 1:200 | MW-citrate | Zeta |
| S-100 protein | PAb, rabbit | 1:400 | MW-citrate | Dako |
| CD21 | MAb, 2G9 | 1:20 | AC-EDTA | NeoMarker |
| CD23 | MAb, SP23 | 1:25 | MW-citrate | NeoMarker |
| CD35 | MAb, KuN241 | 1:20 | AC-EDTA | NeoMarker |
| CD1a | MAb, MTB1 | 1:20 | MW-citrate | Zeta |
| Podoplanin | MAb, D2-40 | 1:50 | AC-EDTA | Zeta |
| p53 protein | MAb, DO-7 | 1:100 | AC-EDTA | NeoMarker |
| Ki-67 antigen | MAb, MIB-1 | 1:200 | AC-EDTA | Zymed |
Antibody suppliers: Dako, Glostrup, Denmark; Invitrogen Corporation, Carlsbad, CA, USA; NeoMarkers Inc., Fremont, CA, USA; Novacastro Laboratories Ltd., Newcastle upon Tyne, UK; Zeta Corporation, Sierra Madre, CA; Zymed Laboratories, San Diego, CA, USA. EMA: Epithelial membrane antigen; MAb: Mouse monoclonal antibody; PAb: Polyclonal antibody; SMA: Smooth muscle-type actin; MW-citrate: Microwaving for 8 min in citrate buffer (pH 6.0); AC-EDTA: Autoclaving for 100 s in EDTA buffer (pH 9.0).
The expression levels were evaluated semiquantitatively according to the reaction intensities and percentages of immunoreactive cells, and expressed as strong (3+), moderate (2+), weak (+) and negative (-). Ki-67 antigen expression levels were assessed based on the number of positive cells/1000 nucleated tumor cells from randomly selected 5 high-power fields, and expressed as Ki-67 antigen-labeling indices (Ki-67-LI) in percentages. The p53 protein expression levels were evaluated by percentages of positive cells as described previously[18] and expressed as high (3+, > 30%), moderate (2+, 5%-30%), low (1+, < 5%) and absent (-, 0%).
In situ hybridization
In situ hybridization was performed in seven cases to demonstrate EBV-encoded RNA (EBER) molecules using a detection kit (Triplex International Biosciences Co. Ltd., Fuzhou, China) according to instructions of the manufacturer. Briefly, sections were treated with proteinase K following deparaffinization and rehydration. After denaturation for 90 min at 55°C, the sections were incubated with a digoxin-labeled DNA probe overnight at 37°C, and washed with phosphate-buffered saline. The hybridization signals were demonstrated by consecutive reactions with a mouse monoclonal antibody against digoxin and the polymerized HRP-labeled anti-mouse immunoglobulin G, and visualized by incubation in a solution containing DAB and hydrogen peroxidase. Finally, the slides were counterstained slightly with hematoxylin and mounted with resin. A case of Hodgkin lymphoma, known to be positive for EBER, was used as a positive control. Yellow or brown staining of tumor cell nuclei was considered positive.
Amplification and sequencing of P53 gene
As most of the documented mutations of P53 gene occur in its exons 5-8[19,20], these regions were examined for mutations by nested (for exons 6 and 7) or semi-nested polymerase-chain reaction (PCR; for exons 5 and 8). The primers used were adopted from literatures[21,22]. Their sequences were as follows: exon5s, 5'-TCTGTCTCCTTCCTCTTCCTA-3'; exon5as, 5'-AACCAGCCCTGTCGTCTCT-3'; exon6s, 5'-TTGCTCTTAGGTCTGGCCCC-3'; exon6as, 5'-CAGACCTCAGGCGGCTCATA-3'; exon7s, 5'-TTGCTCTTAGGTCTGGCCCC-3'; exon7as, 5'-GGGTCAGCGGCAAGCAGAGG-3'; exon8s, 5'-GACAAGGGTGGTTGGGAGTAGATG-3'; exon8as, 5'-GCAAGGAAAGGTGATAAAAGTGAA-3'. Selected tumor tissues were collected from serial paraffin sections of 10 μm in thickness. Following deparaffinization and rehydration, the tissues were digested by incubation with proteinase K (20 mg/mL) at 56°C for 72 h. Genomic DNA was extracted with a kit (QIAamp DNA Mini Kit, Qiagen GmbH, Hiden, Germany) following instructions of the manufacturer. Primer pairs used for the first-round amplification of exons 5, 6, 7 and 8 were exon5s/exon6as, exon5s/exon7as, exon6s/exon8as and exon7s/exon8as, respectively. The reactions were in a mixture of 10 μL containing 100 ng of DNA templates, 0.8 μL of deoxynucleotide triphosphate (2.5 mmol/L each), 1 μL of 1 μmol/L sense and antisense primers each, 1 μL 10× buffer (100 mmol/L Tris-HCl, pH 8.3, containing 500 mmol/L KCl and 20 mmol/L MgCl2) and 0.5 U of Taq DNA polymerase (Takara Biotechnology Co., Ltd., Dalian, China). Amplification was conducted for 35 cycles (94°C, 40 s; 55°C, 40 s; 72°C, 40 s for exons 5 and 7; 1 min for exons 6 and 8) following the initial denaturation at 94°C for 5 min. The final elongation was at 72°C for 10 min. The second-round PCR was performed in a mixture of 50 μL containing 3 μL of products of the first-round reaction and 1 μL of 5 μmol/L sense and antisense inner premiers each. Primer pairs used for amplifying exons 5, 6, 7 and 8 were exon5s/5as, exon6s/6sa, exon7s/7as and exon8s/8as, respectively. The cycling conditions were the same as the first-round reaction. Amplification efficiency and specificity were visualized by electrophoresis on an agarose gel (2%), and the products of exons 5, 6, 7 and 8 migrated at positions of 196, 81, 234 and 277 bp, respectively. The products were subjected to direct sequencing using an automatic system (Prism 3100 Genetic Analyzer, Applied Biosystems, Foster, CA, USA) as described previously[22,23]. The sequences obtained were compared with those from Genbank (www.gdb.org).
Literature review
Search of literatures was performed using MEDLINE on PubMed (www.ncbi.nlm.nih.gov/pubmed) with the terms “follicular dendritic cell tumor” or “follicular dendritic cell sarcoma” combined with “extranodal”. Articles published in Chinese journals were found by searches on Wanfangdata (www.wanfangdata.com.cn). References of the collected articles were also reviewed to identify other relevant publications. Efforts were made to identify cases that were reported more than once in different settings and only one entry with the most updated information was included for such cases. Nodal tumors were not included in this study.
Statistical analysis
Statistical analyses were carried out using the Software Packages for Social Science 13.0 for Windows (SPSS, Inc, Chicago, IL, USA). Survival of patients was assessed using the data obtained from both the current study and the literatures. Overall and recurrence-free survival rates were calculated and analyzed using the Kaplan-Meier method. Associations of different pathological factors with clinical outcomes were described by the Chi-square test. Fisher’s exact test was also used when necessary. P values below 0.05 were considered significant.
RESULTS
Current cases
Ages of the nine patients (five men and four women) ranged from 23 to 63 years (median, 36 years). A local mass was the primary complaint in eight cases (Figure 1), and a sore throat was the first symptom in one patient (Case 2). Sizes of the tumors ranged from 2.0 to 15.0 cm as measured by the longitudinal dimension, averaging 7.2 cm. A diagnosis of FDC sarcoma was made in four of the nine cases following pathologic examination of the resected specimens. For Cases 1, 2, 3, 5 and 7, the resected lesions were misdiagnosed as granulomatous inflammation suspicious of tuberculosis, nasopharyngeal carcinoma (NPC), primitive neuroectodermal tumor (PNET), malignant peripheral nerve sheath tumor (MPNST) and malignant gastrointestinal stromal tumor (GIST), respectively. The diagnosis of FDC sarcoma was established by re-examination and immunohistochemistry with the delayed periods ranging from one to 66 mo. Their clinicopathological features are listed in Table 1. No precursor lesions were recorded in any of them.
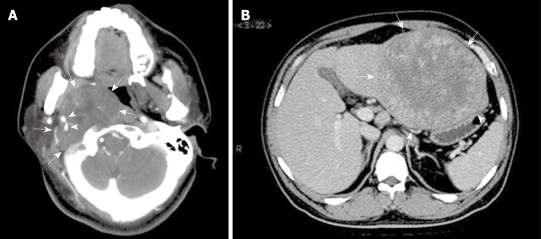
Figure 1.
Radiological features of extranodal follicular dendritic cell (FDC) sarcoma shown by computed tomographic scan. A: Case 8, a tumor (arrows) at the right parapharyngeal space showing soft tissue-like density and an expansive growth pattern, with the internal and external carotid arteries (arrowheads) engulfed; B: Case 6, a well-circumscribed mass (arrows) at the left lobe of liver, showing irregular enhancement at its periphery.
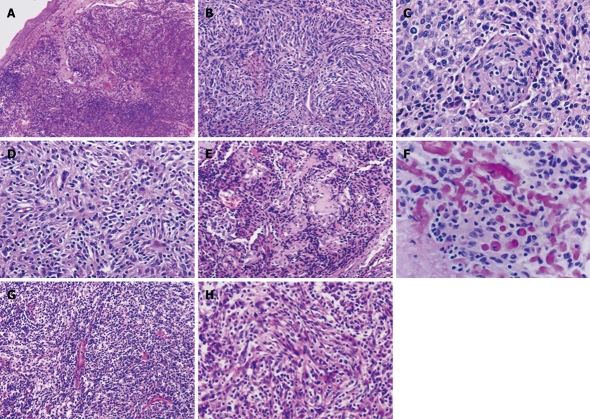
Typically, the lesions were composed of spindle and ovoid tumor cells arranged in whorl, fascicular and storiform patterns, showing mild nuclear atypia and sparkled with small lymphocytes (Figure 2). Of the nine tumors, three (Cases 1, 5 and 6) were composed uniformly of the typical components, and one (Case 3) showed typical components in > 90% of the area and epithelioid appearance in small foci (Figure 3A). In the remaining five cases, large areas of epithelioid (Cases 2 and 8) and pleomorphic tumor cell components (Cases 4, 7-9), arranged in a diffuse or sheet-like pattern and with moderate to marked nuclear atypia, were also identified (Figure 3B-D). Sporadic small lymphocytes were present throughout tumor tissues in all the four typical cases (Figure 2), and this feature was less prominent (Figure 3B) or even absent (Figure 3C and D) in the rest five lesions. Focal coagulative necrosis was identified in six cases (Cases 2, 3, 5-8). The hepatic lesion (Case 6) was composed of spindle cells with mild nuclear atypia and numerous inflammatory cells including plasma cells, resembling an inflammatory pseudotumor (Figure 2G and H). In addition, neutrophils infiltration was observed in two lesions (Cases 3 and 8). In some tumor areas (Case 3), vascular proliferation was prominent with perivascular sclerosis (Figure 2E) and focal deposition of osteoid matrix mimicking osteosarcoma (Figure 2F).
Figure 2.
Typical features of follicular dendritic cell sarcoma of the conventional (A-F) and inflammatory pseudotumor-like types (G and H). Spindle and ovoid tumor cells, frequently growing in nodules as in Case 1 (A), arrange in whorl (Case 3, B and C) and storiform (Case 3, D) and fascicular patterns (Case 6, H), with sprinkling small lymphocytes throughout the former type of lesions and numerous plasma cells and lymphocytes in the latter. Perivascular sclerosis was noted in Case 3 (E), with foci of osteoid matrix deposition resembling osteosarcoma (F). HE: A, × 40; B, E and G, × 100; C, D, F and H, × 200.
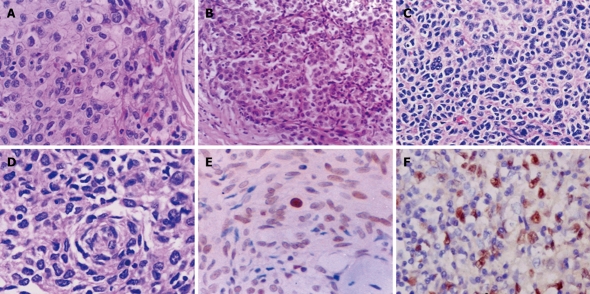
Figure 3.
Atypical morphology of FDC sarcoma (A-D) and expression of p53 protein (E) and Epstein-Barr virus-encoded RNA (EBER) (F) in tumor cells. A-D: Epithelioid (A and B) and pleomorphic tumor cells (C and D) are arranged in a sheet-like or diffuse pattern. Lymphocyte infiltration is less prominent (A and B) or absent (C and D) in these areas. HE: A, C and D, × 400; B, × 200; E: Nuclear immunoreactivity for p53 protein in majority of tumor cells. S-P, × 400; F: In situ hybridization signal for EBER in tumor cells, × 400.
As described above, the histology varies greatly among the lesions. It is conceivable to predict clinical outcomes using pathological parameters. According to our own data and proposals by other authors[3], we defined the criteria for low- and high-grade FDC sarcomas using six parameters. As shown in Table 3, the former four were found to be decisive and regarded as major factors for the grading. The lesions with typical architectural and cellular phenotypes, mitotic counts below 5/10 HPF and Ki-67-LI below 10%, as in Cases 1, 3, 5 and 6, were classified as low-grade tumors, and those with anaplastic morphology, mitotic counts up to 5/10 HPF and/or Ki-67-LI up to 10%, as in Cases 2, 4, 7-9, as high-grade tumors. The rest two parameters, including necrosis and loss or reduction of infiltrating lymphocytes, were found to be useful adjuvant factors, but not prerequisites for establishing a diagnosis of the high-grade lesion.
Table 3.
Histological features of low- and high-grade FDC sarcomas
| Parameters | Low-grade phenotypes | High-grade phenotypes |
| Architectures | Nodular patterns in whorl, storiform and fascicular arrangement | Diffuse and sheet-like patterns |
| Cellular features | Spindle or blunt cells with mild nuclear atypia and a small nucleolus in > 90% of area | Epithelioid or pleomorphic cells with marked nuclear atypia and large nucleoli |
| Mitotic counts | < 5/10 HPF | ≥ 5/10 HPF |
| Ki-67-LI | < 10% | ≥ 10% |
| Necrosis | Absent | Frequently present |
| Lymphocyte infiltration | Sporadic and throughout the lesion | Focal or regional |
As listed in Table 4, all of the nine lesions were positive for vimentin and negative for CK and CD45. Weak immunoreactivity for EMA was observed in two cases. A diagnosis of FDC sarcoma was established by its immunohistochemical phenotypes including positivity for CD21 (9/9, 100%), CD23 (7/7, 100%) and CD35 (6/9, 67%). Podoplanin was detected in three (43%) of the seven cases. CD68 and S100 protein were detected in about half of the lesions, but their expression levels were low to moderate. Nuclear accumulation of p53 protein, albeit at relatively low levels, was observed in most of the tumor cells in eight (89%) of the nine lesions (Figure 3E). Amplification and sequencing were successful for P53 exons 5-8 in one lesion (Case 9), and we failed to show any mutation. Ki-67-LI in these lesions was quite variable, ranging from 1% to 40%. In situ hybridization was performed in seven of the lesions for EBER, with positive signals demonstrated only in the hepatic lesion (Case 6, Figure 3F).
Table 4.
Expression of lineage-specific and kinetic-associated molecules and EBV-encoded RNA (EBER) in nine FDC sarcomas (Cases 1-9)
| Cases | CK | EMA | Vimentin | CD45 | CD21 | CD23 | CD35 | Podoplanin | CD68 | S-100 protein | p53 protein1 | Ki-67-LI (%) | EBER |
| 1 | - | 1+ | 3+ | - | 3+ | NT | 1+ | NT | NT | NT | 3+ | 4 | NT |
| 2 | - | - | NT | - | 2+ | NT | 2+ | NT | NT | - | 2+ | 15 | NT |
| 3 | - | 1+ | 2+ | - | 2+ | 3+ | - | 3+ | NT | - | 2+ | 1 | - |
| 4 | - | - | 3+ | - | 3+ | 2+ | 2+ | - | - | 1+ | 2+ | 8 | - |
| 5 | - | - | 3+ | - | 2+ | 2+ | 3+ | 2+ | 1+ | 2+ | 2+ | 15 | - |
| 6 | - | - | NT | - | 1+ | 2+ | - | - | 1+ | 1+ | - | 10 | + |
| 7 | - | - | 2+ | - | 1+ | 3+ | 3+ | - | - | - | 2+ | 40 | - |
| 8 | - | - | 1+ | - | 3+ | 2+ | - | 2+ | 2+ | 1+ | 2+ | 20 | - |
| 9 | - | - | 2+ | - | 1+ | 2+ | 1+ | - | - | - | 2+ | 35 | - |
Evaluated by the percentages of positive tumor cells, although majority of the positive cells showing weak nuclear immunoreactivity. Immunoreactivities expressed as strong (3+), moderate (2+), weak (1+) and negative (-); NT: Not tested.
As the initial treatment, surgery was performed for all of the lesions, with the tumor masses completely removed in seven cases. In Cases 1 and 8, complete resection was not fulfilled because of the involvement of the internal carotid artery. Adjuvant treatment was given to six of the patients (Cases 1, 3-5, 8 and 9) after the operation. Radiation was administered in all the cases and chemotherapy in three cases (Cases 3, 5 and 8). The remaining three patients (Cases 2, 6 and 7) rejected any adjuvant treatment. Follow-up was carried out in all the cases for a period of 12-86 mo, with a median of 27 mo. As shown in Table 1, seven (78%) patients remained alive, four of them had recurrence or metastasis and three were alive without event, and two (22%) died of the tumor recurrence. Of the six cases with events, two recurred locally and four had distant metastases, with a disease-free period below 3 years.
Clinicopathologic features of extranodal FDC sarcomas
Data of 106 extranodal FDC sarcomas, including nine new cases (Table 1) and 97 from literatures (Table 5), were extracted and reviewed carefully. Of the patients, 56 were men and 50 were women, with a ratio of 1.1:1. Ages of the patients ranged from 9 to 82 years at diagnosis, with their mean being 46 years and median being 44 years. The tumors were identified at different anatomic regions. The abdominal and pelvic region was affected in 46 cases (43%), including 14 in the liver, 13 in abdominal/pelvic soft tissues, five in the spleen, and 14 at other organs including pancreas (n = 3), stomach (n = 3), colon and rectum (n = 3), appendix (n = 2), ampulla of Vater (n = 1), small intestine (n = 1) and adrenal (n = 1). Of the five splenic cases, two also showed liver involvement. These hepatic lesions were regarded as metastasis in this study. Head and neck were another common region for FDC sarcoma (38/106, 36%), with tonsils and parapharyngeal space affected most frequently. Fourteen (13%) of the lesions were identified from thoracic cavity, of which nine were located at mediastinum, three at lung, one at pleura and one at the chest wall. The less frequent sites included breast (n = 3), soft tissues at thigh (n = 2), dura mater (n = 1), groin (n = 1) and skin (n = 1). Tumor sizes ranged from 1.5 to 21 cm as described in longitudinal dimensions, with a mean of 7.4 cm. A broad spectrum of histological phenotypes were described, while the most helpful diagnostic features were the typical arrangement of spindle and ovoid tumor cells with eosinophilic cytoplasm, indistinct cytoplasmic borders and syncytial appearance, and presence of small lymphocytes in the tumor.
Table 5.
Clinicopathologic and therapeutic data of 97 cases of extranodal FDC sarcoma obtained from literatures (Cases 10-106)
| Cases No. | Origins | Age (yr) | Gender | Size (cm) | Sites | Initial treatment (responses) | Recurrence, DFT (mo) | Status | Follow-up (mo) |
| 10 | Chan et al[24], 1994 | 44 | M | 1.5 | Tonsil | Surg | NED | 36 | |
| 11 | Chan et al[24], 1994 | 63 | F | 3 | Palate | Surg + RT | NED | 54 | |
| 12 | Hollowood et al[25], 1995 | 23 | F | 6.5 | Small intestine | Surg | LR (2 times), 6 | AWD | 24 |
| 13 | Hollowood et al[25], 1995 | 63 | M | NA | Pancreas head | Surg | LR, 8 | DOD | 11 |
| 14 | Nayler et al[26], 1996 | 18 | F | 2 | Tonsil | Surg + ChT | NA | NA | |
| 15 | Perez-Ordonez et al[16], 1996 | 40 | F | NA | Abdomen | Surg + ChT | LR and DM (liver), 12 | AWD | 24 |
| 16 | Perez-Ordonez et al[16], 1996 | 62 | F | NA | Tonsil | Surg | NED | 12 | |
| 17 | Perez-Ordonez et al[16], 1996 | 62 | M | NA | Mediastinum | Surg + ChT | DM (lung), 24 | AWD | 24 |
| 18 | Perez-Ordonez et al[16], 1996 | 46 | M | NA | Mediastinum | Surg + RT | NED | 12 | |
| 19 | Perez-Ordonez et al[16], 1996 | 31 | M | NA | Mediastinum | Surg | NED | 10 | |
| 20 | Perez-Ordonez et al[16], 1996 | 75 | F | NA | Spleen | Surg | LR, 11 | DOD | 11 |
| 21 | Selves et al[27], 1996 | 68 | F | NA | Liver | Surg + ChT | NED | 30 | |
| 22 | Shek et al[28], 1996 | 35 | F | NA | Liver | Surg | LR (2 times), 30 | AWD | 60 |
| 23 | Chan et al[3], 1997 | 42 | M | 8 | Mesocolon, involving LN and peritoneum | Surg + ChT | LR, 18 | DOD | 18 |
| 24 | Chan et al[3], 1997 | 32 | M | NA | Tonsil | Surg + RT | LR and DM (LN), 54 | AWD | 54 |
| 25 | Chan et al[3], 1997 | 40 | F | 7 | Parapharyngeal space | Surg | LR, 12 | AWD | 12 |
| 26 | Chan et al[3], 1997 | 17 | M | 11 | Neck soft tissue | Surg + ChT | NA | NA | |
| 27 | Chan et al[3], 1997 | 25 | F | 7 | Neck soft tissue | Surg + RT | DM (lung), 20 | AWD | 20 |
| 28 | Beham-Schmid et al[29], 1998 | 44 | M | 2 | Nasopharynx | Surg + RT + ChT | NED | 20 | |
| 29 | Shek et al[30], 1998 | 37 | M | 1.5 | Liver | Surg | NED | 24 | |
| 30 | Shek et al[30], 1998 | 61 | F | 4 | Ampulla of Vater | Surg | NED | 9 | |
| 31 | Araújo et al[31], 1999 | 14 | M | 1.5 | Hard palate | Surg | NED | 5 | |
| 32 | Desai et al[32], 1999 | 45 | F | NA | Parapharyngeal space | Surg | LR, 31 | NED | 57 |
| 33 | Fisher et al[33], 1999 | 41 | F | 2.5 | Breast | Surg | LR and DM (LN), 36 | NED | 36 |
| 34 | Galati et al[34], 1999 | 65 | F | 1.5 | Thyroid | Surg + RT | NED | 36 | |
| 35 | Schwarz et al[35], 1999 | 62 | M | 15 | Abdominal wall | Surg + RT | NED | 8 | |
| 36 | Choi et al[36], 2000 | 28 | F | 4 | Neck soft tissue | Surg | DM (lung), 324 | AWD | 324 |
| 37 | Choi et al[36], 2000 | 66 | F | NA | Neck soft tissue | Surg + RT | DM (lung), 24 | AWD | 24 |
| 38 | Han et al[37], 2000 | 45 | M | 5 | Stomach | Surg | NED | 10 | |
| 39 | Chan et al[38], 2001 | 34 | M | NA | Nasopharynx | Surg | NED | 36 | |
| 40 | Chang et al[39], 2001 | 37 | F | 5 | Ascending colon | Surg | NED | 7 | |
| 41 | Chen et al[5], 2001 | 57 | F | 9.5 | Liver | Surveillance | AWD | 36 | |
| 42 | Chen et al[5], 2001 | 51 | F | 15 | Liver | Surg | NED | 12 | |
| 43 | Chiaramonte et al[40], 2001 | 39 | M | NA | Retroperitoneum | Surg | DM (LN and lung), 6 | DOD | 6 |
| 44 | Shah et al[41], 2001 | 33 | M | 9.5 | Lung | Surg + ChT | NA | NA | |
| 45 | Biddle et al[11], 2002 | 33 | M | NA | Pharynx | Surg + RT | DM (lung), 10 | AWD | 10 |
| 46 | Biddle et al[11], 2002 | 48 | F | 1.5 | Tonsil | Surg | NED | 6 | |
| 47 | Biddle et al[11], 2002 | 48 | M | 3.5 | Tonsil | Surg | NED | 8 | |
| 48 | Pileri et al[2], 2002 | 42 | M | NA | Mediastinum | Surg | NED | NA | |
| 49 | Vargas et al[42], 2002 | 54 | F | 3 | Tonsil | Surg | NED | 8 | |
| 50 | Vargas et al[42], 2002 | 54 | F | 6 | Left parotid | Surg + RT | LR, 6 | AWD | 8 |
| 51 | Ceresoli et al[43], 2003 | 35 | M | NA | Mediastinum | ChT + RT (Prog) | DOD | 7 | |
| 52 | Satoh et al[44], 2003 | 16 | M | 3 | Tonsil | Surg + RT + ChT | NED | 24 | |
| 53 | Geerts et al[45], 2004 | 40 | F | NA | Stomach, with DM (liver) | Surg | AWD | 5 | |
| 54 | Grogg et al[46], 2004 | 62 | M | NA | Left-thigh soft tissue | Surg | NED | 18 | |
| 55 | Grogg et al[46], 2004 | 57 | F | NA | Tonsil, involving LN | Surveillance | AWD | 8 | |
| 56 | Grogg et al[46], 2004 | 64 | F | 14 | Spleen, with DM (liver) | Surg + ChT | NED | 5 | |
| 57 | Kröber et al[47], 2004 | 76 | M | NA | Mediastinum | Surg + RT | NED | 24 | |
| 58 | O’Malley[48], 2004 | 38 | M | 5 | Cecum | Surg | NA | NA | |
| 59 | Shi et al[49], 2004 | 37 | M | 1.5 | Tonsil | Surg + ChT | NED | 36 | |
| 60 | Bradshaw et al[50], 2005 | 9 | M | 5 | Neck soft tissue | Surg | DM (lung), 60 | NED | 96 |
| 61 | Kazakov et al[13], 2005 | 38 | M | 1.8 | Skin | Surg | NA | NA | |
| 62 | Khalid et al[51], 2005 | 19 | F | 16 | Liver | ChT (PR) | DOD | 24 | |
| 63 | Torres et al[52], 2005 | 82 | M | 15 | Liver | Surg | NED | 18 | |
| 64 | Aydin et al[53], 2006 | 76 | F | 3.5 | Tonsil | Surg + RT | NED | 48 | |
| 65 | Choi et al[54], 2006 | 68 | M | NA | Dura mater | Surg + RT | NED | 7 | |
| 66 | Clement et al[55], 2006 | 27 | F | 4 | Tonsil | Surg + RT | NED | 6 | |
| 67 | Díaz de Liaño et al[56], 2006 | NA | NA | NA | Abdominal cavity | Surg | DM (liver), 18 | NED | 18 |
| 68 | Gan et al[57], 2006 | 32 | F | 4.5 | Parotid | Surg | NED | 20 | |
| 69 | Jiang et al[58], 2006 | 46 | F | 8.4 | Anterior mediastinum | ChT (stable) | DM (bone), 12 | NED | 19 |
| 70 | Jiang et al[59], 2006 | 46 | F | 21 | Pelvic/abdominal cavity, multiple | Surg | NED | 6 | |
| 71 | Kovács et al[60], 2006 | 65 | M | 4 | Lung | Surg | NED | 18 | |
| 72 | Li et al[61], 2006 | 19 | M | NA | Nasal cavity | Surg | NED | 11 | |
| 73 | Lu et al[62], 2006 | 72 | F | 7 | Groin | Surg | NA | NA | |
| 74 | Shen et al[63], 2006 | 64 | M | 10.5 | Pancreas | Surg | DM (liver), 18 | NED | 24 |
| 75 | Shia et al[64], 2006 | 30 | M | NA | Rectum | Surg | LR, 10 | AWD | 15 |
| 76 | Shia et al[64], 2006 | 29 | F | 12 | Lesser omentum | Surg | NED | 17 | |
| 77 | Shia et al[64], 2006 | 69 | F | NA | Tonsil | Surg + RT | DM (hilar LN+lung), 96 | AWD | 108 |
| 78 | Xu et al[65], 2006 | 16 | M | 5 | Adrenal | Surg | NA | NA | |
| 79 | Chang et al[66], 2007 | 64 | M | 16 | Chest wall | Surg | NED | 15 | |
| 80 | Leipsic et al[67], 2007 | 43 | M | 13 | Mediastinum | Surg | NA | NA | |
| 81 | Padilla-Rodríguez et al[68], 2007 | 35 | M | 21 | Retroperitoneal space | Surg + RT | NED | 24 | |
| 82 | Sander et al[69], 2007 | 44 | F | 11 | Spleen | Surg | DM (thorax, liver and kidney), 4 | DOD | 9 |
| 83 | Soriano et al[70], 2007 | 25 | F | 6 | Pelvis | Surg | LR, 2, 2nd Surg+RT | NED | 14 |
| 84 | Soriano et al[70], 2007 | 56 | M | 2 | Pancreas, involving LN | Surg + RT | LR, 2 | AWD | 7 |
| 85 | Soriano et al[70], 2007 | 33 | M | NA | Nasopharynx | Surg + RT | LR, 10 | DOD | 14 |
| 86 | Soriano et al[70], 2007 | 64 | F | 4 | Spleen, with liver involved | Surg + ChT | AWD | 29 | |
| 87 | Soriano et al[70], 2007 | 66 | M | 16 | Pleura | ChT (Prog) | DOD | 7 | |
| 88 | Tu et al[12], 2007 | 63 | M | 15 | Jejunum mesentery | Surg | NA | NA | |
| 89 | Tu et al[12], 2007 | 43 | M | 4 | Appendix | Surg | NA | NA | |
| 90 | Tu et al[12], 2007 | 28 | F | 15 | Stomach | Surg | DM (liver), 3 | AWD | 3 |
| 91 | Yuan et al[71], 2007 | 29 | M | 10 | Liver | Surg | LR, 6 | AWD | 6 |
| 92 | De Pas et al[72], 2008 | 40 | F | 4 | Breast | Surg | NED | 62 | |
| 93 | De Pas et al[72], 2008 | 53 | F | NA | Liver | Surg | DM (LN), 11 | DOD | 22 |
| 94 | De Pas et al[72], 2008 | 64 | F | 2.1 | Breast | Surg | NED | 20 | |
| 95 | Granados et al[73], 2008 | 57 | F | 13 | Liver | Surg | NED | 24 | |
| 96 | Liu et al[74], 2008 | 42 | M | 12 | Gastrocolic omentum | Surg | NA | NA | |
| 97 | Peng et al[75], 2008 | 60 | M | 7 | Mesentery | Surg | NA | NA | |
| 98 | Zhang et al[76], 2008 | 36 | M | NA | Nasopharynx | Surg | NED | 5 | |
| 99 | Zhang et al[76], 2008 | 32 | F | NA | Spleen | Surg | NA | NA | |
| 100 | An et al[77], 2009 | 40 | M | 5 | Liver | Surg | NED | 3 | |
| 101 | Denning et al[78], 2009 | 64 | F | 1.7 | Lung | Surg | NED | 24 | |
| 102 | Liu et al[79], 2009 | 75 | M | 4 | Liver | Surg | NA | NA | |
| 103 | Romero-Guadarrama et al[80], 2009 | 54 | F | 15 | Thigh soft tissue | Surg | LR, 12 | NED | 48 |
| 104 | Shen et al[81], 2009 | 43 | M | 5 | Appendix | Surg | LR, 8 | NED | 8 |
| 105 | Vaideeswar et al[82], 2009 | 50 | M | 2.5 | Tonsil | Surg | NED | 48 | |
| 106 | Xu et al[83], 2009 | 57 | F | 11 | Liver | Surg | NA | NA |
LN: Lymph node; NA: Not available; Prog: Disease progression.
Morphologic information, indicative of the architectural and cellular anaplasia, was collected from the articles and re-evaluated carefully. Of the 63 informative cases, 37 (59%) were described as lesions with mild atypia, and 26 (41%) with moderate to severe cytological atypia throughout or in a certain area of the tumor. In the current study, these two groups were classified into low-grade and high-grade lesions, respectively. All of the tumors occurring in the liver resembled an inflammatory pseudotumor in morphology. Five of the eight informative cases were divided into low-grade group, and the other three into high-grade group due to identification of anaplastic components. Coagulative necrosis was recorded in 29 (45%) of the 64 informative cases. Mitotic counts were provided in 61 cases, with 31 (51%) up to 5/10 HPF. Ki-67-LI was calculated in 20 lesions, with 14 (70%) up to 10%. Preoperative tumor spreading was identified in 15 (23%) of the 66 informative cases through pathological examination of the resected samples, including local lymph node involvement in 11, distant metastasis to liver or peritoneal dissemination in three, and both nodal involvement and peritoneal spreading in one.
Management and clinical outcomes
One hundred and four patients received therapeutic procedures and two rejected any treatment. Of the patients, 100 (94%) were treated surgically to remove the tumor. Adjuvant treatment was administered in 35 cases, including radiation in 20, chemotherapy in 10 and both procedures in five. Chemotherapy was performed in most of the cases based on CHOP regimen including cyclophosphamide, adriamycin, vincristine and prednisone.
Follow-up data were available in 91 cases. The observation periods ranged from 3 to 324 mo, with a mean and a median of 27 and 19 mo, respectively. Overall, 38 patients (42%) had local recurrence and/or distant metastasis during the adjuvant treatment or surveillance period, with the events occurring within 36 mo in most (33/37, 89%) of the cases. Local recurrence occurred in 21 patients (23%) at a median of 12 mo after surgical removal (range, 3-31 mo). Distant metastases occurred in 19 patients (21%) at a median of 14 mo after operation (range, 4-324 mo). Metastatic sites included lung (n = 9), liver (n = 6), lymph node (n = 5), bone (n = 1), thorax (n = 1), ovary (n = 1) and kidney (n = 1). At the last follow-up, 12 patients (13%) died of the tumor, 25 (27%) were alive with disease, and 54 (59%) were alive with no evidence of disease. The 2-year and 5-year overall survival rates for the entire group were 82% and 79%, and their 2-year and 5-year disease-free survival rates were 57% and 32%, respectively (Figure 4).
Figure 4.
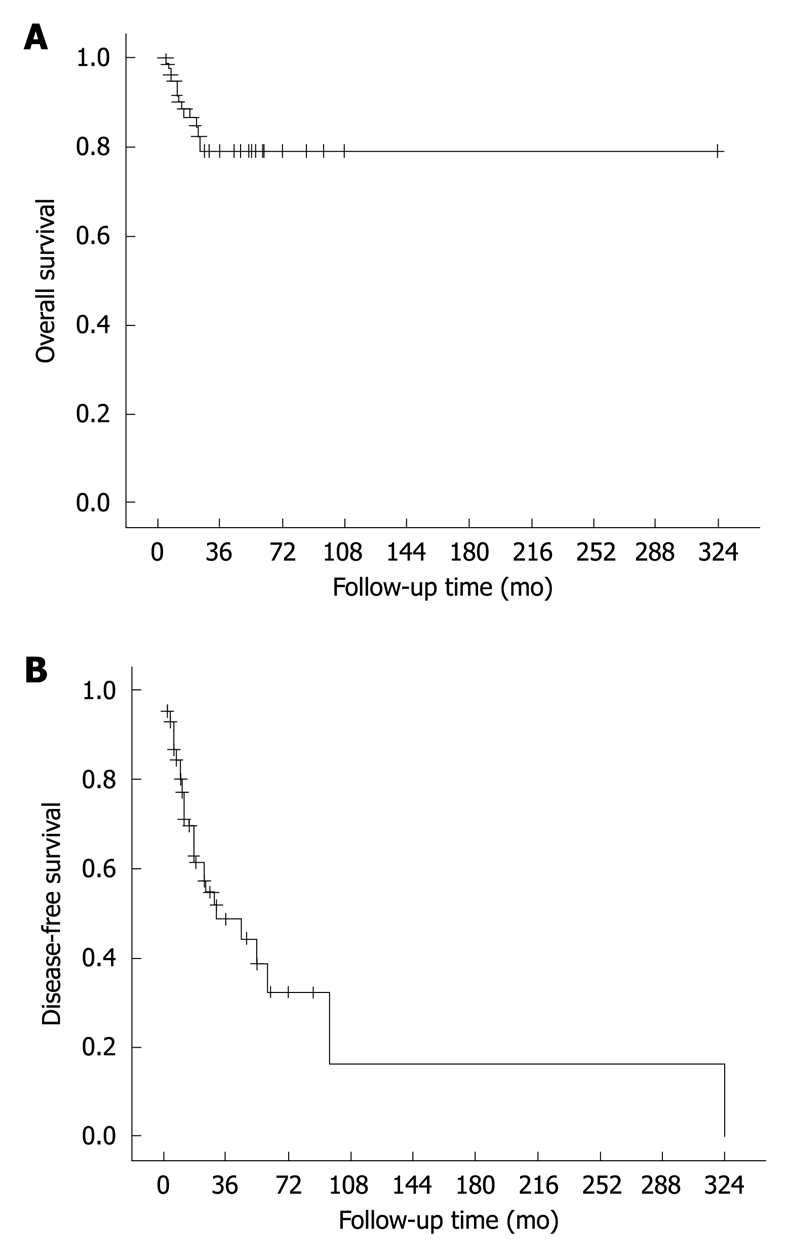
Overall (A) and disease-free survival curves (B) of FDC sarcoma. The results are based on follow-up data of 91 informative cases.
Prognostic factors
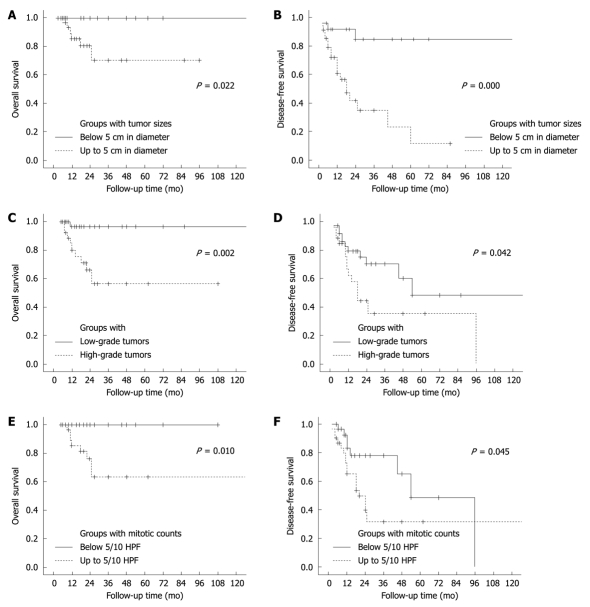
Associations between nine clinicopathological factors and recurrence risk were studied, and the results are summarized in Table 6. Of the 60 informative lesions, 25 (42%) were smaller than 5 cm and 34 (58%) were up to 5 cm in their longitudinal dimensions. Four (16%) of the smaller lesions relapsed (two with local recurrence and two with distant metastasis) and 19 (56%) of the larger lesions relapsed (nine with local recurrence and 10 with metastasis). The recurrence rate was markedly elevated with the increase of tumor size (P = 0.003). In addition, a mortality rate of 17% was observed for cases with the larger lesions, and none of the patients with smaller lesions died of the disease (P = 0.036).
Table 6.
Associations between clinicopathological parameters and recurrence risk in patients with extranodal FDC sarcoma
| Pathologic parameters | Recurrence frequencies (%) | P values |
| Age (yr) | ||
| < 50 | 26/51 (51) | 0.072 |
| ≥ 50 | 11/35 (31) | |
| Gender | ||
| Male | 15/40 (38) | 0.290 |
| Female | 22/46 (48) | |
| Size (cm) | ||
| < 5 | 4/25 (16) | 0.003 |
| ≥ 5 | 19/34 (56) | |
| Histological grades | ||
| Low | 12/37 (32) | 0.046 |
| High | 15/26 (58) | |
| Mitotic counts | ||
| < 5/10 HPF | 8/30 (27) | 0.013 |
| ≥ 5/10 HPF | 18/31 (58) | |
| Ki-67-LI | ||
| < 10% | 2/6 (25) | 0.628 |
| ≥ 10% | 8/14 (57) | |
| Necrosis | ||
| Absent | 17/35 (49) | 0.307 |
| Present | 13/29 (45) | |
| Treatments | ||
| Surgery alone | 22/52 (42) | 0.590 |
| Surgery + adjuvant therapy | 15/31 (48) | |
| Sites | ||
| Abdominal/pelvic | 18/37 (49) | 0.372 |
| Other sites | 20/53 (38) |
For most of the lesions, as listed in Table 5, histological grades were assessed mainly according to descriptions on architectural features and cellular atypia from respective literatures. For this reason, tumor necrosis, mitotic activity and Ki-67-LI were evaluated separately in this study. Of the 63 informative cases, 37 (59%) were evaluated as low-grade and 26 (41%) as high-grade lesions. Twelve (32%) of the low-grade lesions relapsed (seven with local recurrence, four with metastasis and one with both local recurrence and metastasis) and 15 (58%) of the high-grade lesions relapsed (seven with local recurrence, seven with metastasis and one with both local recurrence and metastasis). The recurrence rate was higher in the latter group than in the former (P = 0.046). In addition, the high-grade lesions resulted in death in nine (35%) of the cases, while only one (3%) of the patients with low-grade lesions died of the disease, the mortality rate being closely associated with tumor grade (P = 0.001). Eighteen (58%) of the 31 lesions with a mitotic count ≥ 5/10 HPF relapsed, the frequency being higher than those with a count < 5/10 HPF (8/30, 27%, P = 0.013). The mortality rate (7/31, 23%) was also higher in the former group than in the latter (0/30, P = 0.011). According to the limited number of informative cases, increased Ki-67-LI (≥ 10%) appeared to indicate an unfavorable clinical outcome, but its impact failed to reach statistical significance (P = 0.628). The patients younger than 50 years tended to develop tumor recurrence more frequently (51%) than those over 50 years of age (31%), but without statistically significant difference (P = 0.072). Other factors, including gender, necrosis and therapeutic procedures following surgery, were not related to clinical outcomes (P > 0.05).
The associations of tumor size, histological grade and mitoactivity with overall and disease-free survival are described in Figure 5. Among them, tumor size and histological grade were the most important factors, respectively, for tumor recurrence (Figure 5B) and disease-associated death (Figure 5C). Thus, a model was established for the recurrence risk assessment by combining the former two parameters. Recurrence rates of lesions smaller than 5 cm, the larger lesions (≥ 5 cm) with low-grade histology and those with high-grade histology were 16% (4/25), 46% (11/24) and 73% (8/11), respectively, their difference being significant (P = 0.000). Based on the recurrence potential, these three groups of lesions were designated as low-, intermediate- and high-risk FDC sarcomas, respectively (Figure 6). A markedly higher mortality rate was also observed in cases with a high-risk tumor (45%) than in those with the low-risk (0%, P = 0.001) and intermediate-risk lesions (4%, P = 0.006). The low-risk group had a longer survival and the high-risk group had the most unfavorable clinical outcomes considering both the tumor recurrence and mortality (Figure 7).
Figure 5.
Overall (A, C and E) and disease-free survival curves (B, D and F) of patients with FDC sarcoma, showing data of 120 mo.
Figure 6.
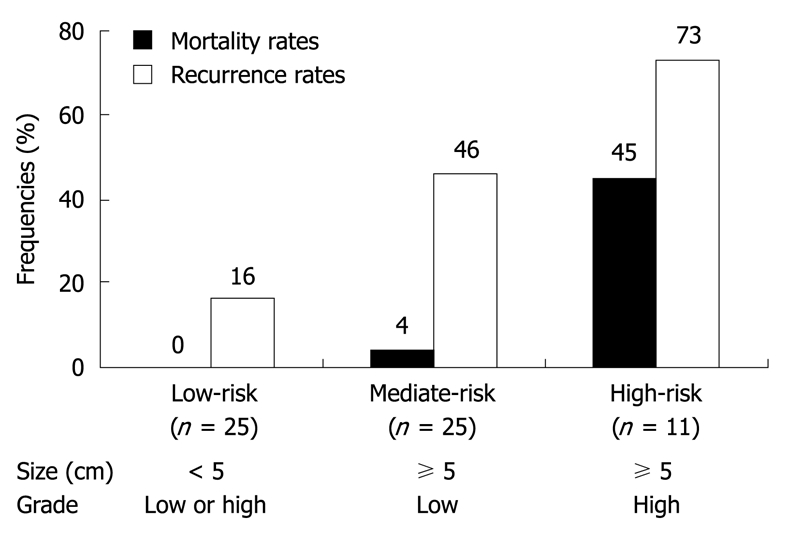
Definitions of low- (low-risk), intermediate- (mediate-risk) and high-risk groups (high-risk) of extranodal FDC sarcomas. Mortality rates: overall, P = 0.000; low-risk vs mediate-risk, P = 1.000; low-risk vs high-risk, P = 0.001; mediate-risk vs high-risk, P = 0.006; Recurrence rates: overall, P = 0.001; low-risk vs mediate-risk, P = 0.032; low-risk vs high-risk, P = 0.002; mediate-risk vs high-risk, P = 0.167.
Figure 7.
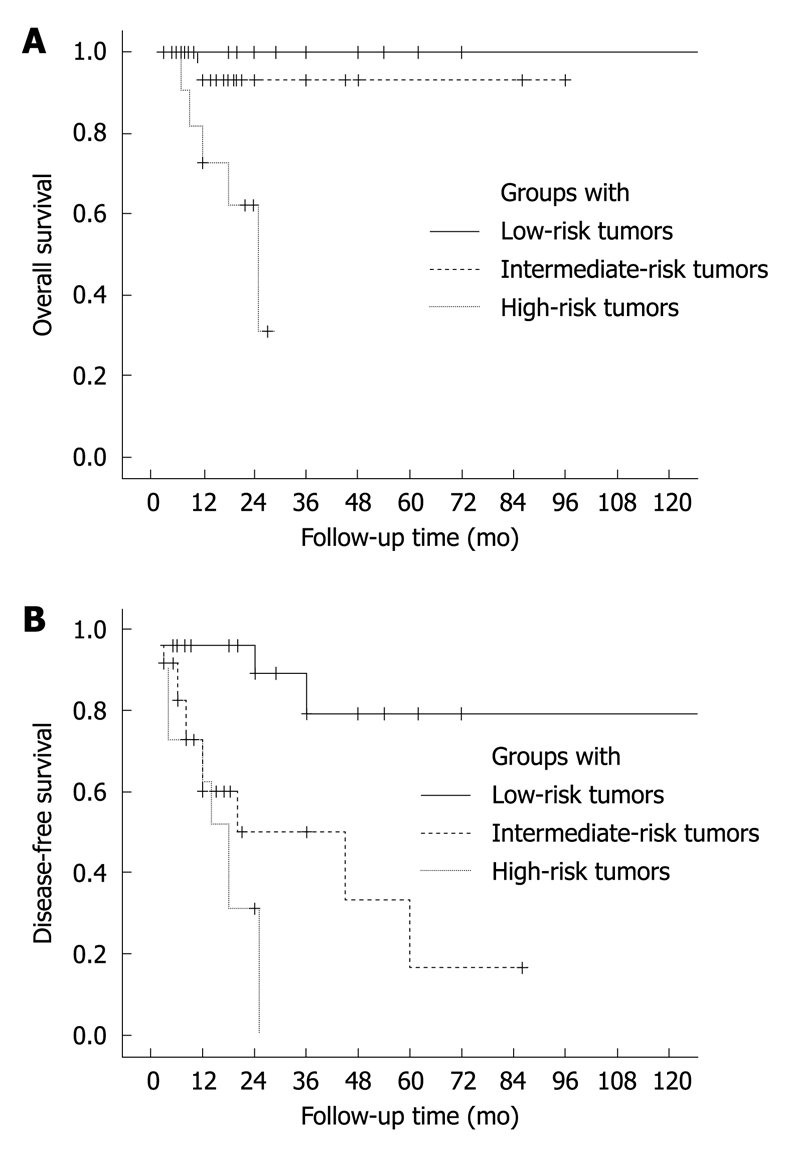
Overall (A) and disease-free survival curves (B) of patients with FDC sarcoma, estimated in groups with low-, intermediate- and high-risk tumors, showing data of 120 mo. A: Overall, P = 0.000; low-risk vs mediate-risk, P = 0.273; low-risk vs high-risk, P = 0.000; mediate-risk vs high-risk, P = 0.017; B: Overall, P = 0.000; low-risk vs mediate-risk, P = 0.003; low-risk vs high-risk, P = 0.000; mediate-risk vs high-risk, P = 0.207.
During the follow-up period, 18 (49%) of the 37 informative lesions from abdominal and pelvic cavities recurred (20/53, 38%, P = 0.302). Of the 37 abdominal/pelvic lesions, 12 occurred in the liver and 25 were identified from other tissues. A lower recurrence risk (3/12, 25%) was observed in hepatic lesions than in the extrahepatic lesions (15/25, 60%, P = 0.046). A more favorable disease-free survival was also observed for the former group (P = 0.004). However, there was no significant difference between the hepatic (2/12, 17%) and extrahepatic groups (5/25, 20%) in mortality (P = 0.372, Figure 8). The size of the hepatic lesions (mean, 9.5 cm; range, 2-21 cm) was comparable to that of the extrahepatic lesions (mean, 10.4 cm; range, 1.5-16 cm, P = 0.644). Morphologically, all of the hepatic lesions belonged to the inflammatory pseudotumor-like variant, and most of the extrahepatic lesions were classified into conventional type. However, we failed to show a significant difference in frequency of occurrence of high-grade component between the hepatic (3/8, 38%) and extrahepatic lesions (7/18, 39%, P = 1.000).
Figure 8.
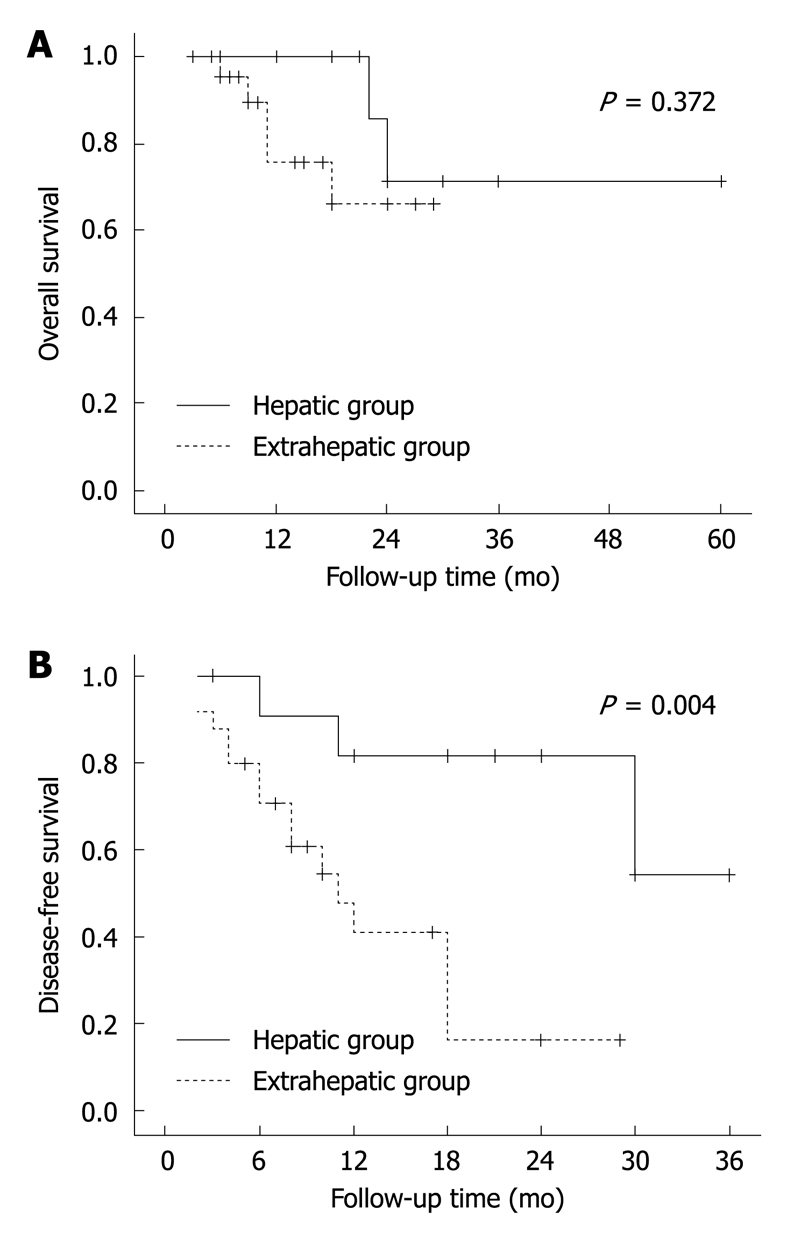
Overall (A) and disease-free survival curves (B) of abdominal/pelvic FDC sarcomas. The lesions are divided into hepatic and extrahepatic groups.
The extrahepatic, abdominal/pelvic lesions were also compared with those occurring out of abdominal/pelvic cavity. Their recurrence rates were 60% (15/25) and 38% (20/53), respectively, with the former lesions recurring more frequently (P = 0.000) and earlier (Figure 9). The former cases tended to have a higher mortality rate (5/25, 20%) than the latter group (5/53, 6%), but without statistically significant difference (P = 0.051, Figure 9). These two groups also showed difference in their sizes, with the average of the former group (9.5 cm) being larger than that of the latter group (5.3 cm, P = 0.003). They were not significantly different in histological grade (P = 0.204), with high-grade phenotypes observed in 57% (26/46) of the former lesions and 39% (7/18) of the latter lesions.
Figure 9.
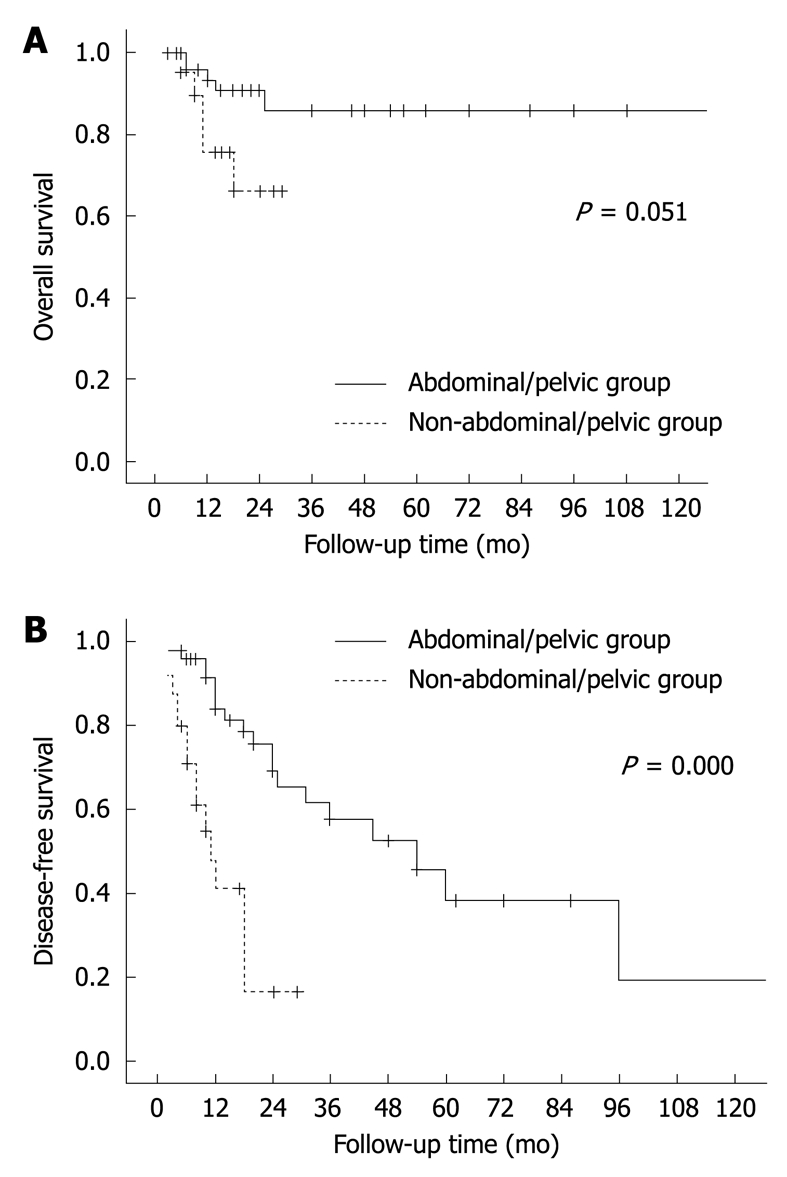
Overall (A) and disease-free survival curves (B) of patients with extrahepatic FDC sarcomas, showing data of 120 mo. The lesions are divided into abdominal/pelvic and non-abdominal/pelvic groups.
DISCUSSION
FDC sarcoma may occur in extranodal tissues from a variety of anatomical sites, including head and neck, liver, spleen, gastrointestinal tract, soft tissue, skin, lung and breast[14]. As shown in this survey, abdominal/pelvic cavity and head/neck areas are affected more frequently, where 43% and 36% of extranodal FDC sarcomas were identified. It usually presents with a solitary tumor, typically composed of spindle and ovoid cells with scattered mature lymphocytes. However, there is a broad spectrum of morphologic variations in this tumor, some of which overlap with those of other neoplasms including ectopic meningioma, thymoma, myoepithelial tumor, inflammatory myofibroblastic tumor and GIST. In our series, osteoid component was found in a low-grade FDC sarcoma (Case 3). This is the first case showing presence of an osteosarcoma-like phenotype (Figure 2F). Misdiagnosis may occur when a pathologist is not aware of the tumor, as in Cases 1 and 2 of the current series (Table 1). The diagnosis can be established by immunohistochemical demonstration of CD21, CD23, CD35[70], podoplanin and clusterin expression[64,84,85].
FDC sarcoma is usually regarded as an indolent tumor with a tendency of local recurrence but a low risk of metastasis, behaving like a low-grade soft tissue sarcoma[15]. In a study of 13 cases, Perez-Ordonez et al[16] observed a substantial risk of metastases, prompting consideration of this malignancy to be of intermediate grade. An analysis by Shia et al[64] found that the 2- and 5-year recurrence-free survival rates were 62% and 27%, respectively. In our study, a total number of 106 extranodal cases were analyzed. The recurrence rate was 42%, including local recurrence in 23% and distant metastasis in 21% of the cases, and the mortality rates were 13% over the periods from 3 to 324 mo (mean, 27 mo; median, 19 mo). The 2-year and 5-year disease-free survival rates were 57% and 32%, and the 2-year and 5-year overall survival were 82% and 79%, respectively. It appears that extranodal FDC sarcomas, as a whole, are more aggressive than a low-grade soft tissue sarcoma.
As demonstrated in this study, pathologic representations of extranodal FDC sarcoma are variable among different cases. New approaches are needed to find most important prognostic factors relevant to their clinical outcomes. It has been proposed that intra-abdominal involvement, a high mitotic count (≥ 5/10 HPF), coagulative necrosis and marked cellular atypia are potentially helpful predictors of an unfavorable outcome[3]. In this study, nine clinicopathological parameters were analyzed, and large tumor size (≥ 5 cm in diameter), high-grade histology and high mitotic counts (≥ 5/10 HPF) were found to be closely associated with the clinical outcomes. The recurrence risk seemed to be increased in lesions with high Ki-67-LI, but the association showed no statistical significance. It needs to be clarified in future studies with a larger number of informative cases.
While some histological phenotypes, including cellular atypia, mitoactivity and coagulative tumor necrosis, were considered important parameters for prognosis assessment[3], a grading model remains to be established. Soriano et al[70] identified five high-grade lesions from 14 FDC sarcomas based on cellular atypia, but they failed to show its relevance of statistical significance to clinical outcomes. In this study, we examined nine new cases from northern China, extracted and reviewed data of 97 cases from literatures, and established histological criteria for low-grade and high-grade FDC sarcomas according to four major parameters including architectural alteration, cellular atypia, mitoactivity and/or Ki-67-LI (Table 3). Using this model, 63 informative cases were classified into low- (n = 37) and high-grade lesions (n = 26). Their recurrence rates were 32% and 58%, and their mortality rates were 3% and 35%, respectively. The results show a significant difference in their recurrence risks and mortality rates. Our data demonstrate that these two groups of lesions behave like low- and high-grade soft tissue sarcomas.
It should be noted that, for reported cases, histological grades were evaluated mainly according to two parameters (the architectural features and cellular atypia) in this study. Mitotic counts were not available for about half of the cases from literatures, and Ki-67-LI was provided in only 21 of the reported cases. For this reason, influence of mitoactivity on clinical outcomes was evaluated separately, with the recurrence rates of the mitotically indolent (< 5/10 HPF) and active lesions (≥ 5/10 HPF) determined to be 27% and 58%, respectively. It is expected that, with the assessment based on all of the six parameters including four major and two adjuvant factors (Table 3), the predicting power of our grading model for tumor recurrence risk would be stronger. This has been reflected in our own cases: the recurrence rates of the low- and high-grade lesions were 25% (1/4) and 100% (5/5), respectively.
Among the three parameters associated with clinical outcomes of extranodal FDC sarcoma, tumor size was shown to be most important. It appears that tumor size is a main factor for predicting its recurrence potential, and histological grade is closely associated with mortality rate mainly in patients with larger tumors (Figures 5 and 6). Considering the independent impact of the size to tumor behaviors and close association between mitoactivity and histological grade, we combined the size and grade factors, and proposed a model for recurrence risk assessment (Figure 6). By this model, FDC sarcomas were classified into low-, intermediate- and high-risk groups. Recurrence rates in the three groups were 16%, 46% and 73%, and the mortality rates were 0%, 4% and 45%, respectively. This will provide a convenient approach for diagnostic pathologists to predict the clinical outcomes of the tumor more accurately following its surgical resection or even with a biopsy. It should be noted that it is impossible to know exactly whether an FDC sarcoma developed from lymph node or extranodal tissues at advanced stages, as in some of the cases in this study. We believe that this model may also be applied for prognosis assessment of the FDC sarcomas which develop from lymph nodes.
Chan et al[3] pointed out that intra-abdominal location was associated with a higher recurrence rate. In this study, the recurrence rates of the intra-abdominal lesions and those from other sites were 49% and 38%, respectively, but their difference did not attain a statistical significance. Interestingly, a lower recurrence rate (25%) and a more favorable clinical course were observed in the hepatic lesions as compared with that of the extrahepatic, abdominal/pelvic tumors (60%). It seems to be true that, for extranodal FDC sarcomas of the conventional type, intra-abdominal lesions recur more frequently than those from other sites. The tumors of the former group were found to be larger at diagnosis (9.5 cm) than the latter lesions (5.3 cm). It is conceivable to ascribe their difference in clinical outcomes to their size difference, considering the fact that abdominal/pelvic FDC sarcomas are frequently concealed and diagnosed later compared with those from other sites including head, neck and some superficial areas.
While its pathogenesis has not been established, several factors were linked to development of FDC sarcoma. Some FDC sarcomas from lymph nodes appear to be associated with hyaline-vascular type Castleman disease[86], and similar changes were also noted in tissues surrounding some rare extranodal tumors[38,87,88]. Several reports have observed FDC proliferation and dysplastic changes in lymphoid tissues with Castleman disease where an FDC sarcoma develops[38,89-91], apparently supporting the hypothesis that FDC sarcoma develops from Castleman disease in a hyperplasia - dysplasia - neoplasia sequence[24]. However, the association was not evident in any lesion of our series.
There have been some data indicative of a link between persistent EBV infection and Castleman disease[92,93]. EBER signals were also demonstrated in some FDC sarcomas, particularly the hepatic and splenic lesions[4-9]. Most of these EBV-positive lesions contain numerous inflammatory cells, including plasma cells and lymphocytes, and dispersed tumor cells, thereby being described as inflammatory pseudotumor-like FDC sarcoma[94]. In this study, EBER was demonstrated in the hepatic inflammatory pseudotumor-like lesion (Case 6), but not in any of the six conventional FDC sarcomas examined. It appears to be true that inflammatory pseudotumor-like FDC sarcomas are related to EBV infection.
Clonal cell composition was described in an extranodal FDC sarcoma[17], but greater efforts are required to elucidate its genetic background and establish its molecular pathogenesis. Nuclear immunoreactivity for p53 protein was noticed fortuitously in a few cases[17,38]. In this series, eight extranodal FDC sarcomas without a background of Castleman disease were tested, and a low-level accumulation of p53 protein was observed in majority of tumor cells. Our data raises a possibility of involvement of the p53-mediated pathway during development or progression of FDC sarcoma. In addition, P53 exons 5-8, where most of the P53 gene mutations had been found[19,95], were examined in one of the lesions, but without any mutation demonstrated. Based on these data, we consider that the nuclear immunoreactivity may reflect accumulation of wild-type p53 protein. This phenomenon may be an adaptive reaction responsive to elevated mitotic activity and/or increased stress for cell survival, as observed in other cell types under neoplastic[96] and non-neoplastic conditions[18,97-99].
COMMENTS
Background
Follicular dendritic cell (FDC) sarcoma is a rare tumor, occurring in lymph nodes and extranodal tissues. It is usually regarded as an indolent tumor with a tendency of local recurrence but a low risk of metastasis. With more cases encountered during pathological practice and reported from literatures, various morphologic and clinical representations were noticed. Because of the rarity of the tumor, assessment of its prognosis remains difficult. Clearly, histological grade of this malignancy remains to be defined and a model is needed to evaluate the recurrence risk of individual tumors within this category.
Research frontiers
While FDC sarcoma is regarded as an indolent tumor, like a low-grade soft tissue sarcoma, a broad spectrum of pathologic phenotypes and more aggressive clinical representations were noticed for this tumor type. Intra-abdominal involvement, elevated mitoactivity, coagulative necrosis and marked cellular atypia were considered potentially helpful predictors of an unfavorable outcome, but a grading model remains to be established. In this study, a total number of 106 extranodal FDC sarcomas, nine new cases and 97 from literatures, were analyzed, histological grades of this malignancy were defined and a model was established for recurrence risk assessment of the tumor.
Innovations and breakthroughs
In this study, data of 106 cases of extranodal FDC sarcoma were extracted and reviewed. Histological criteria for low-grade and high-grade FDC sarcomas were established according to four major parameters including architectural pattern, cellular atypia, mitoactivity and/or Ki-67-labeling index. Using this model, 63 informative cases were divided into low- (n = 37) and high-grade lesions (n = 26). Their recurrence rates were 32% and 58%, and the mortality rates were 3% and 35%, respectively. The results showed a significant difference in their recurrence risks and mortality rates. In addition, nine clinicopathological parameters were analyzed, and large tumor size (≥ 5 cm in diameter), high-grade histology and elevated mitotic counts (≥ 5/10 HPF) were found to be closely associated with the clinical outcomes. According to the tumor size and histological grade, a model was proposed for recurrence risk assessment. By this model, FDC sarcomas were classified into low-, intermediate- and high-risk groups, and their recurrence rates were 16%, 46% and 73%, and the mortality rates were 0%, 4% and 45%, respectively.
Applications
A grading approach was established for evaluating aggressiveness of extranodal FDC sarcomas, and the lesions were divided into low-grade and high-grade categories. According to the tumor size and histological grade, a model was proposed for recurrence risk assessment. This will provide a convenient procedure for diagnostic pathologists to predict the clinical outcomes of the tumor more accurately following its surgical resection or even with a biopsy. This model may also be applied to the prognosis assessment of the FDC sarcomas which develop from lymph nodes.
Peer review
It is a well written and important paper that likely represents the most thorough examination of a rare sarcoma. The authors are to be congratulated for an important work.
Acknowledgments
The authors thank Dr. Ling Li and Dr. Hong-Tu Zhang for the helpful discussions, Dr. Lin Yang for her help in statistical analysis, Guo-Lian Wei, Xiu-Yun Liu, Xin-Hua Xue and Yong-Qiang Xie for their technical assistance.
Footnotes
Supported by Grants from National Natural Science Foundation of China, No. 30171052, 30572125 and 30772508
Peer reviewer: Leonidas G Koniaris, Professor, Alan Livingstone Chair in Surgical Oncology, 3550 Sylvester Comprehensive Cancer Center (310T), 1475 NW 12th Ave., Miami, FL 33136, United States
S- Editor Wang JL L- Editor Ma JY E- Editor Lin YP
References
- 1.Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol. 1986;122:562–572. [PMC free article] [PubMed] [Google Scholar]
- 2.Pileri SA, Grogan TM, Harris NL, Banks P, Campo E, Chan JK, Favera RD, Delsol G, De Wolf-Peeters C, Falini B, et al. Tumours of histiocytes and accessory dendritic cells: an immunohistochemical approach to classification from the International Lymphoma Study Group based on 61 cases. Histopathology. 2002;41:1–29. doi: 10.1046/j.1365-2559.2002.01418.x. [DOI] [PubMed] [Google Scholar]
- 3.Chan JK, Fletcher CD, Nayler SJ, Cooper K. Follicular dendritic cell sarcoma. Clinicopathologic analysis of 17 cases suggesting a malignant potential higher than currently recognized. Cancer. 1997;79:294–313. [PubMed] [Google Scholar]
- 4.Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW, Ng WF, Chan AC, Prat J. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol. 2001;25:721–731. doi: 10.1097/00000478-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Chen TC, Kuo TT, Ng KF. Follicular dendritic cell tumor of the liver: a clinicopathologic and Epstein-Barr virus study of two cases. Mod Pathol. 2001;14:354–360. doi: 10.1038/modpathol.3880315. [DOI] [PubMed] [Google Scholar]
- 6.Horiguchi H, Matsui-Horiguchi M, Sakata H, Ichinose M, Yamamoto T, Fujiwara M, Ohse H. Inflammatory pseudotumor-like follicular dendritic cell tumor of the spleen. Pathol Int. 2004;54:124–131. doi: 10.1111/j.1440-1827.2004.01589.x. [DOI] [PubMed] [Google Scholar]
- 7.Bai LY, Kwang WK, Chiang IP, Chen PM. Follicular dendritic cell tumor of the liver associated with Epstein-Barr virus. Jpn J Clin Oncol. 2006;36:249–253. doi: 10.1093/jjco/hyl001. [DOI] [PubMed] [Google Scholar]
- 8.Laurent C, Meggetto F, de Paiva GR, Selves J, Palasse J, Laurent G, Brousset P. Follicular dendritic cell tumor of the spleen associated with diffuse large B-cell lymphoma. Hum Pathol. 2008;39:776–780. doi: 10.1016/j.humpath.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 9.Gong QX, Fan QH, Zhou ZS, Zhang ZH, Yu MN, Wang Z, Wang C, Zhang WM. [Inflammatory pseudotumor-like follicular dendritic cell tumor of spleen] Zhonghua Binglixue Zazhi. 2008;37:40–44. [PubMed] [Google Scholar]
- 10.Agaimy A, Wünsch PH. Follicular dendritic cell tumor of the gastrointestinal tract: Report of a rare neoplasm and literature review. Pathol Res Pract. 2006;202:541–548. doi: 10.1016/j.prp.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Biddle DA, Ro JY, Yoon GS, Yong YW, Ayala AG, Ordonez NG, Ro J. Extranodal follicular dendritic cell sarcoma of the head and neck region: three new cases, with a review of the literature. Mod Pathol. 2002;15:50–58. doi: 10.1038/modpathol.3880489. [DOI] [PubMed] [Google Scholar]
- 12.Tu XY, Sheng WQ, Lu HF, Wang J. [Clinicopathologic study of intraabdominal extranodal follicular dendritic cell sarcoma] Zhonghua Binglixue Zazhi. 2007;36:660–665. [PubMed] [Google Scholar]
- 13.Kazakov DV, Morrisson C, Plaza JA, Michal M, Suster S. Sarcoma arising in hyaline-vascular castleman disease of skin and subcutis. Am J Dermatopathol. 2005;27:327–332. doi: 10.1097/01.dad.0000171606.55810.86. [DOI] [PubMed] [Google Scholar]
- 14.Youens KE, Waugh MS. Extranodal follicular dendritic cell sarcoma. Arch Pathol Lab Med. 2008;132:1683–1687. doi: 10.5858/2008-132-1683-EFDCS. [DOI] [PubMed] [Google Scholar]
- 15.Chan JKC, Pileri SA, Delsol G, Fletcher CDM, Weiss LM, Grogg KL. Follicular dendritic cell sarcoma. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, et al., editors. WHO classification of tumors of haematopoietic and lymphoid tissues. 4th ed. Lyon: International Agency for Research on Cancer; 2008. pp. 364–365. [Google Scholar]
- 16.Perez-Ordonez B, Erlandson RA, Rosai J. Follicular dendritic cell tumor: report of 13 additional cases of a distinctive entity. Am J Surg Pathol. 1996;20:944–955. doi: 10.1097/00000478-199608000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Su Q, Wang SF, Shang FX, Sun H, Gong L, Duan YY, Yuan LT, Wang YC. Extranodal follicular dendritic cell sarcoma, report of 2 cases and clonality analysis. Zhonghua Binglixue Zazhi. 2004;34:183–185. [Google Scholar]
- 18.Su Q, Schröder CH, Otto G, Bannasch P. Overexpression of p53 protein is not directly related to hepatitis B x protein expression and is associated with neoplastic progression in hepatocellular carcinomas rather than hepatic preneoplasia. Mutat Res. 2000;462:365–380. doi: 10.1016/s1383-5742(00)00026-0. [DOI] [PubMed] [Google Scholar]
- 19.Lamb P, Crawford L. Characterization of the human p53 gene. Mol Cell Biol. 1986;6:1379–1385. doi: 10.1128/mcb.6.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joerger AC, Fersht AR. Wild type p53 conformation, structural consequences of p53 mutations and mechanisms of mutant p53 rescue. In: Hainaut P, Wiman KG, et al., editors. 25 Years of p53. Dordecht: Springer-Verlag; 2005. pp. 377–398. [Google Scholar]
- 21.Chau Y, Hongyo T, Aozasa K, Chan JK. Dedifferentiation of adenoid cystic carcinoma: report of a case implicating p53 gene mutation. Hum Pathol. 2001;32:1403–1407. doi: 10.1053/hupa.2001.28966. [DOI] [PubMed] [Google Scholar]
- 22.Bharaj BS, Angelopoulou K, Diamandis EP. Rapid sequencing of the p53 gene with a new automated DNA sequencer. Clin Chem. 1998;44:1397–1403. [PubMed] [Google Scholar]
- 23.Detwiler MM, Hamp TJ, Kazim AL. DNA sequencing using the liquid polymer POP-7 on an ABI PRISM 3100 Genetic Analyzer. Biotechniques. 2004;36:932–933. doi: 10.2144/04366BM01. [DOI] [PubMed] [Google Scholar]
- 24.Chan JK, Tsang WY, Ng CS. Follicular dendritic cell tumor and vascular neoplasm complicating hyaline-vascular Castleman's disease. Am J Surg Pathol. 1994;18:517–525. doi: 10.1097/00000478-199405000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Hollowood K, Stamp G, Zouvani I, Fletcher CD. Extranodal follicular dendritic cell sarcoma of the gastrointestinal tract. Morphologic, immunohistochemical and ultrastructural analysis of two cases. Am J Clin Pathol. 1995;103:90–97. doi: 10.1093/ajcp/103.1.90. [DOI] [PubMed] [Google Scholar]
- 26.Nayler SJ, Verhaart MJ, Cooper K. Follicular dendritic cell tumour of the tonsil. Histopathology. 1996;28:89–92. doi: 10.1046/j.1365-2559.1996.t01-3-258289.x. [DOI] [PubMed] [Google Scholar]
- 27.Selves J, Meggetto F, Brousset P, Voigt JJ, Pradère B, Grasset D, Icart J, Mariamé B, Knecht H, Delsol G. Inflammatory pseudotumor of the liver. Evidence for follicular dendritic reticulum cell proliferation associated with clonal Epstein-Barr virus. Am J Surg Pathol. 1996;20:747–753. doi: 10.1097/00000478-199606000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Shek TW, Ho FC, Ng IO, Chan AC, Ma L, Srivastava G. Follicular dendritic cell tumor of the liver. Evidence for an Epstein-Barr virus-related clonal proliferation of follicular dendritic cells. Am J Surg Pathol. 1996;20:313–324. doi: 10.1097/00000478-199603000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Beham-Schmid C, Beham A, Jakse R, Auböck L, Höfler G. Extranodal follicular dendritic cell tumour of the nasopharynx. Virchows Arch. 1998;432:293–298. doi: 10.1007/s004280050168. [DOI] [PubMed] [Google Scholar]
- 30.Shek TW, Liu CL, Peh WC, Fan ST, Ng IO. Intra-abdominal follicular dendritic cell tumour: a rare tumour in need of recognition. Histopathology. 1998;33:465–470. doi: 10.1046/j.1365-2559.1998.00547.x. [DOI] [PubMed] [Google Scholar]
- 31.Araújo VC, Martins MT, Salmen FS, Araújo NS. Extranodal follicular dendritic cell sarcoma of the palate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:209–214. doi: 10.1016/s1079-2104(99)70274-x. [DOI] [PubMed] [Google Scholar]
- 32.Desai S, Deshpande RB, Jambhekar N. Follicular dendritic cell tumor of the parapharyngeal region. Head Neck. 1999;21:164–167. doi: 10.1002/(sici)1097-0347(199903)21:2<164::aid-hed11>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 33.Fisher C, Magnusson B, Hardarson S, Smith ME. Myxoid variant of follicular dendritic cell sarcoma arising in the breast. Ann Diagn Pathol. 1999;3:92–98. doi: 10.1016/s1092-9134(99)80036-7. [DOI] [PubMed] [Google Scholar]
- 34.Galati LT, Barnes EL, Myers EN. Dendritic cell sarcoma of the thyroid. Head Neck. 1999;21:273–275. doi: 10.1002/(sici)1097-0347(199905)21:3<273::aid-hed14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 35.Schwarz RE, Chu P, Arber DA. Extranodal follicular dendritic cell tumor of the abdominal wall. J Clin Oncol. 1999;17:2290–2292. doi: 10.1200/JCO.1999.17.7.2290. [DOI] [PubMed] [Google Scholar]
- 36.Choi PC, To KF, Lai FM, Lee TW, Yim AP, Chan JK. Follicular dendritic cell sarcoma of the neck: report of two cases complicated by pulmonary metastases. Cancer. 2000;89:664–672. [PubMed] [Google Scholar]
- 37.Han JH, Kim SH, Noh SH, Lee YC, Kim HG, Yang WI. Follicular dendritic cell sarcoma presenting as a submucosal tumor of the stomach. Arch Pathol Lab Med. 2000;124:1693–1696. doi: 10.5858/2000-124-1693-FDCSPA. [DOI] [PubMed] [Google Scholar]
- 38.Chan AC, Chan KW, Chan JK, Au WY, Ho WK, Ng WM. Development of follicular dendritic cell sarcoma in hyaline-vascular Castleman's disease of the nasopharynx: tracing its evolution by sequential biopsies. Histopathology. 2001;38:510–518. doi: 10.1046/j.1365-2559.2001.01134.x. [DOI] [PubMed] [Google Scholar]
- 39.Chang KC, Jin YT, Chen FF, Su IJ. Follicular dendritic cell sarcoma of the colon mimicking stromal tumour. Histopathology. 2001;38:25–29. doi: 10.1046/j.1365-2559.2001.01035.x. [DOI] [PubMed] [Google Scholar]
- 40.Chiaramonte MF, Lee D, Abruzzo LV, Heyman M, Bass BL. Retroperitoneal follicular dendritic cell sarcoma presenting as secondary amyloidosis. Surgery. 2001;130:109–111. doi: 10.1067/msy.2001.113441. [DOI] [PubMed] [Google Scholar]
- 41.Shah RN, Ozden O, Yeldandi A, Peterson L, Rao S, Laskin WB. Follicular dendritic cell tumor presenting in the lung: a case report. Hum Pathol. 2001;32:745–749. doi: 10.1053/hupa.2001.25595. [DOI] [PubMed] [Google Scholar]
- 42.Vargas H, Mouzakes J, Purdy SS, Cohn AS, Parnes SM. Follicular dendritic cell tumor: an aggressive head and neck tumor. Am J Otolaryngol. 2002;23:93–98. doi: 10.1053/ajot.2002.30781. [DOI] [PubMed] [Google Scholar]
- 43.Ceresoli GL, Zucchinelli P, Ponzoni M, Gregorc V, Bencardino K, Paties CT. Mediastinal follicular dendritic cell sarcoma. Haematologica. 2003;88:ECR04. [PubMed] [Google Scholar]
- 44.Satoh K, Hibi G, Yamamoto Y, Urano M, Kuroda M, Nakamura S. Follicular dendritic cell tumor in the oro-pharyngeal region: report of a case and a review of the literature. Oral Oncol. 2003;39:415–419. doi: 10.1016/s1368-8375(02)00138-0. [DOI] [PubMed] [Google Scholar]
- 45.Geerts A, Lagae E, Dhaene K, Peeters M, Waeytens A, Demetter P, Cuvelier C, Defreyne L, De Vos M, Pattyn P. Metastatic follicular dendritic cell sarcoma of the stomach: a case report and review of the literature. Acta Gastroenterol Belg. 2004;67:223–227. [PubMed] [Google Scholar]
- 46.Grogg KL, Lae ME, Kurtin PJ, Macon WR. Clusterin expression distinguishes follicular dendritic cell tumors from other dendritic cell neoplasms: report of a novel follicular dendritic cell marker and clinicopathologic data on 12 additional follicular dendritic cell tumors and 6 additional interdigitating dendritic cell tumors. Am J Surg Pathol. 2004;28:988–998. doi: 10.1097/01.pas.0000112536.76973.7f. [DOI] [PubMed] [Google Scholar]
- 47.Kröber SM, Marx A, Aebert H, Dohmen BM, Kaiserling E. Sarcoma of follicular dendritic cells in the dorsal mediastinum. Hum Pathol. 2004;35:259–263. doi: 10.1016/j.humpath.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 48.O¡¯Malley DP. Diagnosis: follicular dendritic cell tumor mimicking GI stromal tumor. 2004. Available from: http://www.socforheme.org/case-nov-04.htm.
- 49.Shi QL, Zhou XJ, Ma J, Ma HH, Wu KM, Zhou M, Wang QP. Follicular dendritic cell sarcoma of tonsil: a case report and review of the literature. Zhenduan Binglixue Zazhi. 2004;11:81–83. [Google Scholar]
- 50.Bradshaw EJ, Wood KM, Hodgkinson P, Lucraft H, Windebank KP. Follicular dendritic cell tumour in a 9-year-old child. Pediatr Blood Cancer. 2005;45:725–727. doi: 10.1002/pbc.20446. [DOI] [PubMed] [Google Scholar]
- 51.Khalid T, Folman R. Symptoms in cancer patients and an unusual tumor: Case 3. Follicular dendritic cell sarcoma. J Clin Oncol. 2005;23:9425–9426. doi: 10.1200/JCO.2004.00.9365. [DOI] [PubMed] [Google Scholar]
- 52.Torres U, Hawkins WG, Antonescu CR, DeMatteo RP. Hepatic follicular dendritic cell sarcoma without Epstein-Barr virus expression. Arch Pathol Lab Med. 2005;129:1480–1483. doi: 10.5858/2005-129-1480-HFDCSW. [DOI] [PubMed] [Google Scholar]
- 53.Aydin E, Ozluoglu LN, Demirhan B, Arikan U. Follicular dendritic cell sarcoma of the tonsil: case report. Eur Arch Otorhinolaryngol. 2006;263:1155–1157. doi: 10.1007/s00405-006-0124-9. [DOI] [PubMed] [Google Scholar]
- 54.Choi JW, Lee JH, Kim A, Kim CH, Chae YS, Kim I. Follicular dendritic cell sarcoma arising in the dura mater of the spine. Arch Pathol Lab Med. 2006;130:1718–1721. doi: 10.5858/2006-130-1718-FDCSAI. [DOI] [PubMed] [Google Scholar]
- 55.Clement P, Saint-Blancard P, Minvielle F, Le Page P, Kossowski M. Follicular dendritic cell sarcoma of the tonsil: a case report. Am J Otolaryngol. 2006;27:207–210. doi: 10.1016/j.amjoto.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Díaz de Liaño A, Garde C, Artieda C, Yárnoz C, Flores L, Ortiz H. Intra-abdominal follicular dendritic cell sarcoma. Clin Transl Oncol. 2006;8:837–838. doi: 10.1007/s12094-006-0143-4. [DOI] [PubMed] [Google Scholar]
- 57.Gan MF, Yu CK, Cai JF, Lu HS, Yu XR. Follicular dendritic cell sarcoma of parotid: a case report and review of literatures. Linchuang Yu Shiyan Binglixue Zazhi. 2006;22:204–207. [Google Scholar]
- 58.Jiang L, Admirand JH, Moran C, Ford RJ, Bueso-Ramos CE. Mediastinal follicular dendritic cell sarcoma involving bone marrow: a case report and review of the literature. Ann Diagn Pathol. 2006;10:357–362. doi: 10.1016/j.anndiagpath.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Jiang Y, Yao M, Liu Q. Follicular dendritic cell sarcoma occurring in the pelvic and abdominal cavity, a case report. Shanghai Yixue Yingxiang. 2006;15:174–175. [Google Scholar]
- 60.Kovács RB, Sattar HA, Krausz T, Kas J, Berta M, Sápi Z. Primary follicular dendritic cell sarcoma of the lung. Histopathology. 2006;49:431–433. doi: 10.1111/j.1365-2559.2006.02486.x. [DOI] [PubMed] [Google Scholar]
- 61.Li F, Yu YH, Yao LQ. Follicular dendritic cell sarcoma of nasal cavity: a case report and literature review. Zhongguo Wuzhenxue Zazhi. 2006;6:4715–4717. [Google Scholar]
- 62.Lu CH, Hong M, Luo YD, Zhang ZM, Xu L. Follicular dendritic cell sarcoma occurring in the left groin, a case report. Fujian Yiyao Zazhi. 2006;28:55–56. [Google Scholar]
- 63.Shen SC, Wu CC, Ng KF, Wu RC, Chen HM, Chen TC. Follicular dendritic cell sarcoma mimicking giant cell carcinoma of the pancreas. Pathol Int. 2006;56:466–470. doi: 10.1111/j.1440-1827.2006.01991.x. [DOI] [PubMed] [Google Scholar]
- 64.Shia J, Chen W, Tang LH, Carlson DL, Qin J, Guillem JG, Nobrega J, Wong WD, Klimstra DS. Extranodal follicular dendritic cell sarcoma: clinical, pathologic, and histogenetic characteristics of an underrecognized disease entity. Virchows Arch. 2006;449:148–158. doi: 10.1007/s00428-006-0231-4. [DOI] [PubMed] [Google Scholar]
- 65.Xu KJ, Li HZ, Shi J, Jin HZ, Liu YH. A case of paraneoplastic pemphigus associated with follicular dendritic cell sarcoma. Linchuang Pifuke Zazhi. 2006;35:794–796. [Google Scholar]
- 66.Chang ZP, Liao SL, Jin Y, Song QP, Duan LJ. [Castleman's disease of chest wall complicated by follicular dendritic cell sarcoma/tumor: report of a case] Zhenduan Binglixue Zazhi. 2007;36:430–431. [PubMed] [Google Scholar]
- 67.Leipsic JA, McAdams HP, Sporn TA. Follicular dendritic cell sarcoma of the mediastinum. AJR Am J Roentgenol. 2007;188:W554–W556. doi: 10.2214/AJR.04.1530. [DOI] [PubMed] [Google Scholar]
- 68.Padilla-Rodríguez AL, Bembassat M, Lazaro M, Ortiz-Hidalgo C. Intra-abdominal follicular dendritic cell sarcoma with marked pleomorphic features and aberrant expression of neuroendocrine markers: report of a case with immunohistochemical analysis. Appl Immunohistochem Mol Morphol. 2007;15:346–352. doi: 10.1097/01.pai.0000213113.50849.9e. [DOI] [PubMed] [Google Scholar]
- 69.Sander B, Middel P, Gunawan B, Schulten HJ, Baum F, Golas MM, Schulze F, Grabbe E, Parwaresch R, Füzesi L. Follicular dendritic cell sarcoma of the spleen. Hum Pathol. 2007;38:668–672. doi: 10.1016/j.humpath.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 70.Soriano AO, Thompson MA, Admirand JH, Fayad LE, Rodriguez AM, Romaguera JE, Hagemeister FB, Pro B. Follicular dendritic cell sarcoma: a report of 14 cases and a review of the literature. Am J Hematol. 2007;82:725–728. doi: 10.1002/ajh.20852. [DOI] [PubMed] [Google Scholar]
- 71.Yuan J, Li XH, Lu YL, Song X. Pathologic diagnosis of hepatic inflammatory pseudotumor and inflammatory pseudotumor-like follicular dendritic cell tumor. Linchuang Yu Shiyan Binglixue Zazhi. 2007;23:39–42. [Google Scholar]
- 72.De Pas T, Spitaleri G, Pruneri G, Curigliano G, Noberasco C, Luini A, Andreoni B, Testori A, de Braud F. Dendritic cell sarcoma: an analytic overview of the literature and presentation of original five cases. Crit Rev Oncol Hematol. 2008;65:1–7. doi: 10.1016/j.critrevonc.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 73.Granados R, Aramburu JA, Rodríguez JM, Nieto MA. Cytopathology of a primary follicular dendritic cell sarcoma of the liver of the inflammatory pseudotumor-like type. Diagn Cytopathol. 2008;36:42–46. doi: 10.1002/dc.20744. [DOI] [PubMed] [Google Scholar]
- 74.Liu YH, Wang XH, Lu LM. Pathological features of follicular cell sarcoma of gastrocolic omentum: a case report and review of the literatures. Wannan Yixueyuan Xuebao. 2008;27:118–120. [Google Scholar]
- 75.Peng WJ, Yao LQ. Small mesenteric follicular dendritic cell sarcoma: a case report and literature review. Zhongguo Wuzhenxue Zazhi. 2008;8:3040–3042. [Google Scholar]
- 76.Zhang ZX, Cheng J, Shi QL, Ma J, Zhou XJ, Zhou HB, Ma HH. [Follicular dendritic cell sarcoma: a clinicopathologic study of 8 cases] Zhonghua Binglixue Zazhi. 2008;37:395–399. [PubMed] [Google Scholar]
- 77.An XJ, Zhang ZX, Shi QL, Wu B, Ma J, Zhou HB, Ma HH. Hepatic inflammatory pseudotumor-like follicular dendritic cell tmnor: repart of one case and review of literature. Zhenduanxue Lilun Yu Shijian. 2009;8:63–66. [Google Scholar]
- 78.Denning KL, Olson PR, Maley RH Jr, Flati VR, Myers JL, Silverman JF. Primary pulmonary follicular dendritic cell neoplasm: a case report and review of the literature. Arch Pathol Lab Med. 2009;133:643–647. doi: 10.5858/133.4.643. [DOI] [PubMed] [Google Scholar]
- 79.Liu J, Zhang ZT, Li JS, Wang Y. Hepatic follicular dendritic cell sarcoma: a case report and review of the literatures. Zhongguo Shiyong Waike Zazhi. 2009;29:421–424. [Google Scholar]
- 80.Romero-Guadarrama MB, Reyes-Posada O, Hernández-González MM, Durán-Padilla MA. Follicular dendritic cell sarcoma/tumor: 2 cases of a rare tumor of difficult clinical and pathological diagnosis. Ann Diagn Pathol. 2009;13:257–262. doi: 10.1016/j.anndiagpath.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 81.Shen DP, Ni XZ, Yin XL, Wu ZY. Clinical and pathological features of follicular dendritic cell sarcoma of appendix: a case report. Chin Med J (Engl) 2009;122:1595–1597. [PubMed] [Google Scholar]
- 82.Vaideeswar P, George SM, Kane SV, Chaturvedi RA, Pandit SP. Extranodal follicular dendritic cell sarcoma of the tonsil - case report of an epithelioid cell variant with osteoclastic giant cells. Pathol Res Pract. 2009;205:149–153. doi: 10.1016/j.prp.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 83.Xu YQ, Dai CL, Jia CJ, Yi YF, Shu H, L PS, Li XJ, Zhao L. Follicular dendritic cell sarcoma of the liver. Zhonghua Putong Waikexue Wenxian. 2009;3:48–49. [Google Scholar]
- 84.Xie Q, Chen L, Fu K, Harter J, Young KH, Sunkara J, Novak D, Villanueva-Siles E, Ratech H. Podoplanin (d2-40): a new immunohistochemical marker for reactive follicular dendritic cells and follicular dendritic cell sarcomas. Int J Clin Exp Pathol. 2008;1:276–284. [PMC free article] [PubMed] [Google Scholar]
- 85.Yu H, Gibson JA, Pinkus GS, Hornick JL. Podoplanin (D2-40) is a novel marker for follicular dendritic cell tumors. Am J Clin Pathol. 2007;128:776–782. doi: 10.1309/7P8U659JBJCV6EEU. [DOI] [PubMed] [Google Scholar]
- 86.Cronin DM, Warnke RA. Castleman disease: an update on classification and the spectrum of associated lesions. Adv Anat Pathol. 2009;16:236–246. doi: 10.1097/PAP.0b013e3181a9d4d3. [DOI] [PubMed] [Google Scholar]
- 87.Katano H, Kaneko K, Shimizu S, Saito T, Irié T, Mori S. Follicular dendritic cell sarcoma complicated by hyaline-vascular type Castleman's disease in a schizophrenic patient. Pathol Int. 1997;47:703–706. doi: 10.1111/j.1440-1827.1997.tb04445.x. [DOI] [PubMed] [Google Scholar]
- 88.Kazakov DV, Fanburg-Smith JC, Suster S, Neuhauser TS, Palmedo G, Zamecnik M, Kempf W, Michal M. Castleman disease of the subcutis and underlying skeletal muscle: report of 6 cases. Am J Surg Pathol. 2004;28:569–577. doi: 10.1097/00000478-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 89.Lin O, Frizzera G. Angiomyoid and follicular dendritic cell proliferative lesions in Castleman's disease of hyaline-vascular type: a study of 10 cases. Am J Surg Pathol. 1997;21:1295–1306. doi: 10.1097/00000478-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 90.Cokelaere K, Debiec-Rychter M, De Wolf-Peeters C, Hagemeijer A, Sciot R. Hyaline vascular Castleman's disease with HMGIC rearrangement in follicular dendritic cells: molecular evidence of mesenchymal tumorigenesis. Am J Surg Pathol. 2002;26:662–669. doi: 10.1097/00000478-200205000-00013. [DOI] [PubMed] [Google Scholar]
- 91.Pauwels P, Dal Cin P, Vlasveld LT, Aleva RM, van Erp WF, Jones D. A chromosomal abnormality in hyaline vascular Castleman's disease: evidence for clonal proliferation of dysplastic stromal cells. Am J Surg Pathol. 2000;24:882–888. doi: 10.1097/00000478-200006000-00016. [DOI] [PubMed] [Google Scholar]
- 92.Hernández JL, Gómez-Román J, Ramos-Estébanez C, Nan D, Martín-Oviedo J, Riancho JA, González-Macías J. Human herpesvirus 8 and Epstein-Barr virus coinfection in localized Castleman disease during pregnancy. Haematologica. 2005;90 Suppl:ECR35. [PubMed] [Google Scholar]
- 93.Chen CH, Liu HC, Hung TT, Liu TP. Possible roles of Epstein-Barr virus in Castleman disease. J Cardiothorac Surg. 2009;4:31. doi: 10.1186/1749-8090-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maeda E, Akahane M, Kiryu S, Kato N, Yoshikawa T, Hayashi N, Aoki S, Minami M, Uozaki H, Fukayama M, et al. Spectrum of Epstein-Barr virus-related diseases: a pictorial review. Jpn J Radiol. 2009;27:4–19. doi: 10.1007/s11604-008-0291-2. [DOI] [PubMed] [Google Scholar]
- 95.Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 96.Abdel-Fattah R, Challen C, Griffiths TR, Robinson MC, Neal DE, Lunec J. Alterations of TP53 in microdissected transitional cell carcinoma of the human urinary bladder: high frequency of TP53 accumulation in the absence of detected mutations is associated with poor prognosis. Br J Cancer. 1998;77:2230–2238. doi: 10.1038/bjc.1998.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hainaut P, Milner J. Interaction of heat-shock protein 70 with p53 translated in vitro: evidence for interaction with dimeric p53 and for a role in the regulation of p53 conformation. EMBO J. 1992;11:3513–3520. doi: 10.1002/j.1460-2075.1992.tb05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fritsche M, Haessler C, Brandner G. Induction of nuclear accumulation of the tumor-suppressor protein p53 by DNA-damaging agents. Oncogene. 1993;8:307–318. [PubMed] [Google Scholar]
- 99.Siegel J, Fritsche M, Mai S, Brandner G, Hess RD. Enhanced p53 activity and accumulation in response to DNA damage upon DNA transfection. Oncogene. 1995;11:1363–1370. [PubMed] [Google Scholar]