Abstract
Our objective in this study was to evaluate the safety and efficacy of transurethral cord blood stem cell injection for treatment of stress urinary incontinence in women. Between July 2005 and July 2006, 39 women underwent transurethral umbilical cord blood stem cell injection performed by one operator at a single hospital. All patients had stress urinary incontinence. The patients were evaluated 1, 3, and 12 months postoperatively. No postoperative complications were observed. 28 patients (77.8%) were more than 50% satisfied according to the Patient's Satisfaction results after 1 month, 29 patients (83%) were more than 50% satisfied according to the Patient's Satisfaction results after 3 months, and 26 (72.2%) continuously showed more than 50% improvement after 12 months. Intrinsic sphincter deficiency and mixed stress incontinency improved in the ten patients evaluated by urodynamic study. Our results suggest that transurethral umbilical cord blood stem cell injection is an effective treatment for women with all types of stress urinary incontinence.
Keywords: Urinary Incontinence, Stress; Intrinsic Sphincter Deficiency; Umbilical Cord Blood Stem Cell; Urodynamic Study
INTRODUCTION
Urinary incontinence occurs in 10-40% of women, affecting more than two hundred million women worldwide. Stress urinary incontinence is the most common form, accounting for about 50% of all types of incontinence. Stress urinary incontinence is the involuntary urination resulting from increase in stress, where urine leaks without bladder contraction, and peaks in women aged 45 to 49 yr. Affecting 65% of women aged 45 to 49, urinary incontinence creates hygienic as well as social problems (1, 2).
Depending on the pathophysiology, stress urinary incontinence is divided into anatomic incontinence caused by urethral hypermobility and intrinsic sphincter deficiency (ISD). Surgical treatments for anatomic incontinence include bladder neck suspension performed through the abdomen or vagina, or the midurethral sling operation using tape. Surgical treatments for ISD include sling operation, urethral submucosal injection or creation of an artificial urethral sphincter using fat tissue, teflon, collagen, or silicon. However, a perfect treatment method applicable to all types of incontinence has not yet been created.
Stem cells have recently emerged as a new treatment method for various diseases. Primal undifferentiated cells, stem cells retain the ability to differentiate into other cell types. Stem cells are commonly categorized as embryonic or adult, according to their source. Unlike embryonic stem cells, which are not easily obtained for clinical application, adult stem cells can be extracted from human cord blood without harm, making clinical application relatively easy. Adult stem cells are present in minute quantities as multipotent cells that multiply and differentiate in response to damaged tissue or increased stress. Stem cell therapy for urinary incontinence has been investigated by others. Yiou and his team reported that intrinsic satellite cells in striated muscle are useful in the regeneration of damaged rhabdomyosphincter in mice (3). Yokoyama and his team reported that injection of muscle-derived progenitor cells (MDPCs) into the mouse urethra yielded better durability than injection of bovine collagen (4). Tracy and his team reported improved sphincter contractibility induced by injection of allogeneic muscle derived progenitor cells into the urethra of a mouse with severed nerves (5). Strasser and his team, who were first to conduct a human clinical study, performed transurethral injection of autologous muscle-derived stem cells using transurethral ultrasound and reported improved symptoms, a thickened urethral sphincter, and improved urethral sphincter contractibility (6).
Human cord blood stem cells can transform into other cell types (transdifferentiation) and are expected to be useful in the treatment of patients with dysfunction in periurethral nervous tissue, smooth muscle, striated muscle, urethral mucosa, submucosal connective tissue and various other tissues. Human cord blood stem cells are expected to yield highly efficacious treatments.
Our objective was to evaluate the safety and efficacy of periurethral cord blood stem cell injection for treatment of stress urinary incontinence.
MATERIALS AND METHODS
This study was carried out from July, 2005 to July, 2006 at the CHA Gangnam Medical Center and School of Medicine, CHA University, with IRB approval. The procedure was completed on 39 women with urinary incontinence who had signed informed consent forms. All had previously tried conservative treatment, and one had been treated with other surgical method for the disorder, without success.
All patients were given a Quality-of-life Questionnaire and Incontinence Impact Questionnaire prior to the procedure. History, physical examination, urine test, urine culture, general blood test, routine chemistry, cotton-swab test, urodynamic study, and HLA typing were performed on the patients.
The umbilical cord vein was pricked after normal delivery and then the human umbilical cord blood was collected in an aseptic collecting bag (National Red Cross, Korea) containing 23 mL of citrate-phosphate-dextrose-adenine (CPDA-1) and stored at -70℃. After thawing at 37℃, cord blood cells were separated into a low-density mononuclear fraction (<1,077 g/mL) by Ficoll-Paque Plus (GE Healthcare AB, Uppsala, Sweden, http://www.amersham.com). To briefly summarize the separation method, a small amount of saline was added to the cord blood and mixed well. Diluted cord blood was carefully poured into a 15 mL tube which contained Ficoll (Ficoll:diluted cord blood=3:4). The mixture of diluted cord blood and Ficoll was centrifuged at 1,800 rpm for 30 min and mononuclear cells were carefully drawn out from the top layer. The cells were stored in 50 mL tubes with saline, and the withdrawn mononuclear cells were centrifuged three times at 1,800 rpm for 10 min. The cells were collected afterward and the supernatant was discarded. Saline (50 mL) was mixed with the remaining cell pellet and the process of centrifuging at 1,000 rpm for 10 min was repeated twice. The cell pellet was resuspended with 50 mL saline, and 10 µL was removed. The cells were then counted.
All parts of the transurethral umbilical cord blood stem cell injection were performed by one operator. Antibiotic was given to the patient prior to periurethral umbilical cord blood stem cell (UCBSC) injection. After the vagina and perineum were sterilized, local anesthesia was given in the vicinity of the urethra in the 4 and 8 o'clock positions with 1% lidocaine. Using a 30° 26 French cystoscope, the vesicourethral junction was checked visually. Stem cells removed from human cord blood were verified in the HLA test, and HUCB mononuclear cell suspension in saline solution (mean, 4.3±1.9×108 cells per 2 mL) were injected into the urethra under cystoscope in the 4 and 8 o'clock positions. Two injections per patient (1 mL each) were performed in submucosal area of the proximal-urethra. Injection continued until the urethral mucosa was completely closed, while the inflation of urethra mucosa was observed through the cystoscope. The patient was released the day of the procedure. Patients were informed to immediately visit the clinic in case of any postoperative complications. As follow-up, physical examination and liver function tests were performed on a weekly basis for the following month.
Outcomes were evaluated by comparing patients' voiding diaries and perceptions. To compare patients' perceptions, the Patient Satisfaction Test was performed 1 month, 3 months, and 12 months after the procedure. A 3-month postoperative urodynamic study was also used to monitor the objective improvement of urinary incontinence following periurethral UCBSC injection. A urodynamic study was conducted on ten patients who had a MUCP result of less than 30 cmH2O before the stem cell transplantation 3 months after the procedure. Individual comparisons were made using Student's t-test. A P-value of less than 0.05 was accepted as significant.
RESULTS
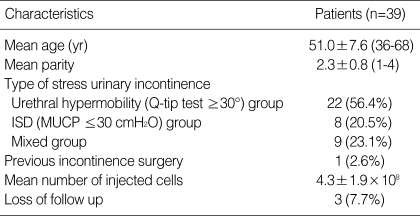
Mean age of the 39 patients treated in the study was 51.5±7.6 yr. All patients had stress urinary incontinence; 22 had urethral hypermobility, eight had intrinsic sphincter deficiency, and nine had mixed stress urinary incontinence. One of the patients had previously undergone a surgical method to treat incontinence (Table 1).
Table 1.
Characteristics of patients undergoing transurethral UCBSC injection
UCBSC, umbilical cord blood stem cell; MUCP, maximal urethral closing pressure.
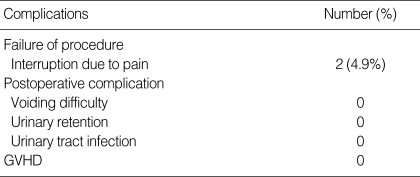
Periurethral UCBSC injection took 15 min. Two patients stopped the injection due to pain during the procedure, but were not included in the study. None of the cases had voiding dysfunction, urinary retention, urinary tract infection or graft versus host disease (GVHD) resulting from the procedure (Table 2).
Table 2.
Complications of the transurethral UCBSC injection
GVHD, graft versus host disease.
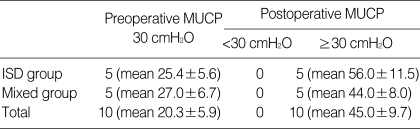
The mean follow-up was 13.0±2.7 months. Three patients discontinued the 1 month postoperative Patient Satisfaction Test. 28 (77.8%) showed more than 50% improvement, and eight (22.2%) reported no improvement in the 1-month postoperative Patient Satisfaction Test. In the 3-month postoperative Patient Satisfaction Test, 29 (80.5%) continuously showed more than 50% improvement. Finally, In the 12-month postoperative Patient Satisfaction Test, 26 (72.2%) continuously showed more than 50% improvement (Table 3). In addition, a 3-month postoperative urodynamic study conducted on five patients with stress urinary incontinence due to ISD and five patients with mixed stress urinary incontinence verified that in all ten patients the maximal urethral closing pressure (MUCP) values were increased by more than 30 cmH2O (Table 4). The MUCP values were significantly improved in the ISD and mixed stress urinary incontinence patients after 3 months.
Table 3.
Postoperative subjective cure rates-Patient Satisfaction
36/39 patients were checked.
Table 4.
Postoperative objective cure rates-Urodynamic study (except for urethral hypermobility group)
MUCP, maximal urethral closing pressure; ISD, intrinsic sphincter deficiency; 10/19 patients were checked.
DISCUSSION
Recently, stem cell therapy has been tried for urinary incontinence under the concept that urinary incontinence is essentially a degenerative disease, so stem cell may play a role in regenerating the damaged sphincter. Various types of tissue have been used as the source of stem cells for the treatment of urinary incontinence. Previous attempts to treat incontinence with stem cells have not been completely successful. First, harvesting stem cells from the aged patient's own muscle is advantageous since rejection complications are minimized. But unfortunately, the restoration efficiency of stem cells decreases with age (7). Renault, Thornell and their team reported that the restoration efficiency of stem cells in skeletal muscle declines in direct proportion with age (4-6% vs. 1-2%) (8,9). Jejurikar and his team reported that stem cell growth and transference also decrease with age (10). Thus, the therapeutic efficacy of transplanted stem cells obtained from the patient's muscle is poor. Second, taking muscle from the patient's arm is an invasive procedure associated with complications of bleeding or infection.
Use of human cord blood overcomes these problems. Gluckman and his team have successfully conducted cord blood transplantation (CBT) procedures since 1988 (11). It also has been investigated in many animal models of various degenerative diseases, such as spinal cord injury model and cerebral ischemia model, with some promising results (12-14). CBT has a low risk of causing GVHD and viral contamination, requires less stringent HLA matching and is easily performed. Furthermore, cord blood stem cells are obtained from a large source of donors.
As far as we know, this is the first use of human cord blood stem cells for treatment of urinary incontinence. Periurethral UCBSC injection is a very short, minimally invasive outpatient procedure that is not associated with complications. Human cord blood stem cell therapy improved different types of stress urinary incontinence 3 months after the procedure in 80% of our study patients. A urodynamic study conducted on ten patients who had a MUCP result of less than 30 cmH2O before the stem cell transplantation showed improved function of greater than 30 cmH2O in MUCP 3 months after the procedure.
Unfortunately, the number of patients studied was too small to allow comparison of therapeutic efficacy for the different types of stress urinary incontinence. Long term follow-up of more than a year and investigation into the mechanism of stem cell therapy are goals for our next study.
In conclusion, periurethral injection of human cord blood stem cells was effective, at least for the short term, with a lower incidence of surgery related complications compared to previous surgical methods.
Footnotes
This study was supported by the Research Fund from Pochon CHA University.
References
- 1.Norton P, Brubaker L. Urinary incontinence in women. Lancet. 2006;367:57–67. doi: 10.1016/S0140-6736(06)67925-7. [DOI] [PubMed] [Google Scholar]
- 2.Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trondelag. J Clin Epidemiol. 2000;53:1150–1157. doi: 10.1016/s0895-4356(00)00232-8. [DOI] [PubMed] [Google Scholar]
- 3.Yiou R, Lefaucheur JP, Atala A. The regeneration process of the striated urethral sphincter involves activation of intrinsic satellite cells. Anat Embryol (Berl) 2003;206:429–435. doi: 10.1007/s00429-003-0313-x. [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama T, Yoshimura N, Dhir R, Qu Z, Fraser MO, Kumon H, de Groat WC, Huard J, Chancellor MB. Persistence and survival of autologous muscle derived cells versus bovine collagen as potential treatment of stress urinary incontinence. J Urol. 2001;165:271–276. doi: 10.1097/00005392-200101000-00077. [DOI] [PubMed] [Google Scholar]
- 5.Cannon TW, Lee JY, Somogyi G, Pruchnic R, Smith CP, Huard J, Chancellor MB. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003;62:958–963. doi: 10.1016/s0090-4295(03)00679-4. [DOI] [PubMed] [Google Scholar]
- 6.Strasser H, Marksteiner R, Margreiter E, Pinggera GM, Mitterberger M, Fritsch H, Klima G, Radler C, Stadlbauer KH, Fussenegger M, Hering S, Bartsch G. Stem cell therapy for urinary incontinence. Urologe A. 2004;43:1237–1241. doi: 10.1007/s00120-004-0700-9. [DOI] [PubMed] [Google Scholar]
- 7.Fulle S, Protasi F, Di Tano G, Pietrangelo T, Beltramin A, Boncompagni S, Vecchiet L, Fano G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol. 2004;39:17–24. doi: 10.1016/j.exger.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Renault V, Thornell LE, Eriksson PO, Butler-Browne G, Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell. 2002;1:132–139. doi: 10.1046/j.1474-9728.2002.00017.x. [DOI] [PubMed] [Google Scholar]
- 9.Thornell LE, Lindstrom M, Renault V, Mouly V, Butler-Browne GS. Satellite cells and training in the elderly. Scand J Med Sci Sports. 2003;13:48–55. doi: 10.1034/j.1600-0838.2003.20285.x. [DOI] [PubMed] [Google Scholar]
- 10.Jejurikar SS, Kuzon WM., Jr Satellite cell depletion in degenerative skeletal muscle. Apoptosis. 2003;8:573–578. doi: 10.1023/A:1026127307457. [DOI] [PubMed] [Google Scholar]
- 11.Gluckman E, Broxmeyer HE, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 12.Dasari VR, Spomar DG, Li L, Gujrati M, Rao JS, Dinh DH. Umbilical cord blood stem cell mediated downregulation of fas improves functional recovery of rats after spinal cord injury. Neurochem Res. 2008;33:134–149. doi: 10.1007/s11064-007-9426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vendrame M, Cassady J, Newcomb J, Butler T, Pennypacker KR, Zigova T, Sanberg CD, Sanberg PR, Willing AE. Infusion of human umbilical cord blood cells in a rat model of stroke dose-dependently rescues behavioral deficits and reduces infarct volume. Stroke. 2004;35:2390–2395. doi: 10.1161/01.STR.0000141681.06735.9b. [DOI] [PubMed] [Google Scholar]
- 14.Lee HH, Kim HG, Jang SK, Choi OH. Cord blood-derived CD34 (+) cells promotes functional recovery in transient middle cerebral artery occlusion model of rat. Korean J Obstet Gynecol. 2007;50:1521–1531. [Google Scholar]