Abstract
Anticancer effects of β-lapachone (β-lap) are due to generation of ROS and metabolic catastrophes as a result of NAD(P)H:quinone oxidoreductase (NQO1)-mediated futile cycling between the oxidized and reduced forms of β-lap. It has been shown that NQO1 is also essential for the TNF-induced activation of NF-κB and that β-lap suppresses the TNF-induced NF-κB activation. We investigated whether or not NQO1 is involved and β-lap suppresses the radiation-induced NF-κB activation using A549 human lung cancer cells and NQO1-knock down A549 cells (shNQO1 A549 cells). Irradiation with 4 Gy markedly increased the DNA binding activity of NF-κB in A549 cells, but not in the shNQO1 A549 cells, thus demonstrating that NQO1 plays a pivotal role in irradiation-induced NF-κB activation. Treatment with 10 µM β-lap for 4 h almost completely abrogated the radiation-induced increase in NF-κB activation and the transcription of NF-κB target genes such as bcl2, gadd45β and cyclinD1. Moreover, β-lap markedly suppressed the activation of IκB kinase γ (IKKγ) and the subsequent phosphorylation of IκBα, thereby inhibiting NF-κB activation. It is concluded that β-lap suppresses the radiation-induced activation of NF-κB by interrupting the involvement of NQO1 in the activation of NF-κB, thereby inhibiting the transcription of survival signals. The radiosensitization caused by β-lap may, in part, be attributed to β-lap-induced suppression of NF-κB activation.
Keywords: ionizing radiation, NAD(P)H:quinone oxidoreductase, NF-κB, β-lapachone
Introduction
β-Lapachone (3,4-dihydro-2,2-dimethyl-2H-naphtho [1,2-b]pyran-5,6-dione) (β-lap), is novel bio-reductive anti-cancer drug (Planchon et al., 1995; Wuerzberger et al., 1998; Planchon et al., 2001). In addition to killing a variety of cancer cells, β-lap has been shown to sensitize cancer cells to radiation by inhibiting the repair of sublethal radiation damage (Boothman et al., 1993; Park et al., 2005b; Suzuki et al., 2006; Choi et al., 2007). The anti-tumor activity and the radiosensitizing activity of β-lap are dependent on the cytosolic flavoenzyme NAD(P)H:quinone oxidoreductase (NQO1, E>C>1.6.99.2), which catalyzes the two-electron reduction of endogenous as well as exogenous quinone compounds to their corresponding hydroquinone forms using H+ from NADH or NADPH (Boothman et al., 1993; Trush et al., 1996; Pink et al., 2000; Planchon et al., 2001; Tagliarino et al., 2001; Park et al., 2005a, 2005b; Suzuki et al., 2006; Bey et al., 2007; Choi et al., 2007; Song et al., 2008). Boothman and his colleagues (Trush et al., 1996; Pink et al., 2000; Planchon et al., 2001; Tagliarino et al., 2001; Park et al., 2005b; Bentle et al., 2006; Bey et al., 2007) proposed that NQO1 mediates the two-electron reduction of β-lap and that the reduced form of β-lap is unstable and thus immediately oxidized to the original oxidized form. The intermediate one-electron reduced form of β-lap generates redox reactions creating cytotoxic oxygen species (ROS) leading to apoptosis. In addition, the futile cycling between the oxidized and reduced forms of β-lap causes depletion of ATP, the elevation of cytosolic Ca2+ and the release of cytochrome C, thereby inducing cell death. Treatment of cells with dicoumarol, an inhibitor of NQO1 (Boothman et al., 1993; Park et al., 2005b; Suzuki et al., 2006; Choi et al., 2007; Song et al., 2008), or with the small interfering RNA (siRNA)-NQO1 (Choi et al., 2007) have been shown to effectively suppress the cytotoxic and radiosensitizing effects of β-lap. Furthermore, NQO1-null-cells were resistant to β-lap and this resistance could be reversed by administration of exogenous NQO1 (Pink et al., 2000). These observations unequivocally demonstrated that NQO1 plays a crucial role in cell death or radiosensitization caused by β-lap. Importantly, the NQO1 content in various human cancer cells is significantly greater than that observed in normal tissues implying that tumors may be preferentially damaged by β-lap. Furthermore, NQO1 expression in cancer cells can be increased by a number of different types of stresses such as ionizing radiation (Choi et al., 2007), heat shock (Park et al., 2005b) and certain chemotherapeutic drugs such as cisplatin (Terai et al., 2009). These observations strongly indicated that conventional cancer treatments may increase the sensitivity of the cancer to β-lap treatment by increasing NQO1 activity in the target tumor cells (Park et al., 2005b; Suzuki et al., 2006; Choi et al., 2007; Song et al., 2008).
NF-κB (nuclear factor-κB) is an important transcription factor that is activated in response to a variety of different types of cellular stress. NF-κB is a heterodimer consisted of p50 and p65, and, under normal condition, is retained in the cytoplasm as a complex with the members of the NF-κB inhibitors family, mainly inhibitor-κBα (IκBα). (Baeuerle and Henkel , 1994). Cellular stress triggers the activation of the IKK (IκB kinase) complex, including IKKα, IKKβ and IKKγ/NEMO (IκB kinase-γ/NF-κB essential modulator), which then phosphorylate IκBα, thereby inducing the ubiquitin-proteasome-dependent degradation of IκBα. This process results in the dissociation of NF-κB, which then translocates into the nucleus, binds DNA as a p50 homodimer or p50-p65 heterodimer and transcribes various target genes including those involved in the survival and proliferation of the cells (Sen and Baltimore, 1986; Ahmed and Li, 2008). It has been demonstrated that the exposure of cells to ionizing radiation (IR) or to certain chemotherapeutic drugs increases the binding of NF-κB to DNA both in vitro and in vivo (Brach et al., 1991; Flynn et al., 2003; Fan et al., 2007), and that blocking the NF-κB-DNA binding event inhibits the adaptive resistance to ionizing radiation and chemotherapeutic drugs (Flynn et al., 2003; Fan et al., 2007).
It was previously reported that the inhibition of NQO1 with dicoumarol effectively suppresses the TNF (tumor necrosis factor)-induced activation of NF-κB (Cross et al., 1999), and that deletion of the nqo1 gene also abolishes the TNF-induced activation of NF-κB (Ahn et al., 2006). These results clearly demonstrated that NQO1 plays an important role in the TNF-induced activation of NF-κB. Interestingly, it was reported that β-lap completely inhibits the TNF-induced activation of NF-κB by inhibiting the TNF-induced degradation of IκBα (Manna et al., 1999). The purpose of the present study was to elucidate whether or not NQO1 is involved in the radiation-induced activation of NF-κB and if β-lap inhibits the radiation-induced activation of NF-κB. We observed that β-lap inhibits the radiation-induced NF-κB activation by interacting with NQO1.
Results
Apoptosis and clonogenic cell death
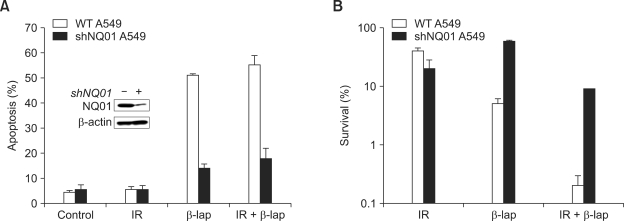
Figure 1 shows the apoptosis and clonogenic death of A549 cells and shNQO1 A549 cells treated with 4 Gy irradiation alone, 4 h incubation with 10 µM β-lap alone or a combination of these two treatments. There were no increases in apoptosis 24 h after 4 Gy irradiation in both A549 cells and shNQO1 A549 cells (Figure 1A). On the other hand, 51% and 55% of A549 cells were in apoptosis 24 h after the β-lap treatment alone or after β-lap treatment in combination with irradiation, respectively. However, in the shNQO1 A549 cells, apoptosis occurred only in about 14% and 18% of the cells 24 h after treating with β-lap alone or with β-lap in combination with irradiation, respectively. The clonogenic survival of A549 cells decreased to 27.2%, 4.0% and 0.2% when treated with irradiation alone, β-lap or a combination of β-lap treatment with irradiation, respectively (Figure 1B). If the combination of β-lap and irradiation killed the cells by additive manner, the clonogenic cell survival would be 1.1% (e.g. 27.2%×4.0%) instead of 0.2%. It may therefore be concluded that β-lap increased the radiosensitivity of cells resulting in the cell death greater than additive. The clonogenic survival of shNQO1 A549 cells was 36.7%, 18.7% and 6.1% after treatment with β-lap alone, irradiation alone or a combination of irradiation and β-lap, respectively (Figure 1B). These results demonstrated that shNQO1 A549 cells were resistant to β-lap treatment or to the combined treatment of β-lap and irradiation as compared with the wild-type A549 cells.
Figure 1.
β-Lap causes cell death and increases cellular radiosensitivity in NQO1 dependent manner. (A) Effects of β-lap on the apoptosis in wild type A549 cells and shQO1 A549 cells. (B) Effects of β-lap on the clonogenic survival of wild type A549 cells and shNQO1 A549 cells. An average of seven experiments ± SEM is shown.
Effects of β-lap on the radiation-induced activation of NF-κB
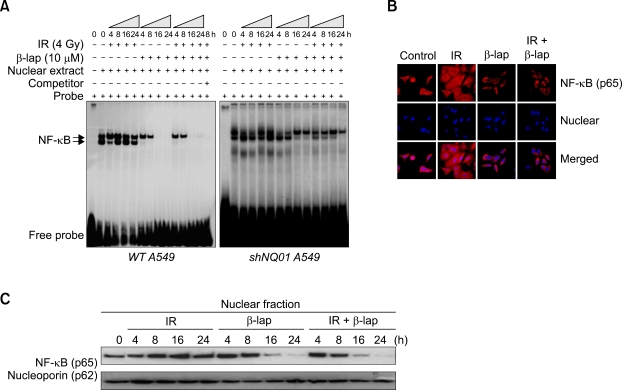
Figure 2A shows the results of the electrophoretic mobility shift assay studies for the changes in NF-κB activation caused by treatment with 4 Gy irradiation alone, incubation with 10 µM β-lap alone for 4 h or a combination of these two treatments. In A549 cells, the basal level of NF-κB activity was considerable and the NF-κB activity significantly increased from 4 to 24 h after irradiation. On the other hand, when the cells were treated with 10 µM β-lap for 4 h, the NF-κB activity was already suppressed at the end of 4 h incubation with B-lap and it was completely abated at 16 h to 24 h. The NF-κB activity in the cells treated with both radiation and β-lap was similar to that in the cells treated with β-lap alone indicating that the radiation-induced activation of NF-κB was completely suppressed by β-lap. The NF-κB activity in the shNQO1 remained unchanged after 4 Gy irradiation but decreased slightly after β-lap treatment. The NF-κB activity in the shNQO1 A549 cells treated with the combination of irradiation and β-lap was similar to that observed in the cells treated with β-lap alone.
Figure 2.
β-Lap inhibits the radiation-induced activation of NF-κB. (A) EMSA for nuclear extract from both wild type A549 cells and shNQO1 A549 cells treated with 4 Gy radiation alone, 10 µM β-lap alone, and combined. (B) Confocal fluorescence microscopic examination of NF-κB (upper) and DAPI staining (lower) of A549 cells treated with 4 Gy radiation alone, 10 µM β-lap alone, and combined. (C) Western blot analysis of NF-κB (p65) in the nuclear fraction of A549 cells treated with the combination of irradiation and β-lap.
Figure 2B shows the change in the nuclear localization of NF-κB (p65) in A549 cells after treatment. The 4 Gy irradiation significantly increased the fluorescence staining for NF-κB (p65) at 24 h. Interestingly, rather strong fluorescence was observed not only in the nucleus but throughout the cells, demonstrating the presence of activated NF-κB not only in the nucleus but also in the cytoplasm. Notably, the β-lap treatment suppressed the fluorescence staining for NF-κB particularly in the nucleus. In the cells treated with irradiation together with β-lap, the fluorescence staining for NF-κB (p65) was similar to that in the cells treated with β-lap alone.
The western blot analysis for the expression of NF-κB (p65) in the protein extracts of the nucleus is shown in Figure 2C. The irradiation with 4 Gy gradually increased the level of NF-κB (p65) while 4 h incubation with 10 µM β-lap almost completely inhibited the expression of NF-κB (p65) in the nuclear fraction of A549 cells. The expression of NF-κB (p65) in the nuclear fraction of the cells treated with the combination of irradiation and β-lap was identical to that observed in the cells treated with β-lap alone.
Effect of β-lap on NF-κB promoter activity and NF-κB target gene expression
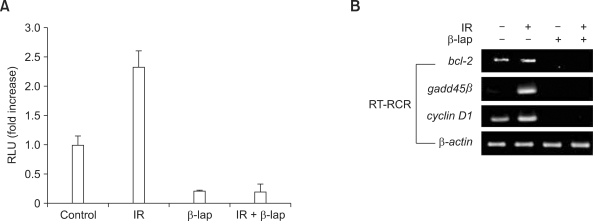
The effect of β-lap on the NF-κB promoter activity, as determined using the luciferase reporter assay, is shown in Figure 3A. The irradiation with 4 Gy significantly increased the NF-κB promoter activity whereas treatment with 10 µM β-lap for 4 h dramatically suppressed the NF-κB promoter activity. In the cells treated with irradiation in combination with β-lap, the NF-κB promoter activity was similar to that in the cells treated with β-lap alone, demonstrating that β-lap treatment completely suppressed the radiation-induced increase in the NF-κB promoter activity.
Figure 3.
β-Lap inhibits NF-κB promoter activity and target gene expression. (A) NF-κB promoter activity, determined using a luciferase reporter assay, in A549 cells treated with the combination of irradiation and β-lap. (B) Expression of NF-κB target genes bcl-2, gadd45β and cyclin D1 determined, as assessed using RT-PCR method, in A549 cells treated with the combination of irradiation and β-lap.
Figure 3B shows the effects of β-lap and irradiation alone or combined on the transcription of anti-apoptotic genes such as bcl2, gadd45β and cyclinD1, by the NF-κB. The irradiation with 4 Gy significantly increased the expression of mRNA for bcl2, gadd45β, and cyclinD1. However, the 10 µM β-lap treatment for 4 h almost completely suppressed the expression of constitutive mRNA for bcl2 and cyclinD1 and nullified the radiation-induced increase in mRNA for bcl2, gadd45β and cyclinD1.
Effects of β-lap on the upstream signaling cascade of NF-κB
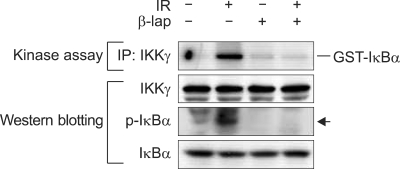
To obtain insights into the mechanisms by which β-lap suppresses NF-κB activity, we determined the effect of β-lap on the kinase activity of IKK in regard to IκBα phosphorylation in A549 cells. Figure 4 shows that 4 Gy irradiation markedly increased the IKK kinase activity as demonstrated by an increase in IκBα phosphorylation. On the other hand, a 4 h incubation with 10 µM β-lap completely abolished the basal level of IKK kinase activity and prevented the radiation-induced activation of IKK kinase. The β-lap treatment also prohibited the radiation-induced activation of IKK.
Figure 4.
In vitro kinase activity of IKK and Western blots for phosphorylated-IκBα and IκBα in A549 cells treated with the combination of irradiation and β-lap.
Discussion
The major conclusions of the present study are, first, the β-lap-induced cell death and the synergistic interaction between β-lap and radiation to induce cell death are NQO1 dependent and, second, the irradiation-induced activation of NF-κB is dependent on NQO1 activity, whereas it is suppressed by β-lap.
In good agreement with previous reports (Boothman et al., 1993; Planchon et al., 1995, 2001; Wuerzberger et al., 1998; Pink et al., 2000; Park et al., 2005a; Suzuki et al., 2006; Choi et al., 2007; Song et al., 2008), we have observed in the present study that not only the apoptosis and clonogenic cell death caused by β-lap but also the synergistic interaction between β-lap and irradiation in causing cell death occurred preferentially in the NQO1-positive A549 cells relative to NQO1-null shNQO1 A549 cells (Figure 1). These results clearly demonstrated that the effect of β-lap and NQO1 activity is closely related.
As shown in Figure 2A, β-lap almost completely abolished the basal level DNA binding activity of NF-κB and also the radiation-induced increase in DNA binding activity of NF-κB in A549 cells. It was probable that the marked decline in the NF-κB activity by β-lap treatment was due to death of the cells. However, the decline in the NF-κB activity was already significant at the end of the 4 h treatment with β-lap when the cell death was insignificant. Note that the NF-κB activity was completely abolished 16-24 h after β-lap treatment while only 50% of cells were apoptotic (Figure 1A). Therefore, it would be reasonable to conclude that the decline in the NF-κB activity by β-lap treatment was not the result of cell death although the decline in the NF-κB activity 16-24 h after β-lap treatment may be attributed in part to cell death. On the other hand, in the shNQO1 A549 cells, irradiation failed to increase the DNA binding activity of NF-κB, and the treatment with β-lap alone or in combination with irradiation slightly suppressed the DNA binding activity of NF-κB. These results clearly demonstrated that the DNA binding activity of NF-κB is strongly dependent on NQO1 activity in these cells. The confocal microscopic study (Figure 2B) coupled with the western blot analysis used to assess the level of NF-κB (p65) expression in the protein extracts of the nucleus (Figure 2C) demonstrated that irradiation markedly increased the nuclear translocation and the activity of NF-κB. It was also apparent that β-lap suppresses the activation and nuclear translocation of NF-κB caused by radiation (Figures 2A, 2B and 2C). In addition, β-lap significantly suppressed both the basal level and the radiation-induced increase in the NF-κB promoter activity (Figure 3A). Likewise, β-lap dramatically suppressed both the basal level and the radiation-induced increase in the mRNA expression of several NF-κB target genes such as bcl2, gadd45β and cyclin D1 (Figure 3B). Therefore, it may be concluded that the basal level as well as the radiation-induced increase in nuclear translocation, DNA binding activity, and transcriptional activity of NF-κB are strongly dependent on NQO1, and that these activities are effectively inhibited by β-lap.
It was previously reported that NQO1 plays an essential role in the TNF-induced activation of NF-κB probably by playing a role in the activation of the IKK, thereby increasing the IκBα phosphorylation, and IκBα degradation leading to liberation of NF-κB (Cross et al., 1999; Ahn et al., 2006). On the other hand, β-Lap has been shown to suppress the TNF-induced NF-κB activation, leading to prohibition of TNF-induced activation of AP-1, c-Jun N-terminal kinase and MARPKK or MEK (Manna et al., 1999). In the present study, β-lap suppressed the radiation-induced activation of NF-κB in A549 cells but not in the shNQO1 cells (Figure 2), and β-lap inhibited the radiation-induced activation of IKK and the IκBα phosphorylation in A549 cells (Figure 4). Taken together, it may be reasonable to conclude that the enzyme-substrate interaction between NQO1 and β-lap restraines the NQO1 from the participation in the upstream signaling pathway such as the activation of IKK complex for the activation of NF-κB. It may also be concluded that the radiosensitization by β-lap is due, at least in part, to the suppression of radiation-induced activation of NF-κB by the β-lap. Further investigation into the role of NQO1 and β-lap in NF-κB activation and the mechanisms underlying the radiosensitization by β-lap is warranted.
Methods
Materials and cells
Antibodies against the phospho-specific anti-IκBα (Ser32), nucleoporin p62 and IKKγ were purchased from Santa Cruz Biotechnology. The anti-NF-κB p65 antibody was purchased from Cell Signaling Technology while anti-β-actin antibody was obtained from Sigma-Aldrich and the anti-NQO1 antibody was purchased from Zymed. β-Lap was purchased from Biomol. All other chemicals were purchased from Sigma-Aldrich. The cell line used in the present study was the human lung carcinoma cell line A549 obtained from the American Type Culture Collection (ATCC). Cells were cultured with RPMI (GIBCO) supplemented with 10% (v/v) FBS (GIBCO) and 10 units/ml penicillin/streptomycin (GIBCO).
Treatment with irradiation and β-lap
Cells in the exponential growth phase in T25-type culture flasks were irradiated using a 137Cs irradiator at 4 Gy/min (CIS Bio International). β-Lap was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 µM, stored at -20℃, thawed immediately before use and diluted to the desired concentrations in RPMI. Cells in the exponential growth phase in T25-type culture flasks were incubated with β-lap at 37℃ under 95% O2 + 5% CO2 atmosphere for 4 h and gently rinsed twice with RPMI. When the cells were treated with both irradiation and β-lap, the cells were first irradiated and then immediately treated with β-lap.
Apoptosis analysis
The cells were stained with propidium iodide (PI, Sigma) and apoptosis was assessed using the FACSCalibur flow cytometer (Beckman Dickinson) (Choi et al., 2007).
Clonogenic survival assay
Cells were cultured for 10 to 14 days under the standard culture conditions and the resultant colonies were stained with 1% crystal violet in 20% ethanol. The numbers of colonies containing more than 50 cells were counted (Park et al., 2005a; Choi et al., 2007).
nqo1 shRNA transfection
For transfection, about 3 × 106 exponentially growing A549 cells were plated in each T25-type culture flask, incubated overnight, and then the cells were incubated for 24 h with the plasmids expressing shRNA for nqo1 (Superarray) in the Fugene HD transfection reagent (Roche). The transfected cells were further incubated with 1,000 µg/ml G418 for 7 days and a single colony of nqo1 knock-down A549 cells (shNQO1 A549) was amplified (Pink et al., 2000; Park et al., 2005b).
Western blot analysis
A549 cells grown in flasks were washed twice with ice-cold PBS, scraped from the dishes and lysed with RIPA buffer (Park et al., 2005b; Suzuki et al., 2006; Choi et al., 2007). The lysates were centrifuged, and an equal amount of protein from each sample was resolved using an 8 to 10% SDS-polyacrylamide gel electrophoresis and transferring to a Hybond-P (Amersham Life Sciences) membrane. The blots were blocked with 5% nonfat dry milk and labeled with primary antibodies and horseradish peroxidase (HRP) conjugated secondary antibodies. The immunoreactive bands were visualized using chemiluminescence (Pierce Biotechnology).
Reporter assay
A549 cells were transfected with the pNF-κB-Luc Cis-Reporter Plasmid (Stratagene) construct using Fugene HD transfection reagent (Roche) according to the manufacturer's protocol. The pCMV-β-Gal reporter vector was also used as an internal control. The transfected cells were incubated overnight under regular culture conditions, treated with β-lap and IR, and incubated for 12 h. The harvested cells were lysed, and the Renilla luciferase activity was measured using the Luciferase Assay System (Promega) and a Model TD-20/20 luminometer (Turner Designs). The luciferase activities were normalized with the internal control β-galatosidase (β-Gal) that was detected using chlorophenol red β-D-galactopyranoside as a substrate. All transfections experiments were repeated three times (Tirumalai et al., 2002; Pircher et al., 2003).
Reverse transcription polymerase chain reaction (RT-PCR)
A549 cells were washed twice with ice-cold PBS and collected in TRizol (Invitrogen) with a scraper. The first-strand cDNA from 1 µg of total Purified RNA was generated using Superscript III reverse transcriptase (Invitrogen). The synthesized cDNA samples were subsequently analyzed using PCR. The target mRNA transcripts were amplified with primers including bcl2 forward (5'-TTGCCCTCAAACAGAACAGC-3'), bcl2 reverse (5'-TGCAGCTCCTCTTGGCTAAA-3'), gadd45β forward (5'-CTGCAAATCCACTTCACGCT-3'), gadd45β reverse (5'-CCTTTGTCATACATGGCAGC-3'), cyclin D1 forward (5'-TGCTGGTTTTCTACCCAACG-3'), cyclin D1 reverse (5'-TTTCTTCTTGACTGGCACGC-3'), β-actin forward (5'-TGTGGCAGCAACTCAACAGA-3') β-actin reverse (5'-TACTTGCGCTCAGGAGGAGC-3'). The PCR reaction mixtures, which consisted of 0.6 µM of each primer, 200 µM of each dNTP, 5 units of exTaq DNA polymerase (Takara) and cDNA, were subjected to 25 cycles (22 cycles for β-actin) of amplification consisting of 30 s at 95℃, 30 s at 55℃, and 30 s at 72℃. The final extension step was performed for 10 min at 72℃. The PCR products were separated by electrophoresis on 2% agarose gels (Yang et al., 2007).
Electrophoretic mobility shift assay
Nuclear extracts were prepared using the CelLytic™ NuCLEAR™ Extraction Kit (Sigma), quantified using the BCA Kit and then incubated with [γ-32P] ATP (Amersham Biosciences) end-labeled NF-κB consensus probe (Santa Cruz Biotechnology) in a 20 µl reaction mixture for 30 min at room temperature. The reactions were terminated with a solution containing EDTA, sodium dodecyl sulfate (SDS) and bromophenol blue, and the samples were run on a non-denaturing 6% polyacrylamide gel and imaged by autoradiography (Byun et al., 2002; Wuerzberger-Davis et al., 2007).
Immunofluorescence microscopy
The NF-κB protein localization in A549 cells was investigated using the immunofluroescence staining method (Park et al., 2005b; Suzuki et al., 2006; Choi et al., 2007). Cells grown on cover glasses were treated with either 4 Gy irradiation, 4 h incubation with 10 µM β-lap or a combination of these two treatments. At 24 h after the treatment, the cells were rinsed with PBS, and fixed with 3.7% paraformaldehye. After blocking with 1% BSA, the cells were incubated with rabbit anti-NF-κB antibody and then labeled with Tex-Red conjugated anti-rabbit secondary antibody (Jackson ImmunoResearch Laboratories). The image was visualized using a laser scanning TE2000-E confocal microscope (Nikon).
In vitro kinase assay for IKK
A549 cells were treated with 4 Gy irradiation alone, 10 µM β-lap or a combination and harvested for in vitro kinase assay. Whole cell lysates were prepared and immunoprecipitated with the IKKγ/NEMO antibody. The activity of IKK in the immunecomplexes were determined using reaction mixtures containing 10 µM ATP, 2-5 µCi [γ-32P]ATP and 2 µg GST-IκBα. Samples were analyzed by 12% SDS-PAGE. Phosphorylated GST-IκBα was visualized by autoradiography (Wuerzberger-Davis et al., 2007).
Acknowledgements
This work was supported by grants from the Korea Health 21 R&D Project (A062254), the Nuclear R&D Program of KOSEF (2009-0062222 and 2009-0078449), and the National Institute of Health of the United States (RO1 CA116721).
Abbreviations
- ATM
ataxia-telangiectasia mutated
- GADD45β
growth arrest DNA damage gene 45β
- IKK
IκBα kinase
- IR
ionizing radiation
- NEMO
NF-κB essential modulator
- NQO1
NAD(P)H:quinone oxidoreductase
References
- 1.Ahmed KM, Li JJ. NF-kappa B-mediated adaptive resistance to ionizing radiation. Free Radic Biol Med. 2008;44:1–13. doi: 10.1016/j.freeradbiomed.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn KS, Sethi G, Jain AK, Jaiswal AK, Aggarwal BB. Genetic deletion of NAD(P)H:Quinone Oxidoreductase 1 abrogates activation of nuclear factor-κBα kinase, c-Jun N-terminal kinase, Akt, p38, and p44/42 mitogen-activated protein kinases and potentiates apoptosis. J Biol Chem. 2006;281:19798–19808. doi: 10.1074/jbc.M601162200. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 4.Bentle MS, Reinicke KE, Bey EA, Spitz DR, Boothman DA. Calcium-dependent modulation of poly(ADP-ribose) polymerase-1 alters cellular metabolism and DNA repair. J Biol Chem. 2006;281:33684–33696. doi: 10.1074/jbc.M603678200. [DOI] [PubMed] [Google Scholar]
- 5.Bey EA, Bentle MS, Reinicke KE, Dong Y, Yang CR, Girard L, Minna JD, Bornmann WG, Gao J, Boothman DA. An NQO1- and PARP-1-mediated cell death pathway induced in non-small-cell lung cancer cells by beta-lapachone. Proc Natl Acad Sci USA. 2007;104:11832–11837. doi: 10.1073/pnas.0702176104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boothman DA, Meyers M, Fukunaga N, Lee SW. Isolation of x-ray-inducible transcripts from radioresistant human melanoma cells. Proc Natl Acad Sci USA. 1993;90:7200–7204. doi: 10.1073/pnas.90.15.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brach MA, Hass R, Sherman ML, Gunji H, Weichselbaum R, Kufe D. Ionizing radiation induces expression and binding activity of the nuclear factor kappa B. J Clin Invest. 1991;88:691–695. doi: 10.1172/JCI115354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byun MS, Jeon KI, Choi JW, Shim JY, Jue DM. Dual effect of oxidative stress on NF-kappaB activation in HeLa cells. Exp Mol Med. 2002;34:332–339. doi: 10.1038/emm.2002.47. [DOI] [PubMed] [Google Scholar]
- 9.Choi EK, Terai K, Ji IM, Kook YH, Park KH, Oh ET, Griffin RJ, Lim BU, Kim JS, Lee DS, Boothman DA, Loren M, Song CW, Park HJ. Upregulation of NAD(P)H:quinone oxidoreductase by radiation potentiates the effect of bioreductive beta-lapachone on cancer cells. Neoplasia. 2007;9:634–642. doi: 10.1593/neo.07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross JV, Deak JC, Rich EA, Qian Y, Lewis M, Parrott LA, Mochida K, Gustafson D, Vande Pol S, Templeton DJ. Quinone reductase inhitiors block SAPK/JNK and NF-κB pathways and potentiate apoptosis. J Biol Chem. 1999;274:31150–31154. doi: 10.1074/jbc.274.44.31150. [DOI] [PubMed] [Google Scholar]
- 11.Fan M, Ahmed KM, Coleman MC, Spitz DR, Li JJ. Nuclear factor-kappaB and manganese superoxide dismutase mediate adaptive radioresistance in low-dose irradiated mouse skin epithelial cells. Cancer Res. 2007;67:3220–3228. doi: 10.1158/0008-5472.CAN-06-2728. [DOI] [PubMed] [Google Scholar]
- 12.Flynn V, Jr, Ramanitharan A, Moparty K, Davis R, Sikka S, Agrawal KC, Abdel-Mageed AB. Adenovirus-mediated inhibition of NF-kappaB confers chemo-sensitization and apoptosis in prostate cancer cells. Int J Oncol. 2003;23:317–323. [PubMed] [Google Scholar]
- 13.Lopes JN, Cruz FS, Docampo R, Vasconcellos ME, Sampaio MC, Pinto AV, Gilbert B. In vitro and in vivo evaluation of the toxicity of 1,4-naphthoquinone and 1,2-naphthoquinone derivatives against Trypanosoma cruzi. Ann Trop Med Parasitol. 1978;72:523–531. doi: 10.1080/00034983.1978.11719356. [DOI] [PubMed] [Google Scholar]
- 14.Manna SK, Gad YP, Mukhopadhyay A, Aggarwal BB. Suppression of tumor necrosis factor-activated nuclear transcription factor-κB, activator protein-1, c-Jun N-terminal kinase, and apoptosis by β-lapachone. Biochem Pharmacol. 1999;57:763–774. doi: 10.1016/s0006-2952(98)00354-2. [DOI] [PubMed] [Google Scholar]
- 15.Park HJ, Ahn KJ, Ahn SD, Choi E, Lee SW, Williams B, Kim EJ, Griffin R, Bey EA, Bornmann WG, Gao J, Boothman DA, Song CW. Susceptibility of cancer cells to beta-lapachone is enhanced by ionizing radiation. Int J Radiat Oncol Biol Phys. 2005a;61:212–219. doi: 10.1016/j.ijrobp.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Park HJ, Choi EK, Choi J, Ahn KJ, Kim EJ, Ji IM, Kook YH, Ahn SD, Williams B, Griffin R, Boothman DA, Lee CK, Song CW. Heat-induced up-regulation of NAD(P)H:quinone oxidoreductase potentiates anticancer effects of beta-lapachone. Clin Cancer Res. 2005b;11:8866–8871. doi: 10.1158/1078-0432.CCR-05-0818. [DOI] [PubMed] [Google Scholar]
- 17.Pink JJ, Planchon SM, Tagliarino C, Varnes ME, Siegel D, Boothman DA. NAD(P)H:Quinone oxidoreductase activity is the principal determinant of beta-lapachone cytotoxicity. J Biol Chem. 2000;275:5416–5424. doi: 10.1074/jbc.275.8.5416. [DOI] [PubMed] [Google Scholar]
- 18.Pircher PC, Kitto JL, Petrowski ML, Tangirala RK, Bischoff ED, Schulman IG, Westin SK. Farnesoid X receptor regulates bile acid-amino acid conjugation. J Biol Chem. 2003;278:27703–27711. doi: 10.1074/jbc.M302128200. [DOI] [PubMed] [Google Scholar]
- 19.Planchon SM, Wuerzberger S, Frydman B, Witiak DT, Hutson P, Church DR, Wilding G, Boothman DA. Beta-lapachone-mediated apoptosis in human promyelocytic leukemia (HL-60) and human prostate cancer cells: a p53-independent response. Cancer Res. 1995;55:3706–3711. [PMC free article] [PubMed] [Google Scholar]
- 20.Planchon SM, Pink JJ, Tagliarino C, Bornmann WG, Varnes ME, Boothman DA. beta-Lapachone-induced apoptosis in human prostate cancer cells: involvement of NQO1/xip3. Exp Cell Res. 2001;267:95–106. doi: 10.1006/excr.2001.5234. [DOI] [PubMed] [Google Scholar]
- 21.Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986;46:705–716. [PubMed] [Google Scholar]
- 22.Song CW, Chae JJ, Choi EK, Hwang TS, Kim C, Lim BU, Park HJ. Anti-cancer effect of bio-reductive drug beta-lapachon is enhanced by activating NQO1 with heat shock. Int J Hyperthermia. 2008;24:161–169. doi: 10.1080/02656730701781895. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki M, Amano M, Choi J, Park HJ, Williams BW, Ono K, Song CW. Synergistic effects of radiation and beta-lapachone in DU-145 human prostate cancer cells in vitro. Radiat Res. 2006;165:525–531. doi: 10.1667/RR3554.1. [DOI] [PubMed] [Google Scholar]
- 24.Tagliarino C, Pink JJ, Dubyak GR, Nieminen AL, Boothman DA. Calcium is a key signaling molecule in beta-lapachone-mediated cell death. J Biol Chem. 2001;276:19150–19159. doi: 10.1074/jbc.M100730200. [DOI] [PubMed] [Google Scholar]
- 25.Terai K, Dong GZ, Oh ET, Park MT, Gu Y, Song CW, Park HJ. Cisplatin enhances the anticancer effect of beta-lapachone by upregulating NQO1. Anticancer Drugs. 2009;20:901–909. doi: 10.1097/CAD.0b013e328330098d. [DOI] [PubMed] [Google Scholar]
- 26.Tirumalai R, Rajesh Kumar T, Mai KH, Biswal S. Acrolein causes transcriptional induction of phase II genes by activation of Nrf2 in human lung type II epithelial (A549) cells. Toxicol Lett. 2002;132:27–36. doi: 10.1016/s0378-4274(02)00055-3. [DOI] [PubMed] [Google Scholar]
- 27.Trush MA, Twerdok LE, Rembish SJ, Zhu H, Li Y. Analysis of target cell susceptibility as a basis for the development of a chemoprotective strategy against benzene-induced hematotoxicities. Environ Health Perspect. 1996;104(Suppl 6):1227–1234. doi: 10.1289/ehp.961041227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wuerzberger SM, Pink JJ, Planchon SM, Byers KL, Bornmann WG, Boothman DA. Induction of apoptosis in MCF-7:WS8 breast cancer cells by beta-lapachone. Cancer Res. 1998;58:1876–1885. [PubMed] [Google Scholar]
- 29.Wuerzberger-Davis SM, Nakamura Y, Seufzer BJ, Miyamoto S. NF-kappaB activation by combinations of NEMO SUMOylation and ATM activation stresses in the absence of DNA damage. Oncogene. 2007;26:641–651. doi: 10.1038/sj.onc.1209815. [DOI] [PubMed] [Google Scholar]
- 30.Yang XY, Yang TT, Schubert W, Factor SM, Chow CW. Dosage-dependent transcriptional regulation by the calcineurin/NFAT signaling in developing myocardium transition. Dev Biol. 2007;303:825–837. doi: 10.1016/j.ydbio.2006.11.036. [DOI] [PubMed] [Google Scholar]