Abstract
The human auditory system perceptually restores short deleted segments of speech and other sounds (e.g. tones) when the resulting silent gaps are filled by a potential masking noise. When this phenomenon, known as ‘auditory induction’, occurs, listeners experience the illusion of hearing an ongoing sound continuing through the interrupting noise even though the perceived sound is not physically present. Such illusions suggest that a key function of the auditory system is to allow listeners to perceive complete auditory objects with incomplete acoustic information, as may often be the case in multisource acoustic environments. At present, however, we know little about the possible functions of auditory induction in the sound-mediated behaviours of animals. The present study used two-choice phonotaxis experiments to test the hypothesis that female grey treefrogs, Hyla chrysoscelis, experience the illusory perceptual restoration of discrete pulses in the male advertisement call when pulses are deleted and replaced by a potential masking noise. While added noise restored some attractiveness to calls with missing pulses, there was little evidence to suggest that the frogs actually experienced the illusion of perceiving the missing pulses. Instead, the added noise appeared to function as an acoustic appendage that made some calls more attractive than others as a result of sensory biases, the expression of which depended on the temporal order and acoustic structure of the added appendages.
Keywords: auditory grouping, auditory induction, auditory scene analysis, continuity illusion, gray treefrog, perceptual restoration, phonemic restoration, sensory bias, temporal induction
Hearing is important for many animal behaviours. It plays critical roles in sexual selection by allowing animals to choose their mates (Andersson 1994; Gerhardt & Huber 2002) and assess their rivals (Wagner 1989; Peake et al. 2002). It also enables animals to recognize other individuals (Beecher 1991; Aubin & Jouventin 2002), capture prey (Tuttle & Ryan 1981; Au 1993; Popper & Fay 1995), avoid predators (Hoy 1992; Bernal et al. 2007) and choose suitable habitats (Ward & Schlossberg 2004; Nocera et al. 2006). Across these diverse behavioural contexts, animals encounter acoustic scenes that comprise multiple, simultaneously active sound sources. While some sources produce behaviourally relevant sounds, others generate what amounts to extraneous noise (Brumm & Slabbekoorn 2005). In the hearing literature, the auditory processes that carry out the perceptual analysis of acoustic scenes are commonly referred to as ‘auditory scene analysis’ (Bregman 1990; Hulse 2002) or ‘auditory grouping’ (Bregman 1990; Darwin 1997). Although understanding these processes remains an important goal of research on human hearing and speech perception, they have thus far received limited attention in the field of animal behaviour (Hulse 2002; Bee & Micheyl 2008).
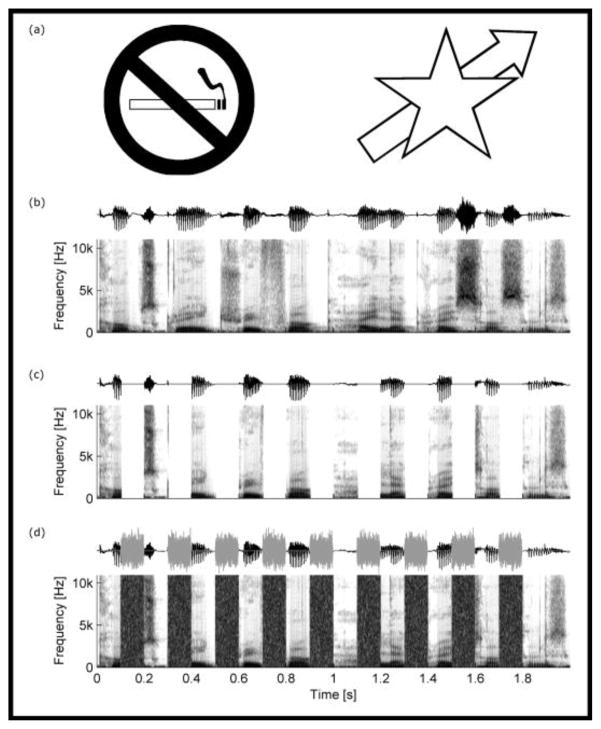
The principles of auditory grouping are similar to those of visual perceptual grouping first enumerated by the Gestalt psychologists (Bregman 1990). In forming visual objects, for example, the principle of ‘good continuation’ is evidenced when an observer recognizes one object that is partially occluded by another (Fig. 1a). Studies of a parallel auditory phenomenon known as ‘auditory induction’ have revealed that under some conditions humans perceive speech as continuing through brief (e.g. 50–200 ms) interruptions by loud noises (e.g. the sound of someone coughing), even when the interrupted speech segments are actually deleted and replaced by silent gaps (Fig. 1b–d; reviewed in: Warren 1999; Kashino 2006; King 2007). The noise filling the gaps induces the illusion of speech continuing through the noise, a phenomenon known as ‘phonemic restoration’ (Warren 1970). Similar ‘continuity illusions’ can also be evoked using noise-filled gaps inserted into nonspeech sounds, such as pure tones or frequency-modulated glides (reviewed in: Bregman 1990; King 2007). Auditory induction is believed to function as an adaptation by which the brain perceptually reconstructs sounds interrupted or masked by other sounds in multisource environments. Previous studies of cats, Felis domesticus (Sugita 1997), cottontop tamarins, Sanguinus oedipus (Miller et al. 2001), rhesus macaques, Macaca mulatta (Petkov et al. 2003, 2007) and European starlings, Sturnus vulgaris (Braaten & Leary 1999; Seeba & Klump 2009), also provide evidence of auditory induction and the perceptual restoration of both artificial sounds (e.g. tones) and conspecific vocalizations. This taxonomic diversity suggests the hypothesis that auditory induction is a widespread hearing mechanism among vertebrates that could influence behaviours that depend on sound perception, such as acoustic communication. Our aim was to test this general hypothesis.
Figure 1.
Examples of the grouping principle of ‘good continuation’. (a) A lit cigarette is perceived as continuing under the ‘no’ symbol in a no smoking sign (left) and an arrow is perceived as continuing under the star (right). These two examples illustrate how the visual system connects spatially separated and discontinuous parts of a scene to create the perception of visual objects that are partially occluded by other objects. (b–d) Spectrograms of a human speech token (‘Cope’s grey treefrog, Hyla chrysoscelis’) that is continuous (b), that contains 100 ms silent gaps in which speech has been deleted (c), and that has the deleted segments filled with short bursts of broadband noise (d). Human listeners typically report the experience of ‘phonemic restoration’ (Warren 1970) and hear ongoing speech continuing through the noise bursts even though no speech sounds are present in the noise-filled gaps.
In this study of Cope’s grey treefrog, Hyla chrysoscelis, we tested the specific hypothesis that auditory induction in the frog auditory system promotes the perceptual restoration of pulsed advertisement calls that are interrupted by brief but loud sounds. Like many other frogs, grey treefrogs commonly breed in large, mixed-species choruses in which males produce loud advertisement calls to attract females (Gerhardt 2001; Gerhardt & Huber 2002). Acoustic interference and auditory masking in the complex acoustic scene of a chorus are two problems that pose potentially serious limitations on treefrog communication (e.g. Gerhardt & Klump 1988; Schwartz et al. 2001; Bee 2008a). Moreover, grey treefrogs commonly breed syntopically with other anurans that have vocal repertoires that include short-duration (< 200 ms), broadband signals, such as green frogs (Bee & Perrill 1996), bullfrogs (Wiewandt 1969), and other species of treefrogs (Gerhardt 2001). Hence, acoustic overlap between the pulsed conspecific advertisement call and other short-duration, broadband sounds may be a common natural occurrence in the multisource environment of a mixed-species chorus.
Here, we report the results of female phonotaxis experiments that tested the auditory induction hypothesis. As stimuli we used synthetic advertisement calls that either comprised a normal complement of pulses or that had silent gaps created by removing pulses. According to the auditory induction hypothesis, our expectation was that filling the silent gaps with noise bursts would lead to the illusory perception of the missing pulses. Our basic experimental approach, creating gaps and filling them with noise, is similar to those of previous studies of auditory induction in humans and other animals. However, there is one potential complication of using this approach coupled with phonotaxis tests to study the perceptual restoration of sexual signals in frogs: the addition of noise bursts to frog advertisement calls can exploit pre-existing sensory biases.
The elaboration of male sexual displays due to the hidden preferences or pre-existing biases of female receivers is well established (Arak & Enquist 1993; Ryan & Rand 1993; Endler & Basolo 1998; Arnqvist 2006). In frogs, the addition of novel acoustic appendages (e.g. ‘chucks’ or bursts of noise) to signals already in the animals’ repertoire can enhance the attractiveness of those signals to females (Rand et al. 1992; Ryan & Rand 1993; Gerhardt et al. 2007). Sensory biases generally select for signals that provide greater sensory stimulation (Ryan & Keddy-Hector 1992). Precisely how such biases are translated into increased preferences, however, may be constrained by rules related to the temporal order and acoustic structure of new appendages relative to established signals already in the species’ repertoire (Gerhardt et al. 2007). Consequently, using noise bursts to test the auditory induction hypothesis in frogs could alter signal attractiveness (and thus phonotaxis behaviour) because of sensory biases and not because of the perceptual restoration of missing or masked sound elements. Therefore, we conducted additional two-choice phonotaxis experiments to evaluate sensory bias as an alternative hypothesis that could explain the results of our tests of the auditory induction hypothesis.
METHODS
Study System
Cope’s grey treefrog is a common frog throughout eastern North America that has been the subject of intensive studies of acoustic communication and sexual selection (reviewed in Gerhardt 2001). We tested females collected between 1 and 17 June 2009 from several ponds and marshes located in the Carver Park Reserve (44°52′49.29″N, 93°43′3.10″W; Carver County, MN, U.S.A.) and the Crow-Hassan Park Reserve (45°11 ′18.71″N, 93°39′9.05″W; Hennepin County, MN, U.S.A.). In these Minnesota populations, the male advertisement call comprises a series of discrete pulses (typically 20–50; average = 32 pulses; SD = 4 pulses; Bee 2008b) that are approximately 11 ms in duration and separated by interpulse intervals of about 11 ms (pulse duty cycle = 0.50, pulse rate = 45.5 pulses/s, corrected to 20 °C; M. A. Bee, unpublished data). Each pulse consists of two harmonically related spectral peaks near 1250 Hz and 2500 Hz, with the latter having a relative amplitude that is typically 5–10 dB higher. Advertisement calls are produced at sound pressure levels of 85–92 dB SPL at a distance of 1 m (re. 20 μPA, fast RMS, C-weighted; Gerhardt 1975). The rate of pulses in a call is an important cue for species recognition (Schul & Bush 2002), and females have directional preferences for longer calls with higher pulse numbers (Gerhardt et al. 1996; Bee 2008b).
General Protocol
All collections, handling and testing of animals were approved by the University of Minnesota’s Institutional Animal Care and Use Committee (No. 0809A46721, 21 November 2008). Our basic collecting and testing procedures followed those recently described elsewhere (Bee 2007, 2008a, b; Bee & Riemersma 2008). Briefly, females were collected in amplexus (total N = 66), returned to the laboratory for testing, and then released at their original location of capture within 1–3 days of collection. We conducted phonotaxis experiments under infrared illumination at a temperature of 20 °C (± 2 °C). We used the same equipment, test arena and temperature-controlled, hemi-anechoic sound chamber described in Bee (2008b; inside dimensions, L × W × H: 220 × 280 × 216 cm). The circular test arena (2 m diameter) had acoustically transparent walls constructed from hardware cloth (60 cm high) covered by black fabric. Two A/D/S L210 speakers were used to broadcast alternating stimuli and were placed just outside the arena wall on the sound chamber floor (2 m and 180° apart) and aimed inward towards a small, acoustically transparent release cage positioned at the centre of the arena.
The two alternating stimuli in each two-choice experiment consisted of various combinations of synthetic advertisement calls and band-limited noise (see Synthetic Calls and Noise Bursts below). Each stimulus alternative repeated with a period of 5 s as a continuous sequence, and its onset was 180° out of phase relative to that of the alternative in the other stimulus sequence. Each of the two stimulus alternatives was the first stimulus presented in tests with 50% of the subjects. We changed the location from which the two alternatives originated each day to control for side bias in the testing chamber. Prior to beginning tests each day (and periodically throughout each testing day), we calibrated the level of the pulsatile portion of each stimulus call to be 79 dB SPL (re. 20 μPa, fast RMS, C-weighted). We calibrated the level of noise bursts by adjusting the level of a 60 s band-limited noise of equivalent spectrum and long-term RMS amplitude to be 85 dB SPL. Hence, the noise bursts had an average overall sound level (85 dB SPL) that was 6 dB greater than that of the pulsed call (79 dB SPL). Consequently, stimuli comprising a combination of pulses and noise had RMS amplitudes that were about 3 dB greater than those comprising pulses alone. Calibrations were performed using a Brüel & Kjær Type 2250 sound level meter and a Type 4189 microphone that was placed at the approximate position of a female’s head in the release cage. The frequency response of the playback system was flat (± 3 dB) between 500 and 4000 Hz.
At the beginning of a phonotaxis test, a female was placed in the release cage and given a 1 min silent acclimatization period, after which we began broadcast of the alternating stimuli. After four repetitions of both alternating stimuli, the female was remotely released from outside the chamber and given up to 5 min to approach either speaker. We scored a response when the female touched the wall of the arena in a 15° arc centred on one of the speakers. In total, we performed 16 separate phonotaxis experiments, each with a minimum sample size of 20 individuals (range 20–24 individuals). Females were tested in multiple experiments (median = 6; range 3–10 experiments) and were given a 5–15 min time-out between experiments (see Gerhardt et al. 2000 for evidence for a lack of carryover effects). The outcome of each two-choice experiment was analysed separately using a two-tailed binomial test of the null hypothesis that 50% of females would choose each alternative (α = 0.05).
Synthetic Calls and Noise Bursts
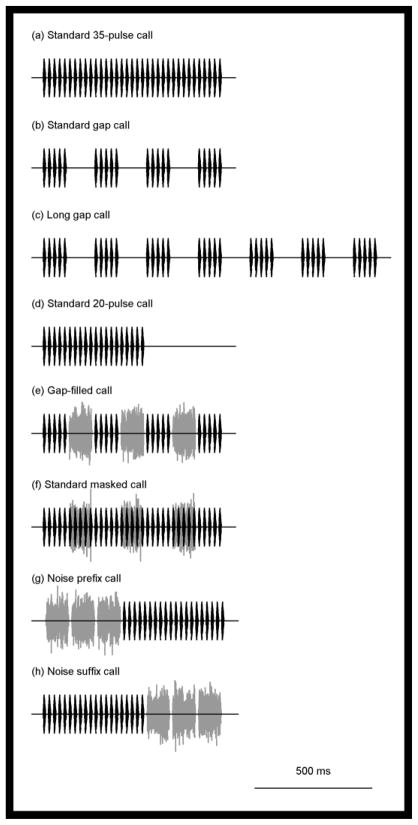
All acoustic stimuli (Fig. 2) were created at a sampling rate of 20 kHz with 16-bit resolution. We used custom software to generate synthetic advertisement calls comprising continuous strings of pulses. A pulse was constructed by adding two phase-locked sinusoids with frequencies (and relative amplitudes) of 1250 Hz (−8 dB) and 2500 Hz (0 dB). Each pulse was 11 ms in duration (pulse duty cycle = 0.50) and had a 4 ms rise time and 7 ms fall time that were shaped to reflect the average pulse envelope in local populations. We used Matlab v7.6.0 (Mathworks, Natick, MA, U.S.A.) to create short bursts of band-limited noise (750–3000 Hz) by first generating spectral buffers with constant magnitude and random phases and then converting these into the time domain using an inverse FFT. Previous studies of auditory induction have shown that the noises most effective at eliciting induction are those that are also the most effective maskers of the signal (Warren 1999). Therefore, in constructing and presenting noise bursts, we chose a bandwidth (2250 Hz) that encompassed the two spectral peaks in each pulse (1250 Hz and 2500 Hz) and a relative sound level (+6 dB) that should have masked a call presented at 79 dB (Bee & Schwartz 2009). Each noise burst was equivalent in duration to five pulses and four interpulse intervals (99 ms) and had onsets and offsets shaped by 4 ms Hanning windows. Within a particular stimulus sequence, each occurrence of a noise burst was generated independently of all other noise bursts used in that sequence and in the alternative stimulus sequence in the same experiment. In some stimuli (Fig. 2), three noise bursts were inserted into three silent gaps introduced into the pulsed advertisement call. In those cases, each noise burst was separated from the pulses that preceded and followed the gap by silence equivalent in duration to one interpulse interval (11 ms). In other stimuli (Fig. 2), three noise bursts were appended to the beginning or ending of a call and were separated from each other, and from the preceding or following pulse, by an 11 ms silent gap.
Figure 2.
Acoustic stimuli used in experiments with female grey treefrogs. Waveforms showing the eight stimuli used in two-choice phonotaxis tests comprising synthetic pulses and short bursts of band-limited noise.
AUDITORY INDUCTION HYPOTHESIS
Predictions, Experiments and Results
We tested the hypothesis that auditory induction results in the restored (i.e. illusory) perception of complete signals that actually contain missing sound elements. In the context of grey treefrog communication, the key prediction of this hypothesis is that replacing pulses in the advertisement call with a potential masking noise results in the illusion of hearing the missing pulses and the perception of an uninterrupted pulsed call continuing through the noise. As a first step in testing this hypothesis, it was necessary to demonstrate that replacing pulses with silence interfered with the perception of an otherwise attractive call. We did this in experiments 1–3 by giving females choices between continuous calls and calls with silent gaps.
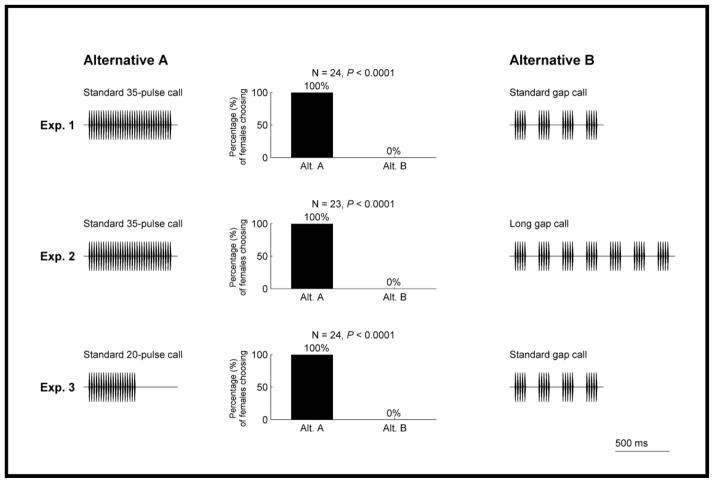
In experiment 1, females were given a choice between a synthetic call with 35 uninterrupted pulses (759 ms) and an alternative of equivalent total duration in which pulses 6–10, 16–20 and 26–30 (and their subsequent interpulse intervals) were replaced by silence. We refer to these two stimuli, respectively, as the ‘standard 35-pulse call’ (Fig. 2a) and the ‘standard gap call’ (Fig. 2b). The standard gap call comprised 20 pulses divided among four groups of five pulses. Each group of five pulses was separated by a silence equivalent in duration to five pulses and six interpulse intervals. While experiment 1 controlled for total stimulus duration (759 ms), the two alternatives differed in pulse number: 35 pulses (about average) versus 20 pulses (about 3 SD below average). In experiments 2 and 3, we controlled for differences in pulse number but not total duration. In experiment 2, we gave females a choice between the standard 35-pulse call (759 ms; Fig. 2a) and a ‘long gap call’ (1419 ms; Fig. 2c) that contained 35 pulses divided among seven groups of five pulses. In the latter stimulus, each pulse group was separated by a silence equivalent in duration to five pulses and six interpulse intervals, as in the standard gap call (cf. Fig. 2b, c). In experiment 3, we gave females a choice between two alternatives with pulse numbers from the low end of the range of natural variation (Bee 2008b). One alternative was a continuous 20-pulse call (429 ms; hereafter termed the ‘standard 20-pulse call’; Fig. 2d) and the other was the standard gap call (Fig. 2b), which also comprised 20 pulses.
In all three experiments, 100% of females chose the continuous standard call over the alternative with silent gaps (Fig. 3). This result was consistent after controlling for differences in both total duration (experiment 1; Fig. 3) and total pulse number (experiments 2, 3; Fig. 3). Together, these results demonstrated that silent gaps rendered calls less attractive than equivalent calls lacking silent gaps. Importantly, the reduction in the attractiveness of the gap calls could not be attributed to a simple reduction in pulse number that resulted from replacing some pulses with silence.
Figure 3.
Results from experiments 1–3. Shown are waveforms of the two paired alternatives, A and B, in each two-choice experiment, and a bar graph of the percentages of females that chose each alternative. P values are from two-tailed binomial tests of the hypothesis that 50% of females would choose each alternative.
Auditory induction prediction 1
Given that introduced silence rendered calls with gaps less attractive than continuous calls, the auditory induction hypothesis makes the following prediction: filling the gaps with noise should at least partially restore the attractiveness of the signal. We tested this prediction in experiments 4–7.
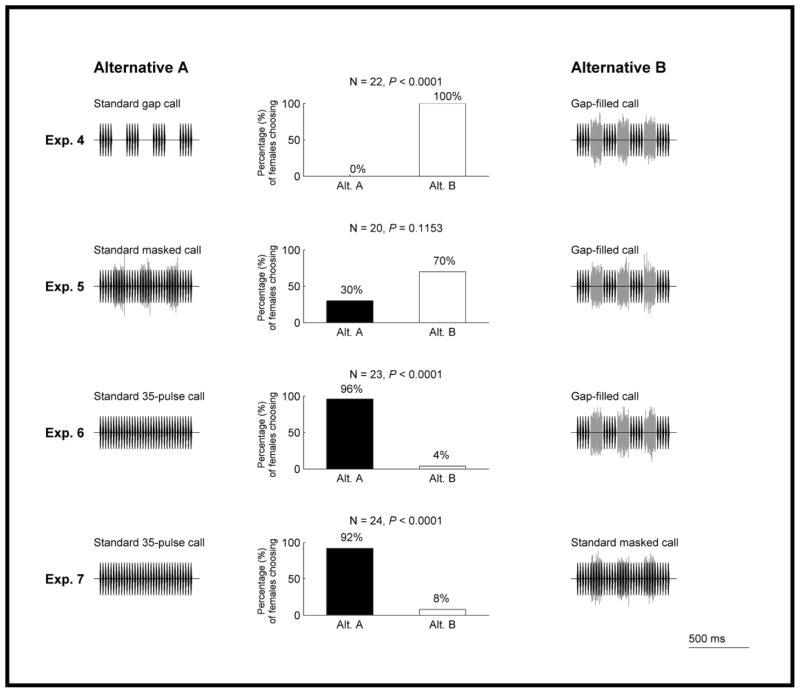
In experiment 4, we paired the standard gap call (Fig. 2b) against an equivalent call after inserting a burst of band-limited noise into each of the three gaps (hereafter termed the ‘gap-filled call’; Fig. 2e). Our expectation was that the addition of noise bursts would restore the robust preference for a continuous signal over one interrupted by silent gaps that was demonstrated in experiment 1 (Fig. 3). As expected, 100% of the females tested in experiment 4 chose the gap-filled call when it was paired against the standard gap call (Fig. 4). Thus, filling the gaps with noise made the signal more attractive than one in which the silent gaps remained.
Figure 4.
Results from experiments 4–7 testing prediction 1 of the auditory induction hypothesis. Other details as in Fig. 3.
We investigated the relative attractiveness of the gap-filled call in experiments 5–7. Our expectation was that the attractiveness of a gap-filled call would be similar to that of a continuous call masked by three equivalent noise bursts, and that both a gap-filled call and a masked call would be either equally as attractive as, or less attractive than, a continuous unmasked call. These expectations are based on the following rationale. In humans, phonemic restoration results in the illusory perception of actual ongoing speech sounds continuing through noise-filled gaps. However, the intelligibility of missing speech segments that are perceptually restored by noise is typically similar to that of continuous speech that has been masked by similar noise, and is generally worse than when listening to normal unmasked speech (e.g. Miller & Licklider 1950).
In experiment 5, we gave females a choice between the gap-filled call (Fig. 2e) and a stimulus of equivalent duration comprising the standard 35-pulse call plus three noise bursts (hereafter termed the ‘standard masked call’; Fig. 2f). The noises in the standard masked call had the same timing relative to call onset as in the gap-filled call (cf. Fig. 2e, f). Our expectation was that females would perceive these two stimuli as being acoustically equivalent. The results were consistent with this expectation; the proportions of females choosing the gap-filled call when it was paired against the standard masked call did not differ significantly from the null expectation of 50:50 (experiment 5; Fig. 4).
In two additional experiments (experiments 6, 7), we gave females a choice between the standard 35-pulse call (Fig. 2a) and either the gap-filled call (Fig. 2e) or the standard masked call (Fig. 2f). Females preferred the continuous call lacking noise bursts (standard 35-pulse call) over both the gap-filled call (experiment 6; Fig. 4) and the standard masked call (experiment 7; Fig. 4).
Auditory induction prediction 2
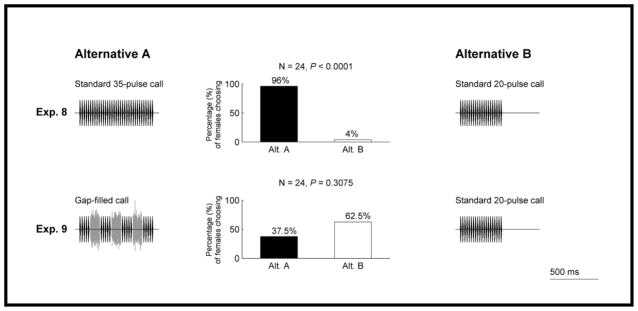
Given that inserting silent gaps rendered calls less attractive, and filling these gaps with noise restored some of the signal’s lost attractiveness, the auditory induction hypothesis would make the following prediction. Any attractiveness restored by filling gaps with noise is due to the illusory perception of actual pulses continuing through the noise-filled gaps. To test this prediction, we gave subjects a choice that required a comparison of differences in pulse number. We did so by exploiting the robust preferences of females for longer calls with higher pulse numbers. Previous studies have shown that female grey treefrogs prefer calls having average or higher-than-average numbers of pulses over calls with lower-than-average numbers of pulses. Discrimination is strongest against the shortest calls in the population (Gerhardt et al. 1996; Schwartz et al. 2001; Bee 2008b). For example, given a choice between a call with 32 pulses (about average length) and one with 24 pulses (about 2 SD below average), females chose the former in significantly greater numbers even when it was attenuated by 6 dB relative to the shorter alternative (Bee 2008b). In addition, females can discriminate differences in pulse number as small as two pulses (e.g. 30 pulses versus 32 pulses; Bee 2008b). Therefore, we predicted that females should prefer the gap-filled call over a shorter-duration continuous call with the equivalent number of actual pulses. We tested this prediction in experiments 8 and 9.
In experiment 8, we paired the standard 35-pulse call (759 ms; Fig. 2a) against the standard 20-pulse call (429 ms; Fig. 2d) to establish the baseline level of preference for longer calls using these two stimulus durations. In experiment 9, we gave females a choice between the gap-filled call (759 ms; Fig. 2e) and the standard 20-pulse call (429 ms; Fig. 2d). Recall that the gap-filled call consisted of 20 pulses (four groups of five pulses) and three noise bursts and had a duration equivalent to that of the standard 35-pulse call (759 ms). Our expectation was that females would discriminate against the standard 20-pulse call in favour of the standard 35-pulse call (experiment 8) and the gap-filled call (experiment 9) in similar proportions.
Females showed the expected preference for a longer continuous call (standard 35-pulse call) over a shorter continuous call (standard 20-pulse call) (experiment 8; Fig. 5). Females did not, however, behaviourally discriminate between a short continuous call (standard 20-pulse call) and a gap-filled call having a total duration equal to that of the standard 35-pulse call (experiment 9; Fig. 5). The proportion of females that chose the standard 35-pulse call over the standard 20-pulse call in experiment 8 (0.958) was significantly greater than the proportion of females that chose the gap-filled call over the standard 20-pulse call in experiment 9 (0.375) (Z test of two proportions: Z = 4.0, P < 0.01).
Figure 5.
Results from experiments 8–9 testing prediction 2 of the auditory induction hypothesis. Other details as in Fig. 3.
Discussion
Interrupting advertisement calls with silent gaps reduced the attractiveness of the signal relative to continuous, uninterrupted alternatives (Fig. 3). Parallel findings have been reported in studies of túngara frogs, Physalaemus pustulosus (Wilczynski et al. 1995), and the eastern grey treefrog, H. versicolor (Schwartz et al., in press). Together, findings from these studies are consistent with the idea that the introduction of temporal gaps into the calls of frogs can impair or interfere with the mechanisms of call recognition and mate choice. According to the auditory induction hypothesis, the insertion of noise bursts into silent gaps should restore a signal’s attractiveness (prediction 1), and this restoration of attractiveness should result because females actually perceive the missing portions of the signal continuing through the interrupted noise (prediction 2). Our results were only partially consistent with these two predictions.
Inserting noise into temporal gaps restored the female preference for continuous signals over interrupted ones. This preference was similar to that elicited by pairing a continuous pulsed call (the standard 35-pulse call) against one with silent gaps (cf. experiments 1, 4; Figs 3, 4). In addition, females failed to discriminate between the gap-filled call and the standard masked call (Fig. 4), suggesting that the noise-filled gaps resulted in a percept equivalent to that elicited by a continuous call in which the pulses were present but masked by noise. Our finding that females still preferred the standard 35-pulse call over the gap-filled call (experiment 6) and the standard masked call (experiment 7) is consistent with results from previous studies of auditory induction, which have shown that perception of continuous signals is better than that of equivalent signals that are masked or manipulated by creating gaps and inserting noise (e.g. Miller & Licklider 1950). Thus, results from experiments 4–7 are broadly consistent with the auditory induction hypothesis.
However, over the range of stimulus parameters tested in this study, we found little direct evidence supporting the prediction that females actually perceived illusory pulses that were not present in the signal. In the key experiment (experiment 9; Fig. 5), there was no evidence that a preference for longer calls with more pulses could be elicited based on the perception of illusory pulses. In fact, females did not behaviourally discriminate between the standard 20-pulse call and the gap-filled call. This is an important result, because the latter also had only 20 actual pulses but had a total duration equivalent to a call comprising 35 pulses. Recall that females strongly discriminate against short calls with low pulse numbers in favour of longer calls with more pulses (Gerhardt et al. 1996; Schwartz et al. 2001; Bee 2008b). The failure of our subjects to behaviourally discriminate between the gap-filled call and the standard 20-pulse call in experiment 9 suggests that females did not actually hear illusory pulses continuing through the noise bursts. As discussed in the next section, the lack of preference for the gap-filled call over the standard 20-pulse call cannot be explained simply as a result of added noise bursts making a stimulus inherently less attractive or repulsive. As an aside, we would also note that human listeners report hearing illusory pulses continuing through noise bursts inserted into the gap-filled call (F. Seeba & M. A. Bee, unpublished data).
Based on our failure to find support for the second prediction of the auditory induction hypothesis, we provisionally conclude that the female grey treefrogs tested in this study did not experience a continuity illusion similar to that reported in previous studies of humans and other animals. Other evidence corroborating this conclusion has recently been reported in a study of the closely related eastern grey treefrog, H. versicolor (Schwartz et al., in press). We offer this provisional conclusion with the following caveats. First, we acknowledge that the results of experiments 1–9 cannot rule out the possibility that other stimulus parameters not tested here might elicit some form of continuity illusion. Second, we acknowledge that we have tested only one frog species. Given the general difficulty of assessing negative results, we believe future tests of auditory induction in additional frog species would be worthwhile and we encourage such studies. We also believe, however, that other processes might better explain why noise bursts could modulate the attractiveness of a pulsed frog call without eliciting the illusory perception of pulses. We investigated sensory bias as one such potential mechanism.
SENSORY BIAS HYPOTHESIS
Predictions, Experiments and Results
In its most basic formulation, the sensory bias hypothesis predicts that when females have directional preferences, they tend to prefer signals with more acoustic energy that provide greater stimulation of the sensory organs (Ryan & Keddy-Hector 1992). The exploitation of such biases can explain the evolution of novel acoustic appendages added to established signals in a species’ repertoire (Rand et al. 1992; Ryan & Rand 1993). More recent work suggests that sensory biases may follow rules that determine the attractiveness of novel acoustic appendages based on their temporal ordering and acoustic structure relative to established signals (Gerhardt et al. 2007). We conducted six additional experiments to test the hypothesis that bursts of band-limited noise have some potential to modulate the attractiveness of an established signal by exploiting sensory biases for signals of greater acoustic energy. We used the standard 20-pulse call as the established signal, as this is about the shortest calls that males in our populations will produce (Bee 2008b). As acoustic appendages, we used either an additional string of 15 pulses (so that the total pulse number was 35) or three consecutive bursts of band-limited noise that were appended to the beginning of the established signal (the ‘noise prefix call’; Fig. 2g) or to the ending of the established signal (the ‘noise suffix call’; Fig. 2h). We made two general predictions based on the sensory bias hypothesis.
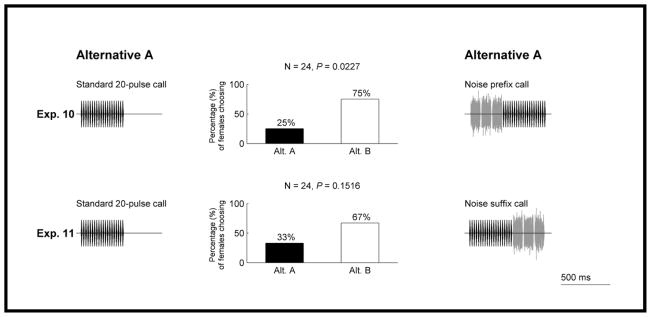
Sensory bias prediction 1
We predicted that, if females showed preferences, these would be in favour of an established signal to which we added acoustic appendages that provided additional stimulation of the sensory organs when paired against the same signal lacking the appendages. As described above (experiment 8; Fig. 5), a call comprising a continuous string of 35 pulses (i.e. the standard 35-pulse call) was significantly preferred over the standard 20-pulse call. We also found that adding three noise bursts to the beginning of the standard 20-pulse call elicited a significant preference for the noise prefix call over the standard 20-pulse call (experiment 10; Fig. 6). In contrast, appending equivalent noise bursts to the ending of the standard 20-pulse call did not elicit a statistically significant preference for the noise suffix call over the standard 20-pulse call alone (experiment 11; Fig. 6). These results are broadly consistent with the sensory bias hypothesis; when there was a directional preference, it was for the signal with greater acoustic energy.
Figure 6.
Results from experiments 10–11 testing prediction 1 of the sensory bias hypothesis. Other details as in Fig. 3.
Sensory bias prediction 2
Our second prediction was that the ability of acoustic appendages to elicit female preferences depended on both their temporal ordering and their spectrotemporal structure, and not simply on the amount of acoustic energy. We tested this prediction by giving females choices between two alternative stimuli that either possessed or lacked three noise bursts, or that varied in the position of the noise bursts. The rules of sensory biases have not been explored in enough detail and in enough species to state directional expected outcomes. Our null expectations were that females would prefer stimuli providing greater acoustic stimulation and that stimuli containing equivalent acoustic energy would elicit equivalent responses (i.e. 50:50 in a two-choice test).
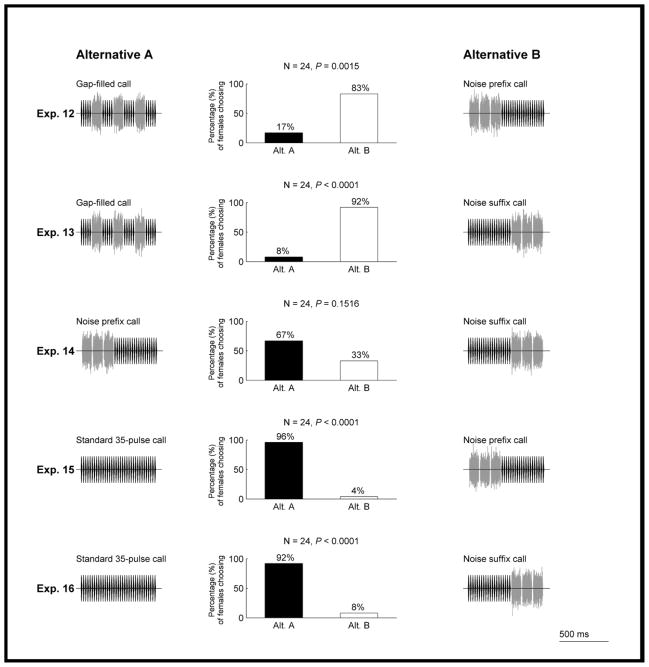
We examined the effects of temporal order in experiments 12 and 13. We gave females a choice between the gap-filled call (Fig. 2e) and either the noise prefix call (Fig. 2g; experiment 12) or the noise suffix call (Fig. 2h; experiment 13). In experiment 14, females were given a choice between the noise prefix and noise suffix calls. It is worth reiterating that in all three of these experiments, the two alternative stimuli comprised 20 pulses and three bursts of band-limited noise, had the same RMS amplitudes, and thus, had equivalent acoustic energy. They differed only in the relative temporal order of pulses and noise bursts. Females preferred both the noise prefix call (experiment 12; Fig. 7) and the noise suffix call (experiment 13; Fig. 7) when they were paired against the gap-filled call, but neither was preferred over the other (experiment 14; Fig. 7).
Figure 7.
Results from experiments 12–16 testing prediction 2 of the sensory bias hypothesis. Other details as in Fig. 3.
We also investigated whether the ability of an acoustic appendage to increase signal attractiveness depended on its spectrotemporal structure and not simply on the total amount of acoustic energy present in the signal. As described above, experiments 6 and 10 established that elongating a 20-pulse call both with 15 additional pulses and with three burst of noise could elicit a preference for the longer alternative. In experiments 15 and 16, we directly compared the relative attractiveness of appendages comprising 15 additional pulses and three noise bursts. We gave females a choice between the standard 35-pulse call (Fig. 2a) and either the noise prefix call (Fig. 2g; experiment 15) or the noise suffix call (Fig. 2h; experiment 16). We found that females preferred the standard 35-pulse call over the noise prefix call (experiment 15; Fig. 7) and the noise suffix call (experiment 16; Fig. 7).
Discussion
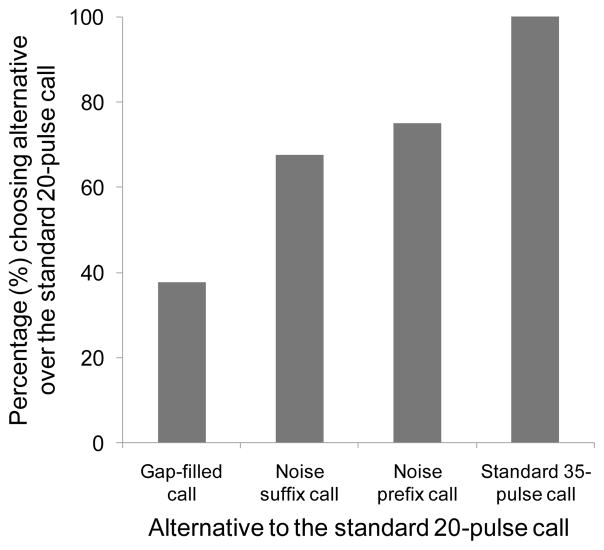
In our tests of the sensory bias hypothesis, females sometimes, but now always, preferred the alternative having the greatest acoustic energy. Our experiments suggest that rule-based sensory biases resulted in a hierarchy of preferences that depended on the temporal ordering and spectrotemporal properties of the acoustic appendages added to an established signal. The most effective way to increase the attractiveness of a shorter-than-average pulsed call was to add more pulses (Fig. 8). As noted above, the standard 35-pulse call was significantly preferred over the standard 20-pulse call (experiment 8). However, in three additional experiments, the standard 35-pulse call was also chosen significantly more often than the gap-filled call, the noise prefix call and the noise suffix call (experiments 6, 15 and 16, respectively). The results of these three experiments are particularly important, because the overall RMS amplitude of the standard 35-pulse call was actually 3 dB less than that of the three stimuli containing noise bursts. Thus females did not simply choose the stimulus providing the greatest acoustic energy, but rather, they preferred one that was elongated through the addition of acoustic elements (pulses) already present in the call. That is, the sensory bias was not just for ‘more’ but for ‘more of the same’. If taxonomically widespread, such a bias might contribute, or have contributed in the past, to selection favouring the evolution of the often long, pulsatile signals prevalent in the repertoires of many insects and anurans (reviewed in Gerhardt & Huber 2002).
Figure 8.
Hierarchical preferences based on the temporal order and spectrotemporal structure of acoustic appendages. Each bar depicts the percentage of females (Y axis) that chose the specified alternative (X axis) when it was paired against the standard 20-pulse call. See text and other figures for the statistical outcomes of individual phonotaxis tests. No omnibus statistical tests were performed because the data depicted here represent partially overlapping subject pools, and hence, a combination of independent and nonindependent data.
Although less effective than additional pulses (Fig. 8), the novel acoustic appendage of noise bursts could also increase the attractiveness of a shorter-than-average call. However, as in studies of túngara frogs by Wilczynski et al. (1999) and grey treefrogs by Gerhardt et al. (2007), the temporal positions of acoustic appendages influenced their impact on the attractiveness of the signal. The addition of three noise bursts to the beginning of a string of 20 pulses elicited a significant preference for the noise prefix call over the standard 20-pulse call (experiment 10), with 75.0% of females choosing the former (Fig. 8). However, the addition of the same noise bursts to the end of the same string of 20 pulses (experiment 11) failed to elicit a statistically significant preference over the standard 20-pulse call at our sample size, although 66.7% of females chose the noise suffix call (Fig. 8). In addition, although females showed no statistically significant preference when the noise prefix call and the noise suffix call were paired against each other (experiment 14), 66.7% of females chose the former. When a call with three noise bursts inserted into three evenly spaced gaps in a call with 20 pulses (i.e. the gap-filled call) was paired against the standard 20-pulse call, there was no statistically significant preference (experiment 9), but only 37.5% of females chose the gap-filled call (Fig. 8). Both the noise prefix and noise suffix calls were significantly preferred when paired against the gap-filled call (experiments 12 and 13). Thus, in terms of a hierarchy of preferential responses (Fig. 8), noise bursts added at the beginning of an established signal were more salient than those added at the end, and noise bursts added in both of these temporal positions were more salient than those inserted into the middle of an established signal. Noise bursts added in all of these positions were less effective than adding additional pulses (Fig. 8).
While generally consistent with the operation of rule-based sensory biases, some of our results contrast with those of Gerhardt et al. (2007) in important ways. Gerhardt et al. found that appendages following, but not preceding, an established signal could increase the signal’s attractiveness in tests with both Cope’s grey treefrog (H. chrysoscelis) and the eastern grey treefrog (H. versicolor) from populations in Missouri. Moreover, appendages that preceded the established signal often decreased, and never increased, the attractiveness of the signal. Our demonstration that leading appendages can also increase a signal’s attractiveness corroborates the conjecture by Gerhardt et al. (2007) that forward masking was not an adequate mechanism to explain their results showing greater salience for appendages that followed the signal. Our results, however, also call into question any generalization that novel sounds added to the end of established signals are somehow better at exploiting sensory biases. It will be important to determine the extent to which methodological differences, population differences, or both, contributed to the differences between our two studies of grey treefrogs.
GENERAL DISCUSSION
Perceptual Restoration or Sensory Bias?
We investigated the extent to which grey treefrogs experience the perceptual illusion of hearing continuous acoustic signals through interrupting sounds. When considered in their entirety, we believe our results fail to provide the robust support necessary to conclude that the frogs tested in this study experienced the illusion of hearing signal elements that had been deleted and replaced with bursts of noise. Instead, the ability of noise bursts to modulate the attractiveness of a signal appears to have resulted from the operation of rule-based sensory biases. Two findings were particularly telling in terms of how well the auditory induction and sensory bias hypotheses might account for our overall pattern of results. First, signals comprising noise bursts appended to both the beginning and the ending of a string of 20 pulses were more attractive than one in which the three noise bursts were evenly distributed throughout the signal (Fig. 7). Second, noise bursts appended to the beginning of a 20-pulse call increased signal attractiveness, but the same noises appended to the end of the call did not (Fig. 6). Contrary to these results, the auditory induction hypothesis would predict that noise bursts added to the beginning of a call should have the lowest ability to induce the perception of an ongoing signal that continued through the noise. Neither of these findings, however, is inconsistent with the operation of rule-based sensory biases.
One possible explanation for our failure to find support for the auditory induction hypothesis concerns the type of signal used. Most previous studies of ‘phonemic restoration’ and the ‘continuity illusion’ in humans have used as the original signal either continuous speech sounds or ongoing tones, respectively (reviewed in: Warren 1999; Kashino 2006; King 2007). In contrast, we used a signal comprising short, discrete sound elements (11 ms pulses) separated by short periods of silence (11 ms interpulse intervals). Thus, a distinction can be made between perceptual restoration of continuous, ongoing sounds (e.g. tones or continuous speech) versus a composite string of discrete sounds, like pulse trains. This distinction highlights the important caveat that induction could be less likely to occur when the to-be-induced signal comprises discrete elements separated by silent intervals. Unfortunately, too few data are available to assess the likelihood of this alternative explanation. As noted earlier, the gap-filled call used in the present study elicited the illusion of a continuous pulse train in human listeners (no such effect occurred with the noise prefix and noise suffix calls; F. Seeba & M. A. Bee, unpublished data). In addition, Plack & White (2000) reported induction in humans when noise was inserted into gaps in unresolved complex tones that had a distinctly pulse-like temporal structure (see their Figure 1). Thus, auditory induction of pulsatile sounds is at least possible in humans. Data from previous animal studies does not shed light on this issue. Both of the studies demonstrating auditory induction in primates inserted silent gaps and gap-filling noises into the middle of longer (e.g. > 300 ms) and continuous sounds. These sounds include the ‘whistle’ component of the combination long call of cottontop tamarins (Miller et al. 2001) and the ‘coo’ vocalization of rhesus macaques (Petkov et al. 2003). In previous studies demonstrating induction of song in songbirds, either gaps were inserted at repeated, periodic intervals within a song (e.g. every 75 ms) without regard for the song’s internal temporal structure (Seeba & Klump 2009), or an entire discrete song element was removed from the middle of a song (Braaten & Leary 1999). Therefore, one important avenue for future comparative research on auditory induction in frogs would be to test species that produce calls comprising longer, continuous (i.e. not pulsatile) sounds, such as the ‘whine’ of the túngara frog (≈ 300 ms in duration; Rand et al. 1992) or the sonorous croaks of the North American bullfrog (≈ 700 ms in duration; Bee & Gerhardt 2002). Until data from such studies are available, any conclusion that frogs in general do not experience illusory sounds as a result of auditory induction must remain provisional.
Possible Mechanistic Explanations
At present, there are simply too few data on the neural mechanisms of auditory induction to draw any conclusions about how they might differ among taxonomic groups as diverse as frogs, birds and mammals. Nevertheless, it is worth speculating why frogs, in contrast to humans, nonhuman primates and songbirds, might not experience auditory induction of vocal communication signals. We offer the following in hopes that one or more of these explanations might be falsified in future studies. One hypothesis is that frogs, songbirds and primates differ in the relative importance of bottom–up and top–down auditory processing in vocal communication (Bee & Micheyl 2008). In humans, auditory scene analysis depends on both bottom–up, data-driven mechanisms that are automatic and obligatory as well as cognitive processes related to learning, attention and listener expectations (Bregman 1990; Carlyon 2004). There is some evidence to suggest that perceptual restoration of speech in humans (Samuel 1996) and of song in songbirds (Seeba & Klump 2009) is more pronounced when subjects have acquired familiarity with the to-be-restored signals through prior experience. These studies thus suggest a potentially important role for top–down processing in the illusory perception of vocal communication signals due to auditory induction. Importantly, both humans and songbirds are also vocal learners (Doupe & Kuhl 1999), and both can learn to recognize other individuals based on their individually distinct vocal signals (for reviews on birds see: Falls 1982; Lambrechts & Dhondt 1995). Although nonhuman primates are not considered vocal learners, acoustic communication plays critically important roles in their complex social behaviours. In addition, primate social behaviours often depend on acquired familiarity with the vocal characteristics of particular individuals (Rendall et al. 1996; Cheney & Seyfarth 1999). Thus, it is perhaps not too surprising that nonhuman primates also show auditory induction with vocal communication signals (Miller et al. 2001; Petkov et al. 2003). At a neurophysiological level, current evidence suggests that neurons in the auditory cortex are important in auditory induction in mammals (Sugita 1997; Micheyl et al. 2003; Petkov et al. 2007; Riecke et al. 2007, 2009).
The vocal behaviour and auditory systems of frogs differ from those of primates and songbirds in a number of important ways. In contrast to songbirds and humans, there is no evidence that frogs learn to produce or recognize their vocal signals (Dawson & Ryan 2009; Baugh & Ryan, in press). Frogs typically do not show the complex, vocally mediated social behaviours characteristic of many birds and primates (Wells 2007). And although some territorial frogs can learn to recognize other individuals by voice (Bee & Gerhardt 2002), there is little evidence to suggest similar capabilities in lek-breeding species with temporary calling sites, like grey treefrogs. Hence, the role of experience-dependent top–down processing of vocal signals in frog behaviour is probably relatively minor compared to that in songbirds and primates.
Instead, call recognition in frogs may depend heavily on bottom–up mechanisms that involve increasing levels of neuronal selectivity for the spectrotemporal properties of the call between the auditory periphery and the midbrain (Pollack 2001; Gerhardt & Huber 2002). Frogs lack the equivalent of a mammalian auditory cortex, and in grey treefrogs, females still show selective phonotaxis to conspecific calls following substantial lesions of the auditory thalamus (Endepols et al. 2003). In Cope’s grey treefrogs, pulse rate is a particularly important temporal cue for call recognition (Schul & Bush 2002), and the recognition of conspecific calls may depend on neurons in the frog midbrain that fire only after integrating over a number of consecutive pulses having the correct pulse rate (e.g. Alder & Rose 1998, 2000; Edwards et al. 2002). Importantly, these integration processes can be completely reset by inserting short silent gaps into strings of pulses with the correct timing (Edwards et al. 2002). It is interesting to speculate that the silent gaps used in our study interrupted ongoing integration by similar neurons in the midbrains of our subjects. Perhaps the addition of noise bursts into these gaps was unable to sustain fully the ongoing integration that might be necessary to give rise to auditory induction and the perception of illusory pulses.
Conclusions
The study of auditory illusions is proving to be important in understanding the mechanisms of auditory grouping and their role in vocal perception in humans, nonhuman primates and songbirds. In addition, our understanding of the evolution of these mechanisms requires broadscale taxonomic comparisons designed to investigate auditory illusions in distantly related taxa. Our study and that of Schwartz et al. (in press) suggest that grey treefrogs may not experience the continuity illusion when it comes to perceiving acoustic communication signals. Robust confirmation of this preliminary conclusion will require additional behavioural and neurophysiological studies of these and other frog species. Such studies could also shed light on why and how sensory biases in these and other species are constrained by rules related to temporal order and acoustic structure. It will be particularly important to determine how long-term neural integration operates in tests of auditory induction and sensory bias, and to what extent it might serve as a possible neural mechanism underlying the sensory biases that give rise to female preferences for calls with greater pulse numbers.
Acknowledgments
We thank Jenna Cook, Jennifer Henderson, Johanna Henly, Shannon Hinrichs, Michael Kuczynski, Ali Leightner, Sarah Markegard, Andrew Morabu, Cathleen Nguyen, Steffen Peterson, Abby Rapacz-Van Neuren, Maria Rodionova, Adam Smith, Keiran Speirs, Alejandro Vélez, and especially Sandra Tekmen, for their assistance with collecting and testing frogs. We thank Carl Gerhardt, Georg Klump, Christophe Micheyl and two anonymous referees for their critical feedback on earlier drafts of the manuscript. We thank Madeleine Linck and the Three Rivers Park District for access to field sites. Animal collections were made under Minnesota Special Use Permit no. 15662. This study was supported by grants from the German Research Foundation (DFG GRK 591 to F.S.), the National Science Foundation (NSF 0342183 to J.J.S.) and the National Institute on Deafness and Other Communication Disorders (DC008396 and DC009582 to M.A.B.) and by a fellowship from the McKnight Foundation (to M.A.B).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alder TB, Rose GJ. Long-term temporal integration in the anuran auditory system. Nature Neuroscience. 1998;1:519–523. doi: 10.1038/2237. [DOI] [PubMed] [Google Scholar]
- Alder TB, Rose GJ. Integration and recovery processes contribute to the temporal selectivity of neurons in the midbrain of the northern leopard frog, Rana pipiens. Journal of Comparative Physiology A. 2000;186:923–937. doi: 10.1007/s003590000144. [DOI] [PubMed] [Google Scholar]
- Andersson M. Sexual Selection. Princeton, New Jersey: Princeton University Press; 1994. [Google Scholar]
- Arak A, Enquist M. Hidden preferences and the evolution of signals. Philosophical Transactions of the Royal Society of London, Series B. 1993;340:207–213. [Google Scholar]
- Arnqvist G. Sensory exploitation and sexual conflict. Philosophical Transactions of the Royal Society B. 2006;361:375–386. doi: 10.1098/rstb.2005.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au WWL. The Sonar of Dolphins. New York: Springer; 1993. [Google Scholar]
- Aubin T, Jouventin P. How to vocally identify kin in a crowd: the penguin model. Advances in the Study of Behavior. 2002;31:243–277. [Google Scholar]
- Baugh AT, Ryan MJ. The development of sexual behavior in túngara frogs (Physalaemus pustulosus) Journal of Comparative Psychology. doi: 10.1037/a0017227. In press. [DOI] [PubMed] [Google Scholar]
- Bee MA. Sound source segregation in grey treefrogs: spatial release from masking by the sound of a chorus. Animal Behaviour. 2007;74:549–558. [Google Scholar]
- Bee MA. Finding a mate at a cocktail party: spatial release from masking improves acoustic mate recognition in grey treefrogs. Animal Behaviour. 2008a;75:1781–1791. doi: 10.1016/j.anbehav.2007.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bee MA. Parallel female preferences for call duration in a diploid ancestor of an allotetraploid treefrog. Animal Behaviour. 2008b;76:845–853. doi: 10.1016/j.anbehav.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bee MA, Gerhardt HC. Individual voice recognition in a territorial frog (Rana catesbeiana) Proceedings of the Royal Society of London, Series B. 2002;269:1443–1448. doi: 10.1098/rspb.2002.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bee MA, Micheyl C. The cocktail party problem: What is it? How can it be solved? And why should animal behaviorists study it? Journal of Comparative Psychology. 2008;122:235–251. doi: 10.1037/0735-7036.122.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bee MA, Perrill SA. Responses to conspecific advertisement calls in the green frog (Rana clamitans) and their role in male–male communication. Behaviour. 1996;133:283–301. [Google Scholar]
- Bee MA, Riemersma KK. Does common spatial origin promote the auditory grouping of temporally separated signal elements in grey treefrogs? Animal Behaviour. 2008;76:831–843. doi: 10.1016/j.anbehav.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bee MA, Schwartz JJ. Behavioral measures of signal recognition thresholds in frogs in the presence and absence of chorus-shaped noise. Journal of the Acoustical Society of America. 2009;126:2788–2801. doi: 10.1121/1.3224707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher MD. Successes and failures of parent–offspring recognition systems in animals. In: Hepper PG, editor. Kin Recognition. Cambridge: Cambridge University Press; 1991. pp. 94–124. [Google Scholar]
- Bernal XE, Rand AS, Ryan MJ. Sexual differences in the behavioral response of túngara frogs, Physalaemus pustulosus, to cues associated with increased predation risk. Ethology. 2007;113:755–763. [Google Scholar]
- Braaten RE, Leary JC. Temporal induction of missing birdsong segments in European starlings. Psychological Science. 1999;10:162–166. [Google Scholar]
- Bregman AS. Auditory Scene Analysis: the Perceptual Organization of Sound. Cambridge, Massachusetts: MIT Press; 1990. [Google Scholar]
- Brumm H, Slabbekoorn H. Acoustic communication in noise. Advances in the Study of Behavior. 2005;35:151–209. [Google Scholar]
- Carlyon RP. How the brain separates sounds. Trends in Cognitive Sciences. 2004;8:465–471. doi: 10.1016/j.tics.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM. Recognition of other individuals’ social relationships by female baboons. Animal Behaviour. 1999;58:67–75. doi: 10.1006/anbe.1999.1131. [DOI] [PubMed] [Google Scholar]
- Darwin CJ. Auditory grouping. Trends in Cognitive Sciences. 1997;1:327–333. doi: 10.1016/S1364-6613(97)01097-8. [DOI] [PubMed] [Google Scholar]
- Dawson B, Ryan MJ. Early experience leads to changes in the advertisement calls of male Physalaemus pustulosus. Copeia. 2009;2009:221–226. [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annual Review of Neuroscience. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Alder TB, Rose GJ. Auditory midbrain neurons that count. Nature Neuroscience. 2002;5:934–936. doi: 10.1038/nn916. [DOI] [PubMed] [Google Scholar]
- Endepols H, Feng AS, Gerhardt HC, Schul J, Walkowiak W. Roles of the auditory midbrain and thalamus in selective phonotaxis in female gray treefrogs (Hyla versicolor) Behavioural Brain Research. 2003;145:63–77. doi: 10.1016/s0166-4328(03)00098-6. [DOI] [PubMed] [Google Scholar]
- Endler JA, Basolo AL. Sensory ecology, receiver biases and sexual selection. Trends in Ecology & Evolution. 1998;13:415–420. doi: 10.1016/s0169-5347(98)01471-2. [DOI] [PubMed] [Google Scholar]
- Falls JB. Individual recognition by sounds in birds. In: Kroodsma DE, Miller EH, editors. Acoustic Communication in Birds. New York: Academic Press; 1982. pp. 237–278. [Google Scholar]
- Gerhardt HC. Sound pressure levels and radiation patterns of vocalizations of some North American frogs and toads. Journal of Comparative Physiology. 1975;102:1–12. [Google Scholar]
- Gerhardt HC. Acoustic communication in two groups of closely related treefrogs. Advances in the Study of Behavior. 2001;30:99–167. [Google Scholar]
- Gerhardt HC, Huber F. Acoustic Communication in Insects and Anurans: Common Problems and Diverse Solutions. Chicago: University of Chicago Press; 2002. [Google Scholar]
- Gerhardt HC, Klump GM. Masking of acoustic signals by the chorus background noise in the green treefrog: a limitation on mate choice. Animal Behaviour. 1988;36:1247–1249. [Google Scholar]
- Gerhardt HC, Dyson ML, Tanner SD. Dynamic properties of the advertisement calls of gray tree frogs: patterns of variability and female choice. Behavioral Ecology. 1996;7:7–18. [Google Scholar]
- Gerhardt HC, Tanner SD, Corrigan CM, Walton HC. Female preference functions based on call duration in the gray tree frog (Hyla versicolor) Behavioral Ecology. 2000;11:663–669. [Google Scholar]
- Gerhardt HC, Humfeld SC, Marshall VT. Temporal order and the evolution of complex acoustic signals. Proceedings of the Royal Society B. 2007;274:1789–1794. doi: 10.1098/rspb.2007.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy RR. The evolution of hearing in insects as an adaptation to predation from bats. In: Webster DB, Fay RR, Popper AN, editors. Evolutionary Biology of Hearing. New York: Springer; 1992. pp. 115–129. [Google Scholar]
- Hulse SH. Auditory scene analysis in animal communication. Advances in the Study of Behavior. 2002;31:163–200. [Google Scholar]
- Kashino M. Phonemic restoration: the brain creates missing speech sounds. Acoustical Science and Technology. 2006;27:318. [Google Scholar]
- King AJ. Auditory neuroscience: filling in the gaps. Current Biology. 2007;17:R799–R801. doi: 10.1016/j.cub.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts MM, Dhondt AA. Individual voice discrimination in birds. In: Power DM, editor. Current Ornithology. New York: Plenum; 1995. pp. 115–139. [Google Scholar]
- Micheyl C, Carlyon RP, Shtyrov Y, Hauk O, Dodson T, Pullvermuller F. The neurophysiological basis of the auditory continuity illuion: a mismatch negativity study. Journal of Cognitive Neuroscience. 2003;15:747–758. doi: 10.1162/089892903322307456. [DOI] [PubMed] [Google Scholar]
- Miller CT, Dibble E, Hauser MD. Amodal completion of acoustic signals by a nonhuman primate. Nature Neuroscience. 2001;4:783–784. doi: 10.1038/90481. [DOI] [PubMed] [Google Scholar]
- Miller GA, Licklider JCR. The intelligibility of interrupted speech. Journal of the Acoustical Society of America. 1950;22:167–173. [Google Scholar]
- Nocera JJ, Forbes GJ, Giraldeau LA. Inadvertent social information in breeding site selection of natal dispersing birds. Proceedings of the Royal Society B. 2006;273:349–355. doi: 10.1098/rspb.2005.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake TM, Terry AMR, McGregor PK, Dabelsteen T. Do great tits assess rivals by combining direct experience with information gathered by eavesdropping? Proceedings of the Royal Society of London, Series B. 2002;269:1925–1929. doi: 10.1098/rspb.2002.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov CI, O’Connor KN, Sutter ML. Illusory sound perception in macaque monkeys. Journal of Neuroscience. 2003;23:9155–9161. doi: 10.1523/JNEUROSCI.23-27-09155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov CI, O’Connor KN, Sutter ML. Encoding of illusory continuity in primary auditory cortex. Neuron. 2007;54:153–165. doi: 10.1016/j.neuron.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plack CJ, White LJ. Perceived continuity and pitch perception. Journal of the Acoustical Society of America. 2000;108:1162–1169. doi: 10.1121/1.1287022. [DOI] [PubMed] [Google Scholar]
- Pollack GS. Analysis of temporal patterns of communication signals. Current Opinion in Neurobiology. 2001;11:734–738. doi: 10.1016/s0959-4388(01)00277-x. [DOI] [PubMed] [Google Scholar]
- Popper AN, Fay RR. Hearing by Bats. New York: Springer-Verlag; 1995. [Google Scholar]
- Rand AS, Ryan MJ, Wilczynski W. Signal redundancy and receiver permissiveness in acoustic mate recognition by the túngara frog, Physalaemus pustulosus. American Zoologist. 1992;32:81–90. [Google Scholar]
- Rendall D, Rodman PS, Emond RE. Vocal recognition of individuals and kin in free-ranging rhesus monkeys. Animal Behaviour. 1996;51:1007–1015. [Google Scholar]
- Riecke L, van Opstal AJ, Goebel R, Formisano E. Hearing illusory sounds in noise: sensory-perceptual transformations in primary auditory cortex. Journal of Neuroscience. 2007;27:12684–12689. doi: 10.1523/JNEUROSCI.2713-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecke L, Esposito F, Bonte M, Formisano E. Hearing illusory sounds in noise: the timing of sensory-perceptual transformations in auditory cortex. Neuron. 2009;64:550–561. doi: 10.1016/j.neuron.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Ryan MJ, Keddy-Hector A. Directional patterns of female mate choice and the role of sensory biases. American Naturalist, Supplement. 1992;139:S4–S35. [Google Scholar]
- Ryan MJ, Rand AS. Sexual selection and signal evolution: the ghost of biases past. Philosophical Transactions of the Royal Society of London, Series B. 1993;340:187–195. [Google Scholar]
- Samuel AG. Does lexical information influence the perceptual restoration of phonemes? Journal of Experimental Psychology-General. 1996;125:28–51. [Google Scholar]
- Schul J, Bush SL. Non-parallel coevolution of sender and receiver in the acoustic communication system of treefrogs. Proceedings of the Royal Society of London, Series B. 2002;269:1847–1852. doi: 10.1098/rspb.2002.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JJ, Buchanan BW, Gerhardt HC. Female mate choice in the gray treefrog (Hyla versicolor) in three experimental environments. Behavioral Ecology and Sociobiology. 2001;49:443–455. [Google Scholar]
- Schwartz JJ, Huth K, Jones SH, Brown R, Marks J. Tests for call restoration in the gray treefrog,Hyla versicolor. Bioacoustics In press. [Google Scholar]
- Seeba F, Klump GM. Stimulus familiarity affects perceptual restoration in the European starling (Sturnus vulgaris) PLoS ONE. 2009;4:e5974. doi: 10.1371/journal.pone.0005974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita Y. Neuronal correlates of auditory induction in the cat cortex. Neuroreport. 1997;8:1155–1159. doi: 10.1097/00001756-199703240-00019. [DOI] [PubMed] [Google Scholar]
- Tuttle MD, Ryan MJ. Bat predation and the evolution of frog vocalizations in the Neotropics. Science. 1981;214:677–678. doi: 10.1126/science.214.4521.677. [DOI] [PubMed] [Google Scholar]
- Wagner WE. Fighting, assessment, and frequency alteration in Blanchard cricket frog. Behavioral Ecology and Sociobiology. 1989;25:429–436. [Google Scholar]
- Ward MP, Schlossberg S. Conspecific attraction and the conservation of territorial songbirds. Conservation Biology. 2004;18:519–525. [Google Scholar]
- Warren RM. Perceptual restoration of missing speech sounds. Science. 1970;167:392–393. doi: 10.1126/science.167.3917.392. [DOI] [PubMed] [Google Scholar]
- Warren RM. Auditory Perception: a New Analysis and Synthesis. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Wells KD. The Ecology and Behavior of Amphibians. Chicago: University of Chicago Press; 2007. [Google Scholar]
- Wiewandt TA. Vocalization, aggressive behavior, and territoriality in the bullfrog, Rana catesbeiana. Copeia. 1969;1969:276–285. [Google Scholar]
- Wilczynski W, Rand AS, Ryan MJ. The processing of spectral cues by the call analysis system of the túngara frog, Physalaemus pustulosus. Animal Behaviour. 1995;49:911–929. [Google Scholar]
- Wilczynski W, Rand AS, Ryan MJ. Female preferences for temporal order of call components in the túngara frog: a bayesian analysis. Animal Behaviour. 1999;58:841–851. doi: 10.1006/anbe.1999.1208. [DOI] [PubMed] [Google Scholar]