Abstract
Left ventricular (LV) remodeling following myocardial infarction (MI) is considered to contribute to cardiac dysfunction. Though myofiber organization is a key component of cardiac structure, functional and anatomical features of injured myofiber during LV remodeling have not been fully defined. We investigated myocyte injury after acute MI in a mouse model. Mice were subjected to surgical coronary occlusion/reperfusion by left anterior descending coronary artery (LAD) ligation and examined at 1 week and 4 weeks post-MI. Magnetic resonance imaging (MRI) analysis demonstrated a significant decrease in systolic regional wall thickening (WT) in the border and remote zones at 4 weeks post-MI compared to that at 1 week post-MI (−86% in border zone, P<0.05, and −77% in remote zone, P<0.05). Histological assays demonstrated that a broad fibrotic scar extended from the initial infarct zone to the remote zone along midcircumferential myofibers. Of particular note was the fact that no fibrosis was found in longitudinal myofibers in the epi- and endomyocardium. This pattern of the scar formation coincided with the helical ventricular myocardial band (HVMB) model, introduced by Torrent-Guasp. MRI analysis demonstrated that the extension of the fibrotic scar along the band might account for the progression in cardiac dysfunction during LV remodeling.
Keywords: Muscle fibers, Ventricular function, Ventricular remodeling, Animals
1. Introduction
Interstitial myocardial fibrosis is an important pathogenic feature of adverse left ventricular (LV) remodeling after myocardial infarction (MI) [1]. Since myocardium architecture, including the geometry of myofibers, accounts for efficient pump function at the global level of the heart [2], distribution of interstitial fibrosis during LV remodeling is a critical factor for prognosis in heart diseases. The helical ventricular myocardial band (HVMB) reported by Torrent-Guasp could explain the myofiber geometry and how its form as a single muscular band relates to cardiac motion [2, 3]. Recent reports, demonstrating the distribution of coronary arteries and pattern of electrophysiological mapping along the muscular band, support the HVMB model’s architectural and anatomical conclusions[4, 5. However, whether the HVMB explains pathophysiological features of LV remodeling after MI has not been explored.
In the present study with a mouse model of MI, we demonstrate that scar formation in the mid-myocardium during LV remodeling is consistent with the HVMB. Magnetic resonance imaging (MRI) analysis shows that a change in regional wall thickness during the cardiac cycle is significantly reduced at the zones possessing mid-myocardial fibrosis. Together, these data imply that the HVMB model accounts for pathological and functional features observed during LV remodeling.
2. Materials and methods
2.1. Animal model of ischemia-reperfusion injury
This study was approved by the Institution Animal Care and Use Committee at Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA. C57BL/6 male mice aged 12–14 weeks (Charles River, Wilmington, MA, USA) were subjected to ischemia-reperfusion injury in cohort with an acute MI model as previously described [6]. The left anterior descending coronary artery (LAD) was ligated with 7-0 silk (occlusion). After 30 min, the LAD ligature was released, and reperfusion was visually confirmed. Based on our previous results from echocardio-graphic studies, which indicated that post-MI mice develop ventricular dilation in a time-dependent manner, 4 of 14 mice subjected to occlusion-reperfusion were examined at 1 week post-MI to study early LV remodeling. The remaining 10 mice were examined at 4 weeks post-MI in order to study late LV remodeling. To evaluate the ischemic area produced by the operation in each heart, fluorescent microspheres were injected during the coronary occlusion, as previously described [6]. We have confirmed that micro-sphere injection did not cause MI, and therefore fibrosis, even at 4 weeks after MI. For sham-operated controls, three mice were operated on using the same protocol as used for the MI model, except without LAD ligation.
2.2. MRI
MRI was acquired with a 4.7 T magnet (Bruker BioSpin, Billerica, MA, USA) using a transmit-receive, bird-cage coil with an inner diameter of 35 mm (Bruker BioSpin, Billerica, MA). Image acquisition was gated to the electrocardiogram (ECG) R wave during the expiratory phase of respiration (BioTrig, Bruker Biospin). A series of gradient echo scout images was generated to determine the long-axis of the heart. Gated gradient echo sequences were used to acquire sequential true short-axis slices 1 mm in thickness and contiguous to each other, typically requiring 7–8 total slices for coverage of the entire LV cavity from apex to LV outflow tract. Ten cine frames encompassing one cardiac cycle were obtained at each slice level with the following sequence parameters: repetition time=ECG R-R interval/10 (typically 5 ms), echo time=2.2 ms, number of repeats=4, field of view=30×30 mm2, matrixs128×128 (in-plane resolution of 234×234 μm2) and flip angle=30°. To assess regional wall thickening (WT), regional spoke lengths at end systole (ES) and end diastole (ED) were acquired at the mid-papillary level. The percentage of WT was calculated with the following equation: %WT=(WTES–WTED)×100/WTED.
2.3. Histological assays
After measuring cardiac function at the end of each time point, the mouse was euthanized with pentobarbital sodium and the heart was harvested for histological assays. In addition to the post-MI mice subjected to MRI study, additional mice were examined for histological assays, such that a total of 4 mice were considered at 1 week post-MI and a total of 10 mice were considered at 4 weeks post-MI. After washing with saline, each heart was fixed with 4% polyformaldehyde and sectioned from apex to base into four 2-mm sections using a tissue slicer (Zivic, Pittsburgh, PA, USA). After confirming the area-at-risk with micro-spheres under UV light, fixed-sections were dehydrated and embedded in paraffin. Serial 5 μm cross sections were then cut from each tissue section. The sections were stained with Masson’s trichrome to show tissue fibrosis or immunostained with anti-dystrophin (Abcam, Cambridge, MA, USA) to identify cardiomyocytes. Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole) (Vector, Burlingame, CA, USA).
2.4. Statistical analysis
Data are represented as the mean±S.E. from at least three independent samples in each group and were compared using a two-tailed Student’s t-test. One-way ANOVA followed by Bonferroni’s multiple comparison test was used for comparison of %WT in MRI analysis. For all analyses, a P-value <0.05 was considered significant.
3. Results
3.1. MRI
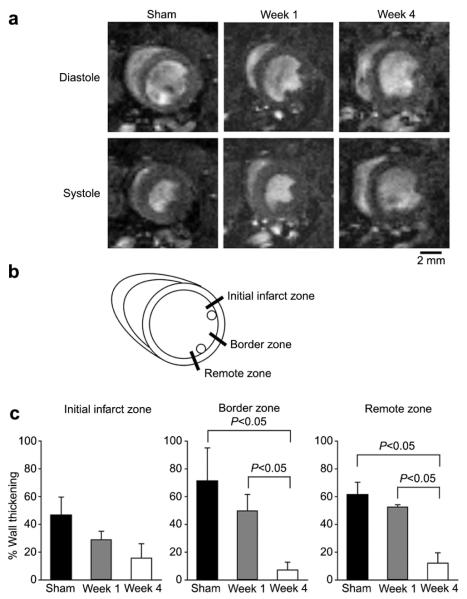
MRI demonstrated a dramatically enlarged LV cavity in ES at week 4 (4 weeks post-MI), compared to a more moderate enlargement at week 1 (1 week post-MI) (Fig. 1a). To evaluate regional wall function in the LV, the percent wall thickening (%WT) was measured in three zones; initial infarct zone, border zone and remote zone (Fig. 1b). MRI analysis demonstrated a significant decrease in regional systolic WT in the border and remote zones at week 4 compared to that at week 1 (7.2±5.7% vs. 50.0±11.7% in the border zone and 12.1±7.5% vs. 52.7±5.6% in the remote zone, week 4 vs. week 1), while no significant change between week 1 and week 4 was seen at the initial infarct zone (caused by the coronary ligation)(Fig. 1c).
Fig. 1.
MRI analysis. (a) Representative MRI of sham, 1 week post-MI (week 1) and 4 weeks post-MI (week 4) mice in full diastolic (Diastole) and full systolic (Systole) phases at the mid-ventricular level. Images are representative from three sham-operated, three week 1 and six week 4 mice. (b) Measured points for wall thickness of MRI images. Front of anterior papillary muscle (Initial infarct zone), between papillary muscles (Border zone), and posterior side of posterior papillary muscle (Remote zone). (c) Systolic wall thickening. The percentage of systolic wall thickening of sham-operated (n=3), week 1 (n=3) and week 4 (n=6) mice was measured as described in the materials and methods. Data are mean±S.E.M.
3.2. Fibrosis in the mid-myocardium
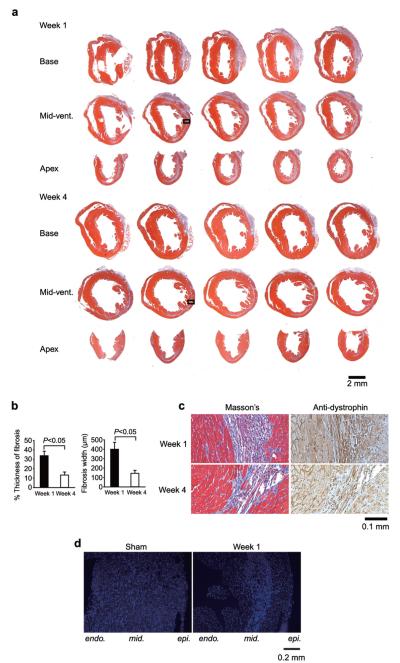
Fluorescent microspheres demonstrated that there was no significant difference in the area-at-risk between week 1 and week 4 hearts (Fig. 2). Masson’s trichrome staining demonstrated broad scar formation, indicated by blue stain, in both week 1 and week 4 mice. Of note, except initial injury that was transmural at the spot of coronary ligation in the anterior wall, all scars in post-MI animals (4 mice at week 1 and 10 mice at week 4) were observed in the mid-myocardium (Fig. 3a). High magnification view of Masson’s trichrome and anti-dystrophin staining demonstrated that the line of damaged myocytes was along the circumferential myofibers rather than the longitudinal orientated myofibers (Fig. 3c). Moreover, the fibrosis ran to the apex with a clear margin (Fig. 3a). The width of scar in the adjacent zone at week 4 was significantly thinner than at week 1 (13.4±3.2% vs. 33.9±4.7% and 145.6±30.2 μm vs. 401.4±73.2 μm, week 4 vs. week 1, P<0.05, Fig. 3b). Granulation tissue including inflammatory cells was seen dominantly in the mid-myocardium at week 1 (Fig. 3c). Nuclear staining at week 1 demonstrated that the cells were accumulated in the mid-myocardium with a clear margin (Fig. 3d).
Fig. 2.
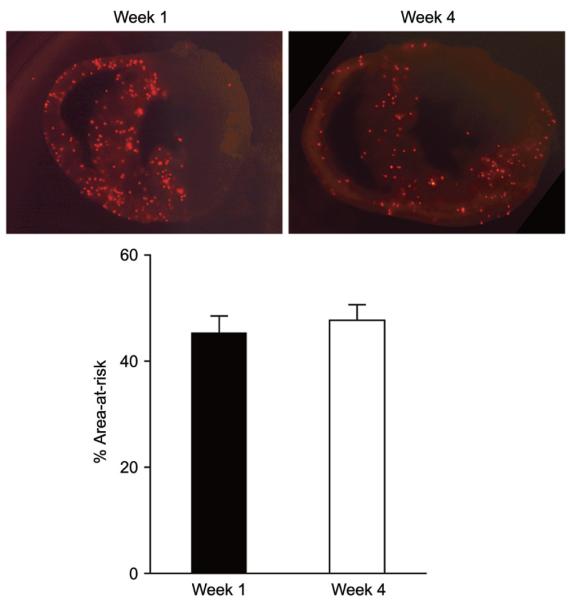
The ischemic are (area-at-risk). Images of fluorescent microspheres are representative from week 1 and week 4 mice (Upper). The percent of the area-at-risk of week 1 (n=3) and week 4 (n=10) mice was measured as previously described [6] (Lower). Data are mean±S.E.M.
Fig. 3.
Histological assays. (a) Representative sections of Masson’s trichrome staining at week 1 and week 4. Serial cross sections were acquired from basal (Base), mid-ventricular (Mid-vent.) and apical (Apex) slice positions. (b) The percent (left) and width (right) of scar fibrosis at week 1 (black) and week 4 (white). The percent thickness of scar fibrosis was measured by the ratio to total wall thickness at the same position. Week 1 mice, n=4 and week 4 mice, n=5. Data are mean±S.E.M. (c) High magnification view of Masson’s trichrome and anti-dystrophin staining. The areas indicated with a square in (a) are shown. (d) Representative nuclear staining. Cross sections from hearts of sham-operated and post-MI (week 1) mice were stained with DAPI. endo., endomyocardium; mid., mid-myocardium; epi., epimyocardium.
4. Discussion
The present study shows that fibrotic scar formation in the process of LV remodeling after surgical coronary occlusion/reperfusion by LAD ligation is located along the mid-myocardium, the endo-myocardium or the epi-myocardium. MRI analysis demonstrates a significant decrease in %WT in the border and remote zones in adverse LV remodeling.
In post-MI patients, scarring along the mid-myocardium (called the subendocardium) has frequently been demonstrated in previous reports using a variety of examinations. Gadolinium-contrast MRI demonstrates MI scar as a late enhancement in the subendocardial layer [7]. However, further examinations of anatomical structure with midmyocardial scar in post-MI have not been discussed. In the border and remote zones of post-MI hearts, we have observed scar extension specifically along myofibers in the mid-myocardium but not along any myofibers of either the epi- or endo-myocardium. Coronary vessels cross the whole LV wall from the epicardium to the endocardium. According to the anatomical distribution, in clinical diagnosis, the assignment of myocardial segments supplied by the three major coronary arteries are delineated with transmural lines [8]. Although total coronary ligation models (no reper-fusion) in mice demonstrated transmural injury that is depicted with a radial line, ischemia-reperfusion injury has always induced myocardial injury in the mid-circumferential myofibers dominantly with survived cardiomyocytes in both the epi- and endo-myocardium. Blood flow only through transmural vessels cannot account for the injury pattern because the pressure dependent blood flow in myocardium cannot make a clear marginal line that we and other groups [9 have seen in ischemia-reperfusion injury. In addition to the scar formation, we and other groups [10 have observed that inflammatory cells were accumulated dominantly in the mid-myocardium with a clear margin. The consistent injury pattern observed in the mid-myocardium suggests that some part of the blood supply to the mid-myocardium flows along circumferential myofibers rather than along radial vessels across the myocardium. The HVMB introduced by Torrent-Guasp describes myocar-dial architecture in the form of a single muscular band that is spatially organized into two distinct helicoids [11]. In the HVMB concept, the LV free wall consists of three layers at the mid-ventricular level [11]. The distribution of scar in the mid-circumferential myofibers that we saw in this study appears to support aspects of the HVMB concept [11]. Recent reports demonstrated that the distribution of coronary arteries and pattern of electrophysiological mapping are observed along the HVMB[4, 5]. Taking everything together, we hypothesize that some of the coronary vessels may progress along the myocardial band in addition to transmural vessels and that tissue fibrosis during LV remodeling would extend along the coronary vessels in accord with the HVMB. In fact, recent reports have frequently discussed the fact that late enhancement of the subendocardium in gadolinium-contrast MRI is a critical indicator for subsequent heart failure [12].
Ventricular WT during systole is an essential mechanism for generating pump function within the myocardium [2]. The percent of regional WT indicated by MRI is a good indicator of regional myocardium function and often is utilized for studies of post-MI LV remodeling in patients [13 and animal models, including mice [14]. In the current study, MRI showed a significant decline in %WT in the border and remote zones at four weeks post-MI. These zones corresponded to the areas where we have seen mid-circumferential scar. In post-MI patients, there is a significant correlation between late enhanced myocardium in gadolinium-contrast MRI and WT [13], suggesting that extension of scar area in myocardium is a critical predictor for adverse LV remodeling in the chronic phase. While considerably more samples will be necessary for complete analysis of LV remodeling, we hypothesize a mechanism for the preservation of %WT in the early stage of LV remodeling (1 week post-MI). Consistent with the prior report [10], we observed a decrease on thickness of fibrotic scar in the mid-myocardium during progression of LV remodeling. As previously reported, systolic slippage of laminar sheets in the myocardium is a critical factor for generating WT [15]. Even if the mid-circumferential myofibers are dysfunctional or injured, slippage of the laminar sheet may generate enough of a longitudinal gap between the epi- and endomyocardial surfaces during a stroke, as long as the width of the laminar sheet is kept at the same range to normal, that the rest of the myocardium (the epi- and endomyocardium) is still functional. Although the injury pattern is a feasible mechanism for the adaptive reaction in early LV remodeling, further experiments will be necessary to address the issue.
In conclusion, the HMVB concept accounts for the localization of fibrotic scar formation that we observed in LV remodeling following acute MI. Understanding the consistent pattern of the scar formation following acute-MI may have important implications not only for cardiac exams such as MRI and echocardiography but also for interventional therapies such as surgery and cell therapy.
References
- [1].Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- [2].Buckberg G, Hoffman JI, Mahajan A, Saleh S, Coghlan C. Cardiac mechanics revisited: the relationship of cardiac architecture to ventricular function. Circulation. 2008;118:2571–2587. doi: 10.1161/CIRCULATIONAHA.107.754424. [DOI] [PubMed] [Google Scholar]
- [3].Torrent-Guasp F, Kocica MJ, Corno AF, Komeda M, Carreras-Costa F, Flotats A, Cosin-Aguillar J, Wen H. Towards new understanding of the heart structure and function. Eur J Cardiothorac Surg. 2005;27:191–201. doi: 10.1016/j.ejcts.2004.11.026. [DOI] [PubMed] [Google Scholar]
- [4].Nagamine H, Sawa S, Ikeda C, Shimada M. Ventricular myocardial band and coronary artery systems. Eur J Cardiothorac Surg. 2007;31:741. doi: 10.1016/j.ejcts.2007.01.021. [DOI] [PubMed] [Google Scholar]
- [5].Boineau JP. Left ventricular muscle band (VMB): thoughts on its physiologic and clinical implications. Eur J Cardiothorac Surg. 2006;29(Suppl 1):S56–S60. doi: 10.1016/j.ejcts.2006.02.045. [DOI] [PubMed] [Google Scholar]
- [6].Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, Liao R, Rosenzweig A. Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart. J Biol Chem. 2002;277:22896–22901. doi: 10.1074/jbc.M200347200. [DOI] [PubMed] [Google Scholar]
- [7].Barbier CE, Bjerner T, Johansson L, Lind L, Ahlstrom H. Myocardial scars more frequent than expected: magnetic resonance imaging detects potential risk group. J Am Coll Cardiol. 2006;48:765–771. doi: 10.1016/j.jacc.2006.05.041. [DOI] [PubMed] [Google Scholar]
- [8].Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- [9].Diwan A, Krenz M, Syed FM, Wansapura J, Ren X, Koesters AG, Li H, Kirshenbaum LA, Hahn HS, Robbins J, Jones WK, Dorn GW. Inhibition of ischemic cardiomyocyte apoptosis through targeted ablation of Bnip3 restrains postinfarction remodeling in mice. J Clin Invest. 2007;117:2825–2833. doi: 10.1172/JCI32490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, Gersch C, Tincey S, Michael LH, Entman ML, Frangogiannis NG. Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am J Pathol. 2004;164:665–677. doi: 10.1016/S0002-9440(10)63154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Torrent-Guasp F, Kocica MJ, Corno A, Komeda M, Cox J, Flotats A, Ballester-Rodes M, Carreras-Costa F. Systolic ventricular filling. Eur J Cardiothorac Surg. 2004;25:376–386. doi: 10.1016/j.ejcts.2003.12.020. [DOI] [PubMed] [Google Scholar]
- [12].Costa MA, Shoemaker S, Futamatsu H, Klassen C, Angiolillo DJ, Nguyen M, Siuciak A, Gilmore P, Zenni MM, Guzman L, Bass TA, Wilke N. Quantitative magnetic resonance perfusion imaging detects anatomic and physiologic coronary artery disease as measured by coronary angiography and fractional flow reserve. J Am Coll Cardiol. 2007;50:514–522. doi: 10.1016/j.jacc.2007.04.053. [DOI] [PubMed] [Google Scholar]
- [13].Ichikawa Y, Sakuma H, Suzawa N, Kitagawa K, Makino K, Hirano T, Takeda K. Late gadolinium-enhanced magnetic resonance imaging in acute and chronic myocardial infarction. Improved prediction of regional myocardial contraction in the chronic state by measuring thickness of nonenhanced myocardium. J Am Coll Cardiol. 2005;45:901–909. doi: 10.1016/j.jacc.2004.11.058. [DOI] [PubMed] [Google Scholar]
- [14].Yang Z, Berr SS, Gilson WD, Toufektsian MC, French BA. Simultaneous evaluation of infarct size and cardiac function in intact mice by contrast-enhanced cardiac magnetic resonance imaging reveals contractile dysfunction in noninfarcted regions early after myocardial infarction. Circulation. 2004;109:1161–1167. doi: 10.1161/01.CIR.0000118495.88442.32. [DOI] [PubMed] [Google Scholar]
- [15].Costa KD, Takayama Y, McCulloch AD, Covell JW. Laminar fiber architecture and three-dimensional systolic mechanics in canine ventricular myocardium. Am J Physiol. 1999;276:H595–H607. doi: 10.1152/ajpheart.1999.276.2.H595. [DOI] [PubMed] [Google Scholar]