Abstract
AIM: To evaluate the efficacy of high-dose proton pump inhibitors (PPIs) vs low-dose PPIs for patients with upper gastrointestinal bleeding.
METHODS: PubMed, Embase, the Cochrane Library, and Web of Science were searched to identify relevant randomized controlled trials (RCTs). Eligible trials were RCTs that compared high-dose PPI with low-dose PPI following endoscopic hemostasis. The primary endpoint was rebleeding; secondary endpoints were patient numbers that needed surgery, and mortality. The meta-analysis was performed with a fixed effects model or random effects model.
RESULTS: Nine eligible RCTs including 1342 patients were retrieved. The results showed that high-dose intravenous PPI was not superior to low-dose intravenous PPI in reducing rebleeding [odds ratio (OR) = 1.091, 95% confidential interval (CI): 0.777-1.532], need for surgery (OR = 1.522, 95% CI: 0.643-3.605) and mortality (OR = 1.022, 95% CI: 0.476-2.196). Subgroup analysis according to different region revealed no difference in rebleeding rate between Asian patients (OR = 0.831, 95% CI, 0.467-1.480) and European patients (OR = 1.263, 95% CI: 0.827-1.929).
CONCLUSION: Low-dose intravenous PPI can achieve the same efficacy as high-dose PPI following endoscopic hemostasis.
Keywords: Meta-analysis, High-dose, Low-dose, Proton pump inhibitors, Gastrointestinal bleeding
INTRODUCTION
Acute upper gastrointestinal bleeding is a prevalent and clinically significant condition[1]. The core management of these patients consists of resuscitation and endoscopic therapy, but rebleeding still occurs after initial control of the bleeding[2]. Currently, the combination of endoscopic hemostasis and administration of proton pump inhibitors (PPIs) is the standard management, and two authoritative consensus guidelines have recommended the use of a high-dose PPI regimen (80 mg stat followed by an infusion of 8 mg/h for 72 h) following endoscopic hemostasis for high-risk peptic ulcer bleeding lesions[3,4]. The possible biological benefit of this high-dose regimen is to promote clot stability by sustaining the intragastric pH above 6[5,6]. However, when high-dose PPI is compared with a low-dose PPI regimen after primary endocopic hemostasis is achieved, some clinical trials[7-10] reported no differences in rebleeding rate and the need for surgery between the high- and low-dose regimens.
This meta-analysis aims to analyze trials of low-dose vs high-dose PPI after endoscopic hemostasis, and to determine whether low-dose PPI administration can produce the same effect as high-dose after endoscopic hemostasis. This study was performed according to the QUOROM (Quality of Reporting Of Meta-analysis) statement, and the QUOROM statement checklists were all answered as “Yes”[11].
MATERIALS AND METHODS
Literature search
A comprehensive literature search was performed to identify all randomized controlled trials (RCTs). Trials were identified by systematically searching the electronic databases PubMed, EMBASE, the Cochrane Library, and Web of Science in any language. The search was conducted using the following key words: omeprazole, lansoprazole, esomeprazole, pantoprazole, dexlansoprazole, rabeprazole, PPI, and bleeding. The last search was done in September 2009. In addition, an extensive manual search was also performed by using references from each retrieved study or review article.
Study selection
Trials were included using the following criteria: (1) RCT; (2) comparing high-dose with low-dose PPI following endoscopic hemostasis for acute upper non-variceal gastrointestinal bleeding; and (3) availability of adequate data (persistent or recurrent bleeding, need for surgery, or mortality). Subsequently, trials were excluded if the PPI treatment was initiated prior to endoscopic hemostasis. The study selection was done by two reviewers independently, and any difference of opinion was settled by discussion. Publications identified as duplicates were excluded, and when one study had substantial overlap in terms of author, institution or study period with another study, the one which was more recent and of better quality was included.
Regarding the definitions of high and low-dose PPIs, high-dose was not limited to the regimen of 80 mg iv bolus followed by an infusion of 8 mg/h for 72 h. In our study, dosage of PPI was considered high-dose if at least twice the low-dose of any of the PPIs was used during the 72 h following endoscopic hemostasis.
Qualitative assessment
The quality of the studies was assessed and graded by two reviewers independently according to criteria described in The Cochrane Handbook 5.0.1[12]. The criteria included: (1) sequence generation; (2) allocation concealment; (3) blinding of participants, personnel and outcome assessors; (4) incomplete outcome data; and (5) selective outcome reporting. Each criteria was categorized as “yes”, “no”, or “unclear”, and the summary assessments of the risk of bias for each important outcome within and across studies was categorized as “low risk of bias”, “unclear risk of bias” and “high risk of bias”[12].
Data extraction
Two reviewers independently extracted data by using a standardized form. If there was inconsistency, the original papers were retrieved and jointly investigated to resolve the disagreement. Data were extracted regarding the details of all therapeutic interventions, including the specific kind of PPI, the dose and administration method (oral, intermittent bolus, or continuous infusion). We also extracted the first author, publication year, patients age, sex ratio, region, numbers assigned to each group, numbers with comorbid conditions, the site of bleeding ulcer (duodenal, gastric or esophageal), signs of recent hemorrhage (spurting, oozing, non-bleeding vessel, and adherent clot). Our primary endpoint was persistent or recurrent ulcer bleeding, and secondary endpoints included patient numbers that needed surgery and mortality.
Statistical analysis
The pooled odds ratio (OR) and 95% confidence intervals (CI) were calculated from the original study data by using the Mantel-Haenszel method (fixed effects model) if no heterogeneity was detected throughout the studies[13]; in other words, if there was no variability among the studies in the meta-analysis because all the studies could be assumed to come from the same population. Statistical heterogeneity was assessed using the forest plot and inconsistency statistic (I2). If any heterogeneity existed (P < 0.1, OR I2 > 50%), then use of the fixed effects model might have been invalid. Any heterogeneity was explored by subgroup analysis and by a sensitivity analysis done by excluding the studies which potentially biased the results. If the investigations of the cause of the heterogeneity showed that this was more appropriate, then the Der Simonian and Laird method (random effects model) was applied. A random effects model assumes that each study has a different underlying effect and this leads to wider confidence intervals than with the fixed effects model[13]. Egger’s test was applied to determine the symmetry of the funnel plot, to identify any publication bias, P < 0.05 indicated bias, and P > 0.05 indicated no publication bias[14].
All data analyses were performed using the statistical software Stata 9.2 (Stata Co., College Station, Texas, USA).
RESULTS
Searches of the PubMed and Embase databases identified 583 and 267 abstracts, respectively. After screening the titles and the abstracts, 12 studies from PubMed and Embase and their full texts were retrieved for further review[2,8,10,15-23], and 3 studies were found from reference lists[7,9,24]. Thus, in total, 15 studies were retrieved for further review. Two studies were excluded because no adequate data were available[18,22], one study was excluded because not all the patients received endoscopic therapy[20], one study was excluded because the total dose of PPI was the same across the different groups[23], one study was excluded because the PPI in the intravenous group was regular dose[15], one study was excluded because not all patients had endoscopic intervention[10]. Thus, 9 studies and 3 abstracts[7,9,24] were included in this meta-analysis. The flow chart of the literature search of this meta-analysis is shown in Figure 1.
Figure 1.
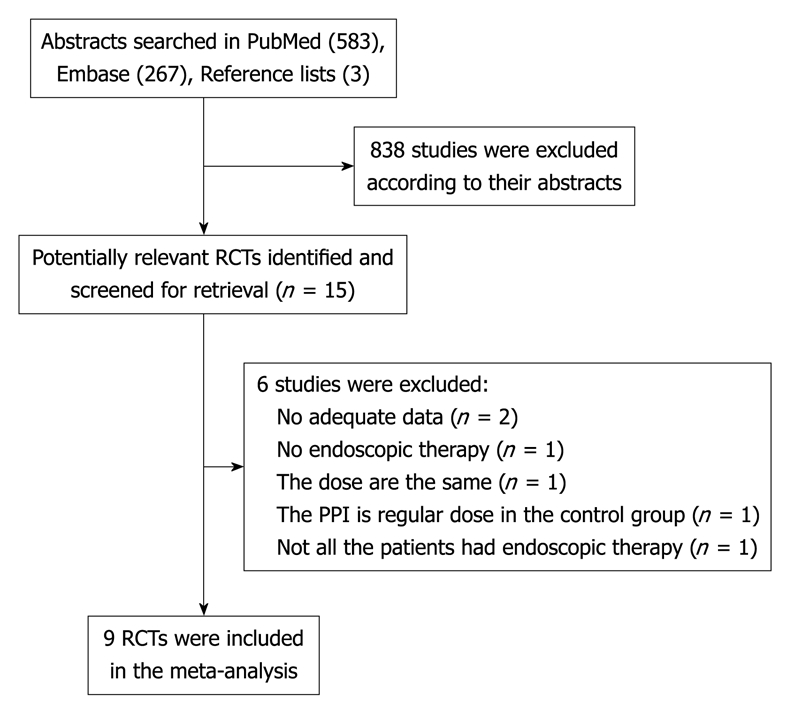
Flow chart of the literature search for this meta-analysis.
The qualities of the included studies are shown in Table 1. Thus, 4 studies are at low risk of bias[16,17,21,22], 2 studies are at unclear risk of bias[9,19], and the other 3 studies are at high risk of bias[2,7,24], including two which could not be accessed as full texts. Thus, 4 studies’ allocation sequence were adequately generated[2,8,16,17], 5 studies’ allocation were adequately concealed[8,16,17,19,21], 3 studies’ blinding of participants, personnel and outcome assessors were adequately carried out[8,16,21], 5 studies’ incomplete outcome data were adequately addressed[8,16,17,19,21], 2 studies were free of other bias[16,17].
Table 1.
Summary assessments of the risk of bias with studies
| Author | Yr | Adequate sequence generation? | Allocation concealment? | Blinding? | Incomplete outcome data addressed? | Free of other bias? | Risk of bias |
| Andriulli et al[16] | 2008 | Yes | Yes | Yes | Yes | Yes | Low |
| Yüksel et al[17] | 2008 | Yes | Yes | Unclear | Yes | Yes | Low |
| Hung et al[2] | 2007 | Yes | Unclear | Unclear | No | No | High |
| Lin et al[19] | 2006 | Unclear | Yes | Unclear | Yes | Unclear | Unclear |
| Cheng et al[21] | 2005 | Unclear | Yes | Yes | Yes | Unclear | Low |
| Dokas et al[9] | 2004 | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Chilovi et al[24] | 2003 | Unclear | Unclear | Unclear | Unclear | Unclear | High |
| Udd et al[8] | 2001 | Yes | Yes | Yes | Yes | Unclear | Low |
| Schonekas et al[7] | 1999 | Unclear | Unclear | Unclear | Unclear | Unclear | High |
Patients were of European origin in 6 studies[7-9,16,17,24] and Asian in 3 studies[2,19,21]. Baseline characteristics and PPI regimens of the included studies are shown in Tables 2 and 3, respectively. Thus, 9 studies including 1342 patients were analyzed in this meta-analysis, and the mean number of patients per study was 149, ranging from 24 to 474. The mean age of patients was ≥ 60 years in 6 studies, in the other three studies the age of the patients could not be ascertained. The number of patients who had gastric ulcers was 435, while that of duodenal ulcers was 600, included in the 6 studies from which adequate data could be extracted[2,8,16,17,19,21]. The PPI used in the included studies was omeprazole or pantoprazole. In fact, 4 studies used omeprazole[8,9,19,21], 3 studies used pantoprazole[2,7,17], while the other 2 studies used both drugs[16,24].
Table 2.
Baseline characteristics of the included studies
| Author | Yr | Region | Arm | No. | Age (yr) | Male/female | NSAID | Aspirin | Antico-agulant | H. pylori positive | Comorbid disease | Gastric ulcer | Duodenal ulcer | Other ulcer3 | Study population |
| Andriulli et al[16] | 2008 | European | High | 238 | 66.3 ± 15.6 | 151/87 | 75 | 72 | NA | NA | 19 | 88 | 150 | 0 | High-risk group1 |
| Low | 236 | 66.8 ± 16.7 | 156/80 | 74 | 82 | NA | NA | 30 | 93 | 143 | 0 | ||||
| Yüksel et al[17] | 2008 | European | High | 48 | 60.4 ± 14.0 | 38/10 | 19 | 22 | NA | NA | 21 | 16 | 32 | 0 | High-risk group1 |
| Low | 49 | 56.3 ± 17.6 | 36/13 | 20 | 25 | NA | NA | 16 | 16 | 33 | 1 | ||||
| Hung et al[2] | 2007 | Asia | High | 54 | 63.7 | 32/22 | 6 | 9 | NA | 30 | NA | 19 | 35 | 0 | High-risk group1 |
| Low | 49 | 57.8 | 35/14 | 6 | 5 | NA | 33 | NA | 14 | 35 | 0 | ||||
| Lin et al[19] | 2006 | Asia | High | 67 | 67 | 58/9 | NA | NA | NA | 48 | 53 | 26 | 35 | 6 | High-risk group1 |
| Low | 66 | 71 | 57/9 | NA | NA | NA | 51 | 50 | 29 | 33 | 4 | ||||
| Cheng et al[21] | 2005 | Asia | High | 52 | 62.5 ± 12.5 | 36/16 | 13 | 1 | 2 | 27 | 52 | 28 | 19 | 5 | Comorbid illnesses |
| Low | 53 | 65.8 ± 13.8 | 31/22 | 12 | 3 | 1 | 21 | 53 | 29 | 20 | 4 | ||||
| Dokas et al[9] | 2004 | European | High | 10 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | High-risk group1 |
| Low | 14 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||
| Chilovi et al[24] | 2003 | European | High | 46 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Low | 45 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||||
| Udd et al[8] | 2001 | European | High | 69 | 63.4 ± 15.7 | 41/28 | 37 | 7 | 10 | 46 | 55 | 39 | 30 | 0 | Unselected2 |
| Low | 73 | 66.0 ± 13.3 | 44/29 | 41 | 16 | 4 | 45 | 54 | 38 | 35 | 0 | ||||
| Schonekas et al[7] | 1999 | European | High | 82 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Low | 86 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
Included peptic ulcer patiens with spurting artery, ooze, non-bleeding visible vessel or adherent clot;
Included peptic peptic ulcer patiens with spurting artery, ooze, non-bleeding visible vessel, adherent clot or black base;
Include both gastral and duodenal ulcer, esophagal ulcer. H. pylori: Helicobacter pylori; NSAID: Non-steroidal anti-inflammatory drug; NA: Not available.
Table 3.
Proton pump inhibitor regimen of the included studies
| Author | Arm | Endoscopic therapy | Schedule of the PPIs |
| Andriulli et al[16] | High | Epinephrine injection or thermal or mechanical therapy | OME or PAN 80 mg iv bolus, 8 mg/h infusion × 3 d |
| Low | OME or PAN 40 mg iv bolus 24 h × 3 d | ||
| Yüksel et al[17] | High | Epinephrine injection and thermal therapy | PAN 80 mg iv bolus, 8 mg/h infusion × 3 d |
| Low | PAN 40 mg iv bolus Q12 h × 3 d | ||
| Hung et al[2] | High | Epinephrine injection or thermal therapy | PAN 80 mg iv bolus, 8 mg/h infusion × 3 d |
| Low | PAN 80 mg iv bolus, 40 mg iv bolus Q12 h × 3 d | ||
| Lin et al[19] | High | Epinephrine injection | OME 40 mg infusion Q6 h × 3 d |
| Low | OME 40 mg infusion Q12 h × 3 d | ||
| Cheng et al[21] | High | Epinephrine injection or thermal therapy | OME 80 mg iv bolus, 200 mg infusion 24 h × 3 d |
| Low | OME 80 mg iv bolus, 80 mg infusion 24 h × 3 d | ||
| Dokas et al[9] | High | Epinephrine injection or mechanical therapy | OME 80 mg iv bolus, 160 mg infusion Q24 h × 3 d |
| Low | OME 40 mg iv bolus Q12 h × 3 d | ||
| Chilovi et al[24] | High | NA | PAN 80 mg iv bolus, 40 mg iv bolus Q8 h |
| Low | OME 80 mg iv bolus, 40 mg | ||
| Udd et al[8] | High | Epinephrine injection or thermal or mechanical therapy | OME 80 mg iv bolus, 8 mg/h infusion × 3 d |
| Low | OME 20 mg iv bolus 24 h × 3 d | ||
| Schonekas et al[7] | High | NA | PAN 40 mg iv bolus 8 mg/h infusion × 3 d |
| Low | PAN 40 mg iv bolus Q24 h × 3 d |
PPIs: Proton pump inhibitors; OME: Omeprazole; PAN: Pantoprazole.
Rebleeding
Nine studies including 1342 patients evaluated the rate of rebleeding as an outcome measure; 666 patients in the high-dose group and 676 patients in the low-dose group. The summary OR for rebleeding was 1.091 (95% CI: 0.777-1.532, P = 0.617) suggesting the result was not statistically significant when comparing high-dose with low-dose PPI following endoscopic hemostasis in reducing the risk of rebleeding (Figure 2A). The χ2 test for heterogeneity for the high-dose effect in rebleeding was not significant (I2 = 0.0%, P = 0.498). The subgroup analysis according to different region did not indicate any benefit in either European or Asian population with high-dose PPI regimen compared with low-dose regimen following endoscopic hemostasis; for Asian patients the summary OR was 0.831 (95% CI: 0.467-1.480, P = 0.529), while for European patients the summary OR was 1.263 (95% CI: 0.827-1.929, P = 0.279). No heterogeneity was detected in those two subgroup analyses, with P-values of 0.143 and 0.812, respectively. The summary OR for rebleeding from 4 studies using omeprazole[8,9,19,21] (OR = 0.926, 95% CI: 0.550-1.557) and for the remaining 5 studies using pantoprazole/omeprazole[2,7,16,17,24] (OR = 1.234, 95% CI: 0.786-1.936) did not differ in rebleeding rate. The summary OR for rebleeding from 5 studies[2,9,16,17,19] with endoscopic high-risk stigmata for rebleeding revealed no reduction in the high-dose PPI group (OR = 0.991, 95% CI: 0.628-1.565, P = 0.970). The summary OR for rebleeding from 4 studies[16,17,21,22] with low risk of bias revealed no reduction in the high-dose PPI group (OR = 1.479, 95% CI: 0.963-2.269, P = 0.999). When excluding the 3 abstracts, the summary OR for rebleeding from the remaining 6 studies revealed no reduction in the high-dose PPI group (OR = 1.130, 95% CI: 0.827-1.544, P = 0.335). In addition, the time-frame of rebleeding rate should be taken into consideration, because one study reported early rebleeding (within first 72 h)[17], two studies reported in-hospital occurrence of rebleeding[8,25], one study reported rebleeding before discharge and within 14 d[19], and 2 studies reported rebleeding within 30 d[21,26]; the time-frame of rebleeding was not reported in the remaining 3 studies[7,9,24].
Figure 2.
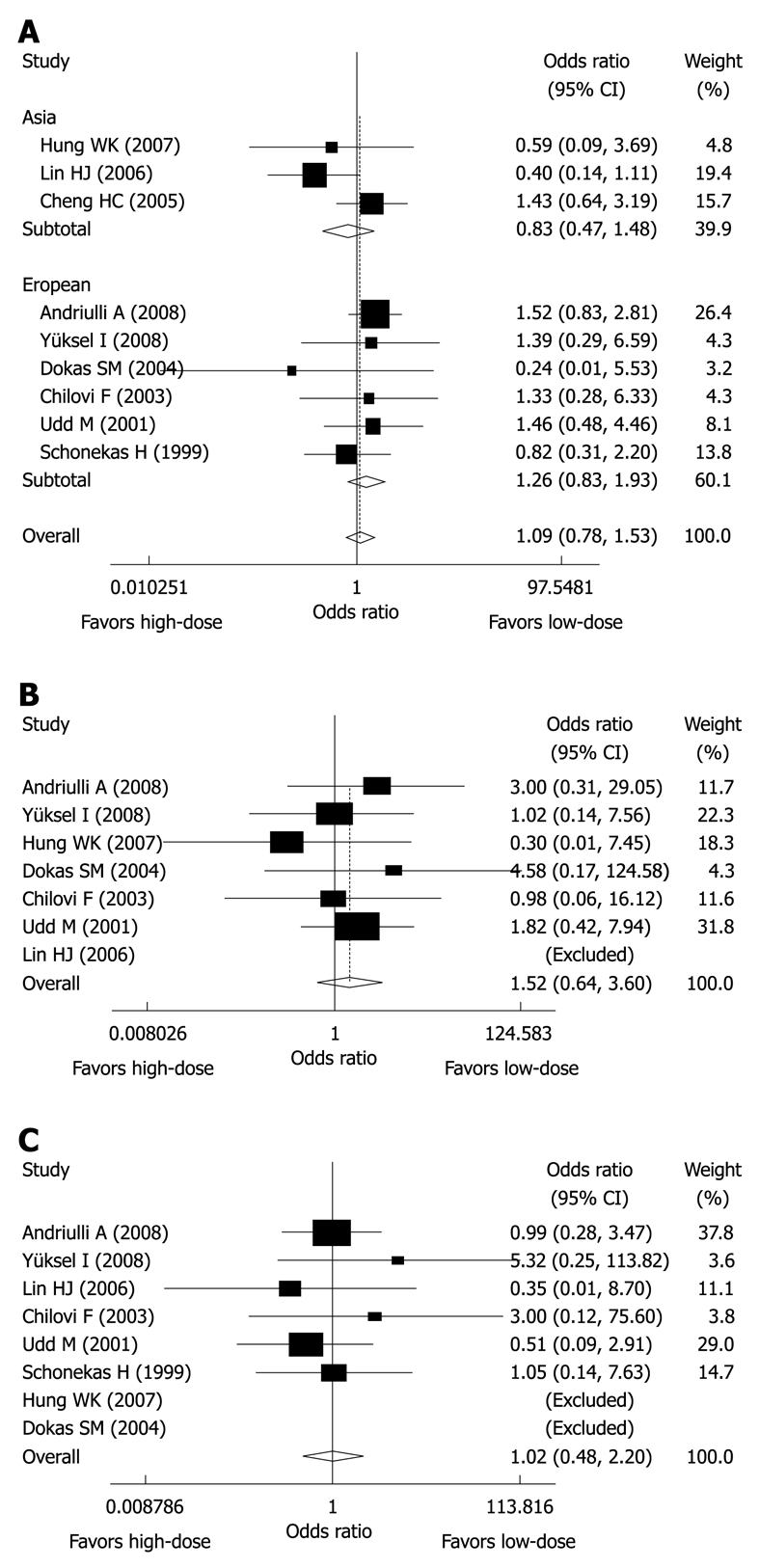
Meta-analysis results of randomized clinical trials comparing high-dose proton pump inhibitor (PPI) vs low-dose PPI in rebleeding (A), surgery (B) and mortality (C).
Surgery
Seven studies[2,8,9,16,17,19,24] including 1225 patients evaluated the rate of surgery as an outcome measure; 532 patients in the high-dose group and 537 in the low-dose group. Thus, the need for surgery was reported in 2.3% of the patients treated with high-dose PPI, and in 1.5% of the patients treated with low-dose PPI. The summary OR for surgery was 1.522 (95% CI: 0.643-3.605, P = 0.340), indicating the result was not statistically significant when comparing high-dose with low-dose PPI following endoscopic hemostasis in reducing the need for surgery (Figure 2B). The χ2 test for heterogeneity for the high-dose effect in reducing the need for surgery was not significant (I2 = 0.0%, P = 0.840). The summary OR for surgery from 5 studies[2,9,16,17,19] with endoscopic high-risk stigmata for rebleeding revealed no reduction in the high-dose PPI group (OR = 1.465, 95% CI: 0.461-4.655, P = 0.517). The summary OR for surgery from 4 studies[16,17,21,22] with low risk of bias revealed no reduction in the high-dose PPI group (OR = 1.760, 95% CI: 0.626-4.946, P = 0.780). When excluding the 2 abstracts, the summary OR for surgery from the remaining 5 studies revealed no reduction in the high-dose PPI group (OR = 1.422, 95% CI: 0.569-3.556, P = 0.676).
Mortality
Eight studies[2,7-9,16,17,19,24] including 1237 patients evaluated the rate of mortality as an outcome measure; 614 patients in the high-dose group and 623 patients in the low-dose group. Thus, mortality was reported in 2.0% of the patients treated with high-dose PPI, and in 1.9% of the patients treated with low-dose PPI. The summary OR for mortality was 1.022 (95% CI: 0.476-2.196, P = 0.955), indicating the result was not statistically significant comparing high-dose with low-dose PPI following endoscopic hemostasis in reducing overall mortality (Figure 2C). The χ2 test for heterogeneity for the high-dose effect of affecting overall mortality was not significant (I2 = 0.0%, P = 0.765). Six studies[2,8,9,16,17,19] reported causes of death, and rebleeding caused 8 deaths (4 in the high-dose PPI group and 4 in the low-dose group); the summary OR for rebleeding-related deaths was 1.008 (95% CI: 0.250-4.067, P = 0.991), suggesting no benefit with high-dose PPI therapy. No studies stated the time-frame of mortality rate clearly, so they were assumed to be in-hospital mortality rates.
Publication bias
The P-values from Egger’s test in rebleeding, the need for surgery, and mortality were of 0.183, 0.674, and 0.488, respectively. Thus, no publication bias exists in this meta-analysis.
DISCUSSION
Rebleeding has remained the most important adverse prognostic factor contributing to morbidity and mortality after upper gastrointestinal hemorrhage[25]. Previous consensus groups have recommended that an intravenous bolus followed by continuous-infusion PPI is effective in decreasing rebleeding in patients who have undergone successful endoscopic therapy[4]. However, this recommendation of a high-dose of PPI is in conflict with some previous studies[7,16], which reported no difference in the magnitude of risk reduction between high-dose and low-dose regimens. Therefore, this meta-analysis set out to evaluate the efficacy of a high-dose PPI regimen vs a low-dose PPI regimen in reducing rebleeding, the need for surgery and the mortality rate following endoscopic hemostasis for upper gastrointestinal bleeding.
The findings of this meta-analysis suggest that a high-dose PPI regimen for patients who have undergone successful endoscopic therapy is not superior to a low-dose PPI regimen for the parameters of rebleeding (OR = 1.091, 95% CI: 0.777-1.532, P = 0.617), the need for surgery (OR = 1.522, 95% CI: 0.643-3.605, P = 0.340), and mortality (OR = 1.022, 95% CI: 0.476-2.196, P = 0.955) and rebleeding-related deaths (OR = 1.008, 95% CI: 0.250-4.067). Also, no heterogeneity exists, suggesting that the studies for these endpoints were homogenous.
It has been hypothesized that the efficacy of a high-dose PPI regimen may vary depending on physiological measures, pharmacodynamic profiles (metabolism of proton-pump inhibitors through the cytochrome P4502C19 genetic polymorphism), and prevalence rates of Helicobacter pylori infection in different ethnicities[26], so subgroup analysis was carried out to determine whether low-dose PPI can have the same efficacy in patients from different regions. This subgroup analysis did not find any difference in rebleeding rate between Asian and European patients using a low-dose PPI regimen. However, the need for surgery and mortality cannot be assessed in this subgroup analysis due to limited studies. Thus, a low-dose PPI regimen can achieve the same efficacy in reducing rebleeding as a high-dose regimen in both Asian (OR = 0.831, 95% CI: 0.467-1.480, P = 0.529) and European patients (OR = 1.263, 95% CI: 0.827-1.929, P = 0.279). The endoscopic therapy technique was epinephrine injection, plus if indicated thermal or mechanical therapy, and this technique was applied in 5 studies[2,8,9,16,21]. This endoscopic therapy technique is practical and widely used in most hospitals, so the endoscopic therapy technique was homogenous across the included studies. In this present meta-analysis, four studies evaluated omeprazole, while the other 5 studies evaluated pantoprazole or both drugs together. Subgroup analysis according to different PPI showed similar results to those found in all 9 studies, so this effect of PPI seems to be a class effect.
This meta-analysis showed that the high-dose PPI regimen did not affect overall mortality (OR = 1.022, 95% CI: 0.476-2.196, P = 0.955) when compared with the low-dose PPI regimen. A total of 8 deaths were rebleeding-related (50% in the high-dose group and 50% in the low-dose group), and 11 deaths were non-ulcer deaths (45.5% in the high-dose group and 54.5% in the low-dose group). The data in this present meta-analysis indicated that the high-dose group had a similar mortality rate as the low-dose group independent of the outcome of comorbid diseases. However, this mortality result is not reliable, because it has been estimated that in order to detect clinically important effects on mortality, at least 1000 high-risk patients should be included in each of the treatment and control groups[27]; thus the number of high-risk patients included in this meta-analysis is not enough. Nonetheless, since high-dose regimens can produce similar reductions in rebleeding and need for surgery as low-dose regimens, maybe the effect of a high-dose PPI regimen on mortality is the same as a low-dose PPI regimen.
Summary improvements in transfusion requirements and duration of hospital stay have not been analyzed in this meta-analysis, because these improvements are of lesser clinical magnitude and are based on weaker source study methodology[28].
A previous meta-analysis revealed that PPI therapy is only efficacious for patients who had endoscopic high-risk stigmata for rebleeding[29]. In addition, subgroup analysis has shown that in this particular patient group, low-dose PPI is as effective as high-dose PPI. Future studies should stratify the management of patients according to low-, intermediate-, and high-risk endoscopic lesions with relevant strategies that include low-dose PPI regimens.
Cost-effectiveness analyses using decision modeling and direct costing from studies have demonstrated that the use of high-dose PPI following endoscopic hemostasis saves money for health care systems in most clinical scenarios[30,31]. Thus, if low-dose PPI is as effective as high-dose, it is assumed that this can result in savings in health care costs. The most recent randomized trials comparing the use of intravenous regular-dose omeprazole with oral rabeprazole revealed that these drugs are equally effective in preventing rebleeding in patients with high-risk bleeding peptic ulcers after successful endoscopic injection with epinephrine[15]. If intravenous PPIs could be replaced by oral PPIs, this could result in significant savings in healthcare resources[32]. This trial was performed in an Asian population, however, so additional data from randomized clinical trials comparing the use of intravenous PPIs with that of oral PPIs are required in western patient populations.
In this meta-analysis, the modality of endoscopic intervention and the type of PPI used have varied across studies. Regarding the claim that monotherapy with epinephrine is inferior to dual endoscopic therapies[33], published data are not consistent. Pharmacologic data indicate a class effect of PPI therapy as inhibitors of gastric secretion, and one study did find similar rebleeding rates in a comparative trial of omeprazole vs pantoprazole[24]. Therefore, the differences in practice patterns across different studies might constitute a particular strength of this meta-analysis, because it better approximates the real-life practice due to availability of different PPIs and endoscopic interventions in different hospitals. Thus, the differences in the modality of endoscopic intervention and the type of PPI would not have affected the overall validity of our findings.
There are some limitations in this meta-analysis: firstly, a total of nine studies were included, in which only four studies were at low risk of bias, and three studies could not be accessed as full texts, so the included studies are not quite adequate; secondly, although subgroup analysis revealed that the summary OR for rebleeding remained statistically stable (similar to summary OR obtained from 9 studies), it does not prove the stability of our results; thirdly, the time-frame of rebleeding rate varied between different studies, so the results for rebleeding should be interpreted with caution, and further subgroup analyses were not performed in this meta-analysis; fourthly, studies included in this meta-analysis were carried out with varying doses of PPI, different populations with different risks of rebleeding and varying proportions of associated comorbid diseases. It is inevitable in clinical practice to encounter different patients, so this should not affect the overall validity of the findings.
In conclusion, a low-dose PPI regimen is as efficacious as a high-dose PPI regimen following endoscopic hemostasis in upper gastrointestinal bleeding patients, and this beneficial effect seems to be similar in both Asian and European patients. Future comparative trials are warranted to compare oral PPI with intravenous PPI following endoscopic hemostasis in western patient populations.
COMMENTS
Background
The combination of endoscopic hemostasis and the administration of proton pump inhibitors (PPIs) is the current standard management for upper gastrointestinal hemorrhage. A low-dose of PPIs may achieve the same efficacy as high-dose PPIs following endoscopic hemostasis.
Research frontiers
This meta-analysis investigated the rebleeding rate, the need for surgery and mortality outcomes in randomized controlled trials (up to 9 studies).
Innovations and breakthroughs
Compared with high-dose PPIs following endoscopic hemostasis, low-dose PPIs can achieve the same effect in reducing rebleeding, the need for surgery and mortality. This beneficial effect seems to be consistent in both Asian and European patients.
Applications
The study can be applied as guidance for using low-dose PPI regimens following endoscopic hemostasis for gastrointestinal bleeding.
Peer review
This is a good quality work on a subject of everlasting interest.
Footnotes
Supported by First Affiliated Hospital, Guangxi Medical University
Peer reviewers: Anastasios Koulaouzidis, MD, MRCP (UK), Day Case and Endoscopy Unit, Centre of Liver and Digestive Disorders, Royal Infirmary of Edinburgh, 51 Little France Crescent, Edinburgh, EH16 4SA, Scotland; Guy D Eslick, PhD, MmedSc (Clin Epi), MmedStat, Department of Medicine, The University of Sydney Nepean Hospital, Level 5, South Block, PO Box 63, Penrith, Sydney, NSW 2751, Australia
S- Editor Wang YR L- Editor Logan S E- Editor Zheng XM
References
- 1.Rockall TA, Logan RF, Devlin HB, Northfield TC. Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom. Steering Committee and members of the National Audit of Acute Upper Gastrointestinal Haemorrhage. BMJ. 1995;311:222–226. doi: 10.1136/bmj.311.6999.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hung WK, Li VK, Chung CK, Ying MW, Loo CK, Liu CK, Lam BY, Chan MC. Randomized trial comparing pantoprazole infusion, bolus and no treatment on gastric pH and recurrent bleeding in peptic ulcers. ANZ J Surg. 2007;77:677–681. doi: 10.1111/j.1445-2197.2007.04185.x. [DOI] [PubMed] [Google Scholar]
- 3.British Society of Gastroenterology Endoscopy Committee. Non-variceal upper gastrointestinal haemorrhage: guidelines. Gut. 2002;51 Suppl 4:iv1–iv6. doi: 10.1136/gut.51.suppl_4.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barkun A, Bardou M, Marshall JK. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139:843–857. doi: 10.7326/0003-4819-139-10-200311180-00012. [DOI] [PubMed] [Google Scholar]
- 5.Green FW Jr, Kaplan MM, Curtis LE, Levine PH. Effect of acid and pepsin on blood coagulation and platelet aggregation. A possible contributor prolonged gastroduodenal mucosal hemorrhage. Gastroenterology. 1978;74:38–43. [PubMed] [Google Scholar]
- 6.Patchett SE, Enright H, Afdhal N, O'Connell W, O'Donoghue DP. Clot lysis by gastric juice: an in vitro study. Gut. 1989;30:1704–1707. doi: 10.1136/gut.30.12.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schonekas H, Ahrens H, Pannewick U, Ell C, Koop H, Petrisch W, Klein M, Fischer R. Comparison of two doses of intravenous pantoprazole in peptic ulcer bleeding. Gastroenterology. 1999;116:A305. [Google Scholar]
- 8.Udd M, Miettinen P, Palmu A, Heikkinen M, Janatuinen E, Pasanen P, Tarvainen R, Kairaluoma MV, Lohman M, Mustonen H, et al. Regular-dose versus high-dose omeprazole in peptic ulcer bleeding: a prospective randomized double-blind study. Scand J Gastroenterol. 2001;36:1332–1338. doi: 10.1080/003655201317097218. [DOI] [PubMed] [Google Scholar]
- 9.Dokas SM, Lazaraki GI, Kontoninas Z, Kouklakis GS, Adamidou A, Tsiaousi E, Christoforidis C, Ziakas G. Bolus intravenous omeprazole b.i.d vs. continuous intravenous omeprazole infusion combined with endoscopic hemostasis in the treatment of peptic ulcer bleeding. Preliminary results. Gut. 2004;53 Suppl VI:A290. [Google Scholar]
- 10.Bajaj JS, Dua KS, Hanson K, Presberg K. Prospective, randomized trial comparing effect of oral versus intravenous pantoprazole on rebleeding after nonvariceal upper gastrointestinal bleeding: a pilot study. Dig Dis Sci. 2007;52:2190–2194. doi: 10.1007/s10620-006-9282-2. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. QUOROM Group. Br J Surg. 2000;87:1448–1454. doi: 10.1046/j.1365-2168.2000.01610.x. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JPT, Green S, editors . Cochrane handbook for systematic reviews of interventions. Version 5.0.2 [updated September 2009]. The Cochrane Collaboration. 2009. Available from: http://www.cochrane-handbook.org. [Google Scholar]
- 13.Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Smith GD, Altman DG, editors. Systematic reviews in health care: meta-analysis in context. 2nd ed. London: BMJ Books; 2001. pp. 285–312. [Google Scholar]
- 14.Egger M, Dickersin K, Smith GD. Problems and limitations in conducting systematic reviews. In: Egger M, Smith GD, Altman DG, editors. Systematic reviews in health care: metaanalysis in context. 2nd ed. London: BMJ Books; 2001. pp. 43–68. [Google Scholar]
- 15.Tsai JJ, Hsu YC, Perng CL, Lin HJ. Oral or intravenous proton pump inhibitor in patients with peptic ulcer bleeding after successful endoscopic epinephrine injection. Br J Clin Pharmacol. 2009;67:326–332. doi: 10.1111/j.1365-2125.2008.03359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andriulli A, Loperfido S, Focareta R, Leo P, Fornari F, Garripoli A, Tonti P, Peyre S, Spadaccini A, Marmo R, et al. High- versus low-dose proton pump inhibitors after endoscopic hemostasis in patients with peptic ulcer bleeding: a multicentre, randomized study. Am J Gastroenterol. 2008;103:3011–3018. doi: 10.1111/j.1572-0241.2008.02149.x. [DOI] [PubMed] [Google Scholar]
- 17.Yüksel I, Ataseven H, Köklü S, Ertuğrul I, Başar O, Odemiş B, Ibiş M, Saşmaz N, Sahin B. Intermittent versus continuous pantoprazole infusion in peptic ulcer bleeding: a prospective randomized study. Digestion. 2008;78:39–43. doi: 10.1159/000158227. [DOI] [PubMed] [Google Scholar]
- 18.Oh JH, Choi MG, Dong MS, Park JM, Paik CN, Cho YK, Jeong JJ, Lee IS, Kim SW, Han SW, et al. Low-dose intravenous pantoprazole for optimal inhibition of gastric acid in Korean patients. J Gastroenterol Hepatol. 2007;22:1429–1434. doi: 10.1111/j.1440-1746.2007.05059.x. [DOI] [PubMed] [Google Scholar]
- 19.Lin HJ, Lo WC, Cheng YC, Perng CL. Role of intravenous omeprazole in patients with high-risk peptic ulcer bleeding after successful endoscopic epinephrine injection: a prospective randomized comparative trial. Am J Gastroenterol. 2006;101:500–505. doi: 10.1111/j.1572-0241.2006.00399.x. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz S, Bayan K, Tüzün Y, Dursun M, Canoruç F. A head to head comparison of oral vs intravenous omeprazole for patients with bleeding peptic ulcers with a clean base, flat spots and adherent clots. World J Gastroenterol. 2006;12:7837–7843. doi: 10.3748/wjg.v12.i48.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng HC, Kao AW, Chuang CH, Sheu BS. The efficacy of high- and low-dose intravenous omeprazole in preventing rebleeding for patients with bleeding peptic ulcers and comorbid illnesses. Dig Dis Sci. 2005;50:1194–1201. doi: 10.1007/s10620-005-2759-6. [DOI] [PubMed] [Google Scholar]
- 22.Udd M, Töyry J, Miettinen P, Vanninen E, Mustonen H, Julkunen R. The effect of regular and high doses of omeprazole on the intragastric acidity in patients with bleeding peptic ulcer treated endoscopically: a clinical trial with continuous intragastric pH monitoring. Eur J Gastroenterol Hepatol. 2005;17:1351–1356. doi: 10.1097/00042737-200512000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Tseng GY, Lin HJ, Lin HY, Perng CL, Lee FY, Lo WC, Chang FY, Lee SD. The influence of intravenous omeprazole on intragastric pH and outcomes in patients with peptic ulcer bleeding after successful endoscopic therapy--a prospective randomized comparative trial. Hepatogastroenterology. 1999;46:2183–2188. [PubMed] [Google Scholar]
- 24.Chilovi F, Piazzi L, Zancanella L, De Guelmi A, Grasso T, Di Fede F, Bertozzo A, Amplatz S, Farris P, Benvenuti S, et al. Intravenous omeprazole and pantoprazole after endoscopic treatment of bleeding peptic ulcers. Gastrointest Endosc. 2003;57:AB150. [Google Scholar]
- 25.Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316–321. doi: 10.1136/gut.38.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leontiadis GI, Sharma VK, Howden CW. Systematic review and meta-analysis: enhanced efficacy of proton-pump inhibitor therapy for peptic ulcer bleeding in Asia--a post hoc analysis from the Cochrane Collaboration. Aliment Pharmacol Ther. 2005;21:1055–1061. doi: 10.1111/j.1365-2036.2005.02441.x. [DOI] [PubMed] [Google Scholar]
- 27.Langman MJ. Problems in assessing pharmacologic treatment of acute upper gastrointestinal bleeding. Hepatogastroenterology. 1990;37 Suppl 1:29–30. [PubMed] [Google Scholar]
- 28.Leontiadis GI, Sharma VK, Howden CW. Systematic review and meta-analysis: proton-pump inhibitor treatment for ulcer bleeding reduces transfusion requirements and hospital stay--results from the Cochrane Collaboration. Aliment Pharmacol Ther. 2005;22:169–174. doi: 10.1111/j.1365-2036.2005.02546.x. [DOI] [PubMed] [Google Scholar]
- 29.Khuroo MS, Khuroo MS, Farahat KL, Kagevi IE. Treatment with proton pump inhibitors in acute non-variceal upper gastrointestinal bleeding: a meta-analysis. J Gastroenterol Hepatol. 2005;20:11–25. doi: 10.1111/j.1440-1746.2004.03441.x. [DOI] [PubMed] [Google Scholar]
- 30.Barkun AN, Herba K, Adam V, Kennedy W, Fallone CA, Bardou M. High-dose intravenous proton pump inhibition following endoscopic therapy in the acute management of patients with bleeding peptic ulcers in the USA and Canada: a cost-effectiveness analysis. Aliment Pharmacol Ther. 2004;19:591–600. doi: 10.1046/j.1365-2036.2004.01808.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee KK, You JH, Wong IC, Kwong SK, Lau JY, Chan TY, Lau JT, Leung WY, Sung JJ, Chung SS. Cost-effectiveness analysis of high-dose omeprazole infusion as adjuvant therapy to endoscopic treatment of bleeding peptic ulcer. Gastrointest Endosc. 2003;57:160–164. doi: 10.1067/mge.2003.74. [DOI] [PubMed] [Google Scholar]
- 32.Spiegel BM, Dulai GS, Lim BS, Mann N, Kanwal F, Gralnek IM. The cost-effectiveness and budget impact of intravenous versus oral proton pump inhibitors in peptic ulcer hemorrhage. Clin Gastroenterol Hepatol. 2006;4:988–997. doi: 10.1016/j.cgh.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Marmo R, Rotondano G, Piscopo R, Bianco MA, D'Angella R, Cipolletta L. Dual therapy versus monotherapy in the endoscopic treatment of high-risk bleeding ulcers: a meta-analysis of controlled trials. Am J Gastroenterol. 2007;102:279–289; quiz 469. doi: 10.1111/j.1572-0241.2006.01023.x. [DOI] [PubMed] [Google Scholar]


