Abstract
The aim of this study was to evaluate the predictive value of the polymorphism Glutathione S-transferase P1 (GSTP1) Ile105Val on oxaliplatin/5-FU-based chemotherapy in advanced gastric cancer. Patients with advanced gastric cancer accepted oxaliplatin/5-FU-based chemotherapy as first-line chemotherapy were investigated. GSTP1 Ile105Val polymorphism was detected by TaqMan-MGB probe allelic discrimination method. Response to treatment was assessed by disease controlled rate. Time to progression, overall survival and toxicities were recorded. Final patient outcomes were as follows: the allele frequencies of GSTP1 were 105Ile/105Ile 52%, 105Ile/105Val 41% and 105Val/105Val 7%. For patients with 105Ile/105Ile and those with at least one 105Val allele, disease control rate was 39% and 71% (P=0.026), respectively; median time to progression was 4.0 and 7.0 months (P=0.002); median overall survival time was 7.0 and 9.5 months (P=0.002). Neurological toxicity was more frequently occurred in patients with two 105Ile alleles (P=0.005). In conclusion, patients with at least one 105Val allele have better prognosis and response to oxaliplatin/5-FU-based regimen as first-line treatment for patients with advanced gastric cancer.
Keywords: Polymorphism, Glutathione S-Transferase pi, Oxaliplatin, Stomach Neoplasms
INTRODUCTION
Gastric cancer is one of the most common adult malignant tumors. Gastric cancer is the fourth common type of cancer and the second most frequent cause of death from cancer, worldwide, with the highest incidence in China, Japan and Eastern European countries (1). Cisplatin is commonly used with 5-fluorouracil (5-FU) as chemotherapy doublets in the treatment of advanced gastric cancer (2). During the last few years additional drugs were introduced into chemotherapy regimens for gastric cancer such as oxaliplatin, taxanes and epirubicin. However, the response rates of these drugs or their combinations were still less than 50% (3-5). One of the remaining challenges is the development of predictive marker profiles to identify patients who will derive both minimal toxicity and maximum benefit from certain chemotherapy.
There is a growing body of evidence suggesting that genetic polymorphisms in genes involved in metabolism, signalling, DNA-repair and cellular response pathways all contribute to inter-patient variability of drug response and toxicity (6, 7). Therefore, pharmacogenetic analyses appear to be a promising tool to develop individualized treatment plans. Individual chemotherapy based on pharmacogenetics and pharmacogenomics has shown a potentially predictive role to achieve superior outcome in retrospective and perspective studies in lung cancer (8, 9). However, little is known in gastric cancer pharmacogenetics.
Oxaliplatin, carrying a 1,2-diamino-cyclohexane ring, leads to platinum-DNA adducts, which are more cytotoxic than adducts formed from other platinum agents and more effective at blocking DNA replication (10). With the wider use of oxaliplatin in gastric cancer, predictive markers for clinical outcome to this platinum agent may help to identify prospectively those patients who are more likely to benefit from the treatment. Resistance to platinum agents has been attributed to enhanced tolerance to platinum DNA adducts, decreased drug accumulation, or enhanced DNA repair (11). Variable chemosensitivity to oxaliplatin may depend on detoxification pathways, including the glutathione S-transferase (GST) family of isoenzymes (12, 13). Glutathione S-transferase P1 (GSTP1) is a member of a superfamily of dimeric phase II metabolic enzymes that play an important role in the cell defense system. GSTP1 directly participates in the detoxification of platinum compounds and is an important mediator of both intrinsic and acquired resistance to platinum (14). A single nucleotide substitution (A→G) at position 313 (resulting in replacing isoleucine with valine at codon 105) has been found to modify the enzyme's activity and affinity for electrophilic substrates (15). Previous studies showed the expression level and the polymorphic Ile105Val of GSTP1 had linked with the sensitivity of cancer cells to platinum (16). Clinical studies also have implicated GSTP1 as a predictive marker for clinical outcome in patients with cancer treated with platinum-based chemotherapy (17, 18). However, the predictive values in advanced gastric cancer with oxaliplatin-based chemotherapy are still not sufficient.
Given the further biochemical evidence that GSTP1 mediates inactivation of platinum drugs, we analyzed the common polymorphism in GSTP1-105 in patients with advanced gastric cancer to determine whether the presence of the polymorphism is associated with clinical outcome to oxaliplatin/5-FU-based first-line chemotherapy in Chinese population.
MATERIALS AND METHODS
Study subjects
From July 2006 to December 2008, 163 patients with advanced gastric cancer were treated at the Cancer Treatment Center in the Affiliated Hospital of Medical College, Qingdao University (China). The criteria for acceptance of the patients were as follows: 1) all patients had bi-dimensionally measurable disease; 2) each participant had gastric adenocarcinoma with stage IV disease, definitely diagnosed by histology; 3) patients' performance status was classified according to Eastern Cooperative Oncology Group (ECOG) criteria, and each patient's ECOG status was not greater than 2; 4) patients had not been treated with chemotherapy previously; 5) each participant's hemogram, hepatic function, renal function, and electrocardiogram were normal before chemotherapy. According to the criteria mentioned-above, 92 patients were eligible for the study. All the 92 patients signed an informed consent form before entering the study. Specimens (blood samples) of the patients were obtained before treatment.
Chemotherapy
All participants received the following oxaliplatin/5-FU-based combination therapy regimen as first-line treatment. All patients administered oxaliplatin plus 5-FU regimen once every two weeks: oxaliplatin (Jiangsu Heng-rui Express, China; H20040817) 85 mg/m2 as a 2-hr infusion on day 1, and then calcium folinate (Jiangsu Heng-rui Express) 200 mg/m2 as a 2-hr infusion on days 1-2, combined with bolus 5-FU (Tianjin Jin-yao Express, China; H12020959) 300 mg/m2 and continuous infusion of 5-FU 600 mg/m2 on days 1-2. Treatment was continued until documented disease progression, unacceptable toxic effects or patient's refusal.
Clinical evaluation
All patients for analyses were required to have completed at least four cycles of chemotherapy. Computed tomography imaging was performed every 6 weeks. Objective response to the treatment corresponded to a decrease in tumor burden (the sum, over all measurable lesions, of the products of the largest diameter and its perpendicular diameter) of 50% or more for at least 6 weeks (partial response, or complete response). And disease control includes complete response (CR), partial response (PR) and stable disease (SD, ≤25% progression, <50% shrinkage) for at least 6 weeks. Progressive disease (PD) was defined as an enlargement in tumor burden of 25% or more (compared with the smallest measurement) or appearance of new lesions. We calculated the disease control rate (the percent of CR+PR+SD) to evaluate patients' response to chemotherapy. Time to progression (TTP) was calculated from the time of initial treatment to disease progression. Overall survival (OS) was calculated from the time of initial treatment to death or the last follow-up. Neuropathy was scored according to the oxaliplatin-specific scale reported by Caussanel et al. (19). On this oxaliplatin-specific scale, the grade of cumulative neuropathy depends on the duration and intensity of symptoms (grade 1, paresthesia, dysesthesia of short duration; grade 2, paresthesia, dysesthesia persisting between cycles; grade 3, paresthesia, dysesthesia causing functional impairment; grade 4, paralysis). Other reliable adverse events were evaluated using the National Cancer Institute-Common Toxicity Criteria (NCI-CTC) version 2.0 (grade 1, mild adverse event; grade 2, moderate adverse event; grade 3, severe and undesirable adverse event; grade 4, life-threatening or disabling adverse event).
DNA extraction and genotyping
Genomic DNA was isolated from 2 mL peripheral blood, using Blood Genomic DNA Isolation Kit (Watson Biotechnologies, Inc., Taipei, Taiwan). The Ile105Val polymorphism was analyzed with TaqMan genotyping assays, using the Rotergene real time 36-well polymerase chain reaction (PCR) system (Gene Company Limited, Hong Kong, China). Probes, primer and TaqMan universal PCR master mix were purchased from Applied Biosystems Inc. (ABI). The TaqMan assay were performed in 25 µL reaction solution containing 1.25 µL TaqMan SNP Genotyping Assay Mix (consisting of 20×mix of unlabeled PCR primers and TaqMan MGB probe, FAM and VIC dye-labeled), 12.5 µL PCR mixture reagent and 20 ng genomic DNA. The polymerase chain reaction consisted of an initial step at 95℃ for 10 min and 48 cycles of denaturing at 92℃ for 15 sec and annealing 60℃ for 1 min. SDS allelic discrimination software provided by Gene Company Limited was used for analysis of the PCR genotyping results. 30 randomly selected DNA samples were genotyped at least twice to confirm the results.
Statistical analysis
Initially, disease controlled rate, time to progression, overall survival, and reliable toxicity were the end points considered in this analysis. Patients' characteristics were compared across genotypes using chi-square test. GSTP1 genotype distribution gained in the study was tested by the Hardy-Weinberg equilibrium law. The disease controlled rate were compared by the chi-square test and Fisher exact test between the patients with 105Ile/105Ile GSTP1 genotype and those with at least one 105Val allele. The Cox proportional hazards model was used to determine the association of TTP and OS with the following baseline prognostic factors: histology, site of tumor, number of metastatic sites, performance status, age at enrollment of study, and gender. The odds ratio (OR) and 95% confidence intervals (95% CI) were calculated at the same time. The log-rank test and Kaplan-Meier plots were used to evaluate the association of overall survival and time to progression with GSTP1 polymorphism. Treatment-related toxicity was compared by the Mann-Whitney test between the patients with GSTP1 wild genotype and patients with GSTP1 mutant genotype at codon 105.
All P values reported here were two-sided, and a probability of 0.05 or smaller was considered statistically significant. Statistical analyses were performed using SPSS software, Version 16.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
GSTP1 polymorphism
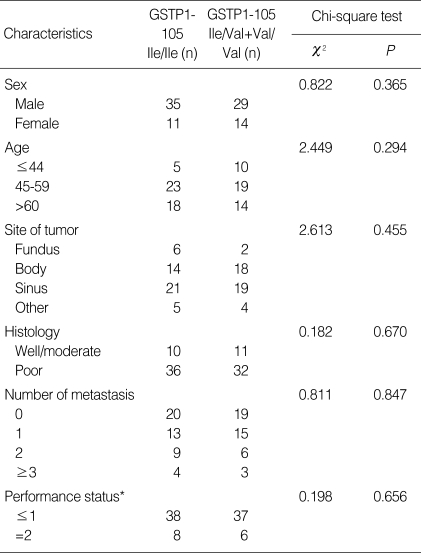
Ninety-seven percent (89/92) of the patients' genetic polymorphism was determined successfully. GSTP1 genotype was analyzed for 89 patients, of which 46 (52%) were homozygous for the 105Ile/105Ile GSTP1 genotype, 37 (41%) were heterozygous for 105Ile/105Val, and 6 (7%) were homozygous for the 105Val/105Val. The allele frequency for the GSTP1 105Val allele in our study was 48% (43/89). The allelic distribution was in Hardy-Weinberg equilibrium (χ2=0.156, P=0.692). The 89 patients consisted of 25 females (28%) and 64 males (72%), with a median age of 55 yr old (range 32 yr old to 77 yr old). No clear patterns for significant associations between the polymorphism and any of the other demographic (gender), clinical (performance status, site of the tumor, and number of metastases), or pathological characteristics (tumor differentiation) were observed (Table 1).
Table 1.
Demographic, clinical, and pathological characteristics compared between patients with different GSTP1 genotype
*Performance status was classified according to Eastern Cooperative Oncology Group (ECOG) criteria.
GSTP1, Glutathione S-transferases P1.
Response to chemotherapy
GSTP1-105 genotype analyses were successful for 89 patients of which 85 were eligible for response analyses. Four patients withdrew from the study too early to evaluate their response. The four patients refused to continue chemotherapy. The median cycles of chemotherapy were 5 cycles for the remaining 85 patients. Among these 85 patients, 12 (14%) of the patients showed PR, 34 (40%) SD, 39 (46%) showed PD, and no patients showed CR. The association between the response to treatment and the GSTP1 polymorphisms was shown in Table 2.
Table 2.
Association between GSTP1 polymorphism and response to treatment
GSTP1, Glutathione S-transferases P1; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease, OR, odds ratio; CI, confidence intervals.
Time to progression
The 85 patients mentioned-above were eligible for survival analysis since the four patients took off study too early to evaluate their survival time. From July 2006 to April 2009, 83 patients (98%) were followed up, with a median follow-up period of 11.6 months (range 3.5 to 23 months). The survival time of the 2 patients who lost follow-up was treated as censored data.
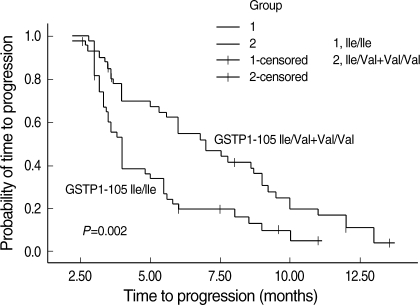
Among the 85 patients, eighty-seven percent (74/85) of the participants' disease had progression during the follow-up period. Then the TTP of the 9 patients without disease progression and the 2 patients with lost of follow up was censored. Compared with patients with a homozygous 105Ile/105Ile GSTP1 genotype, patients with one or two 105Val alleles had increased the median time to progression (MTTP). The MTTP for patients with a homozygous 105Ile/105Ile GSTP1 genotype was 4.0 months (95% CI 3.649-4.351), whereas that for patients with one or two 105Val GSTP1 genotype was 7.0 months (95% CI 5.360-8.640; χ2=10.043, P=0.002) (Fig. 1).
Fig. 1.
Time to progression curves of patients with advanced gastric cancer according to GSTP1 Ile105Val polymorphism.
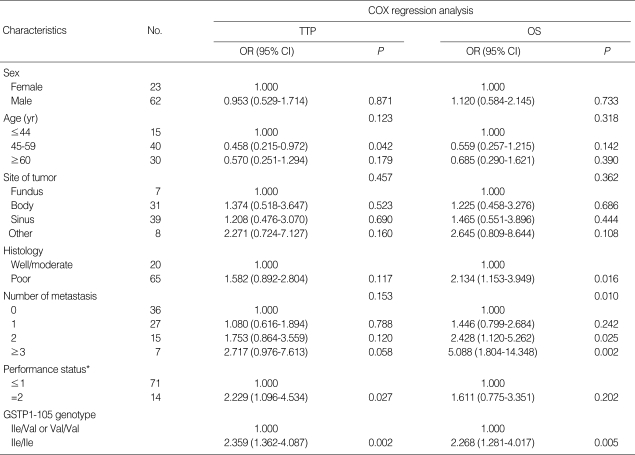
The MTTP for all 85 patients was 5.3 months (95% CI 4.431-6.169). Homozygosity for 105Ile/105Ile GSTP1 genotype was associated with a significantly increased risk of progression compared with at least one 105Val allele (OR 2.359; 95% CI 1.362-4.087; P=0.002). Patients with a performance status ECOG=2 showed an increased risk of progressing (P=0.027) when compared with patients who showed a superior performance status (ECOG 0-1). Age, sex, tumor histologic grade, metastases, as well as site of tumor was not associated with TTP in this series of patients (Table 3).
Table 3.
Association between patients' characteristics and TTP, OS after oxaliplatin/5-FU-based chemotherapy
*Performance status was classified according to Eastern Cooperative Oncology Group (ECOG) criteria.
TTP, time to progression; OS, overall survival; OR, odds ratio; CI, confidence intervals; GSTP1, Glutathione S-transferases P1.
Overall survival time
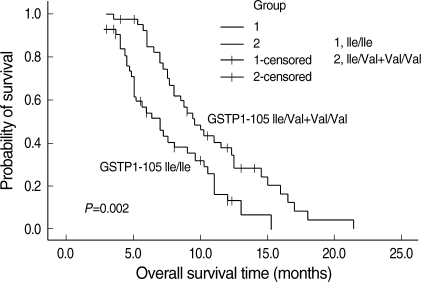
Of the 85 patients, 14 patients were still alive. The survival time of the 14 patients and the two patients with lost of follow up was treated as censored data. The median overall survival (MOS) for patients with a homozygous 105Ile/105Ile GSTP1 genotype was 7.0 months (95% CI 5.137-8.863), whereas that for patients with one or two 105Val GSTP1 genotype was 9.5 months (95% CI 7.847-11.153; χ2=9.223, P=0.002) (Fig. 2).
Fig. 2.
Overall survival curves of patients with advanced gastric cancer according to GSTP1 Ile105Val polymorphism.
The MOS for all 85 patients was 8.0 months (95% CI 6.623-9.377). Patients with 105Ile/105Ile genotype had a higher risk of death compared with patients with one or two 105Val alleles (OR 2.268; 95% CI 1.281-4.017; P=0.005). Age, sex, site of tumor, and performance status were not associated with patient survival. However, patients with more metastases seemed to have a shorter survival time (P=0.010). Otherwise, 65 patients with a poorly differentiated tumor also tended to have a higher risk of death (P=0.016). The association of demographic, clinical, and pathological characteristics and clinical outcome was listed in Table 3.
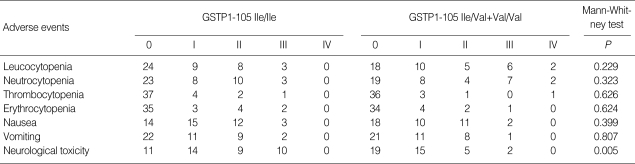
Toxicity
All 85 patients were evaluated toxicity from the time of initial chemotherapy to end of follow-up. No treatment-related deaths occurred. The most common adverse events of the treatment were hematological and neurological toxicity. Grade 3 or 4 cumulative peripheral neuropathy was significantly more common in patients with 105Ile/105Ile GSTP1 genotype (10 of 44 patients, 23%) compared with patients with 105Ile/105Val or 105Val/105Val GSTP1 genotype (2 of 41 patients, 5%; 2=5.577, P=0.018). Meanwhile, the overall incidence of neurological toxicity in patients with homozygous 105Ile/105Ile GSTP1 genotype was significantly higher than that in patients with one or two 105Val GSTP1 genotype (P=0.005) (Table 4).
Table 4.
Association between GSTP1 polymorphism and treatment-related toxicity
GSTP1, Glutathione S-transferases P1.
DISCUSSION
We attempted to identify predictive genetic polymorphisms for response and survival to oxaliplatin/5-FU chemotherapy in patients with advanced gastric cancer. Our analysis of the common polymorphism for the GSTP1 in patients with advanced gastric cancer demonstrates that relevant alteration of a protein's function can result in differences of defined clinical endpoints. In this study, patients with at least one 105Val allele had a better prognosis and response to the oxaliplatin/5-FU-based regimen as first-line treatment for advanced gastric cancer.
The identification of molecular variables that predict either resistance or sensitivity to chemotherapy is of major interest in selecting the most likely effective first-line treatment, avoiding adverse side effects of a toxic but inactive therapy. Glutathione S-transferases catalyze the first step in the formation of mercapturic acids, initiating a pathway that results mostly in the elimination of toxic compounds. Previous studies revealed that substitution of isoleucine with valine at codon 105 alters the function of the GSTP1 protein (20).
It is known that the allele frequencies of metabolic genes are not equally distributed throughout the human population but follow diverse ethnic and/or geographic-specific patterns. An American population-based study reported, for GSTP1, the prevalence rates of 105Ile/105Val heterozygosity and 105Val/105Val homozygosity were found to be 30% to 51% and 7% to 19%, respectively (15). For gastric cancer patients, a Chinese study showed the rates of GSTP1 105Ile/105Val genotype and 105Val/105Val genotype were 30.6% and 0% (21). In our study, 41% of the participants were heterozygous for 105Ile/105Val, and 7% were homozygous for the 105Val/105Val. This frequency is similar to the frequencies above.
Stoehlmacher et al. (17) reported clinical response was not statistically associated with GSTP1 genotypes in patients with colorectal cancer. Ott et al. (18) reported GSTP1 genotypes were not correlated with response to neoadjuvant chemotherapy in gastric cancer. Cabelguenne et al. (22) could not observe a correlation between GSTP1 genotypes and response to cisplatin neoadjuvant chemotherapy in head and neck squamous cell cancer (HNSCC) patients. While another study showed patients possessing the GSTP1-105 Val/Val genotype showed a significant superior response in gastric cancer (23). Our results demonstrated patients with at least one 105Val allele had a better disease control rate compared with patients with homozygous 105Ile/105Ile genotype. There were some controversial reports about the impact of GSTP1 on clinical response, indicating that other potential factors may affect the response to chemotherapy, such as ethnic background, geographic pattern or other potential genetic factors.
Our study suggested patients with GSTP1 105Val genotype had a better clinical outcome to oxaliplatin/5-FU-based chemotherapy in advanced gastric cancer patients. GSTP1 Ile105Val polymorphism was studied widely in various tumors, such as prostate cancer, colorectal cancer, breast cancer, esophageal cancer and gastric cancer. Reports in colorectal cancer patients demonstrated a significantly improved time to progression and overall survival for carriers of the GSTP1 105Val allele after 5-FU/oxaliplatin chemotherapy (17, 24). Goekkurt et al. reported that patients with GSTP1 105Val/105Val genotype demonstrated significant superior median survival time after 5-FU/cisplatin chemotherapy for gastric cancer (23). In the present study, we demonstrated that patients with two 105Ile alleles had a significantly increased risk of progression and death. In summary, the results we report here are in strong agreement with the current understanding of GSTP1 involvement in platinum detoxification. Our findings support the hypothesis that increased platinum sensitivity may in part be due to impaired GSTP1 enzyme function.
Recent clinical reports provided evidence for an association between GSTP1 genotype and cytotoxicity of chemotherapeutic agents (25). A study reported that oxaliplatin-related cumulative neuropathy scored grade 3 was significantly more frequent in patients homozygous for the GSTP1 105Ile allele than in patients homozygous or heterozygous for the GSTP1 105Val allele (OR 5.75; 95% CI 1.08-30.74; P=0.02) (26). Another report showed Patients with GSTP1 105Ile/105Ile genotype had a higher risk of developing a grade >or =2 docetaxel-induced peripheral neuropathy (25). Similarly, our study implied patients with two GSTP1 105Ile alleles had a higher risk of developing neurological toxicity, especially in grade 3 neuropathy. It was reported that individuals with two GSTP1 105Val alleles had a lower catalytic activity compared with individuals with two GSTP1 105Ile alleles (15). In particular, there was in vitro evidence that GSTP1 105Val allele is much more active than the wild-type alleles against cisplatin and carboplatin (27). Thus, the increasing rate of grade 3 neuropathy in patients with 105Ile/105Ile GSTP1 genotype could be explained by the diminished capacity of detoxifying oxaliplatin. And the involvement of GSTP1 in cellular apoptosis could be another hypothesis to explain the protective effect of the variant genotype.
In conclusion, we demonstrated that among patients with gastric cancer who received 5-FU/oxaliplatin chemotherapy as first-line treatment, those possessing the GSTP1 105Val variant allele show a statistically significant benefit for both time to progression and overall survival. Our results support the hypothesis that the effects of chemotherapeutic drugs may be altered when enzymes that could enhance the elimination of these drugs show a reduced activity. However, more perspective multicentric randomized studies are required to confirm our preliminary results for patients with advanced gastric cancer in Chinese population.
Footnotes
This work was supported in part by grants from Shandong Province Natural Science Foundation (Y2008C126).
References
- 1.Varadhachary G, Ajani JA. Gastric cancer. Clin Adv Hematol Oncol. 2005;3:118–124. [PubMed] [Google Scholar]
- 2.Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, Planker M, Santos JG, Piedbois P, Paillot B, Bodenstein H, Schmoll HJ, Bleiberg H, Nordlinger B, Couvreur ML, Baron B, Wils JA. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000;18:2648–2657. doi: 10.1200/JCO.2000.18.14.2648. [DOI] [PubMed] [Google Scholar]
- 3.Sadighi S, Mohagheghi MA, Montazeri A, Sadighi Z. Quality of life in patients with advanced gastric cancer: a randomized trial comparing docetaxel, cisplatin, 5-FU (TCF) with epirubicin, cisplatin, 5-FU (ECF) BMC Cancer. 2006;6:274. doi: 10.1186/1471-2407-6-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sumpter K, Harper-Wynne C, Cunningham D, Rao S, Tebbutt N, Norman AR, Ward C, Iveson T, Nicolson M, Hickish T, Hill M, Oates J. Report of two protocol planned interim analyses in a randomised multicentre phase III study comparing capecitabine with fluorouracil and oxaliplatin with cisplatin in patients with advanced oesophagogastric cancer receiving ECF. Br J Cancer. 2005;92:1976–1983. doi: 10.1038/sj.bjc.6602572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E, Risse ML, Ajani JA. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 6.Allen WL, Johnston PG. Role of genomic markers in colorectal cancer treatment. J Clin Oncol. 2005;23:4545–4552. doi: 10.1200/JCO.2005.19.752. [DOI] [PubMed] [Google Scholar]
- 7.Yamayoshi Y, Iida E, Tanigawara Y. Cancer pharmacogenomics: international trends. Int J Clin Oncol. 2005;10:5–13. doi: 10.1007/s10147-004-0467-4. [DOI] [PubMed] [Google Scholar]
- 8.Rosell R, Cecere F, Santarpia M, Reguart N, Taron M. Predicting the outcome of chemotherapy for lung cancer. Curr Opin Pharmacol. 2006;6:323–331. doi: 10.1016/j.coph.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Olaussen KA, Dunant A, Fouret P, Brambilla E, Andre F, Haddad V, Taranchon E, Filipits M, Pirker R, Popper HH, Stahel R, Sabatier L, Pignon JP, Tursz T, Le Chevalier T, Soria JC. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–991. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- 10.Galanski M. Recent developments in the field of anticancer platinum complexes. Recent Pat Anticancer Drug Discov. 2006;1:285–295. doi: 10.2174/157489206777442287. [DOI] [PubMed] [Google Scholar]
- 11.Raymond E, Faivre S, Woynarowski JM, Chaney SG. Oxaliplatin: mechanism of action and antineoplastic activity. Semin Oncol. 1998;25(2 Suppl 5):4–12. [PubMed] [Google Scholar]
- 12.Marsh S, McLeod HL. Cancer pharmacogenetics. Br J Cancer. 2004;90:8–11. doi: 10.1038/sj.bjc.6601487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagasubramanian R, Innocenti F, Ratain MJ. Pharmacogenetics in cancer treatment. Annu Rev Med. 2003;54:437–452. doi: 10.1146/annurev.med.54.101601.152352. [DOI] [PubMed] [Google Scholar]
- 14.Goto S, Iida T, Cho S, Oka M, Kohno S, Kondo T. Overexpression of glutathione S-transferase pi enhances the adduct formation of cisplatin with glutathione in human cancer cells. Free Radic Res. 1999;31:549–558. doi: 10.1080/10715769900301121. [DOI] [PubMed] [Google Scholar]
- 15.Watson MA, Stewart RK, Smith GB, Massey TE, Bell DA. Human glutathione S-transferase P1 polymorphisms: relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis. 1998;19:275–280. doi: 10.1093/carcin/19.2.275. [DOI] [PubMed] [Google Scholar]
- 16.Booton R, Ward T, Heighway J, Ashcroft L, Morris J, Thatcher N. Glutathione-S-transferase P1 isoenzyme polymorphisms, platinum-based chemotherapy, and non-small cell lung cancer. J Thorac Oncol. 2006;1:679–683. [PubMed] [Google Scholar]
- 17.Stoehlmacher J, Park DJ, Zhang W, Groshen S, Tsao-Wei DD, Yu MC, Lenz HJ. Association between glutathione S-transferase P1, T1, and M1 genetic polymorphism and survival of patients with metastatic colorectal cancer. J Natl Cancer Inst. 2002;94:936–942. doi: 10.1093/jnci/94.12.936. [DOI] [PubMed] [Google Scholar]
- 18.Ott K, Lordick F, Becker K, Ulm K, Siewert J, Hofler H, Keller G. Glutathione-S-transferase P1, T1 and M1 genetic polymorphisms in neoadjuvant-treated locally advanced gastric cancer: GSTM1-present genotype is associated with better prognosis in completely resected patients. Int J Colorectal Dis. 2008;23:773–782. doi: 10.1007/s00384-008-0490-4. [DOI] [PubMed] [Google Scholar]
- 19.Caussanel JP, Levi F, Brienza S, Misset JL, Itzhaki M, Adam R, Milano G, Hecquet B, Mathe G. Phase I trial of 5-day continuous venous infusion of oxaliplatin at circadian rhythm-modulated rate compared with constant rate. J Natl Cancer Inst. 1990;82:1046–1050. doi: 10.1093/jnci/82.12.1046. [DOI] [PubMed] [Google Scholar]
- 20.Srivastava SK, Singhal SS, Hu X, Awasthi YC, Zimniak P, Singh SV. Differential catalytic efficiency of allelic variants of human glutathione S-transferase Pi in catalyzing the glutathione conjugation of thiotepa. Arch Biochem Biophys. 1999;366:89–94. doi: 10.1006/abbi.1999.1217. [DOI] [PubMed] [Google Scholar]
- 21.Liu B, Wei J, Zou Z, Qian X, Nakamura T, Zhang W, Ding Y, Feng J, Yu L. Polymorphism of XRCC1 predicts overall survival of gastric cancer patients receiving oxaliplatin-based chemotherapy in Chinese population. Eur J Hum Genet. 2007;15:1049–1053. doi: 10.1038/sj.ejhg.5201884. [DOI] [PubMed] [Google Scholar]
- 22.Cabelguenne A, Loriot MA, Stucker I, Blons H, Koum-Besson E, Brasnu D, Beaune P, Laccourreye O, Laurent-Puig P, De Waziers I. Glutathione-associated enzymes in head and neck squamous cell carcinoma and response to cisplatin-based neoadjuvant chemotherapy. Int J Cancer. 2001;93:725–730. doi: 10.1002/ijc.1392. [DOI] [PubMed] [Google Scholar]
- 23.Goekkurt E, Hoehn S, Wolschke C, Wittmer C, Stueber C, Hossfeld DK, Stoehlmacher J. Polymorphisms of glutathione S-transferases (GST) and thymidylate synthase (TS)--novel predictors for response and survival in gastric cancer patients. Br J Cancer. 2006;94:281–286. doi: 10.1038/sj.bjc.6602891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoehlmacher J, Park DJ, Zhang W, Yang D, Groshen S, Zahedy S, Lenz HJ. A multivariate analysis of genomic polymorphisms: prediction of clinical outcome to 5-FU/oxaliplatin combination chemotherapy in refractory colorectal cancer. Br J Cancer. 2004;91:344–354. doi: 10.1038/sj.bjc.6601975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mir O, Alexandre J, Tran A, Durand JP, Pons G, Treluyer JM, Goldwasser F. Relationship between GSTP1 Ile(105)Val polymorphism and docetaxel-induced peripheral neuropathy: clinical evidence of a role of oxidative stress in taxane toxicity. Ann Oncol. 2009;20:736–740. doi: 10.1093/annonc/mdn698. [DOI] [PubMed] [Google Scholar]
- 26.Lecomte T, Landi B, Beaune P, Laurent-Puig P, Loriot MA. Glutathione S-transferase P1 polymorphism (Ile105Val) predicts cumulative neuropathy in patients receiving oxaliplatin-based chemotherapy. Clin Cancer Res. 2006;12:3050–3056. doi: 10.1158/1078-0432.CCR-05-2076. [DOI] [PubMed] [Google Scholar]
- 27.Ishimoto TM, Ali-Osman F. Allelic variants of the human glutathione S-transferase P1 gene confer differential cytoprotection against anticancer agents in Escherichia coli. Pharmacogenetics. 2002;12:543–553. doi: 10.1097/00008571-200210000-00006. [DOI] [PubMed] [Google Scholar]