Abstract
Aging is associated with protein damage and imbalance in redox status in a variety of cells and tissues, yet little is known about the extent of age-related oxidative stress in the peripheral nervous system. Previously, we showed a drastic decline in the expression of glial and neuronal proteins in myelinated peripheral nerves with age, which is significantly ameliorated by lifelong calorie restriction. The age-related decline in functional molecules is associated with alterations in cellular protein homeostatic mechanisms, which could lead to a buildup of damaged, aggregated proteins. To determine the extent of oxidative damage within myelinated peripheral nerves, we studied sciatic nerves from rats of four different ages (8, 18, 29, and 38 months) maintained on an ad libitum or a 40% calorie-restricted diet. We found a prominent accumulation of polyubiquitinated substrates with age, which are associated with the conglomeration of distended lysosomes and lipofuscin adducts. The occurrence of these structures is notably less frequent within nerves of age-matched rodents kept on a lifelong reduced calorie diet. Markers for lipid peroxidation, inflammation, and immune cell infiltration are all elevated in nerves of ad libitum–fed rats, whereas food restriction is able to attenuate such deleterious processes with age. Together these results show that dietary restriction is an efficient means of defying age-related oxidative damage and maintaining a younger state in peripheral nerves.
Introduction
Aging of organ systems is associated with the accumulation of oxidatively damaged polynucleotides, proteins, carbohydrates, and lipids that compromise cellular function. This is considered the “Oxidative Stress Theory of Aging” whereby age-related loss of proper physiological function is due to the accumulation of oxidative damage.1 Long-lived postmitotic cells, such as neurons, are at higher risk and accrue greater amounts of damaged waste than short-lived cells.2,3 In addition, Schwann cells, the myelinating glia of the peripheral nervous system (PNS), are rich in polyunsaturated fatty acids,4 which serve as a substrate for reactive oxygen species– (ROS) mediated lipid peroxidation.5,6 Together, the buildup of age-related damaged molecules along with their inefficient removal by homeostatic mechanisms become a concern in the vulnerable neurons and Schwann cells of peripheral nerves.
Intracellular proteolytic mechanisms, including the ubiquitin–proteasome system (UPS) and the autophagy–lysosomal pathway (macroautophagy) are responsible for degradation and removal of damaged cellular material. The accumulation of waste material is not only harmful due to its interference with biological functions, but also for imparting toxicity via lipid peroxidation products such as malondialdehyde (MDA), 4-hydroxynonenal (HNE), and nitrotyrosine.7,8
Although the precise mechanisms underlying the Oxidative Stress Theory of Aging are still largely unknown, there is accumulating evidence to support the involvement of the inflammatory response.9 An increase in the levels of serum cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-6, and interferon are commonly associated with senescence and play integral roles in activating inflammation and innate immunity.10 Free radicals are known to modulate this stress-induced activation of inflammation through the regulator of innate immunity and of the macrophage inflammatory response, the nuclear factor κB (NF-κB).11–13 Activation of NF-κB by degradation of its bound inhibitory protein, IκB, has also been found to upregulate the expression of the oxidizing agent, inducible nitric oxide synthase (iNOS).9,11
Restriction of calorie intake is a widely accepted approach to lower levels of oxidative stress and slow age-associated changes, as well as to extend life span in mammals.14,15 Previous studies have shown that calorie restriction (CR) decreases mitochondrial ROS generation and oxidative damage to DNA, proteins and lipids.16 CR has been found to activate the autophagic protein degradative pathway in aging rats17,18 and to reduce markers of age-related chronic inflammation, like TNF-α, NF-κB, and iNOS.9,14 Although there have been extensive studies on the ability of CR to reduce age-related oxidative damage in the central nervous system (CNS),14 little is known about the beneficial effects of CR on age-associated oxidative stress and inflammation within the PNS.
In this study, we asked whether CR can relieve the oxidative stress placed upon the PNS during aging. We found that a lifelong CR diet decreases the steady-state levels of undegraded polyubiquitinated substrates and oxidative damage markers of proteins and lipids in myelinated peripheral nerves. Furthermore, CR relieves the chronic inflammation commonly associated with age.
Experimental Procedures
Animals and diet
The use of animals in this study was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Florida. Ad libitum–fed and calorie-restricted (CR) male Fischer 344 × BN (Brown Norway) rats (n = 3 per group) of ages 8, 18, 29, and 38 months were obtained from the National Institute on Aging colony at Harlan Sprague Dawley Inc. (Indianapolis, IN). The animals were housed in a temperature- and light-controlled environment and had water available at all times. Rats fed ad libitum had free access to NIH-31 nutrient composition pellets, while the CR group received fortified pellets once daily 1 h before the onset of the dark cycle. Calorie reduction began at 14 weeks of age with 10% restriction, increased to 25% at 15 weeks, and was maintained at 40% from 16 weeks of age until sacrificed at 8, 18, 29, and 38 months of age. Data on survival characteristics and physical performance of the same colony of rats have been previously reported.19,20 The survival percentages of the male Fischer 344 × BN rats in the ad libitum group for the ages 8, 18, 29, and 38 months were 100%, 98%, 70%, and 30%, respectively. In comparison, those for the CR group for the same ages were 100%, 100%, 90%, and 70%, respectively.20
Biochemical analyses
Rats of the above-mentioned ages kept under ad libitum or CR diets were sacrificed as per IACUC protocols. The proximal ends (approximately 5-cm-long piece) of the left and right sciatic nerves were removed surgically within 5 min of decapitation, frozen immediately, and stored in liquid nitrogen. The nerves from the right sides were used for biochemical analysis. Whole nerve lysates (including myelin and axonal proteins) were prepared separately from individual nerves in sodium dodecyl sulfate (SDS) sample buffer (62.5 mM Tris, pH 6.8, 10% glycerol, 3% SDS) supplemented with phosphatase inhibitors, phenylmethylsulfonyl fluoride (PMSF; both from Sigma-Aldrich, St. Louis, MO) and complete protease inhibitor (Roche, Indianapolis, IN). The lysates were assayed for protein content using the BCA kit (Pierce, Rockford, IL) and then separated on polyacrylamide gels and transferred to nitrocellulose membranes for western blotting. Primary antibodies in blocking buffer (5% milk in phosphate-buffered saline [PBS] were applied to the membranes overnight at 4°C (see Table 1). Membranes were then incubated with horseradish peroxidase-conjugated secondary antibodies directed against the primary antibody, including anti-rabbit, anti-mouse (Cell Signaling technology, Inc., Danvers, MA), anti-rat, or anti-goat (Sigma-Aldrich, St. Louis, MO). Membranes were then reacted with an enhanced chemiluminescent substrate (Perkin Elmer, Boston, MA). A GS-800 densitometer (Bio-Rad, Hercules, CA) was used to digitally image the films. In each experiment, the densitometric value of the ad libitum 8-month sample was set as 1, and the values of other age-diet combination were determined with respect to this. One-way analysis of variance (ANOVA) followed by Fisher protected least significant difference (PLSD) analysis was performed using the StatView program, to compare the normalized densitometric values of proteins (dependent variables) between ad libitum or CR diet with age (factor). For each analysis, the p value and significance (#, ##, ###) for Fisher PLSD analysis was determined by comparing the densitometric values of 8-month-old ad libitum samples with the older ages (18, 29, or 38 months) of the ad libitum group. Similarly, in the CR group, the 8-month-old CR sample was compared with older ages. To determine statistical significance between samples, an unpaired t-test was performed using GraphPad Prism v5.0 software. Differences were considered significant at p < 0.05. For each analysis, nerve samples from 3 individual rats were used.
Table 1.
Primary Antibodies Used
| |
|
|
Dilution |
|
|---|---|---|---|---|
| Species | Antigen | Source and catalog number | WB | IS |
| Rabbit | Nitrotyrosine | Calbiochem, San Diego, CA, 487924 | 1:400 | n/a |
| Rabbit | Malondialdehyde (MDA) | Abcam, Cambridge, MA, 27642 | 1:400 | n/a |
| Mouse | 4-Hydroxynonenal (HNE) | Abcam, 48506 | 1:80 | n/a |
| Mouse | GAPDH | Encor Biotechnology Inc., FL, USA; MCA-1D4 | 1:5000 | n/a |
| Mouse | Tubulin | Sigma, St Louis, MO, USA, T6199 | 1:5000 | n/a |
| Mouse | CD11b | Serotec, Raleigh, NC, MCA275GA | n/a | 1:500 |
| Rabbit | NF-κB | Stressgen; Victoria British Columbia, Canada; KAP-TF112 | 1:1000 | n/a |
| Mouse | NF-κB | Santa Cruz Biotechnology, Inc., CA, USA; sc-109 | 1:1000 | n/a |
| Mouse | Phospho-IκB (Ser 32/36) | Cell Signaling Technology, Inc. Boston, MA, USA; 9246 | 1:1000 | n/a |
| Rabbit | TNF-α | Millipore, Temecula, CA, USA; AB1837P | 1:2000 | n/a |
| Rabbit | Ubiquitin | Dako, Carpinteria, CA, Z0458 | 1:500 | 1:100 |
| Mouse | LAMP1 | Stressgen, VAM-EN001 | 1:2000 | 1:500 |
| Rat | CD11b | Serotec, Raleigh, NC, MCA711 | 1:500 | n/a |
WB, Western blot; IS, immunostaining; n/a, nonapplicable; GADPH, glyceraldehyde-3-phosphate dehydrogenase; TNF-α, tumor necrosis factor-α.
Immunolabeling of nerve samples
The proximal ends of each left sciatic nerve (n = 3 animals for each age and diet group) were sectioned at 5-μm thickness and dried for 1 h at room temperature on Superfrost/Plus microslides (Fisher Scientific, Pittsburg, PA). Sections for HNE staining were fixed and permeabilized with 1% paraformaldehyde and 90% ethanol for 2 min at 25°C. Sections analyzed for lysosome-associated membrane protein 1 (LAMP1), pUbi, CD11b, and NF-κB were fixed with 4% paraformaldehye in PBS for 30 min followed by permeabilization with ice-cold methanol for 5 min.21 Following fixation, slides were blocked in 20% normal goat serum in PBS, and then incubated with primary antibodies overnight at 4°C (see Table 1). Bound antibodies were detected using Alexa Fluor 594 or Alexa Fluor 488 goat anti-rabbit or anti-mouse immunogloBulin G (IgG) secondary antibodies (Molecular Probes, Eugene, OR). Hoechst dye (Molecular Probes) was included with the secondary antibodies to visualize nuclei. Slides were mounted with coverslips using the ProLong Antifade kit (Molecular Probes) and imaged with a SPOT camera attached to a Nikon Eclipse E800 microscope (Melville, NY). Images were formatted using Adobe Photoshop 5.5. For quantification of macrophages, CD11b+ cells were counted per field of view in longitudinal sections (5 μm thickness) of sciatic nerves from two different depths and eight random visual fields (0.1 mm2) per animal (n = 3). To determine statistical significance between samples, an unpaired t-test was performed using GraphPad Prism v5.0 software. Differences were considered significant at p < 0.05.
4-[2-(6-Dibutylamino)-2-naphthalenyl)ethenyl]-1-(3-sulfopropyl) hydroxide labeling
For labeling of accumulated oxidized material, 4-[2-(6-dibutylamino)-2-naphthalenyl)ethenyl]-1-(3-sulfopropyl) hydroxide (di-8 ANEPPS), a lipophilic dye that recognizes lipofuscin adducts,22 was used. Frozen nerve sections (as above) were fixed with 4% paraformaldehye in PBS for 10 min followed by permeabilization with 0.2% Triton X-100 in PBS for 15 min at 25°C. Samples were blocked in 20% normal goat serum in PBS and then incubated with 2 nM di-8 ANEPPS in PBS for 30 min at 25°C. Slides were mounted with coverslips and imaged, as above. Mean di-8 ANEPPS pixels per fixed area (0.1 mm2) were determined using the mean gray value tool in the National Institutes of Health (NIH) Image J software. Statistical analysis was performed by an unpaired t-test using GraphPad Prism software v5.0, and differences were considered significant at p < 0.05.
Results
A CR diet slows protein damage within peripheral nerves during aging
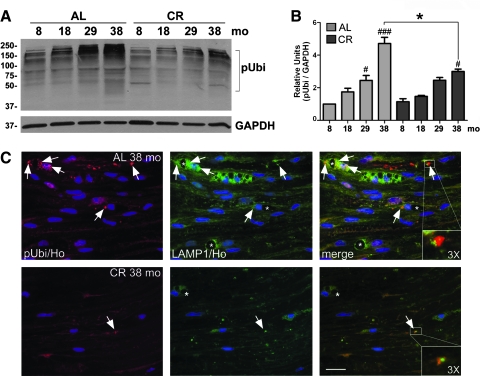
As a marker of damaged and/or aggregated proteins, we compared the steady-state levels of anti-ubiquitin reactive molecules between sciatic nerve lysates from 8- 18-, 29-, and 38-month-old rats (Fig. 1A). There is a gradual accumulation of slow-migrating polyubiquitinated substrates in the nerves of ad libitum–fed rodents, which becomes prominent by 29 months (Fig. 1A). In comparison, the levels of ubiquitin-reactive proteins remain remarkably low in nerves of CR-fed rats, even at 38 months (Fig. 1A). Quantification of blots after correcting for GAPDH from three independent experiments reveals a significant increase in polyubiquitin reactive proteins by 29 months of age in the ad libitum group, which is absent from the CR animals (Fig. 1B).
FIG. 1.
A calorie-restricted (CR) diet minimizes accrual of damaged proteins within peripheral nerves during aging. (A) Western blot analysis of whole sciatic nerve lysates (20 μg/lane) from rats of the indicated months (mo) fed ad libitum (AL) or CR diets probed with an anti-ubiquitin antibody. Slow-migrating polyubiquitinated (pUbi) protein substrates are marked by a square bracket. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is shown as a protein loading control. Molecular mass in kDa is indicated on the left. (B) Quantification of pUbi band intensity normalized to GAPDH is shown (#p < 0.05, ###p < 0.001, Fisher protected least significant difference [PLSD] analysis; *p < 0.05, unpaired t-test, mean ± standard error of the mean [SEM], n = 3). In this and subsequent figures, the p value for Fisher protected least significant difference (PLSD) analysis is determined by comparing the 8-month-old sample with the older ages (18, 29, or 38 months) for each diet group. (C) Longitudinal sciatic nerve sections from 38-month-old animals fed ad libitum or CR diets were analyzed for ubiquitin- (red) and lysosomal associated membrane protein 1– (LAMP1) like (green) immunoreactivity. Arrows point to ubiquitin-positive protein aggregates adjacent to LAMP1-positive lysosomes (magnified 3 × in insets). Asterisks indicate intracytoplasmic vacuoles. Nuclei are labeled with Hoechst dye (blue). Scale bar, 20 μm.
To examine if lysosomes are recruited to ubiquitin aggregates in aged nerves, we coimmunolabeled longitudinal sciatic nerve sections for ubiquitin and LAMP1 (Fig. 1C). As suggested by the western blots (Fig. 1A), nerve samples from 38-month ad libitum–fed rats contain numerous ubiquitin-positive protein aggregates (Fig. 1C), as well as enlarged lysosomes and vacuoles (Fig. 1C, green, arrows).18 Many of the ubiquitin-reactive aggregates are surrounded by LAMP1-positive lysosomes, which are revealed by yellow color on merged images (Fig. 1C, 3 × magnifications). On sections from 38-month-old CR animals, the abundance and apparent size of ubiquitin-positive aggregates are dramatically lower (Fig. 1C, red, arrows), which agree with the biochemical data (Fig. 1A). Correspondingly, these nerves show an overall decrease in LAMP1-like immunoreactivity and fewer vacuoles (Fig. 1C, bottom panel). The CR diet also attenuates the age-related increase the number of nuclei per fixed area of nerve tissue (Fig 1C, blue), as shown previously.18
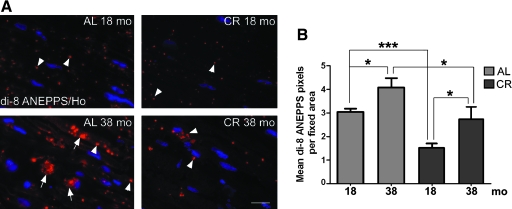
In aged postmitotic mammalian tissues, besides undegraded ubiquitin-tagged substrates, there is a buildup of oxidized and cross-linked proteins and lipids, known as lipofuscin.23 In accordance, sciatic nerve sections from 38-month-old ad libitum–fed rodents stain prominently with di-8 ANEPPS (Fig. 2A, arrows), a lipophilic dye that recognizes lipofuscin adducts,22 as compared to samples from 18-month-old rats (Fig. 2A). Nerve tissues of diet-restricted rats contain fewer and apparently smaller di-8 ANEPPS-positive structures at both ages examined (Fig. 2A, arrowhead). Quantification of the mean di-8 ANEPPS pixels per fixed area shows a statistically significant increase with age from 18 to 38 months in samples from ad libitum rats (Fig. 2B). When compared across ages and diet groups, the accrual is significantly reduced by CR diet (Fig. 2B, *p < 0.05, ***p < 0.001). Together, these results emphasize that a lifelong CR diet is effective in reducing the accumulation of oxidized and cross-linked substances in peripheral nerves, either by lowering damage across life span and/or enhancing the activity of protective mechanisms, such as the ubiquitin-proteasome and/or autophagy–lysosomal pathways.
FIG. 2.
Accumulation of lipofuscin is curtailed by a calorie-restricted (CR) diet with aging. (A) Sciatic nerve sections from 18- and 38-month-old animals fed ad libitum (AL) or CR diets were stained with 4-[2-(6-dibutylamino)-2-naphthalenyl)ethenyl]-1-(3-sulfopropyl) hydroxide (di-8 ANEPPS) dye, which labels lipofuscin content (red). Large (∼10 μm) and smaller (<10 μm) di-8 ANEPPS-positive adducts are marked with arrows and arrowheads, respectively. Nuclei are labeled with Hoechst dye (blue). Scale bar, 20 μm. (B) Quantification of mean di-8 ANEPPS dye-positive pixels per fixed area is shown (*p < 0.05, ***p < 0.001, unpaired t-test, mean ± standard error of the mean [SEM], n = 3). (Color images available online at www.liebertonline.com/rej)
Lipid oxidation-mediated protein damage within myelinated peripheral nerves
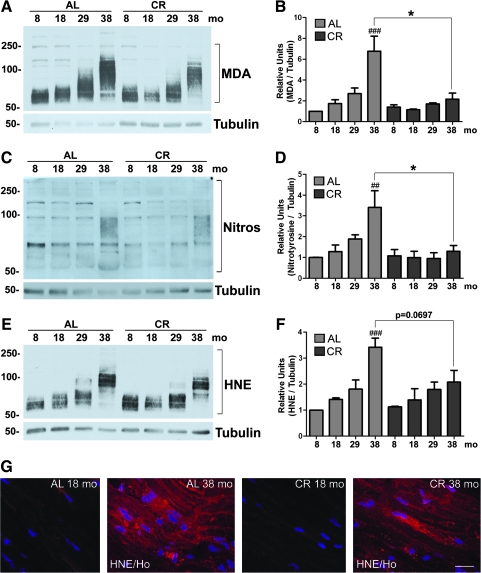
Oxidation of polyunsaturated fatty acids of myelin lipids4 results in the formation of hydroperoxides and hydroxyalkenals, like MDA and HNE, which then can react with proteins and alter their surface hydrophobicity.24,25 To examine lipid modification during aging of myelinated nerves, we analyzed tissue lysates with antibodies to MDA (Fig. 3A). As shown on the western blot, there is a gradual increase in MDA-adducted proteins with age, detected as a smear, in the ad libitum samples (Fig. 3A, square bracket). In addition, the mobility of MDA-positive proteins is slowed in the AL 38-month-old lysate, likely representing aggregates (Fig. 3A). In comparison, we find a marked decrease in MDA-adducted proteins within nerves from animals on CR diets (Fig. 3A, square bracket). Quantification after correction for tubulin reveals a significant increase (###p < 0.01) in MDA-adducted proteins in sciatic nerves of AL fed animals from 29 to 38 months, a trend that is attenuated by the CR intervention (Fig. 3B). Furthermore, the differences are significant when comparing across diet groups at the 38-month time point (Fig. 3B, *p < 0.05). We also measured the levels of protein nitrosylation within whole nerve lysates and found a similar increasing pattern across the ages in ad libitum samples (Fig. 3C,D ##p < 0.01). In contrast, significantly lower levels (*p < 0.05) of nitrosylated proteins are detected in nerves of animals maintained on lifelong CR (Fig. 3D). As both axonal and glial proteins are vulnerable to HNE modification,26 we also quantified HNE adducts by biochemical analysis and found a significant increase from 29 to 38 months in the ad libitum–fed group (Fig. 3E,F, ##p < 0.01). Although the CR diet lowers the levels of HNE-reactive proteins across life span, statistical analysis between ad libitum and CR 38-month samples did not yield significance (p = 0.0697) (Fig. 3F).
FIG. 3.
Lipid peroxidation-associated modifications of proteins with age are relieved in sciatic nerve with a calorie-restricted (CR) diet. (A) Whole rat sciatic nerve lysates (20 μg/lane) were probed with a rabbit anti-malondialdehyde (MDA) antibody to detect MDA adducts to proteins (square bracket). Tubulin is shown as a protein loading control. (B) Quantification of MDA adduct band intensities normalized to tubulin is shown (###p < 0.001, Fisher protected least significant difference (PLSD) analysis; *p < 0.05, unpaired t-test, mean ± SEM, n = 3). (C) Biochemical analysis of tyrosine residues (Nitros) on proteins (square bracket) using an anti-nitrosylation antibody in the same lysates as in A. Tubulin is shown as a protein loading control. (D) Quantification of nitrotyrosine band intensities from Western blots normalized to tubulin is shown (##p < 0.01, Fisher's PLSD analysis; *p < 0.05, unpaired t-test, mean ± standard error of the mean [SEM], n = 3). (E) Western blot analysis of 4-hydroxynonenal (HNE) in the same lysates used in A and C. Tubulin is shown as a protein loading control. In A, C, and E, molecular mass is shown on the left in kDa. (F) Quantification of HNE adduct band intensities from western blots normalized to tubulin is shown (###p < 0.001, Fisher PLSD analysis; p = 0.0697, unpaired t-test, mean ± SEM, n = 3). (G) Immunohistochemical staining of sciatic nerve from 18- and 38-month-old animals fed ad libitum (AL) and CR diets with an antibody against HNE. Nuclei are labeled with Hoechst dye (blue). Scale bar, 20 μm. (Color images available online at www.liebertonline.com/rej)
To examine the distribution of oxidatively damaged proteins within peripheral nerves, we immunostained sciatic nerves with the same anti-HNE antibody that was used for the western blots (Fig. 3G). There is a prominent increase in HNE-like immunoreactivity with age within the nerve tissues of ad libitum–fed rats, with diffuse localization throughout axonal and glial compartments (Fig. 3G). As suggested by the western blots, there are fewer HNE-positive areas within the 38-month CR counterpart. Although the anti-MDA and anti-nitrotyrosine antibodies are unsuitable for immunolocalization studies, we predict that those antibodies would show a similar diffuse immunoreactivity.
Age-related activation of proinflammatory pathways is attenuated by a lifelong CR diet
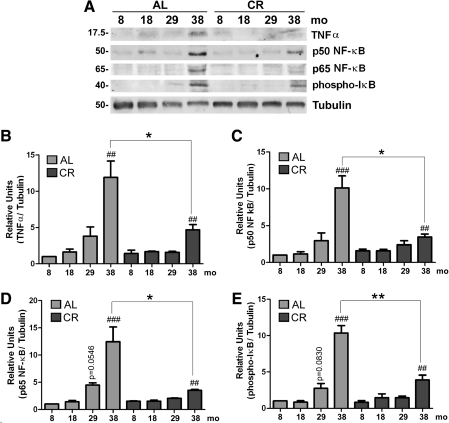
The inflammatory response appears to play a critical role in decreasing redox status and increasing oxidative damage observed with aging.12,27 Therefore, we analyzed sciatic nerve lysates for the levels of markers of chronic inflammation, including TNF-α, the p50 and p65 subunits of proinflammatory NF-κB, and phospho-IκB. For each of these proteins, we found an increasing trend with age, which is attenuated by the CR intervention (Fig. 4A). Quantification of blots from three different groups of animals revealed a significant increase in TNF-α from 8 months to 38 months (##p < 0.01) in animals receiving an ad libitum diet (Fig. 4B). The expression of TNF-α remains low in the CR group, with only significant increase in the oldest animals (##p < 0.01). Additionally, diet restriction significantly reduced TNF-α levels in 38-month-old nerves when compared to their ad libitum–fed counterparts (Fig. 4B, *p < 0.05).
FIG. 4.
Age-related increase in proinflammatory mediators is attenuated by a lifelong calorie-restricted (CR) diet. (A) Total sciatic nerve lysates (20 μg/lane) from the indicated ages and diet were analyzed with antibodies against tumor necrosis factor-α (TNF-α, p50 and p65 subunits of NF-κB, and phospho-IκB. Tubulin is shown as a loading control. Molecular mass is shown on the left in kDa. (B) Quantification of TNF-α band intensities after normalization to tubulin is represented (##p < 0.01, Fisher protected least significant difference (PLSD) analysis; *p < 0.05, unpaired t-test, mean ± standard error of the mean [SEM], n = 3). (C) Quantification of the p50 subunit of NF-κB band intensities normalized to tubulin is shown (##p < 0.01, ###p < 0.001, Fisher PLSD analysis; *p < 0.05, unpaired t-test, mean ± SEM, n = 3). (D) Quantification of the p65 subunit of NF-κB band intensities normalized to tubulin is shown (p = 0.0546, ##p < 0.01, ###p < 0.001, Fisher PLSD analysis; *p < 0.05, unpaired t-test, mean ± SEM, n = 3). (E) Phospho-IκB levels were also analyzed within the same lysates and quantification of band intensities normalized to tubulin was performed (p = 0.0830, ##p < 0.01, ###p < 0.001, Fisher PLSD analysis; **p < 0.01, unpaired t-test, mean ± SEM, n = 3). AL, Ad libitum.
Similar to TNF-α, there is an increase in the expression of both p50 and p65 subunits of proinflammatory NF-κB with age (###p < 0.001 between 8 months and 38 months; Fig. 4A,C,D) in the ad libitum group. Strikingly, the steady-state levels of p50 subunit of NF-κB in the sciatic nerve of 38-month-old CR rats is significantly lower (*p < 0.05) as compared to age-matched ad libitum–fed rats. An analogous trend is observed for the p65 subunit of NF-κB protein in nerves of ad libitum rats with age, and the attenuation of this rise with diet restriction (*p < 0.05; Fig. 4D). We analyzed the steady-state levels of phosphorylated IκB (phospho-IκB), which also shows an increasing pattern with age (Fig. 4E). In the CR group, the increase is still significant at the oldest age examined (##p < 0.01); however, a marked reduction in normalized phospho-IκB protein level is apparent when compared across diet groups (**p < 0.01) (Fig. 4E).
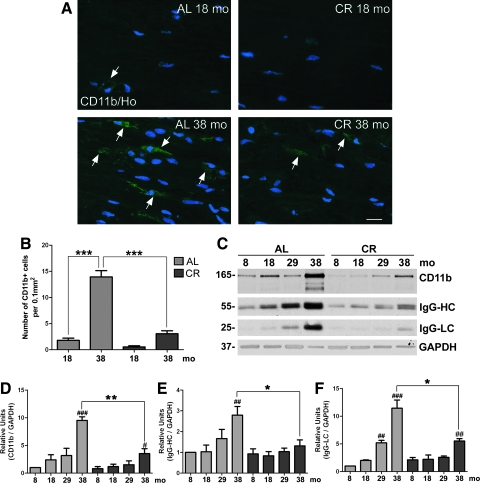
The main source for inflammatory mediators within myelinated peripheral nerves is infiltrating macrophages.28 Hence, we tested whether a CR diet would influence the number of infiltrating immune cells associated with aging in sciatic nerves. We used an anti-CD11b antibody to stain for macrophages,29 which appear to increase in numbers from 18 to 38 months on longitudinal nerve sections from ad libitum–fed rats (Fig. 5A). Quantification of CD11b+ immune cells reveals a significant age-related increase (***p < 0.001) in the ad libitum group from 18 to 38 months old (Fig. 5B). On the other hand, the number of macrophages is low in nerves of the CR rats, particularly when compared at the 38-month time point (***p < 0.001) (Fig. 5B). Accordingly, western blot analysis of whole nerve lysates reveals a pronounced increase in CD11b protein levels in 38-month-old ad libitum samples as compared to 8 months (Fig. 5C), whereas the levels remain low across the tested ages in the diet-restricted group (Fig. 5C,D). In agreement with the increased inflammatory response, biochemical studies with an anti-rat IgG antibody detect a marked increase in endogenous nerve immunoglobulins, specifically IgG heavy chain (HC) and light chain (LC) with age. The dietary modulation notably alleviates the increase in the levels of endogenous nerve IgGs (Fig. 5C,E,F).
FIG. 5.
A calorie-restricted (CR) diet diminishes macrophage infiltration of peripheral nerves with age. (A) Cryosections of sciatic nerves from 18- and 38-month-old rats either maintained on ad libitum (AL) or CR diet were immunostained with an antibody against CD11b (green). Nuclei are stained with Hoechst dye (blue). Scale bar, 20 μm. (B) Quantification of CD11b+ cells per 0.1 mm2 of nerve tissue area is shown for 18- and 38-month-old animals fed ad libitum or CR diets (***p < 0.001, unpaired t-test, mean ± standard error of the mean (SEM), n = 3). (C) Western blot analysis of sciatic nerve lysates (20 μg/lane) for steady-state levels of CD11b and endogenous immunoglobulin G (IgG) (heavy chain [HC] and light chain [LC]) from ad libitum and CR rats is shown. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is used as a protein loading control. Molecular mass in kDa is indicated on the left. (D) Quantification of the CD11b band intensities normalized to GAPDH from western blot analyses from whole sciatic nerve lysates is shown (#p < 0.05, ###p < 0.001, Fisher PLSD analysis; **p < 0.01, unpaired t-test, mean ± SEM, n = 3). (E) Quantification of the IgG-HC band intensities normalized to GAPDH from whole sciatic nerve lysates of ad libitum and CR rats of the indicated ages is shown (##p < 0.01, Fisher PLSD analysis; *p < 0.05, unpaired t-test, mean ± SEM, n = 3). (F) Quantification of band intensities of IgG-LC normalized to GAPDH was performed (##p < 0.01, ###p < 0.001, Fisher PLSD analysis; *p < 0.05, unpaired t-test, mean ± SEM, n = 3). (Color images available online at www.liebertonline.com/rej)
Discussion
Because physical barriers against circulating toxic oxidants such as capillary endothelia and the choroid plexus are absent within the periphery,30 the PNS is a vulnerable target for oxidative damage. Here we show a pronounced accumulation of ubiquitinated and oxidatively damaged proteins with age within myelinated peripheral nerves, which prompts an immunologic response from the host. In comparison, nerves of rats kept on a lifelong CR diet accumulate lower levels of modified proteins and the inflammatory response is muted. Whereas a lifelong CR might not be practical for humans, this study clearly shows the power of this intervention in preventing age-associated damage in peripheral nerves. On the basis of the findings presented here, we hypothesize that the PNS would also respond to a more acute, short-term CR or a long-term, mild CR. Both of these approaches have been explored in other organ systems with success,11,31 but their effects on peripheral nerves are not known.
Proteomics studies of aging rat brain have revealed a decrease in proteins required for proper ubiquitin–proteasomal degradation, resulting in a buildup of undegraded substrates.32 Due to sample size limitations, we were unable to test the levels of proteasomal constituents; however, we detected the accumulation of slow-migrating polyubiquiti-nated substrates within nerves of ad libitum–fed aged rats, suggestive of compromised proteasome activity. Significantly, the levels of such ubiquitin-reactive proteins are reduced in nerves of CR rats (Fig. 1). This effect could be due to less protein damage throughout life span, and/or sustained proteasomal processing of ubiquitinated substrates and/or their removal by an alternative mechanism, such as autophagy. Although we do not know yet which of these possibilities is influenced by the lifelong diet restriction, a contribution from the autophagy–lysosomal pathway is likely.18 With normal aging, lysosomes overwhelmed with lipofuscin still receive proteolytic enzymes, which leads to their depletion and decrease in overall autophagic capacity of the cell.33 Diet restriction is known to activate the autophagy–lysosomal pathway and assist in removal of damaged proteins.17,34 Thus, transgenic mice with compromised autophagic activity accumulate high levels of aggregated proteins that are reactive for ubiquitin.35,36 These findings, together with our previous reports,17,18,34 suggest that dietary restriction is beneficial for the activity of protein degradative pathways, such as the UPS and the autophagy–lysosomal system.
High levels of HNE, nitrotyrosine, and other irreversible protein adducts have been observed in age-related neurodegenerative conditions of the CNS,37 yet their contribution to peripheral nerve disease is unclear. Given that lipids are sensitive to free radical damage,38 myelinated nerves provide an opportune environment for lipid peroxidation-mediated oxidative damage to organelles and proteins. Indeed, we observed a gradual increase in lipid peroxidation products in our ad libitum samples with age, including MDA, HNE, and nitrotyrosine (Fig. 3). Oxidative modifications in cellular proteins lead to increased proteolytic susceptibility due to the hydrophobicity.8,24,39 This increase in proteolytic recognition, however, functions only for mildly oxidized substrates, as extensively oxidized proteins tend to aggregate and covalently cross-link.24,40,41 Such changes are suggested by the accumulation of slow-migrating proteins in nerve samples from old ad libitum rodents (Figs. 1 and 3). Besides protein degradation mechanisms, cells use antioxidant cascades to modulate oxidative damage. With age, both of these processes become less efficient, which can lead to further accumulation of lipid peroxidation, protein oxidation, and other deleterious cellular modifications.22,40 Our results suggest that a CR diet attenuates lipid peroxidation (Fig. 3) and thereby decreases oxidative damage within peripheral nerves with age.
Age-associated oxidant stress is known to activate the inflammatory process via the NF-κB transcription factor and associated target genes, including TNF-α.9 In human and rodent studies, the circulating levels of this proinflammatory cytokine TNF-α increase with age, which in rodents is significantly attenuated by CR.42,43 In agreement with these findings, we found age-associated increase in TNF-α expression in peripheral nerves, a trend that was attenuated up to 38 months of age by CR (Fig. 4). One of the upstream modulators of NF-κB activity is IκB kinase, which is activated by redox imbalance and oxidative stress.9 The phosphorylation of the IκB subunit of NF-κB/IκB complex triggers the degradation of IκB, which then leads to the activation of NF-κB.44,45 Studies in different organ systems have shown an increase in NF-κB activity with age and in disease.9,46 Corresponding to the increase in NF-κB binding activity with age, the levels of p65 and p50 subunits of NF-κB, as well as phospho-IκB are elevated,47 a pattern that is reproduced in myelinated peripheral nerves (Fig. 4). The upregulation of NF-κB in sciatic nerves of old ad libitum–fed rats could be a response to the severe demyelination,18 in combination with an inflammatory response (Fig. 5).48 In inflammatory demyelinating neuropathies, macrophages infiltrated into the nerves are the primary expressors of NF-κB48 and are the likely source for this cytokine in aged nerves as well. Although due to antibody binding specificities we could not perform colocalization studies for macrophages and NF-κB in our rat nerve samples, the significant attenuation of macrophages in CR samples in association with a decrease in proinflammatory NF-κB levels supports this theory.
Infiltration of peripheral nerve tissue by CD11b+ macrophages in inflammatory nerve disease and hereditary demyelinating neuropathies take part in the pathogenic cascade.29,48,49 Upon analyses of longitudinal sections of sciatic nerves, we observed a significant increase (***p < 0.001) in the number of CD11b+ macrophages in ad libitum–fed animals from age 18 to 38 months (Fig. 5), which could contribute to the pronounced morphologic changes observed.18 Indeed, ultrastructural studies of senescent paranodal junctions of rat peripheral nerves have detected myelin fragment-filled macrophages between the ages of 24 and 31 months.50 In addition to a possible role in myelin damage, a pronounced inflammatory response is also evidenced by accumulation of immunoglobulins within nerves, targets of which antibodies are unknown. Together, our studies support the activation of the inflammatory response through oxidative damage within aged peripheral nerves, a detrimental cascade that is muted in nerves of calorie-restricted rodents.
In summary, our studies show evidence of pronounced oxidative damage within myelinated peripheral nerves with age and associated activation of proinflammatory events. In comparison, lifelong CR thwarts such detrimental changes, likely by attenuating the levels of damaging molecules and allowing the cells to maintain endogenous protective mechanisms.14,18 These findings offer novel insights into possible mechanisms of age-associated decline in neural function and provide targets for therapeutic interventions.
Acknowledgments
This research was supported by grants to L.N. (National Muscular Dystrophy Association and U.S. National Institutes of Health NS041012 grants) and C.L. (NIA R01-AG17994 and AG21042) and supported by the University of Florida Institute on Aging and Claude D. Pepper Older Americans Independence Center (1 P30 AG028740). The authors wish to thank Dr. Susie Zoltewicz for a critical reading of this manuscript.
Author Disclosure Statement
No competing financial interests exist
References
- 1.Bokov A. Chaudhuri A. Richardson A. The role of oxidative damage and stress in aging. Mech Ageing Dev. 2004;125:811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal S. Sohal RS. DNA oxidative damage and life expectancy in houseflies. Proc Natl Acad Sci USA. 1994;91:12332–12335. doi: 10.1073/pnas.91.25.12332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohal RS. Agarwal S. Candas M. Forster MJ. Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech Ageing Dev. 1994;76:215–224. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- 4.Garbay B. Heape AM. Sargueil F. Cassagne C. Myelin synthesis in the peripheral nervous system. Prog Neurobiol. 2000;61:267–304. doi: 10.1016/s0301-0082(99)00049-0. [DOI] [PubMed] [Google Scholar]
- 5.Blair I. A. Lipid hydroperoxide-mediated DNA damage. Exp Gerontol. 2001;36:1473–1481. doi: 10.1016/s0531-5565(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 6.Smith KJ. Kapoor R. Felts PA. Demyelination: The role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine RL. Stadtman ER. Oxidative modification of proteins during aging. Exp Gerontol. 2001;36:1495–1502. doi: 10.1016/s0531-5565(01)00135-8. [DOI] [PubMed] [Google Scholar]
- 8.Grune T. Davies KJ. The proteasomal system and HNE-modified proteins. Mol Aspects Med. 2003;24:195–204. doi: 10.1016/s0098-2997(03)00014-1. [DOI] [PubMed] [Google Scholar]
- 9.Chung H. Y. Cesari M. Anton S. Marzetti E. Giovannini S. Seo AY. Carter C. Yu BP. Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spaulding CC. Walford RL. Effros RB. Calorie restriction inhibits the age-related dysregulation of the cytokines TNF-alpha and IL-6 in C3B10RF1 mice. Mech Ageing Dev. 1997;93:87–94. doi: 10.1016/s0047-6374(96)01824-6. [DOI] [PubMed] [Google Scholar]
- 11.Seo AY. Hofer T. Sung B. Judge S. Chung HY. Leeuwenburgh C. Hepatic oxidative stress during aging: Effects of 8% long-term calorie restriction and lifelong exercise. Antioxid Redox Signal. 2006;8:529–538. doi: 10.1089/ars.2006.8.529. [DOI] [PubMed] [Google Scholar]
- 12.Salminen A. Ojala J. Huuskonen U. Kauppinen A. Suuronen T. Kaarniranta K. Interaction of aging-associated signaling cascades: inhibition of NF-kappaB signaling by longevity factors FoxOs and SIRT1. Cell Mol Life Sci. 2008;65:1049–1058. doi: 10.1007/s00018-008-7461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y. Kawamura N. Schmelzer JD. Schmeichel AM. Low PA. Decreased peripheral nerve damage after ischemia-reperfusion injury in mice lacking TNF-alpha. J Neurol Sci. 2008;267:107–111. doi: 10.1016/j.jns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin B. Mattson MP. Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006;5:332–353. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sohal RS. Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert AJ. Portero-Otin M. Pamplona R. Merry BJ. Effect of ageing and caloric restriction on specific markers of protein oxidative damage and membrane peroxidizability in rat liver mitochondria. Mech Ageing Dev. 2004;125:529–538. doi: 10.1016/j.mad.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Wohlgemuth SE. Julian D. Akin DE. Fried J. Toscano K. Leeuwenburgh C. Dunn WA., Jr Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007;10:281–292. doi: 10.1089/rej.2006.0535. [DOI] [PubMed] [Google Scholar]
- 18.Rangaraju S. Hankins D. Madorsky I. Madorsky E. Lee WH. Carter CS. Leeuwenburgh C. Notterpek L. Molecular architecture of myelinated peripheral nerves is supported by calorie restriction with aging. Aging Cell. 2009;8:178–191. doi: 10.1111/j.1474-9726.2009.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J. Knutson MD. Carter CS. Leeuwenburgh C. Iron accumulation with age, oxidative stress and functional decline. PLoS One. 2008;3:e2865. doi: 10.1371/journal.pone.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turturro A. Witt WW. Lewis S. Hass BS. Lipman BD. Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 21.Rangaraju S. Hankins D. Madorsky I. Madorsky E. Lee WH. Carter CS. Leeuwenburgh C. Nottepek L. The molecular architecture of myelinated peripheral nerves is supported by calorie restriction with aging. Aging Cell. 2009;8:178–191. doi: 10.1111/j.1474-9726.2009.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grune T. Jung T. Merker K. Davies KJ. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and 'aggresomes' during oxidative stress, aging, and disease. Int J Biochem Cell Biol. 2004;36:2519–2530. doi: 10.1016/j.biocel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Jung T. Bader N. Grune T. Lipofuscin: formation, distribution, and metabolic consequences. Ann NY Acad Sci. 2007;1119:97–111. doi: 10.1196/annals.1404.008. [DOI] [PubMed] [Google Scholar]
- 24.Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- 25.Adams S. Green P. Claxton R. Simcox S. Williams MV. Walsh K. Leeuwenburgh C. Reactive carbonyl formation by oxidative and non-oxidative pathways. Front Biosci. 2001;6:A17–A24. doi: 10.2741/adams. [DOI] [PubMed] [Google Scholar]
- 26.Gard AL. Solodushko VG. Waeg G. Majic T. 4-Hydroxynonenal, a lipid peroxidation byproduct of spinal cord injury, is cytotoxic for oligodendrocyte progenitors and inhibits their responsiveness to PDGF. Microsc Res Tech. 2001;52:709–718. doi: 10.1002/jemt.1055. [DOI] [PubMed] [Google Scholar]
- 27.Beckman KB. Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 28.Hartung HP. Jung S. Stoll G. Zielasek J. Schmidt B. Archelos JJ. Toyka KV. Inflammatory mediators in demyelinating disorders of the CNS and PNS. J Neuroimmunol. 1992;40:197–210. doi: 10.1016/0165-5728(92)90134-7. [DOI] [PubMed] [Google Scholar]
- 29.Misko A. Ferguson T. Notterpek L. Matrix metalloproteinase mediated degradation of basement membrane proteins in Trembler J neuropathy nerves. J Neurochem. 2002;83:885–894. doi: 10.1046/j.1471-4159.2002.01200.x. [DOI] [PubMed] [Google Scholar]
- 30.Samson FE. Nelson SR. The aging brain, metals and oxygen free radicals. Cell Mol Biol (Noisy-le-grand) 2000;46:699–707. [PubMed] [Google Scholar]
- 31.Ingram DK. Young J. Mattison JA. Calorie restriction in nonhuman primates: assessing effects on brain and behavioral aging. Neuroscience. 2007;145:1359–1364. doi: 10.1016/j.neuroscience.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 32.Yang S. Liu T. Li S. Zhang X. Ding W. Que H. Yan X. Wei K. Liu S. Comparative proteomic analysis of brains of naturally aging mice. Neuroscience. 2008;154:1107–1120. doi: 10.1016/j.neuroscience.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Brunk UT. Terman A. The mitochondrial-lysosomal axis theory of aging: Accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem. 2002;269:1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- 34.Madorsky I. Opalach K. Waber A. Verrier JD. Solmo C. Foster T. Dunn WA., Jr Nottepek L. Intermittent fasting alleviates the neuropathic phenotype in a mouse model of Charcot-Marie-Tooth disease. Neurobiol Dis. 2009;34:146–154. doi: 10.1016/j.nbd.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizushima N. Hara T. Intracellular quality control by autophagy: how does autophagy prevent neurodegeneration? Autophagy. 2006;2:302–304. doi: 10.4161/auto.2945. [DOI] [PubMed] [Google Scholar]
- 36.Komatsu M. Waguri S. Ueno T. Iwata J. Murata S. Tanida I. Ezaki J. Mizushima N. Ohsumi Y. Uchiyama Y. Kominami E. Tanaka K. Chiba T. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beal MF. Oxidatively modified proteins in aging and disease. Free Radic Biol Med. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 38.Pamplona R. Portero-Otin M. Riba D. Requena JR. Thorpe SR. Lopez-Torres M. Barja G. Low fatty acid unsaturation: a mechanism for lowered lipoperoxidative modification of tissue proteins in mammalian species with long life spans. J Gerontol A Biol Sci Med Sci. 2000;55:B286–B291. doi: 10.1093/gerona/55.6.b286. [DOI] [PubMed] [Google Scholar]
- 39.Grune T. Merker K. Sandig G. Davies KJ. Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem Biophys Res Commun. 2003;305:709–718. doi: 10.1016/s0006-291x(03)00809-x. [DOI] [PubMed] [Google Scholar]
- 40.Grune T. Shringarpure R. Sitte N. Davies K. Age-related changes in protein oxidation and proteolysis in mammalian cells. J Gerontol A Biol Sci Med Sci. 2001;56:B459–B467. doi: 10.1093/gerona/56.11.b459. [DOI] [PubMed] [Google Scholar]
- 41.Farout L. Friguet B. Proteasome function in aging and oxidative stress: Implications in protein maintenance failure. Antioxid Redox Signal. 2006;8:205–216. doi: 10.1089/ars.2006.8.205. [DOI] [PubMed] [Google Scholar]
- 42.Bruunsgaard H. The clinical impact of systemic low-level inflammation in elderly populations. With special reference to cardiovascular disease, dementia and mortality. Dan Med Bull. 2006;53:285–309. [PubMed] [Google Scholar]
- 43.Phillips T. Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by lifelong calorie restriction. FASEB J. 2005;19:668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- 44.Zandi E. Rothwarf DM. Delhase M. Hayakawa M. Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 45.Karin M. Role for IKK2 in muscle: Waste not, want not. J Clin Invest. 2006;116:2866–2868. doi: 10.1172/JCI30268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandrasekar B. Freeman G. L. Induction of nuclear factor kappaB and activation protein 1 in postischemic myocardium. FEBS Lett. 1997;401:30–34. doi: 10.1016/s0014-5793(96)01426-3. ). [DOI] [PubMed] [Google Scholar]
- 47.Kim HJ. Yu BP. Chung HY. Molecular exploration of age-related NF-kappaB/IKK downregulation by calorie restriction in rat kidney. Free Radic Biol Med. 2002;32:991–1005. doi: 10.1016/s0891-5849(02)00798-0. [DOI] [PubMed] [Google Scholar]
- 48.Andorfer B. Kieseier BC. Mathey E. Armati P. Pollard J. Oka N. Hartung HP. Expression and distribution of transcription factor NF-kappaB and inhibitor IkappaB in the inflamed peripheral nervous system. J Neuroimmunol. 2001;116:226–232. doi: 10.1016/s0165-5728(01)00306-x. [DOI] [PubMed] [Google Scholar]
- 49.Ceballos D. Cuadras J. Verdu E. Navarro X. Morphometric and ultrastructural changes with ageing in mouse peripheral nerve. J Anat. 1999;195(Pt 4):563–576. doi: 10.1046/j.1469-7580.1999.19540563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugiyama I. Tanaka K. Akita M. Yoshida K. Kawase T. Asou H. Ultrastructural analysis of the paranodal junction of myelinated fibers in 31-month-old-rats. J Neurosci Res. 2002;70:309–317. doi: 10.1002/jnr.10386. [DOI] [PubMed] [Google Scholar]