Abstract
Mast cells play a central role in the initiation and development of allergic diseases through release of various mediators. Tryptase has been known to be a key mediator in mast cell-mediated inflammatory reactions. In the present study, we investigated whether the transcription of tryptase gene in human mast cells was induced by microphthalmia (mi)-associated transcription factor (MITF). We observed that the human CD34+ progenitor-derived cultured mast cells and human mast cell line HMC-1 expressed strongly the transcripts of tryptase-beta1 and MITF-A, which is a MITF alterative splicing isoform. The transcriptional activity of tryptase gene was specifically higher in HMC-1 cells compared to the tryptase-negative cells. Using mutant constructs of tryptase promoter, we observed that two E-box (CANNTG) motifs including between -817 to -715 and -421 to -202 are able to involve in the transactivation of tryptase gene by MITF-A. In addition, the binding of these motifs-containing oligonucleotides to MITF proteins was detectable by EMGA using the nuclear extracts of HMC-1 cells and anti-MITF mAb. The overexpression of MITF-A elevated tryptase production by HMC-1 cells, while the introduction of specific siRNA against MITF attenuated the expression and enzymatic activity of tryptase. These data suggest that MITF might play a role in regulating the transcription of tryptase gene in human mast cells.
Keywords: alternative splicing, gene expression regulation, mast cells, microphthalmia-associated transcription factor, tryptases
Introduction
Mast cells play an important role as the primary responders in immediate-type hypersensitivity reactions, orchestrating strong responses to allergens (Galli, 2000; Galli et al., 1984). Mast cells are developed from CD34+ hematopoietic progenitors found in peripheral blood and bone marrow (Kitamura et al., 1989). In humans, the mast cells have been distinguished to two types on the basis of unique natural proteases expression; the mucosal mast cells (MCT) defined as those containing tryptase alone, and the connective tissue mast cells (MCTC) as those containing both tryptase and chymase. Tryptase is one of reliable markers of mast cell degranulation and comprised in a family of trypsin-like serine proteases (Irani et al., 1986; Sato et al., 1997). Human tryptase has the antigenic and enzymatic properties resulting in the proteolytic activation by cleavage of the N-terminus of protease-activated receptor 2 (PAR-2) (Irani et al., 1992).
The expression of tryptase in mast cells is influenced not only by extracellular factors, including cytokines and tissue environments, but also by intracellular factors such as the transcription factors. It has been known well that this expression in human is increased by incubation in vitro with recombinant human stem cell factor (rhSCF) and IL-6 (Kirshenbaum et al., 1991; Levi-Schaffer et al., 2003). However the transcriptional mechanism of tryptase gene in human mast cells remains to be determined.
Microphthalmia-associated transcription factor (MITF) is a member of the basic/helix-loop-helix/leucine zipper (b-HLH-Zip) protein of transcription factors (Hemesath et al., 1994). The b-HLH-Zip proteins including MITF, TFE3, TFEB and TFEC recognize the E-box (CANNTG) motifs in the promoter region of target genes. To date, nice isoforms of MITF have been found in human, they are called MITF-A, -B, -C, -D, -E, -H, -M, and -J. For most of isoforms, the initial exon is spliced onto the later part of exon 1 and then to the common exons 2-9, which encode the functionally important motifs, including b-HLH-Zip, transactivation domains, and various phosphorylation consensus sequences (Hershey and Fisher, 2005). The recent studies have reported that the peritoneal and cultured mast cells from mice expressed several isoforms, such as MITF-A, -E, -H, and -Mc (Oboki et al., 2002; Takemoto et al., 2002). We previously have demonstrated that MITF-A, -E, and -Mc were strongly expressed in the bone marrow-derived mast cells cultured in the presence of IL-3, but MITF-H and -J were slightly detected (Lee et al., 2008). Most of MITF isoforms has similar transcriptional activity on the expression of mouse mast cell-specific protease 6 (MMCP-6), which is a major trypsin-like serine protease in mice. In particular, SCF-stimulated mast cells expressed MITF-A, but not other isoforms. However, the expression pattern of MITF isoforms and their transcriptional activity for tryptase gene in human mast cells remains to be studied.
In the present study, we examined the isoforms of MITF preferentially expressed in the human cultured mast cells and HMC-1 cells. We also investigated the involvement of MITF isoforms on the transactivation of tryptase gene.
Results
Expression of MITF-A and tryptase-beta1 in the human mast cells
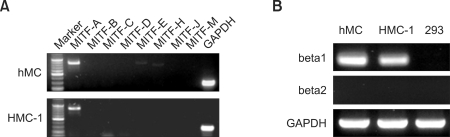
We examined the mRNA expression of MITF isoforms in the human CD34+ progenitor derived mast cells (hMCs) and HMC-1 cells using the specific primers shown in Supplemental Data Table S1. Total RNAs extracted from hMCs and HMC-1 cells were used to synthesize cDNA by RT-PCR. Both hMCs and HMC-1 cells strongly expressed the transcript of MITF-A. The transcripts of MITF-E and -H were expressed weakly in HMC-1 cells, but not detected in hMCs (Figure 1A).
Figure 1.
Expression of MITF isoforms and beta-tryptase genes in HMC-1 cells and hMC. Total RNA was extracted from HMC-1, hMC and 293 cells as a tryptase-negative cell line. The transcripts of MITF isoforms (A) and tryptase beta1 and beta 2 (B) were analyzed by RT-PCR. PCR products were electrophoresed in 1% agarose gel.
Several tryptase genes are clustered on chromosome 16p13.3. Tryptase-beta is the main trypsin-like serine proteases expressed in mast cells, whereas tryptase-alpha predominate in basophiles (Galli, 2000). We thus examined the expression of tryptase-beta in the human mast cells, HMC-1 cells and the hMCs, by RT-PCR. The transcript of tryptase-beta1 was remarkably expressed in both hMCs and HMC-1 cells, but tryptase-beta2, a alterative splicing isoform, was not detected (Figure 1B). In addition, tryptase beta1 was negative in 293 cells, non-mast cell line. The expression level of tryptase-beta1 mRNA in hMCs was higher than that in HMC-1 cells.
The transactivation of tryptase gene in the human mast cells
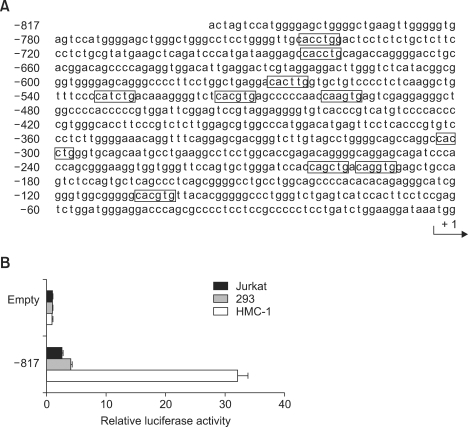
To examine the transcription of tryptase gene in the human mast cells, we cloned the 5'-upstream region of the tryptase beta1 gene, as shown in Figure 2A, and constructed the reporter plasmid which contains the promoter of tryptase gene starting from -817 on the upstream of luciferase gene. After the transfection of reporter plasmid or empty vector into tryptase-positive HMC-1 cells and tryptase-negative cell lines, Jurkat and 293 cells, the transcription of tryptase gene was assayed by the luciferase activity assay. The luciferase activity of reporter plasmid in HMC-1 cells was increased over 30 fold compared to that of empty control vector. In addition, the luciferase activity was strongly upregulated in HMC-1 cells, but not Jurkat and 293 cells (Figure 2B).
Figure 2.
The transcriptional activity of beta tryptase gene in human mast cell line HMC-1. (A) The nucleotide sequence of 5' flanking region of the tryptase gene. The CANNTG motif was boxed. A part of the first exon is shown by capitals, and the 5' flanking region is shown by lower case. The transcription initiation site was numbered as + 1. (B) The reporter plasmids (0.5 µg) were transfected into HMC-1 by electroporation, but they (1 µg) were into Jurkat, and 293 cells by transfection reagent. Luciferase activity assay was performed as described in the Methods. Data revealed as the relative luciferase activity divided by a value from the empty plasmid.
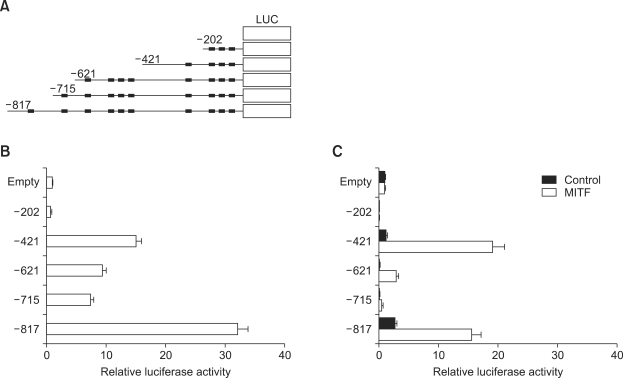
To examine the main elements for transactivation in promoter region of the tryptase-beta1 gene, we synthesized the deletion mutant constructs starting from -817, -715, -621, -421 or -202 of tryptase promoter by PCR amplification (Figure 3A) and then their reporter plasmids were transfected into HMC-1 cells. When the -817 construct was transfected, the luciferase activity was the highest (over 30-fold) compared to that of empty control. The -421 construct revealed the 15-fold increase in luciferase activity. However, the luciferase activity from -202 construct was compareable to that of empty control (Figure 3B).
Figure 3.
The transcriptional activity of deletion mutants in 5' flanking region of beta tryptase gene in the HMC-1 and Jurkat cells. (A) The tryptase promoter region (-817~ + 24) was obtained from the genomic DNA of HMC-1 by PCR. The deleted constructs of beta-tryptase promoter were synthesized and inserted into upstream of luciferase gene in pGL3-basic plasmid as reporter for transcriptional activity assay. (B) The reporter plasmids (0.5 µg) were transfected into HMC-1 cells. (C) The reporter plasmids (0.5 µg) were cotransfected to Jurkat cells with or without MITF expression vector (0.5 µg). The luciferase activity assay was performed as described in the Methods. Data revealed as the relative luciferase activity divided by the value of the empty reporter (pGL3- basic).
We also examined whether overexpression of MITF-A regulates the transactivation of the tryptase promoter in Jurkat cells, which does not express both tryptase and MITF (Figure 3C). All of deletion mutant constructs introduced without MITF-A into the Jurkat cells showed only a low level of luciferase activities. When the -817 and -421 constructs were cotransfected with MITF-A expression vector, the luciferase activity was remarkably increased to over 15-fold and 19-fold, respectively, compared to that of empty control. In contrast, nt -715, -621 and -221 constructs showed no effect on the upregulation of luciferase activity by MITF-A. These data indicate that the MITF-dependent elements might be conserved between -817 to -715 and -421 to -202 in promoter region of the tryptase-beta1 gene.
Transcription of tryptase gene through MITF binding sites
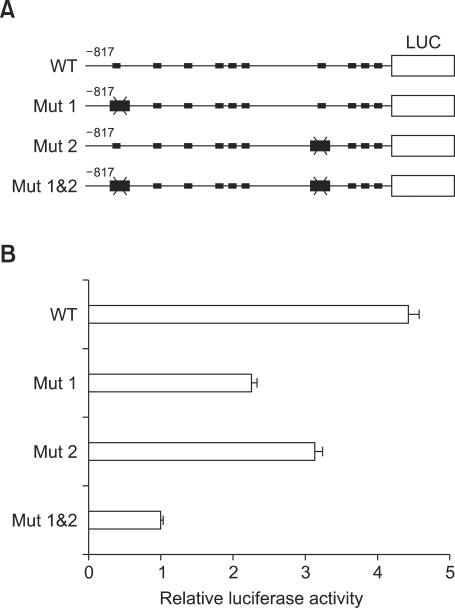
As MITF is a family of the bHLH-Zip proteins recognizing the CANNTG motif, we examined the MITF binding sites on the transcription of tryptase gene in human mast cells. Although there are ten CANNTG motifs in the tryptase promoter region to -817, two CACCTG motifs were found in two regions between -817 to -715 and -421 to -202 and selected as potential transactivating sites based on the luciferase activity of the deletion constructs of tryptase gene. To examine the involvement of two CACCTG motifs on the transcription of tryptase gene in the mast cells, the CACCTG motifs were changed to CTCCAG by PCR using each pair of primers. Thus we prepared three mutation constructs, Mut 1, Mut 2, and Mut 1&2, in the tryptase promoter starting from -817 and transfected them into the HMC-1 cells (Figure 4A). Luciferase activity was significantly decreased in the transfectants of single mutation construct Mut 1 or Mut 2 compared to that Wilde type reporter plasmid. In addition, the transcription activity was dramatically abolished in the transfectants of double mutation construct Mut 1&2 (Figure 4B), suggesting that both CACCTG motifs play a key role in the transcription of tryptase gene in the mast cells.
Figure 4.
Regulation of tryptase transcriptional activity via CACCTG motifs (E-boxes). (A) Diagrams show point mutations of MITF-binding sites in the tryptase promoter constructs used for the luciferase activity assay. (B) To examine beta-tryptase promoter activity via two E-boxes, mutations of MITF-binding sites were constructed by PCR and inserted into upstream of luciferase gene in pGL3-basic plasmid. The reporter plasmids were introduced into HMC-1 cells, and the cells were incubated for 40 h before luciferase activity assay.
Binding activity of MITF-A to CACCTG motifs in the promoter region of tryptase gene
To examine whether the MITF-A protein practically binds to two CACCTG motifs conserved in the promoter region (-817 to -715 and -421 to -202) of tryptase gene, we extracted the nuclear proteins from HMC-1 cells which express MITF-A protein. The DNA binding activity of nuclear extracts was performed by EMSA using the oligonucleotides containing CACCTG motifs. The oligonucleotides used were synthesized as shown in Figure 5, and WT-1 oligonucleotide (probe 1: the hexametric motif shown in the box) and WT-2 oligonucleotide (probe 2) were labeled as the probe. Several DNA-protein complexes were formed in both probes after the reaction with the nuclear extracts. In particular, most of DNA-protein complexes were supershifted by addition of anti-MITF antibody in both reaction using probe 1 and probe 2. Both DNA-binding activities were abolished by addition of the excess non-labeled oligonucleotide, as a competitor, but not that of mutant oligonucleotide.
Figure 5.
Binding activity of MITF extracted from human mast cells to CACCTG motifs in the 5 flanking region of the tryptase gene. The nuclear extracts purified from HMC-1 cells were incubated with biotinylated Oligo-1 (WT-1) or -3 (WT-2) containing the potential MITF-binding site as probe for EGMSA. After electroporesis, DNA-protein complex was transferred to nylon membrane and blotted with avidin-HRP for visualization. CACCTG motifs of WT-1 and -2 were modified as shown in Mut-1 and -2 for competitive DNA binding assay, respectively, and anti-MITF mAb was added for supershift, as described in the Methods.
The increased expression of tryptase by overexpression of MITF-A
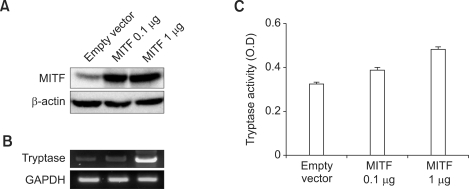
To examine the direct effect of MITF-A protein on the expression of tryptase mRNA and protein in mast cells, we transfected the MITF-A expression vector (0.1 or 1 µg) into the HMC-1 cells and analyzed the level of MITF protein by western blot. The expression of MITF protein was slightly detected in empty vector-introduced transfectants and markedly increased by the transfection of MITF-A vector (Figure 6A). In addition, the increased level of MITF protein enhanced simultaneously the expression of tryptase mRNA in a dose dependent manner (Figure 6B). The enzymatic activity of tryptase also was significantly elevated by the overexpression of MITF in the mast cells (Figure 6C).
Figure 6.
The increased expression of tryptase by MITF overexpression in HMC-1 cells. (A) After transfection of MITF-A (0.1 or 1 µg) or empty expression vector (1 µg) into HMC-1 cells, the expression of MITF protein were analyzed by western blot analysis using anti-human MITF monoclonal antibody. (B) The expression of tryptase mRNA was analyzed using RT-PCR, and (C) tryptase enzymatic activity was measured using a substrate tosyl-gly-pro-lys-pNA (Sigma) using precision microplate reader, as described in the Methods.
The decreased expression of tryptase by MITF depletion
To examine whether the depletion of MITF-A protein influences the expression of tryptase mRNA and protein in the mast cells, we introduced MITF-specific siRNA (0.2 or 1 µg) or control siRNA (1 µg) into HMC-1 cells. We observed that the expression of MITF protein was significantly reduced by MITF siRNA in a dose-dependent manner, but not control siRNA (Figure 7A). The mRNA expression of tryptase-beta1 also was decreased by transfection of MITF siRNA, but not control siRNA (Figure 7B). In addition, the enzymatic activity of tryptase subsequently was reduced by depletion of MITF in the mast cells (Figure 7C).
Figure 7.
The decreased expression of tryptase by MITF depletion in HMC-1 cells. After transfection of human MITF-A specific siRNA (1 or 0.2 µg) or control siRNA (1 µg) into HMC-1 cells, (A) the depletion of MITF were analyzed by western blot analysis, and (B) the expression of tryptase mRNA and (C) tryptase enzymatic activity were measured as described in the Methods.
Discussion
Human tryptases comprise a family of trypsin-like serine proteinases that are encoded by the alleles of these genes located on chromosome 16p13.3. Tryptases identified at the cDNA exhibit a various sequence variation to be divided into three groups, such as alpha, beta, and gamma showing a high degree of sequence identity. Tryptase-beta has been known to be a major variant expressed in mast cells, whereas tryptase alpha predominates in basophiles. In addition, tryptase-bata also includes the variant isoforms, beta1 and beta2 (Galli, 2000, Sommerhoff, 2001). However, the exact variant isoforms of tryptase-bata in human mast cells were unclearly known. In this study, we showed that hMCs and HMC-1 cells expressed tryptase-beta1 as the major isoenzyme. Although the mast cells has been known to express all of them, our result implicates that tryptase-beta1 might be a main type stored in mast cell secretory granules.
MITF has been reported to regulate the development of mast cells as well as melanocytes in mice (Hemesath et al., 1994). In the recent study, the expression of the mMCP-6 gene, which is the main trypsin-like serine proteinases detectable in mice, was deficient in MITF-mutant mice-derived cultured mast cells (Morri et al., 1996). The overexpression of wild type MITF but not mutant MITF was able to transactivate the mMCP-6 promoter through binding to GACCTG and CANNTG motifs, implicating that MITF plays an essential role in the transcription of mMCP-6 (Pejler et al., 2007). In the previous study, we demonstrated that MITF-A is a major isoform that regulates mMCP-6 expression in the mice-derived cultured mast cells (Lee et al., 2008). As reported in mice, our result showed that the human mast cells expressed strongly MITF-A. Moreover, the transcription of tryptase gene was inducible specifically by the mast cells that express MITF-A, but not by non-mast cells. The expression of tryptase in the mast cells also was increased by overexpression of MITF-A, while MITF depletion reduced the expression of tryptase. It implies that MITF-A probably is an important transcription factor to regulate the expression of tryptase by the human mast cells, as well as mice.
The mi locus of mice encodes a member of bHLH-Zip protein family of transcription factors called as mi transcription factor (MITF). The basic domain permits MITF proteins to bind to the CANNTG motifs (E-box) (Hodgkinson et al., 1993; Hughes et al., 1993; Eirikur et al., 1994). When the deleted reporter plasmids were transfected into HMC-1 cells, -817 reporter plasmids appeared to induce the highest luciferase activity compared to other reporter. In addition, the luciferase activity in MITF- and tryptase-negative Jurkat cells was increased when -817 and -421 reporter plasmids were cotransfected with MITF. We identified two CACCTG motifs between -817 to -751 and -421 to -221 in the promoter region of tryptase gene. Their mutation in -817 reporter plasmids significantly reduced the luciferase activity in HMC-1 cells, even through double mutation didn't showed complete abolishment of it. These results indicate that two CACCTG motifs are necessary for the transcription activity of tryptase gene. EMSA also showed that MITF proteins contain in the nuclear extracts isolated from mast cells, and they could form DNA-binding complexes through CACCTG motifs in the promoter region of tryptase gene. However, the deletion constructs starting from -751 and -621 showed the silence on MITF-induced transactivation of tryptase gene in tryptase-negative Jurkat cells. It suggests that the inhibitory molecules expressing in tryptase-negative cells may interact some motifs conserved between -751 to -421 in promoter region. The inhibitory molecules and binding motifs of tryptase tansactivation remain to be studied.
Tryptase has been known to regulate allergic and inflammatory responses, including recruitment of neutrophils, Th2 cells, and basophils (Lee et al., 1998; Pejler et al., 2007). The cultured mast cells derived from bone marrow of transgene-insertional vga9/vga9 mutant mice, which do not express MITF, showed abnormal phenotypes including deficiencies in a various gene expression, including mMCP-4, -6, -7, granzyme B, prostaglandin D2, and tryptophan hydroxylase (Hodgkinson et al., 1993; Ito et al., 1998; Jippo et al., 1999; Ogihara et al., 2001; Mori et al., 2004). However, we can not exclude the involvement of other transcriptional mechanism by MITF-A to induce the expression of human tryptase gene compared to that of MMCP-6 gene expressing in the mouse connective tissue mast cells. Most of human mast cells express abundant tryptase, and the promoter region of tryptase gene showed no apparent homology with that of MMCP-6. MITF has been reported to interact with other transcription factors, TFE3, TFEB and TFEC, all of which belong to the bHLH-Zip family, through the HLH-Zip domains (Eirikur et al., 1994). The region aa 123-127 of MITF was important for the PEBP2 interaction (Sato et al., 1997). In melanocytes, the transcriptional coactivator CBP/p300 selectively associates with MITF to coactivate the transcriptional function of MITF (Hemesath et al., 1998; Price et al., 1998). Therefore, it is possible that the other transcriptional factor might associate with MITF-A for the transcription of human tryptase gene.
In conclusion, our data showed that MITF-A is a major isoform in the human mast cells and plays an important role in regulating the transcription of tryptase beta 1 gene through binding to two CACCTG motifs in the promoter region, suggesting that MITF-A is an essential factor in the expression of human mast cell tryptase. However the progressive studies are required to demonstrate MITF-associating coactivators regulating the restricted transcription of beta-tryptase genes in the human mast cells. Moreover, the regulating mechanism of MITF-A transcription in the mast cells remains to be studied. Such identification would promote further analysis of the development of mast cells and would be useful in evaluating the physiological roles of mast cell-derived proteases.
Methods
Cells and reagents
The isolation of human CD34+ progenitor cells was performed by EasySep human CD34+ selection cocktail (StemCell Tech., Vancouver, Canada) from human umbilical cord blood collected in tubes containing 10 U/ml heparin. Human Mast cells were developed from CD34+ cells cultured with Iscove's Modified Dulbecco's Medium (IMDM; GIBCO BRL), supplemented with 100 ng/ml rhSCF (ATGen, Gyeonggi-do, KOREA ), 50 ng/ml IL-6 (R&D sytems, Minneapolis, MN), 10% FBS (Hyclone, Logan, UT), 50 µM 2-mercaptoethanol and 100 U/ml penicillin/streptomycin up to 8 weeks in 5% CO2 at 37℃ in humidified atmosphere. Half of the media was exchanged every 2 days during the differentiation of mast cells. Mast cells were identified by toluidine blue staining and the purity was more than 95%. HMC-1 cells were maintained in IMDM containing 10% FBS and 100 U/ml penicillin/streptomycin. Jurkat and 293 cells were cultured in DMEM and RPMI1640 containing 10% FBS, respectively. Other reagents were purchased from Sigma (St. Louis, MO).
Reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was extracted from the cells using Trizol reagent (Invitrogen, Carlsbad, CA). For cDNA synthesis, RNA was reverse-transcribed with the superscript One-Step RT-PCR kit (Invitrogen) for 1 h at 42℃ according the manufacturer's instruction. To examine the expression levels of the genes in the human mast cells, PCR was done with the platinum high fidelity Taq DNA polymerase system using a pair of primers as shown in Supplemental Data Table S1. PCR amplification was performed as follows: denaturation (94℃, 30 s), annealing (58℃, 30 s), and extension (72℃, 50 s). The PCR products were electrophoresed on 1.2% agarose gel in 1×TAE buffer and photographed on UV lamp.
Construction of the expression and reporter plasmids
Full length cDNA of MITF-A was synthesized from total RNA extracted from HMC-1 cells by RT-PCR using the following pair of primers and then inserted into the EcoRI site of the pcDNA3.1(+) mammalian expression vector (Invitrogen). All sequences of MITF-A cDNA were identified by sequencing analysis. For the reporter plasmids, the tryptase-beta1 promoter region between nt -1557 and nt +4 was synthesized by PCR using the genomic DNA extracted from HMC-1 cells. DNA fragments containing a promoter region of tryptase-beta1 gene were cloned into the upstream of luciferase gene in pGL3-basic plasmid (Promega, Madison, WI). The deletion mutants of tryptase promoter region were prepared by PCR using each pair of primers. The point mutation of the selective consensus motifs was generated using a Quick Change II site-Direct Mutagenesis kit (Stratagene, La Jolla, CA) with mismatch sense and antisense primers as following; Mut 1, 5'-GGCAGCCAGGCCTCCAGGGTGCAGCAAT-3' (-306 to -279); Mut 2, 5'-TCCTGGGGTTGCTCCAGCACTCCTCTCT-3' (-747 to -719). The deleted and mutated constructs were verified by DNA sequencing.
Transfection and luciferase activity assay
The MITF-A expression vector (0.5 or 1 µg) and reporter plasmid (0.5 or 1 µg) were transfected into HMC-1 (2 × 105 in 6 cm dish) and Jurkat cells (5 × 105 in 6 cm dish) by electroporation using MicrOperator (Soulin Bio, Seoul, Korea) according the manufacturer's instruction. The trans-LT1 transfection reagent (Mirus, Pittsburgh, PA) was used to tansfect them into 293 cells (5 × 105 in 6 cm dish). After incubation for 24 h, the cells were harvested and lysed with luciferase lysis reagent (Promega). The soluble supernatants were separated by centrifugation at 12,000 × g for 20 min and used to determine the luciferase activity using the luminometer LB96P (Berthold GmbH, Wilbad, Germany). Luciferase activity was relatively normalized by β-galactosidase and total protein concentration. Each data were shown as relative values calculated by that of a control expression vector.
Western blot analysis
To selectively silence human MITF gene expression in HMC-1 cells, MITF siRNA (Santa Cruz Biotech, CA) or the negative control siRNA was transfected into HMC-1 cells using MicrOperator. After the incubation for 48 h, the cells were lysed in ice-cold protein extraction buffer (iNtRON Biotech, Gyeonggi-do, Korea) for 20 min and centrifuged to separate the soluble supernatants. Their protein concentrations were measured using a bicinchoninic acid. Cell lysates (25 µg protein per lane) were separated by 10% SDS-PAGE and transferred to PVDF membranes. After blocking with 5% skimmed milk for 1 h, the membrane was blotted with anti-MITF mAb (Abcam, Cambridge, Massachusetts) overnight at 4℃, washed with 1×TBS containing 0.1% Tween 20, and incubated with HRP-conjugated secondary antibody for 45 min. Finally, proteins were visualized using an enhanced chemiluminescence (Amersham Pharmacia Biotech, CA).
Tryptase activity assay
Tryptase activity was measured to determine tryptase production in the mast cells. Cells were homogenized in 10 volumes of 20 mM Na-phosphate buffer (pH 7.4). After centrifugation, the supernatants were used as the extract containing tryptase. The enzyme activity of tryptase was determined using the substrate tosyl-gly-pro-lys-pNA (Sigma) containing 50 µg/ml heparin. Mixture of sample and reaction buffer was incubated for 2 h at 37℃, and the optical density was measured at 405 nm in microplate reader.
Electrophoretic mobility gel shift assay (EMSA)
The cells were washed twice with ice-cold PBS and suspended in 500 µl of hypotonic buffer (10 mM HEPES pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 1 mM EDTA, 1 mM DTT, and 0.5 mM PMSF). After swelling of cells for 15 min on ice, 30 µl of 10% NP-40 solution was added to shake for 5 min. The mixture was then centrifuged at 3000 rpm for 2 min and the pellets of nuclei were washed once with 500 µl of PBS before suspending with 65 µl of buffer B (20 mM HEPES pH 7.9, 25% glycerol, 400 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.2 mM EGTA, 1 mM DTT) for 30 min on ice. After centrifugation for 15 min at 15000 rpm, the supernatant containing nuclear proteins was stored -70℃ until the experiments. The pairs of unique oligonucleotides comprising wild type or mutant in CANNTG sites were annealed for DNA binding assay. The probe was labeled with Biotin-14-CTP by BIOPRIME DNA labeling system (Invitrogen). The labeled DNA probe (25 ng) was mixed with the nuclear extract (10 µg of protein) in a 20 µl reaction mixture containing 10 mM Tris-HCl (pH 8.0), 1 mM EDTA, 75 mM KCl, 1 mM DTT, 4% Ficoll type 400, and 2 µg/ml of poly (dI-dC). The verification of specificity and identity of MITF were carried out through the competition with mutant oligonuclotide and supershift assays with anti-MITF antibody, respectively. After incubation at room temperature for 15 min, the reaction mixture was subjected to electrophoresis on a 5% polyacrylamide gel in 0.25 × TBE buffer (pH 8.3) and transferred to nylon transfer membrane. The membrane was incubated with blocking buffer (1 × TBS buffer, 0.5% gelatin, 5% SDS, 0.05% Tween 20) for 1 h and than subsequently with HRP-conjugated streptavidin (ZyMED, San Francisco, CA) for 40 min at room temperature. After washing with TBS containing 0.1% Tween 20, DNA-protein complexes were visualized using an enhanced chemiluminescence.
Statistical analysis
Data from the experiments were described to mean ± S.E.M. Statistical significance was determined using the Student's t-test to express the difference between groups. All p-values <0.05 were considered to reflect a statistically significant difference.
Supplemental data
Supplemental Data include one table and can be found with this article online at http://e-emm.or.kr/article/article_files/SP-42-5-06.pdf.
Acknowledgments
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD)"(KRF-2006-E00103).
Abbreviations
- hMCs
human CD34+ progenitor-derived mast cells
- MITF
microphthalmia-associated transcription factor
- SCF
stem cell factor
Supplementary Material
Supplemental Data
References
- 1.Eirikur S, Karen JM, Lynn L, Adrian RF, Stephen KB, Debra CSZ, Loren CS, Colin AH, Heinz A, Neal GC, Nancy AJ. Molecular basis of mouse microphthalmia (mi) mutations helps explain their developmental and phenotypic consequences. Nat Genet. 1994;8:256–263. doi: 10.1038/ng1194-256. [DOI] [PubMed] [Google Scholar]
- 2.Galli SJ. Mast cells and basophils. Curr Opin Hematol. 2000;7:32–39. doi: 10.1097/00062752-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Galli SJ, Dvorak AM, Dvorak HF. Basophils and mast cells: Morphologic insights into their biology, secretory patterns, and function. Prog Allergy. 1984;34:1–141. [PubMed] [Google Scholar]
- 4.Hemesath TJ, Steingrímsson E, McGill G, Hansen MJ, Vaught J, Hodgkinson CA, Arnheiter H, Copeland NG, Jenkins NA, Fisher DE. Microphthalmia, a critical factor in melanocyte development, defines a discrete transcription factor family. Genes Dev. 1994;8:2770–2780. doi: 10.1101/gad.8.22.2770. [DOI] [PubMed] [Google Scholar]
- 5.Hemesath TJ, Price ER, Takemoto C, Badalian T, Fisher DE. MAP kinase links the transcription factor Microphthalmia to c-kit signalling in melanocytes. Nature. 1998;391:298–301. doi: 10.1038/34681. [DOI] [PubMed] [Google Scholar]
- 6.Hershey CL, Fisher DE. Genomic analysis of the Microphthalmia locus and identification of the MITF-J/Mitf-J isoform. Gene. 2005;347:73–82. doi: 10.1016/j.gene.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Hodgkinson CA, Moore KJ, Nakayama A, Steingrímsson E, Copeland NG, Jenkins NA, Arnheiter H. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993;74:395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- 8.Hughes MJ, Lingrel JB, Krakowsky JM, Anderson KP. A helix-loop-helix transcription factor-like gene is located at the mi locus. J Biol Chem. 1993;268:20687–20690. [PubMed] [Google Scholar]
- 9.Irani AA, Schechter NM, Craig SS, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA. 1986;83:4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irani AM, Nilsson G, Miettinen U, Craig SS, Ashman LK, Ishizaka T, Zsebo KM, Schwartz LB. Recombinant human stem cell factor stimulates differentiation of mast cells from dispersed human fetal liver cells. Blood. 1992;80:3009–3021. [PubMed] [Google Scholar]
- 11.Ito A, Morii E, Maeyama K, Jippo T, Kim DK, Lee YM, Ogihara H, Hashimoto K, Kitamura Y, Nojima H. Systematic method to obtain novel genes that are regulated by mi transcription factor: impaired expression of granzyme B and tryptophan hydroxylase in mi/mi cultured mast cells. Blood. 1998;91:3210–3221. [PubMed] [Google Scholar]
- 12.Jippo T, Lee YM, Katsu Y, Tsujino K, Morii E, Kim DK, Kim HM, Kitamura Y. Deficient transcription of mouse mast cell protease 4 gene in mutant mice of mi/mi genotype. Blood. 1999;93:1942–1950. [PubMed] [Google Scholar]
- 13.Kirshenbaum AS, Kessler SW, Goff JP, Metcalfe DD. Demonstration of the origin of human mast cells from CD34+ bone marrow progenitor cells. J Immunol. 1991;146:1410–1415. [PubMed] [Google Scholar]
- 14.Kitamura Y. Heterogeneity of mast cells and phenotypic change between subpopulations. Annu Rev Immunol. 1989;7:59–76. doi: 10.1146/annurev.iy.07.040189.000423. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, Guan XY, Kim DK. Alternative isoforms of the mi transcription factor (MITF) regulate the expression of mMCP-6 in the connective tissue-type mast cells cultured with stem cell factor. J Life Sci. 2008;18:1348–1354. [Google Scholar]
- 16.Lee YM, Jippo T, Kim DK, Katsu Y, Tsujino K, Morii E, Kim HM, Adachi S, Nawa Y, Kitamura Y. Alteration of protease expression phenotype of mouse peritoneal mast cells by changing the microenvironment as demonstrated by in situ hybridization histochemistry. Am J Pathol. 1998;153:931–936. doi: 10.1016/S0002-9440(10)65634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levi-Schaffer F, Piliponsky AM. Tryptase, a norvel link between allergic inflammation and fibrosis. Trends Immunol. 2003;24:158–161. doi: 10.1016/s1471-4906(03)00058-9. [DOI] [PubMed] [Google Scholar]
- 18.Morii E, Tsujimura T, Jippo T, Hashimoto K, Takebayashi K, Tsujino K, Nomura S, Yamamoto M, Kitamura Y. Regulation of mouse mast cell protease 6 gene expression by transcription factor encoded by the mi locus. Blood. 1996;88:2488–2494. [PubMed] [Google Scholar]
- 19.Morii E, Oboki K. MITF is necessary for generation of prostaglandin D2 in mouse mast cells. J Biol Chem. 2004;279:48923–48929. doi: 10.1074/jbc.M407026200. [DOI] [PubMed] [Google Scholar]
- 20.Oboki K, Morii E, Kataoka TR, Jippo T, Kitamura Y. Isoforms of mi transcription factor preferentially expressed in cultured mast cells of mice. Biochem Biophys Res Commun. 2002;290:1250–1254. doi: 10.1006/bbrc.2002.6332. [DOI] [PubMed] [Google Scholar]
- 21.Ogihara H, Morii E, Kim DK, Oboki K, Kitamura Y. Inhibitory effect of transcription factor encoded by mutant mi microphthalmia allele on transactivation of mouse mast cell protease 7gene. Blood. 2001;97:645–651. doi: 10.1182/blood.v97.3.645. [DOI] [PubMed] [Google Scholar]
- 22.Pejler G, Abrink M, Ringvall M, Wernersson S. Mast Cell Proteases. Adv Immunol. 2007;95:167–255. doi: 10.1016/S0065-2776(07)95006-3. [DOI] [PubMed] [Google Scholar]
- 23.Price ER, Ding HF, Badalian T, Bhattacharya S, Takemoto C, Yao TP, Hemesath TJ, Fisher DE. Lineage-specific signaling in melanocytes. C-kit stimulation recruits p300/CBP to microphthalmia. J Biol Chem. 1998;273:17983–17986. doi: 10.1074/jbc.273.29.17983. [DOI] [PubMed] [Google Scholar]
- 24.Saito H, Ebisawa M, Tachimoto H, Shichijo M, Fukagawa K, Matsumoto K, Iikura Y, Awaji T, Tsujimoto G, Yanagida M, Uzumaki H, Takahashi G, Tsuji K, Nakahata T. Selective growth of human mast cells induced by Steel factor, IL-6, and prostaglandin E2 from cord blood mononuclear cells. J Immunol. 1996;157:343–350. [PubMed] [Google Scholar]
- 25.Sato S, Roberts K, Gambino G, Cook A, Kouzarides T, Goding CR. CBP/p300 as a co-factor for the Microphthalmia transcription factor. Oncogene. 1997;14:3083–3092. doi: 10.1038/sj.onc.1201298. [DOI] [PubMed] [Google Scholar]
- 26.Sommerhoff AP. Mast cell tryptases and airway remodeling. Am J Respir Crit Care Med. 2001;164:S52–S58. doi: 10.1164/ajrccm.164.supplement_2.2106058. [DOI] [PubMed] [Google Scholar]
- 27.Takemoto CM, Yoon YJ, Fisher DE. The identification and functional characterization of a novel mast cell isoform of the microphthalmia-associated transcription factor. J Biol Chem. 2002;277:30244–30252. doi: 10.1074/jbc.M201441200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data