Abstract
Central retinal artery occlusion (CRAO) causes severe visual loss in affected eye and vision does not recover in more than 90% of the patients. It is believed that it occurs by occlusion of the central retinal artery with small emboli from atherosclerotic plaque of internal cerebral artery. Retina is a part of the brain, thus basically CRAO is corresponding to acute occlusion of intracerebral artery and retinal ischemia is to cerebral stroke. Therefore, intra-arterial thrombolysis (IAT) has been considered as a treatment method in CRAO. Recently, we treated 2 patients diagnosed as CRAO and could achieve complete recanalization on fundus fluorescein angiogram with IAT. Of them, one recovered visual acuity to 20/25. We report our 2 CRAO cases treated with IAT and discuss technical aspects for IAT and management of patient. To the best of our knowledge, this is the first Korean report of IAT for CRAO.
Keywords: Central Retinal Artery Occlusion, Thrombolysis
INTRODUCTION
Central retinal artery occlusion (CRAO) causes painless and sudden visual loss in the affected eye resulting in infarction of the retina with subsequent necrosis. It accounts for about 1/10,000 visits to ophthalmologists each year (1, 2). Generally, it has a very poor prognosis. A meaningful recovery of visual acuity occurred in only <10% of patient (3, 4). Since von Graefe first described the CRAO in 1859, various treatments have been advocated but there is none that has proven effective.
The pathophysiology of CRAO is similar with that of cerebral infarction. CRAO occurs by occlusion of the central retinal artery with small emboli from atherosclerotic plaque of internal carotid artery. Based on this principle, thrombolysis was suggested for restoring retinal blood flow in CRAO through dissolving the thromboemboli (5-10). We report our CRAO cases that were treated with intra-arterial thrombolysus (IAT). To our knowledge, this is the first case report in Korea.
CASE REPORTS
Case 1
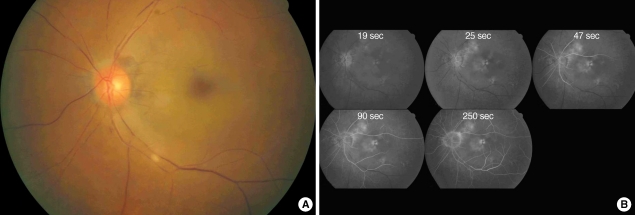
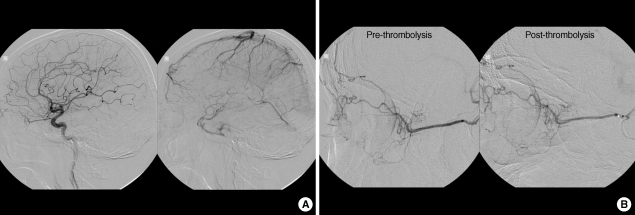
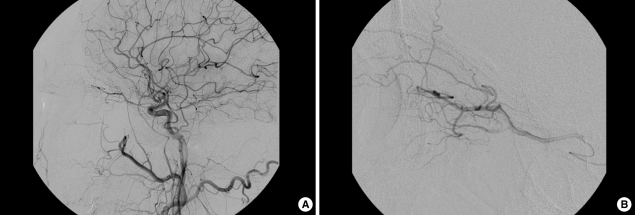
A 69-yr-old man with a history of hypertension and diabetes mellitus was transferred to our hospital for sudden visual loss in his left eye which had occurred 9 hr ago. In medical history, he had no ophthalmic problem and had maintained good visual acuity. On examination, vision was light-perception in left and 20/20 in right eye. Fundus examination revealed white edematous retina and typical "cherry-red spot" as well as vessel fragmentations suggesting CRAO (Fig. 1A). Perfusion of retina was markedly diminished with arteriovenous (AV) transit time of 5 min while choroidal perfusion was normal on fundus fluorescein angiography of left eye (Fig. 1B). There was no strabismus and ocular movement was normal. Intraocular pressure was 9 mmHg and slit lamp examination of anterior segment, lid, and orbit showed normal findings. Because the neurological examination showed no abnormality and he had not recently experienced any neurological symptoms, we skipped the brain imaging studies which were usually performed in the stroke patients. Ten hours after symptom onset, we performed cerebral angiography with biplane angiographic unit (Integris Allura; Philips Medical Systems, Eindhoven, Netherlands). There was no atherosclerotic lesion in distal carotid and proximal cervical internal carotid artery on left carotid angiogram. Internal carotid angiogram showed no occlusion of left ophthalmic artery up to distal segment and normal choroidal blush (Fig. 2A). We changed diagnostic catheter for 6F guiding catheter (Envoy; Cordis, Warren, New Jersey). On selective angiogram at ophthalmic artery using a microcatheter (Excelsior SL-10; Boston Scientifics, Natick, Massachusetts), there was no definite occlusion in branches including ciliary arteries and muscular branches from ophthalmic artery (Fig. 2B). We could not confirm the central retinal artery on angiogram. After the microcatheter was placed in proximal segment of the ophthalmic artery, urokinase (Green Cross pharmacy, Yongin, Korea) and Abxicimab (Reopro; Lilly, Indianapolis, Indiana) which are usually used for IAT in intracerebral artery occlusion were hand-injected slowly up to 400,000 units and 4 mg, respectively. Urokinase was administrated firstly for finbrinolysis and Abxicimab was added for preventing from further platelet aggregation. Their doses were reduced due to small size and territory of central retinal artery and for safety. Compared with before preoperative angiograms, post-IAT angiography did not show the definite changes (Fig. 2B). There was no procedure-related complication or event.
Fig. 1.
Fundus photography (A) and fluorescein angiography (B) before intra-arterial thrombolysis in case 1. There was white edematous retina with typical cherry-red spot and fragmentation of retinal vessels compatible with central retinal artery occlustion (A). Retinal arterial filling was markedly delayed with arterio-venous transit time of 5 min. The choroidal perfusion was normal (B).
Fig. 2.
Arterial phase and venous phase of left internal carotid angiogram (A) in case 1. Ophthalmic artery and chroidal blush were observed. Selective angiogram before and after intra-arterial thrombolysis (B). There was no definite occlusion in ciliary arteries and other muscular branches from ophthalmic artery. Intra-arterial thrombolysis was performed with urokinase and abxicimab.
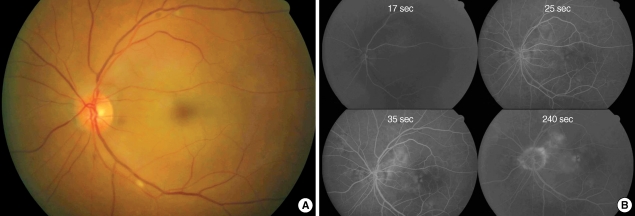
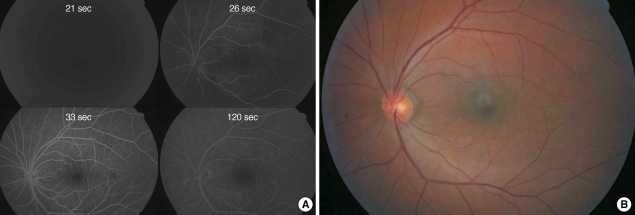
During the procedure, we could not find any improvement of retinal perfusion on fundus examination and the patient had no subjective improvement of vision. After IAT, anticoagulation was not done, systemically. One hour after thrombolysis, the patient experienced marked restoration of vision and visual field. At one day after thrombolysis, his visual acuity was hand motion while he could see 20/100 using his temporal visual field. The fundus fluorescein angiography showed normalized retinal arterial filling with AV transit time of 18 sec (Fig. 3). The b wave amplitude of maximal combined response of electroretinogram was 331.7 uV in his right eye and 250.2 uV in his left eye. However, he had severe macular edema on optical coherence tomography and showed no further improvement of vision thereafter. Because there was no newly developed neurologic sign implying brain lesions, the brain imaging study was not performed, additionally.
Fig. 3.
Fundus photography (A) and fluorescein angiography (B). One day after intra-arterial thrombolysis in case 1. Perfusion of retinal vessels were normalized and retinal color was restored to normal orange hue. However, there still remained macular edema (A). Retinal arterial and venous filling was normalized with arterio-venous transit time was 18 sec (B).
Case 2
A 47-yr-old man presented with sudden decrease of visual acuity in left eye for 10 hr. The best corrected visual acuity of his left eye was 20/70. On ophthalmic examination 1 month ago, he could have seen 20/20 with his left eye. Eight years ago, he had also experienced sudden visual loss in his right eye for CRAO. At that time, because he had visited at hospital 2 days after onset, he had not treated for visual loss and visual acuity was no light perception.
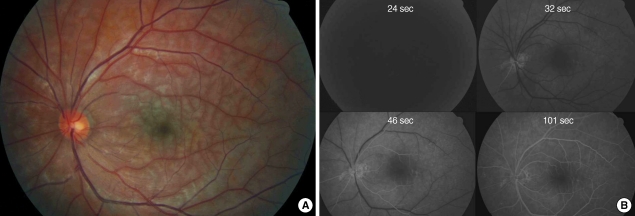
Fundus examination revealed edematous retina without fragmentation of vessels (Fig. 4A). On fundus fluorescein angiography, there was a delay in arterial filling and AV transit time was 70 sec (Fig. 4B). He was diagnosed as having CRAO and agreed to undergo IAT.
Fig. 4.
Fundus photogrphy (A) and fluorescein angiography (B) before intra-arterial thrombolysis in case 2. There is diffuse ischemic retinal edema throughout the entire fundus (A). The visual acuity was 20/70. There is delay in arterial filling and the arteriovenous transit time is 70 sec (B).
There was no steno-occlusive and remarkable lesion on carotid angiogram (Fig. 5A). The selective angiogram of ophthalmic artery showed a choroidal blush and no occlusion in its branches. A microcatheter was placed at the origin of ophthalmic artery and urokinase was hand-injected. During the injection, his visual acuity improved and retinal perfusion increased on fundus examination, so we stopped the IAT after the injection of 300,000 units of urokinase (Fig. 5B).
Fig. 5.
Common carotid angiogram in case 2 (A). There was no definite occlusion and remarkable lesion in ophthalmic artery and main trunk of intracerebral arteries. Selective angiogram of ophthalmic artery after intra-arterial thrombolysis (B). The muscular branches and ciliary arteries were well shown.
One day after IAT, his vision improved to 20/40 and fundus fluorescein angiography showed normal retinal perfusion with AV transit time of 12 seconds (Fig. 6A). Two weeks after IAT, his visual acuity improved to 20/25 and there was no more retinal edema (Fig. 6B).
Fig. 6.
Fluorescein angiography 1day after intra-arterial thrombolysis in case 2 (A). The retinal perfusion restored to normal and arteriovenous transit time is 12 sec. Fundus photography 2 weeks after thrombolysis (B). There is no more retinal edema suggesting previous ischemia. The vision improved to 20/25.
DISCUSSION
In results of meta-analysis of 158 IAT patients for CRAO (11), visual improvement occurred on average in 147 patients (93%), with 13% achieving 20/20 or better, 25% achieving 20/40 or better, and 41% achieving 20/200 or better. The overall complication rate was 4.5% (10/220), including 1 local hemorrhage, 5 transient ischemic attacks, 1 hypertensive crises, 1 intracerebral hemorrhage, and 2 strokes. This result suggested the benefit of IAT compared with natural history and conservative treatment of CRAO. The therapeutic time window for better outcome is unknown. Although some author suggested that there was no correlation between outcome and time to treatment (6), 6-12 hr of time window is generally accepted (12-14). Visual prognosis is often poor when thrombolysis is administered more than 20 hr after CRAO (12-16). In case 1, although we recanalized the central retinal artery within 12 hr after symptom onset, the improvement of functional visual outcome was limited. To our thought, the delay from symptom onset to procedure was too long to make significant functional improvement. In addition, the patient in case 1 could see better immediately after thrombolysis and his vision deteriorated after one night, which suggests that reperfusion injury on retinal neurons could be another cause of limited restoration. Interestingly, case 2 showed better visual outcome than 1, although the time to treatment was similar. The severity of retinal ischemia determined by pre-treatment visual acuity and degree of arterial occlusion by fundus angiography could be the major reason for better visual outcome in case 2. Therefore, we suggest that the better visual outcome might be achieved in cases with the shorter time window and with milder arterial obstruction and good pre-IAT visual acuity.
Even though we have experienced small number of cases of CRAO treated with IAT, the team approach appears to be critical in terms of managing CRAO patients. Ophthalmologists can confirm CRAO with fundus examination and fluorescein angiography and rule out other diseases which can make the sudden monocular blindness. In addition, visual outcome should be evaluated frequently by ophthalmologists before, during, and after IAT. The North American Symptomatic Carotid Endarterectomy Trial has shown the risk of transient monocular visual loss to be about 10% after 3 yr for patients with ipsilateral carotid artery stenosis (17, 18). Therefore, the patients with CRAO are likely to have the risk of future cerebral stroke. Although the risk of cerebral infarction is lower after retinal transient ischemic attack (TIA) than that after hemispheric TIA in this study (17), neurologists should plan the prevention strategies including control and treatment of stroke risk factors (19).
Compared to acute stroke, it is relatively easy to perform IAT for CRAO. The microcatheter with S-shaped distal tip is adequate for ophthalmic artery selection and maintenance of stability during procedures. The microwires used for routine intracerebral procedures which have ≤0.014" in diameter can be introduced into the main trunk of ophthalmic artery. Fibrinolytic and antiplatelet agents can be also chosen like as IAT for acute stroke. According to reports, the doses of urokinase and tPA were 100,000-1,200,000 units and 10-40 mg, respectively (11, 16). It should be usually adjusted on the basis of revascularization of the retinal arteries evaluated on ophthalmoscopic examination during procedures, but it is advisable that the maximum dose is reduced for safety compared with intracerebral stroke although the recanalization may be not confirmed during IAT. Mechanical thrombolysis using microwire with soft tip can be done in case of occlusion in main trunk of ophthalmic artery. Other author suggested that thrombolytics had to be administrated in a branch of external carotid artery in case of ipsilateral internal carotid artery occlusion or severe stenosis (16), but its efficiency remained unknown. The recanalization of central retinal artery is not often visualized due to small size on angiogram. Improvement of retinal perfusion even without recanalization of this artery can be achieved by making CRAO into branch retinal artery occlusion and augmentation of the flow to retina via anastomosis if it may recanalize other branches.
The detailed technical aspects, indications, and clinical efficacy of IAT for CRAO is still not fully elucidated. The EAGLE (European Assessment Group for Lysis in the Eye) (20), the first prospective randomized clinical trial evaluating the effect of IA tPA, is ongoing. The results of this study will be published after a few more years.
IAT might be a sole effective treatment for CRAO. Therefore, endovascular surgeons should have an interest in this disabling disease and participate in treatment. Technically, IAT for CRAO is more feasible than intracerebral artery occlusion but clinical significance is much different, so that it should be performed within appropriate range of safety.
Footnotes
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health, Welfare and Family Affairs, Republic of Korea (grant no: A06-0171-B51004-06N1-00040B).
References
- 1.Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol. 2005;140:376–391. doi: 10.1016/j.ajo.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 2.Rumelt S, Dorenboim Y, Rehany U. Aggressive systematic treatment for central retinal artery occlusion. Am J Ophthalmol. 1999;128:733–738. doi: 10.1016/s0002-9394(99)00359-1. [DOI] [PubMed] [Google Scholar]
- 3.Atebara NH, Brown GC, Cater J. Efficacy of anterior chamber paracentesis and Carbogen in treating acute nonarteritic central retinal artery occlusion. Ophthalmology. 1995;102:2029–2034. doi: 10.1016/s0161-6420(95)30758-0. [DOI] [PubMed] [Google Scholar]
- 4.Mueller AJ, Neubauer AS, Schaller U, Kampik A. Evaluation of minimally invasive therapies and rationale for a prospective randomized trial to evaluate selective intra-arterial lysis for clinically complete central retinal artery occlusion. Arch Ophthalmol. 2003;121:1377–1381. doi: 10.1001/archopht.121.10.1377. [DOI] [PubMed] [Google Scholar]
- 5.Arnold M, Koerner U, Remonda L, Nedeltchev K, Mattle HP, Schroth G, Sturzenegger M, Weber J, Koerner F. Comparison of intra-arterial thrombolysis with conventional treatment in patients with acute central retinal artery occlusion. J Neurol Neurosurg Psychiatry. 2005;76:196–199. doi: 10.1136/jnnp.2004.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beatty S, Au Eong KG. Local intra-arterial fibrinolysis for acute occlusion of the central retinal artery: a meta-analysis of the published data. Br J Ophthalmol. 2000;84:914–916. doi: 10.1136/bjo.84.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butz B, Strotzer M, Manke C, Roider J, Link J, Lenhart M. Selective intraarterial fibrinolysis of acute central retinal artery occlusion. Acta Radiol. 2003;44:680–684. doi: 10.1080/02841850312331287829. [DOI] [PubMed] [Google Scholar]
- 8.Framme C, Spiegel D, Roider J, Sachs HG, Lohmann CP, Butz B, Link J, Gabel VP. Central retinal artery occlusion. Importance of selective intra-arterial fibrinolysis. Ophthalmologe. 2001;98:725–730. doi: 10.1007/s003470170079. [DOI] [PubMed] [Google Scholar]
- 9.Kattah JC, Wang DZ, Reddy C. Intravenous recombinant tissue-type plasminogen activator thrombolysis in treatment of central retinal artery occlusion. Arch Ophthalmol. 2002;120:1234–1236. [PubMed] [Google Scholar]
- 10.Schmidt DP, Schulte-Monting J, Schumacher M. Prognosis of central retinal artery occlusion: local intraarterial fibrinolysis versus conservative treatment. AJNR Am J Neuroradiol. 2002;23:1301–1307. [PMC free article] [PubMed] [Google Scholar]
- 11.Noble J, Weizblit N, Baerlocher MO, Eng KT. Intra-arterial thrombolysis for central retinal artery occlusion: a systematic review. Br J Ophthalmol. 2008;92:588–593. doi: 10.1136/bjo.2007.133488. [DOI] [PubMed] [Google Scholar]
- 12.Plant GT, Landau K. Thrombolysis for central retinal artery occlusion. J Neurol Neurosurg Psychiatry. 2005;76:160–161. doi: 10.1136/jnnp.2004.045583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayreh SS. Retinal arterial occlusion with LIF using rTPA. Ophthalmology. 1999;106:1236–1239. doi: 10.1016/S0161-6420(99)10101-5. [DOI] [PubMed] [Google Scholar]
- 14.Schumacher M, Schmidt D, Wakhloo AK. Intra-arterial fibrinolytic therapy in central retinal artery occlusion. Neuroradiology. 1993;35:600–605. doi: 10.1007/BF00588405. [DOI] [PubMed] [Google Scholar]
- 15.Rumelt S, Brown GC. Update on treatment of retinal arterial occlusions. Curr Opin Ophthalmol. 2003;14:139–141. doi: 10.1097/00055735-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Biousse V, Calvetti O, Bruce BB, Newman NJ. Thrombolysis for central retinal artery occlusion. J Neuroophthalmol. 2007;27:215–230. doi: 10.1097/WNO.0b013e31814b1f66. [DOI] [PubMed] [Google Scholar]
- 17.Benavente O, Eliasziw M, Streifler JY, Fox AJ, Barnett HJ, Meldrum H. Prognosis after transient monocular blindness associated with carotid-artery stenosis. N Engl J Med. 2001;345:1084–1090. doi: 10.1056/NEJMoa002994. [DOI] [PubMed] [Google Scholar]
- 18.Streifler JY, Eliasziw M, Benavente OR, Harbison JW, Hachinski VC, Barnett HJ, Simard D. The risk of stroke in patients with first-ever retinal vs hemispheric transient ischemic attacks and high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Arch Neurol. 1995;52:246–249. doi: 10.1001/archneur.1995.00540270034016. [DOI] [PubMed] [Google Scholar]
- 19.Biousse V, Trobe JD. Transient monocular visual loss. Am J Ophthalmol. 2005;140:717–721. doi: 10.1016/j.ajo.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Feltgen N, Neubauer A, Jurklies B, Schmoor C, Schmidt D, Wanke J, Maier-Lenz H, Schumacher M. Multicenter study of the European Assessment Group for Lysis in the Eye (EAGLE) for the treatment of central retinal artery occlusion: design issues and implications. EAGLE Study report no. 1: EAGLE Study report no. 1. Graefes Arch Clin Exp Ophthalmol. 2006;244:950–956. doi: 10.1007/s00417-005-0140-2. [DOI] [PubMed] [Google Scholar]