Abstract
For three indolylfulgides the quantum efficiency of the ring-opening reaction upon pre-excitation is investigated in a multipulse experiment. The quantum efficiency grows by factor of up to 3.4, when the pre-excitation pulse immediately precedes the excitation process. The change in quantum efficiency after pre-excitation is discussed as a function of reaction time, steady-state quantum efficiency and energetic barriers in the excited electronic state. The observed differences can be explained by the molecular properties of the investigated indolylfulgides.
Keywords: indolylfulgide, ultrafast spectroscopy, pump-repump spectroscopy, photochromism
1. Introduction
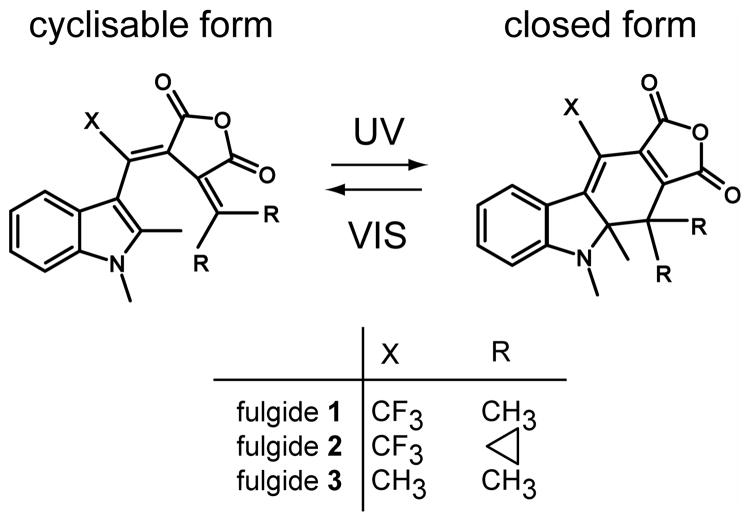
Fulgides are photochromic molecules that can undergo ultrafast pericyclic ring-opening and ring-closure reactions (see Scheme). This and especially their high thermal and photochemical stability make fulgides interesting candidates for applications in molecular memories or as molecular switches [1–10]. Recent studies have shown that the ring-closure reaction of indolylfulgides is a very rapid, non-activated process with a time constant in the range between 200 – 400 fs [11–15]. In contrast, the ring-opening reaction of these molecules is slower and occurs with time constants between 2 and 20 ps [11,14–21]. To summarise these studies: it was found that the ring-opening reaction is thermally activated. It becomes faster and more efficient, if thermal energy is supplied [11,20]. From these experiments it was concluded that two barriers exist in the excited state. Furthermore the ring-opening reaction depends on illumination wavelength, chemical surrounding and substitution of the molecule [16,17]. The following reaction model has been proposed [11,21] for the ring-opening reaction (all values are given for fulgide 1 in the solvent 1,4-dioxane at room temperature without pre-excitation):
Scheme.
Molecular structures of the cyclisable open and the closed form of the three investigated indolylfulgides.
Photoexcitation of the closed form by light in the visible spectral range populates the Franck-Condon state. Subsequently wave packet motion and initial solvent dynamics (solvation) occur on a time scale of several hundred femtoseconds leading to the formationh of a relaxed S1 state. The depopulation of the first electronically excited state to the ground state of the product, the cyclisable open form (yield about 4%), or back to the reactant, the ground state of the closed form (yield about 96%), proceeds on the time scale of about 9 ps. Cooling dynamics of the fulgides in the ground state and heat transfer to the surrounding solvent are observed in the same time range. Hereby two different reaction channels exist from the relaxed S1 state of the closed isomer: The so-called nonreactive pathway with a small barrier of about 375 cm−1 (pre-exponential factor of 0.63 ps−1) leads exclusively back to the ground state of the closed form. The reactive channel, which also allows access to the cyclisable open form, proceeds via a higher barrier of about 1055 cm−1 (pre-exponential factor of 0.63 ps−1) [17]. The described reaction model can be applied to all three fulgides 1, 2 and 3, considered in this study (for reaction parameters see Table 1). Previous investigations have shown that the ultrafast reaction times for ring-closure and ring-opening are faster (ring-closure) than or at least in the same time range (ring-opening) as vibrational relaxation and intermolecular cooling. Therefore the photochromic reactions proceed essentially from vibrationally unrelaxed electronic states. This finding makes indolylfulgides ideal candidates for investigations aiming to unravel the influence of vibrational excess population on pericyclic reactions. In recent experiments using pump-repump schemes, where the ring-opening reaction followed a preceding ring-closure reaction, the importance of vibrational excess population has been demonstrated [21–23]. The ring-opening reaction of fulgides was strongly accelerated and the quantum efficiency for ring-opening was increased by more than a factor of 3, when the reaction started from a light-generated non-equilibrium state [21]. The thorough analysis of the non-exponential kinetics of the ring-opening after pre-excitation showed that the observed increase in reactivity is larger than expected from a model where a temperature dependent reaction from a hot ground state in thermal equilibrium is assumed.
Table 1.
Properties of the ring-opening reaction of the three indolylfulgide compounds. The steady-state ring-opening quantum efficiency ηO, excited state life time τS1, energy barriers for the photochemistry and internal conversion pathway EA,PC and EA,IC and pre-exponential factors APC and AIC are listed for the fulgides at 302 K in the solvent 1,4-dioxane. The quantum efficiency after pre-excitation ηmax was averaged between Δt1 = 1.5 – 2.3 ps, the enlargement factor α = ηmax/ηO and the decay time constant of the quantum efficiency after pre-excitation τη are also given.
In this paper we investigate the ring-opening quantum efficiency after pre-excitation of indolylfulgides with a novel experimental technique [23]. We present data from pre-excitation experiments of two fluorinated indolylfulgides [11] and one non-fluorinated indolylfulgide [23]. We will show that the increase in reaction yield differs for the three molecules and that the observations can be correlated with the specific molecular properties of the three indolylfulgides.
2. Materials and Methods
2.1 The multipulse detection scheme
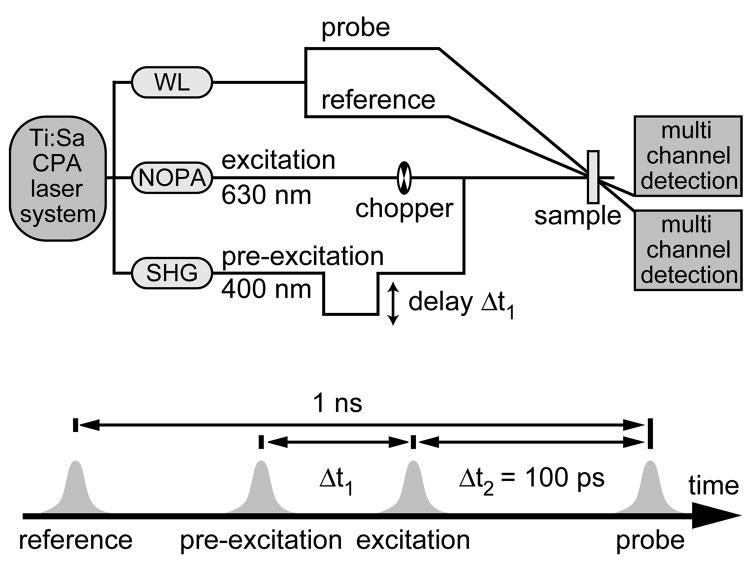
In this publication changes in the quantum efficiency induced by vibrational non-equilibrium of the reactant are investigated. For this purpose we use a multipulse investigation scheme (Figure 1), where the first pre-excitation pulse prepares the reactant (in our case fulgides in the closed form) in a vibrational non-equilibrium. The reactivity of this ensemble is monitored subsequently by a delayed (Δt1) pump-probe experiment. In details:
Figure 1.
Top: Scheme of the setup for the transient absorption pump-repump-probe experiment. Bottom: Pulse sequence and corresponding delay times used in the pump-repump-probe experiment. Delay time Δt1 was varied between −4 and 100 ps. Further details see text.
Pre-excitation process: Fulgide molecules in the cyclisable open form are excited by the pre-excitation pulse in the near UV. A fraction of the excited molecules performs rapid ring-closure reaction to the closed form on the time scale of a few hundred femtoseconds. The majority of fulgides undergoes internal conversion back to the ground state of the open form. The molecules in the closed form are initially in a non-equilibrium vibrational state, which thermalises and cools down by heat transfer to the surroundings on the 10 ps time scale. Only these molecules in the closed form exhibit pronounced absorption in the visible part of the spectrum.
Pump-probe-experiment of pre-excited fulgides in the closed form: At a time Δt1 after the pre-excitation process a second light pulse, the excitation pulse, enters the sample. This excitation pulse is tuned to the red, i.e. to a spectral region, where fulgides in the closed form are excited exclusively. The absorption changes induced by the excitation pulse are sampled by the third, the probe pulse, which enters the sample at a time Δt2 after the excitation pulse. Since the aim of the present investigation is to monitor the reaction yield of the ring-opening reaction as a function of pre-excitation delay, we perform probing at a fixed and late delay time of Δt2 = 100 ps, when the ring-opening reaction (including vibrational cooling in the electronic ground state) is finished, and in a spectral region (λprobe = 547 nm), where the absorption changes monitor the amount of closed form fulgides reconverted to the open form.
Special features of the multipulse experiment have to be considered for the practical realisation: (i) A sufficiently large fraction of fulgide molecules has to be converted to the closed form in order to allow accurate determination of the yield of the cycloreversion. This requires considerable energy of the pre-excitation pulse. (ii) The duration of the pre-excitation pulse must be short enough in order to allow experiments on the time scale of vibrational relaxation. (iii) Optical nonlinearities induced by the pre-excitation process should be avoided. Therefore the duration of the pre-excitation pulse should be sufficiently long to avoid excessive intensities. In order to obtain a compromise between the conflicting requirements (i) to (iii) we chose a pulse duration of the pre-excitation pulse of about 1 ps. (iv) For an accurate determination of the ring-opening yield as a function of Δt1, the absorption changes occurring at the excitation wavelength after pre-excitation have to be considered and the resulting signals have to be scaled to the amount of molecules in the vibrationally excited closed form at the time Δt1. (v) For the precise determination of the ring-opening yield remaining absorption transients from pre-excitation process at the probing wavelength have to be eliminated. This was done by recording the data with and without excitation by chopping the excitation beam and appropriate referencing [23].
2.2 Sample preparation and steady-state spectroscopy
The fluorinated indolylfulgides 1 and 2 (structures see Scheme) were synthesised as described in [3,6,24,25] and indolylfulgide 3 was prepared according to [26]. The molecules were dissolved in 1,4-dioxane purchased from Sigma Aldrich Chemie GmbH and used without further purification. For defined experimental conditions the samples were kept in the photostationary state PSS-550, where nearly all fulgides are in the cyclisable open form [25]. To obtain PSS-550 the sample was first transferred into the closed form (PSS-435) by a Hg(Xe)-lamp (Hamamatsu, 8251) and optical filters BG3 (1 mm, Schott) and GG420 (3 mm, Schott). Afterwards the sample was illuminated by a cold light source (KL 1500 electronic, Schott) with an optical filter OG550 (3 mm, Schott) to form PSS-550. This PSS-550 state was preserved during the experiment by illuminating the sample reservoir by the cold light source using filter OG550. The temperature dependent steady-state quantum yield ηO of the ring-opening reaction of all indolylfulgides was measured by a procedure described recently [11,20]. As illumination source of these quantum yield determinations a HeNe laser (JDSU, 1677P, 2 mW) at 594 nm was used for fulgides 2 and 3 and a frequency-doubled Nd:YAG laser (Laser 2000, LCM-T-111, 1 mW) at 532 nm for fulgide 1.
2.3 Transient absorption measurements
For the time resolved experiments a home-built Ti:sapphire laser system was used, which is described in references [11,27]. The experimental procedure for the transient absorption measurements of the ring-opening reaction with variable sample temperature has been described in detail in reference [11]. For the measurements of the quantum efficiency after optical pre-excitation the experimental pump-repump-probe setup presented in reference [23] and depicted in Figure 1 was used. Its essential features here in short:
The pre-excitation pulse (triggering of the ring-closure reaction) was generated via second harmonic generation (SHG) from the fundamental laser pulse to 400 nm. To avoid sample decomposition and multi-photon processes these pulses were stretched in a 250 mm fused silica glass block (GVD at 400 nm: 97 fs2/mm [28]). The duration of the pre-excitation pulse in this experiment was determined to be 1050 fs (FWHM) at a pulse energy of 1 μJ.
The excitation pulses (used for triggering of the ring-opening reaction) at 630 nm with a pulse duration of 35 fs (FWHM) and pulse energy of 45 nJ were generated by a NOPA [29,30]. A white light continuum (WL) served to monitor the concentration of the closed isomers in a multi-channel detection setup [31]. Temporal resolution, time zero and quantum efficiency were deduced from the multipulse data as described recently [23].
The delay times Δt1 between pre-excitation and excitation pulse and Δt2 between excitation and probe pulse were adjusted by two independent mechanical delay stages. In the presented experiment, the delay time Δt2 between excitation and probe pulse was kept fixed at Δt2 = 100 ps and only Δt1 between pre-excitation and excitation pulse was varied. By this way the excitation induced absorption changes at late delay times, which reflect the quantum efficiency upon the excitation process are monitored as a function of Δt1. The accuracy of the experiment was improved by using a chopper blocking every second excitation pulse. This allowed to measure the difference between the transient absorption signal under “pre-excitation pulse only”-condition and the signal under “pre-excitation and excitation pulse”-condition with reduced noise [23].
3. Experimental Results und Discussion
3.1 Experimental results
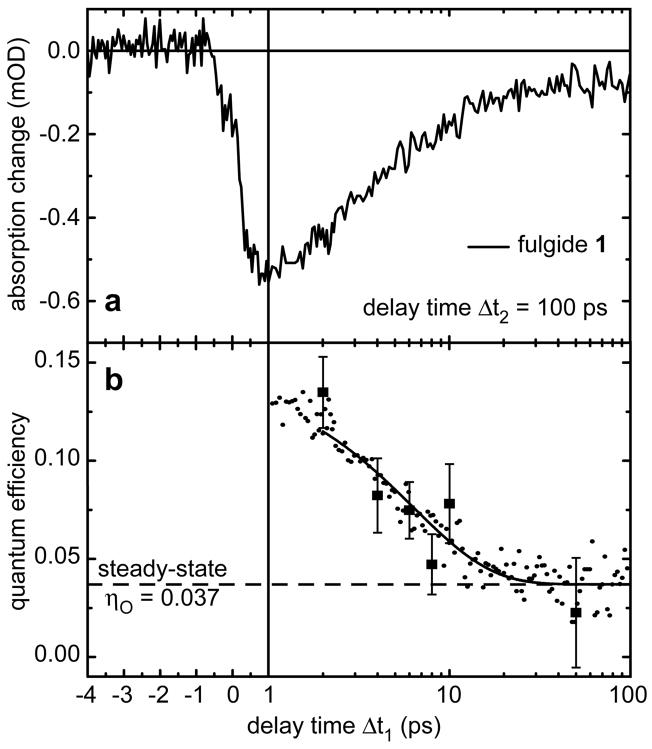
In Figure 2a transient absorption data for fulgide 1 at a probe wavelength of 547 nm are shown. The time axis displays the delay time Δt1 between pre-excitation and excitation pulse on a linear-logarithmic scale and the data points represent the transient absorption signal at the fixed time Δt2 = 100 ps after the excitation pulse. Negative delay times in Figure 2 indicate that the pre-excitation pulse arrives at the sample after the excitation pulse. So the excitation pulse (630 nm) is focussed on a sample consisting of open isomers exclusively, which only absorb at wavelengths < 450 nm, and thus has no effect. As expected, no signal can be observed. For positive delay times below than 1.5 ps signal contribution from open form molecules in the excited state are expected. For longer positive delay times, when the excitation pulse arrives at the sample after the pre-excitation pulse, a negative signal amplitude is observed. Here a small fraction of the cyclisable open isomers were converted by the pre-excitation pulse within about 0.3 ps to the closed form. These fulgides can now absorb the excitation pulse (λexc = 630 nm) and may be reconverted back to the open form. In this reaction the concentration of the closed isomers in the sample is reduced. The negative signal in Figure 2a reflects this reconversion to the open form. The absorption decrease is proportional to the ring-opening quantum efficiency of the newly formed (vibrationally hot) closed isomers. For short delay times Δt1 around 1.5 ps this quantum efficiency reaches an optimal value (minimum of the negative absorption signal) and becomes smaller for longer delay times. For delay times Δt1 > 40 ps between pre-excitation and excitation pulse the signal reaches a constant negative offset. This amplitude can be correlated to the steady-state quantum efficiency of the ring-opening reaction for fulgides in thermal equilibrium, i.e. at room temperature.
Figure 2.
(a) Transient absorption change of fulgide 1 detected at 547 nm and recorded at fixed time delay Δt2 = 100 ps between excitation and probe pulse on a linear-logarithmic scale. The signal is plotted against the time delay Δt1 between pre-excitation and excitation pulse. (b) Quantum efficiency after pre-excitation of fulgide 1. The dashed line indicates the steady-state quantum efficiency ηO = 3.7% at 302 K. The squares are the values taken from [21]. Dots are the measured values deduced from Figure 2a. Both measurements agree very well. The line is the fit of Figure 3. For short delay times Δt1 a strong improvement of the quantum efficiency is found compared to the steady-state value.
In Figure 2b the quantum efficiency η of the ring-opening reaction is plotted as a function of Δt1. η was calculated from the experimental transient absorption signal for fulgide 1 from Figure 2a as described in reference [23]: In this conversion process one had to consider that the absorption of hot molecules in the electronic ground state of the closed form at the moment of the excitation pulse is different from the absorption of thermalised molecules. This influences the fraction of molecules excited by the red excitation pulse. To consider this effect the data were scaled to the amplitude of a transient absorption reference measurement at a probing wavelength 630 nm which was performed with the same setup while keeping the excitation pulse blocked [23].
The quantum efficiency η of fulgide 1 is plotted in Figure 2b (small dots). The largest values of η (of up to 13%) are found at short times around Δt1 = 1 ps. Subsequently the efficiency decays and reaches the steady-state quantum efficiency of 3.7% (dashed line, value for fulgide 1 in 1,4-dioxane at 302 K, see Table 1) after approximately 20 ps. The results show that the quantum efficiency found at early times is increased by a factor of > 3 with respect to the stationary value. This agrees very well with the values found earlier for the same molecule, where a different detection scheme was used [21]. The squares (with arrow bars) plotted in Figure 2b denote the quantum efficiency values taken from this earlier experiment [21].
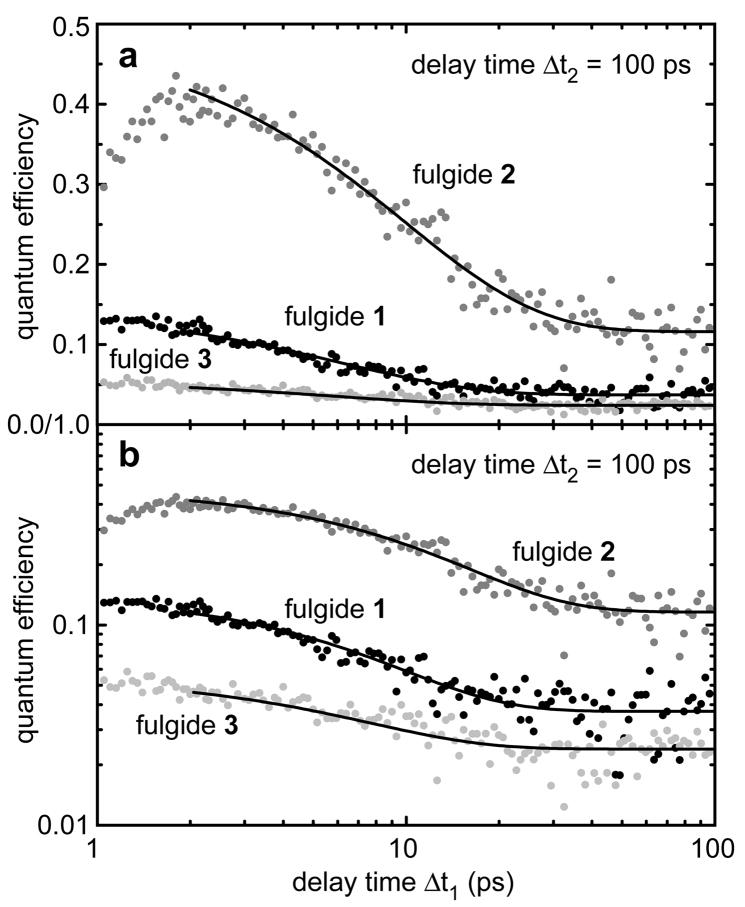
Figure 3a shows data from the multipulse experiments on the three molecules denoted in the Scheme. In Figure 3b a logarithmic scaling is used to allow a ready comparison of the three molecules having different values of the stationary quantum efficiency. For all three investigated fulgides the quantum efficiency of the ring-opening reaction is strongly increased for shorter time delays Δt1 between pre-excitation and excitation pulse. For fulgide 1 a gain in efficiency from ηO = 3.7% up to about ηmax = 12.2% is observed (averaged between Δt1 = 1.5 – 2.3 ps, see Table 1). Fulgide 2 shows an increase from 11.6% up to about 40%. For fulgide 3 an increase from 2.5% up to about 5% is found. The enlargement factor α = ηmax/ηO for the quantum efficiency induced by pre-excitation varies between 1.9 (fulgide 3) to 3.4 (fulgide 2). The time dependences of the quantum efficiencies of the three compounds were fitted by mono-exponential functions (solid curves in Figure 3). For fulgides 1 and 3 decay times τη with very similar values of 6.3 ps and 5.8 ps, respectively, were found (see Table 1). Fulgide 2 shows a longer decay time τη of 10.0 ps.
Figure 3.
Quantum efficiency after pre-excitation for all investigated indolylfulgide compounds on a linear (a) and a logarithmic scale (b). The time dependences of quantum efficiencies at later times Δt1 can be fitted by a mono-exponential decay, time constants see Table 1.
3.2 Discussion of the experimental results
The investigations have shown clearly that the yield for the ring-opening reaction is influenced by the pre-excitation pulse. The three fulgides show a strong increase in reaction yield for short delay times between pre-excitation and excitation pulse. In general, changes in the reaction yield upon pre-excitation are expected when the photochemical reaction proceeds via a non-thermalised excited electronic state. Three situations should be discussed here:
When pre-excitation and excitation promote the molecules to critical locations on the potential energy landscape of the excited electronic state (e.g. towards a conical intersection) the reaction can occur with high efficiency. The use of shaped pulses or adaptive optimisation of the pulses have been discussed in this respect [32,33]. The success of this type of femtochemistry strongly depends on the time scales of the involved processes. Since vibrational relaxation prevents selective population of specific locations on the excited state potential surface, the photochemical reaction has to proceed faster than the vibrational relaxation time.
A second situation may involve selective reactivity from vibrationally excited states. Here the specific population of reactive vibrations by pre-excitation and excitation processes may change the reaction yield. However, the relaxation in the vibrational system (thermalisation) and intramolecular vibrational energy redistribution (IVR) reduce this selectivity. As a consequence a change in reaction yield is only expected when the reaction time is on the same order as vibrational relaxation: τS1 < τvib.
Energetic barriers between the initially populated Franck-Condon region on the excited electronic potential surface and locations on the reactive path may increase the influence of specific excitation and pre-excitation conditions. Molecules carrying high excitation in the promoting modes will react preferentially.
For situation (i) pre-excitation and excitation pulse have to act on the molecule in the excited state and a well-defined phase relation is necessary to steer wave packets in the desired region of the excited state surface. In the described experiment, however, the ring-closure reaction occurs between pre-excitation and excitation pulse and any phase relation is lost due to relaxation processes. Also situation (ii) does not apply here, as a selective excitation of specific vibrational modes as demonstrated e.g. for chromenes [34] would require selective tuning of the excitation wavelength. Therefore, according to the nature of the presented multipulse experiment situation (iii) is valid here. This will be discussed in detail below.
The structural differences of the three investigated indolylfulgides lead directly to changes in the potential energy surfaces and also energetic barriers involved in the ring-opening reaction. The influence on the photochemistry by the trifluoromethyl substitution at position X and the introduction of bulky side groups (cyclopropyl) at position R was already studied by Yokoyama and coworkers [35–37]. Here the observed differences are associated to the reaction/cooling dynamics and reaction barriers. Fulgide 2 is the molecule with the highest steady-state efficiency for ring-opening, ηO = 11.6% and with the shortest lifetime of the excited electronic state τS1 = 3.8 ps. The analysis of temperature dependencies of fulgide 2 [11] revealed low barriers in the excited electronic state. In this molecule thermalisation (occurring on the time scale of τtherm ≈ 10 ps) is slower than the decay of the excited electronic state. Therefore a strong influence of vibrational pre-excitation is expected and observed. Indeed the yield of the ring-opening reaction is increased at short delay times Δt1 by a factor α = 3.4 to ηmax = 39.9%. The pre-excitation gain α = 3.4 decreases slowly with a decay time τη = 10 ps, a time constant similar to vibrational cooling of fulgides. The rather long decay time may be explained by the small height of the reaction barrier. For low energy barriers in the reactive channel improvements of the reactivity can occur even for the small increase in vibrational excess energy found late in the vibrational relaxation process. In fulgide 2 the steric effects from the cyclopropyl groups accelerate the ring-opening reaction by lowering the reactive barrier [11]. The associated fast reaction times which are faster than vibrational relaxation lead to a pronounced influence of pre-excitation.
Fulgide 1 shows a longer excited state lifetime of τS1 = 9 ps and a lower reaction yield ηO = 3.7% [21]. The gain in reaction efficiency is large (α = 3.3) however, its decay time of τη = 6.3 ps is considerably shorter. In this molecule the reaction barrier (EA,PC = 1055 cm−1) is higher by nearly 2 kBT than in fulgide 2 [11]. As a consequence a gain in reaction yield can only occur during the short period when high vibrational excess energies allow the efficient crossing of the large reactive barrier and no gain in reaction yield will prevail late in the thermalisation process. This explains the accelerated decay of the reaction yield with τη = 6.3 ps. The overall reduction of quantum efficiency of fulgide 1 relative to fulgide 2 is connected with differences in the height of the energetic barriers: the reduced steric effects in fulgide 1 decrease the efficiency of the photochemical active channel relative to that of internal conversion.
Fulgide 3 has the smallest steady-state reaction yield ηO = 2.5% and the longest decay time of the excited electronic state τS1 = 14 ps [23]. In this fulgide a methyl group is attached as substituent X (see Scheme). In fulgides 1 and 2 X represents a trifluormethyl group. In fulgide 3 the lack of the electron withdrawing trifluormethyl group is connected with a reduction of the ring-opening quantum yield [38]. In this molecule the influence of pre-excitation is reduced (α = 1.9) and restricted to short times (τη = 5.8 ps). These values are well explained by an excited electronic state persisting considerable longer than the time required for vibrational cooling. As a consequence many molecules react only after vibrational cooling and therefore a small pre-excitation effect is observed.
The comparison of the three molecules reveals the role of the different reaction parameters for pre-excitation induced gain of reaction efficiency: (i) most important is a short excited state lifetime (τS1 < τvib), which guarantees that vibrationally hot molecules are present during the occupation of the reactive excited electronic state. When (ii) the height of the barrier for the reactive channel is larger than that of the non-reactive channel, photochemistry is privileged by an excess of vibrational excitation and the reaction efficiency is improved. The experiments also reveal the dominant role of the potential energy landscape in the excited electronic state. It controls product yield not only by the existence of specific regions (conical intersections) and their branching ratios to product and reactant states but also the probability to reach them selectively. In the case of the investigated fulgides selectivity is tuned by the height of the energetic barriers. For the molecules studied here, pre-excitation pulses allowed a strong improvement of the reaction yield by up to a factor of 3.4.
4. Conclusion
In this publication we investigated the ring-opening quantum efficiency after a preceding ring-closure reaction of three indolylfulgide compounds. An increase of the quantum efficiency by a factor of up to 3.4 was found (fulgide 2) for shorter delay time between pre-excitation and excitation pulse. A fast ring-opening reaction time, a high quantum efficiency and small barriers in the excited state energy surface make the pre-excitation energy available to improve the quantum efficiency during a few picoseconds.
Acknowledgments
The work was supported by Deutsche Forschungsgemeinschaft through the DFG-Cluster of Excellence Munich-Centre for Advanced Photonics and the SFB 749. Financial support to WJL from the NIH/NIGMS programs (SC3GM084752) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yokoyama Y. Chem Rev. 2000;100:1717–1739. doi: 10.1021/cr980070c. [DOI] [PubMed] [Google Scholar]
- 2.Ramsteiner IB, Hartschuh A, Port H. Chem Phys Lett. 2001;343:83–90. [Google Scholar]
- 3.Wolak MA, Thomas CJ, Gillespie NB, Birge RR, Lees WJ. J Org Chem. 2003;68:319–326. doi: 10.1021/jo026374n. [DOI] [PubMed] [Google Scholar]
- 4.Inada T, Uchida S, Yokoyama Y. Chem Lett. 1997:321–322. [Google Scholar]
- 5.Otto B, Rück-Braun K. Eur J Org Chem. 2003:2409–2417. [Google Scholar]
- 6.Wolak MA, Gillespie NB, Thomas CJ, Birge RR, Lees WJ. J Photochem Photobiol A-Chem. 2001;144:83–91. [Google Scholar]
- 7.Geppert D, Seyfarth L, de Vivie-Riedle R. Appl Phys B-Lasers Opt. 2004;79:987–992. [Google Scholar]
- 8.Geppert D, de Vivie-Riedle R. J Photochem Photobiol A-Chem. 2006;180:282–288. [Google Scholar]
- 9.Malkmus S, Koller F, Draxler S, Schrader TE, Schreier WJ, Brust T, DiGirolamo JA, Lees WJ, Zinth W, Braun M. Adv Funct Mat. 2007;17:3657–3662. [Google Scholar]
- 10.Siewertsen R, Renth F, Temps F, Sonnichsen F. Phys Chem Chem Phys. 2009;11:5952–5961. doi: 10.1039/b821344e. [DOI] [PubMed] [Google Scholar]
- 11.Brust T, Draxler S, Popp A, Chen X, Lees WJ, Zinth W, Braun M. Chem Phys Lett. 2009;477:298–303. doi: 10.1016/j.cplett.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordes T, Herzog TT, Malkmus S, Draxler S, Brust T, DiGirolamo JA, Lees WJ, Braun M. Photochem Photobiol Sci. 2009;8:528–534. doi: 10.1039/b817627b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koller FO, Schreier WJ, Schrader TE, Malkmus S, Schulz C, Dietrich S, Rück-Braun K, Braun M. J Phys Chem A. 2008;112:210–214. doi: 10.1021/jp073545p. [DOI] [PubMed] [Google Scholar]
- 14.Heinz B, Malkmus S, Laimgruber S, Dietrich S, Schulz C, Rück-Braun K, Braun M, Zinth W, Gilch P. J Am Chem Soc. 2007;129:8577–8584. doi: 10.1021/ja071396i. [DOI] [PubMed] [Google Scholar]
- 15.Draxler S, Brust T, Malkmus S, Koller FO, Heinz B, Laimgruber S, Schulz C, Dietrich S, Rück-Braun K, Zinth W, Braun M. J Mol Liq. 2008;141:130–136. [Google Scholar]
- 16.Brust T, Malkmus S, Draxler S, Ahmed SA, Rück-Braun K, Zinth W, Braun M. J Photochem Photobiol A-Chem. 2009;207:209–216. [Google Scholar]
- 17.Cordes T, Malkmus S, DiGirolamo JA, Lees WJ, Nenov A, de Vivie-Riedle R, Braun M, Zinth W. J Phys Chem A. 2008;112:13364–13371. doi: 10.1021/jp807097w. [DOI] [PubMed] [Google Scholar]
- 18.Malkmus S, Koller FO, Heinz B, Schreier WJ, Schrader TE, Zinth W, Schulz C, Dietrich S, Rück-Braun K, Braun M. Chem Phys Lett. 2006;417:266–271. doi: 10.1021/jp0657787. [DOI] [PubMed] [Google Scholar]
- 19.Koller FO, Schreier WJ, Schrader TE, Sieg A, Malkmus S, Schulz C, Dietrich S, Rück-Braun K, Zinth W, Braun M. J Phys Chem A. 2006;110:12769–12776. doi: 10.1021/jp0657787. [DOI] [PubMed] [Google Scholar]
- 20.Brust T, Draxler S, Malkmus S, Schulz C, Zastrow M, Rück-Braun K, Zinth W, Braun M. J Mol Liq. 2008;141:137–139. [Google Scholar]
- 21.Draxler S, Brust T, Malkmus S, DiGirolamo JA, Lees WJ, Zinth W, Braun M. Phys Chem Chem Phys. 2009;11:5019–5027. doi: 10.1039/b819585d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Draxler S, Malkmus S, Brust T, DiGirolamo JA, Lees WJ, Braun M, Zinth W. In: Corkum P, et al., editors. Ultrafast Phenomena XVI; Proceedings of the 16th International Conference; Berlin: Springer; 2009. pp. 379–381. [Google Scholar]
- 23.Draxler S, Brust T, Eicher J, Zinth W, Braun M. Opt Commun. 2010;283:1050–1054. [Google Scholar]
- 24.Thomas CJ, Wolak MA, Birge RR, Lees WJ. J Org Chem. 2001;66:1914–1918. doi: 10.1021/jo005722n. [DOI] [PubMed] [Google Scholar]
- 25.Islamova NI, Chen X, Garcia SP, Guez G, Silva Y, Lees WJ. J Photochem Photobiol A-Chem. 2008;195:228–234. doi: 10.1016/j.jphotochem.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zarwell S, Dietrich S, Schulz C, Dietrich P, Michalik F, Rück-Braun K. Eur J Org Chem. 2009:2088–2095. [Google Scholar]
- 27.Brust T, Draxler S, Rauh A, Silber MV, Braun P, Zinth W, Braun M. Chem Phys. 2009;357:28–35. [Google Scholar]
- 28.Zinth W, Zinth U. Optik. Oldenbourg Wissenschaftsverlag; München: 2005. [Google Scholar]
- 29.Wilhelm T, Piel J, Riedle E. Opt Lett. 1997;22:1494–1496. doi: 10.1364/ol.22.001494. [DOI] [PubMed] [Google Scholar]
- 30.Riedle E, Beutter M, Lochbrunner S, Piel J, Schenkl S, Spörlein S, Zinth W. Appl Phys B-Lasers Opt. 2000;71:457–465. [Google Scholar]
- 31.Seel M, Wildermuth E, Zinth W. Meas Sci Technol. 1997;8:449–452. [Google Scholar]
- 32.Weinacht TC, Ahn J, Bucksbaum PH. Nature. 1999;397:233–235. [Google Scholar]
- 33.Herek JL, Fraser NJ, Pullerits T, Martinsson P, Polivka T, Scheer H, Cogdell RJ, Sundström V. Biophys J. 2000;78:2590–2596. doi: 10.1016/S0006-3495(00)76803-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker RS, Dolan E, Balke DE. J Chem Phys. 1969;50:239–245. [Google Scholar]
- 35.Uchida S, Yamada S, Yokoyama Y, Kurita Y. Bull Chem Soc Jpn. 1995;68:1677–1682. [Google Scholar]
- 36.Uchida S, Yokoyama Y, Kiji J, Okano T, Kitamura H. Bull Chem Soc Jpn. 1995;68:2961–2967. [Google Scholar]
- 37.Yokoyama Y, Tanaka T, Yamane T, Kurita Y. Chem Lett. 1991:1125–1128. [Google Scholar]
- 38.Yokoyama Y, Takahashi K. Chem Lett. 1996:1037–1038. [Google Scholar]