Abstract
Background
Studies of the effect of right ventricular ejection fraction (RVEF) on outcomes in heart failure (HF) are limited by small sample size and short follow-up.
Methods and Results
We examined the effect of baseline RVEF on outcomes in 2008 Beta-Blocker Evaluation of Survival Trial participants with HF and left ventricular ejection fraction (LVEF) ≤35% during 24 months of mean follow-up. RVEF, estimated by gated-equilibrium radionuclide ventriculography, was used to categorize patients into four RVEF groups: ≥40% (n=733), 30–39% (n=531), 20–29% (n=473) and <20% (n=271). Unadjusted rates for all-cause mortality in patients with RVEF ≥40%, 30–39%, 20–29% and <20% were 27%, 32%, 35% and 47%, respectively. When compared to patients with RVEF ≥40%, unadjusted hazard ratios (HR) and 95% confidence intervals (CI) for all-cause mortality for those with RVEF 30–39%, 20–29% and <20% were 1.19 (0.97–1.46; P=0.087), 1.45 (1.17–1.78; P=0.001) and 1.98 (1.59–2.47; P<0.0001) respectively. Respective multivariable-adjusted HR’s (95% CI’s) for all-cause mortality associated with RVEF 30–39%, 20–29% and <20% were 1.07 (0.87–1.32; P=0.518), 1.12 (0.89–1.40; P=0.328) and 1.32 (1.02–1.71; P=0.034) respectively. Adjusted HR’s (95% CI’s) for other outcomes associated with RVEF <20% (compared to ≥40%) were: cardiovascular mortality, 1.33 (1.01–1.76; P=0.041); HF mortality, 1.61 (1.03–2.52; P=0.037); sudden cardiac death, 1.29 (0.87–1.91; P=0.212); all-cause hospitalization, 1.21 (1.00–1.47; P=0.056) and HF hospitalization, 1.39 (1.10–1.77; P=0.007).
Conclusions
Baseline RVEF <20% is a significant independent predictor of mortality and HF hospitalization in systolic HF.
Keywords: Heart Failure, Right Ventricle, Mortality, Morbidity
The impact of a reduced left ventricular (LV) ejection fraction (EF) on outcomes in heart failure (HF) is well documented in the literature.1–4 However, little is known about the impact of a reduced right ventricular (RV) EF on outcomes in chronic systolic HF.5–7 Most studies of RVEF in HF are limited by small sample size, short follow-up or potential bias due to confounding variables.8–15 Of the 2708 patients with HF and LVEF ≤35% in the Beta-Blocker Evaluation of Survival Trial (BEST), 2008 had data on baseline RVEF along with data on a large number of other baseline characteristics and long-term follow-up.16 Therefore, the objective of the present study was to examine the effect of baseline RVEF on outcomes in BEST patients.
Methods
Study design
We obtained a public-use copy of the BEST dataset from the National Heart, Lung, and Blood Institute (NHLBI).17 The BEST was sponsored by the NHLBI and the Department of Veterans Affairs Cooperative Studies Program. The rationale, design and results of the BEST have been previously reported.16, 18 Briefly, 2708 chronic HF patients were randomly assigned to bucindolol or placebo between May 1995 and December 1998 at 90 clinical sites in the United States and Canada and followed for a mean duration of 24 months. All but one participant consented to be included in the public-use copy of the database used for the current analysis.
Patients
At randomization, all HF patients in the BEST study had LVEF ≤35%, had a mean duration of 49 months of HF and all had New York Heart Association (NYHA) functional class III (92%) or IV (8%) symptoms. Most patients were receiving angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (> 90%), diuretics (> 90%) and digoxin (> 90%). The protocol was approved by each participating site’s institutional review board and all patients gave written informed consent.
Estimation of LVEF and RVEF
Data on baseline LVEF and RVEF were collected before randomization by gated-equilibrium radionuclide ventriculography using standard techniques at each of the sites. If a patient did not have a LVEF and RVEF by radionuclide ventriculography at a BEST site during the 60 days before randomization, a study was performed at the time of randomization. For quality control purposes, the first two examinations at each site were sent for re-reading at a core laboratory. Thereafter, a random sample of 5% of all the examinations was sent to the core laboratory for quality control.19 Valid measurements of RVEF were available for 2008 patients. The lower limit of normal RVEF by gated-equilibrium radionuclide ventriculography is 40%.20, 21 For the current analysis, we categorized patients into four RVEF groups: ≥40% (n=733 or 37%), 30–39% (n=531 or 26%), 20–29% (n=473 or 24%) and <20% (n=271 or 13%).
Study outcomes
In the current study, primary outcomes were all-cause mortality (the BEST primary outcome) and HF hospitalization (a BEST secondary outcome). Our secondary outcomes were cardiovascular and HF mortality, sudden cardiac death, and all-cause hospitalization.
Statistical analysis
We used chi-square tests and analysis of variance tests, as appropriate, for descriptive analyses to compare baseline characteristics between the four RVEF groups. Kaplan-Meier plots were constructed to determine associations of RVEF groups with all-cause mortality and HF hospitalization. Associations of various RVEF categories with outcomes were determined using Kaplan-Meier survival analysis and Cox proportional hazard models. RVEF category ≥40% was used as the reference category and dummy variables were used for RVEF categories 30% 39%, 20% 29% and <20%. Variables were entered into the model in multiple steps in the following order: step 1 (unadjusted: dummy variables for RVEF 30–39%, 20–29% and <20%), and step 2 (step 1 plus LVEF), step 3 (step 2 plus demographics), step 4 (step 3 plus medical history), step 5 (step 4 plus medications), step 6 (step 5 plus clinical findings), and step 7 (step 6 plus laboratory findings). The same model was used for all the outcomes. We confirmed the assumption of proportional hazards by a visual examination of the log (minus log) curves. All statistical tests were evaluated using two-tailed 95% confidence levels and tests with p-value <0.05 were considered significant. Data analyses were performed using SPSS for Windows, Rel. 15. 2006. Chicago: SPSS Inc.
Results
Baseline characteristics
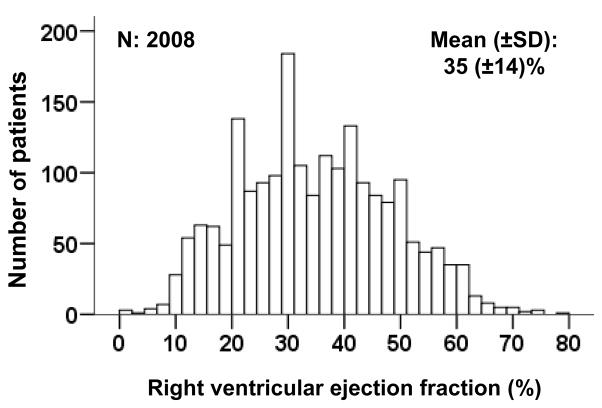
Patients had a mean age of 60 (±12) years, 21% were women and 21% were African Americans. Compared to patients with normal RVEF, those in the lower RVEF categories had lower mean age, fewer women and more African Americans (Table 1). They also had lower mean LVEF, longer HF duration, and a higher prevalence of NYHA functional class IV (Table 1). The distribution of RVEF among the study participants is displayed in Figure 1.
Table 1.
Baseline patient characteristics by right ventricular ejection fraction (RVEF)
| n (%) or mean (±SD) | RVEF ≥40% (n=733) | RVEF 30–39% (n=531) | RVEF 20–29% (n=473) | RVEF <20% (n=271) | P-value |
|---|---|---|---|---|---|
| Age, years | 61 (±12) | 60 (±12) | 61 (±12) | 58 (±13) | 0.010 |
| Female | 184 (25) | 108 (20) | 84 (18) | 38 (14) | <0.0001 |
| African American | 119 (16) | 109 (20) | 112 (24) | 88 (32) | <0.0001 |
| Current smoker | 127 (17) | 91 (17) | 85 (18) | 37 (14) | 0.470 |
| New York Heart Association class III | 687 (94) | 488 (92) | 424 (90) | 229 (84) | <0.001 |
| Body mass index, kg/m2 | 36 (±8) | 37 (±9) | 36 (±8) | 36 (±8) | 0.018 |
| Heart rate, beats per minute | 81 (±13) | 81 (±13) | 82 (±13) | 87 (±14) | <0.0001 |
| Systolic blood pressure, mm Hg | 121 (±18) | 116 (±18) | 114 (±18) | 112 (±17) | <0.0001 |
| Diastolic blood pressure, mm Hg | 71 (±11) | 71 (±11) | 70 (±11) | 72 (±11) | 0.050 |
| Left ventricular ejection fraction, % | 26 (±6.6) | 23 (±6.8) | 21 (±6.7) | 17 (±6.1) | <0.0001 |
| Right ventricular ejection fraction, % | 49 (±7.3) | 34 (±3.0) | 25 (±2.9) | 14 (±3.3) | <0.0001 |
| Past medical history | |||||
| Duration of heart failure, months | 47 (±45) | 48 (±46) | 56 (±56) | 50 (±49) | 0.012 |
| Idiopathic dilated cardiomyopathy | 220 (30) | 148 (28) | 113 (24) | 83 (31) | 0.096 |
| Coronary artery disease | 428 (58) | 310 (58) | 299 (63) | 161 (59) | 0.342 |
| Evidence of prior inferior/posterior myocardial infarction | 110 (15) | 77 (15) | 62 (13) | 41 (15) | 0.807 |
| Coronary artery bypass surgery | 205 (28) | 161 (30) | 164 (35) | 74 (27) | 0.063 |
| Percutaneous coronary intervention | 116 (16) | 81 (15) | 73 (15) | 38 (14) | 0.919 |
| Hypertension | 419 (57) | 313 (59) | 261 (55) | 165 (61) | 0.427 |
| Diabetes mellitus | 246 (34) | 189 (36) | 163 (34) | 103 (38) | 0.597 |
| Hyperlipidemia | 343 (47) | 241 (45) | 176 (37) | 109 (40) | 0.005 |
| Atrial fibrillation | 153 (21) | 145 (27) | 134 (28) | 57 (21) | 0.005 |
| Chronic kidney disease* | 281 (38) | 186 (35) | 189 (40) | 107 (39) | 0.384 |
| Medications | |||||
| Bucindolol | 362 (49) | 276 (52) | 233 (49) | 146 (54) | 0.506 |
| Angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers | 705 (96) | 513 (97) | 455 (96) | 258 (95) | 0.809 |
| Digitalis | 668 (91) | 491 (92) | 447 (94) | 458 (95) | 0.056 |
| Diuretics | 668 (91) | 496 (93) | 451 (95) | 262 (97) | 0.003 |
| Vasodilators | 340 (46) | 255 (48) | 200 (42) | 127 (47) | 0.305 |
| Anticoagulants | 400 (55) | 337 (63) | 286 (60) | 163 (60) | 0.012 |
Estimated glomerular filtration rate <60 ml/min per 1.73 m2 of body surface area
Figure 1.
Histogram displaying frequency distribution of right ventricular ejection fraction (%)
Association between RVEF and mortality
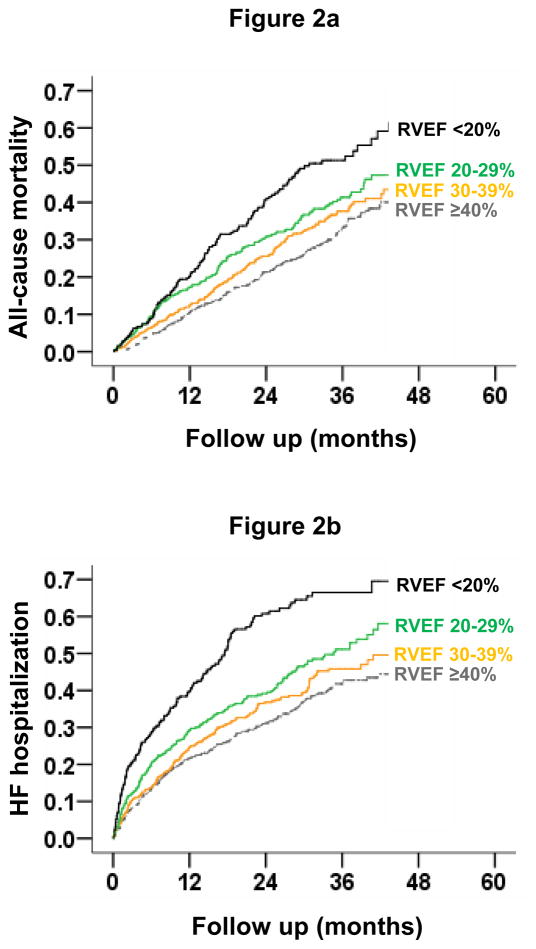
All-cause mortality occurred in 27%, 32%, 35% and 47% of patients with RVEF ≥40%, 30–39%, 20–29% and <20%, respectively (Table 2 and Figure 2a). When compared to patients with RVEF ≥40%, unadjusted hazard ratios (HR) and 95% confidence intervals (CI) for all-cause mortality for those with RVEF 30–39%, 20–29% and <20% were 1.19 (0.97–1.46; P=0.087), 1.45 (1.17–1.78; P=0.001) and 1.98 (1.59–2.47; P<0.0001) respectively. Respective adjusted HR’s for all-cause mortality for those with RVEF 30–39%, 20–29% and <20% were 1.07 (95% CI, 0.87–1.32; P=0.518), 1.12 (95% CI, 0.89–1.40; P=0.328) and 1.32 (95% CI, 1.02–1.71; P=0.034) respectively. Unadjusted and adjusted HR’s (95% CIs) for cause-specific mortalities are displayed in Table 3.
Table 2.
Associations of right ventricular ejection fraction (RVEF) with all-cause mortality
| Hazard ratio (95% confidence interval); P value | ||||
|---|---|---|---|---|
| RVEF ≥40% (n=733) | RVEF 30–39% (n=531) | RVEF 20–29% (n=473) | RVEF <20% (n=271) | |
| All-cause mortality (%) | 27% | 32% | 35% | 47% |
| Step 1:Unadjusted | 1.00 (Reference) | 1.19 (0.97–1.46); P =0.087 | 1.45 (1.17–1.78) P =0.001 | 1.98 (1.59–2.47) P <0.0001 |
| Step 2: Step 1 + LVEF* | 1.00 (Reference) | 1.12 (0.91–1.37); P =0.295 | 1.27 (1.02–1.57) P =0.031 | 1.52 (1.20–1.94) P =0.001 |
| Step 3: Step 2 + demographics** | 1.00 (Reference) | 1.14 (0.93–1.40); P =0.217 | 1.26 (1.02–1.56) P =0.036 | 1.62 (1.27–2.06) P <0.0001 |
| Step 4: Step 3 + medical history*** | 1.00 (Reference) | 1.06 (0.86–1.31); P =0.563 | 1.18 (0.95–1.47) P =0.139 | 1.52 (1.19–1.95) P =0.001 |
| Step 5: Step 4 + medications**** | 1.00 (Reference) | 1.06 (0.86–1.30); P =0.597 | 1.17 (0.94–1.45) P =0.169 | 1.49 (1.16–1.91) P =0.002 |
| Step 6: Step 5 + clinical findings***** | 1.00 (Reference) | 1.05 (0.85–1.30); P =0.640 | 1.07 (0.86–1.34) P =0.542 | 1.37 (1.06–1.76) P =0.015 |
| Step 7: Step 6 + laboratory findings****** | 1.00 (Reference) | 1.07 (0.87–1.32); P =0.518 | 1.12 (0.89–1.40) P =0.328 | 1.32 (1.02–1.71) P =0.034 |
LVEF=left ventricular ejection fraction
Demographics: age, sex, and race.
Medical history: duration of smoking, duration of heart failure, New York Heart Association class, coronary artery disease, angina pectoris, diabetes mellitus, hypertension, hyperlipidemia, peripheral vascular disease, atrial fibrillation, >70% coronary artery stenosis, positive stress perfusion test, and electrocardiographic evidence of anterior, lateral and inferior-posterior myocardial infarction
Medications: bucindolol, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, digitalis, diuretics, and anticoagulants
Clinical findings: body mass index, heart rate, systolic and diastolic blood pressure, S3 gallop, pulmonary rales, and x-ray findings of cardiothoracic ratio and pulmonary edema
Laboratory findings: creatinine, potassium, sodium, magnesium, blood urea nitrogen, glucose, uric acid, total cholesterol, albumin, hemoglobin, white blood cells, and platelets
Figure 2.
Kaplan-Meier plots for (2a) all-cause mortality and (2b) heart failure hospitalization by right ventricular ejection fraction (RVEF) categories
Table 3.
Associations of right ventricular ejection fraction (RVEF) with cause-specific outcomes
| Events (%) | Unadjusted hazard ratio (95% confidence interval); P value | Adjusted hazard ratio* (95% confidence interval); P value | |
|---|---|---|---|
| Cardiovascular mortality | |||
| RVEF ≥40% | 23% | 1.00 (Reference) | 1.00 (Reference) |
| RVEF 30–39% | 25% | 1.13 (0.90–1.41); P =0.299 | 0.98 (0.78–1.24); P =0.885 |
| RVEF 20–29% | 30% | 1.46 (1.17–1.83); P =0.001 | 1.09 (0.86–1.39); P =0.487 |
| RVEF <20% | 41% | 2.07 (1.64–2.63); P <0.0001 | 1.33 (1.01–1.76); P =0.041 |
| Heart failure mortality | |||
| RVEF ≥40% | 8% | 1.00 (Reference) | 1.00 (Reference) |
| RVEF 30–39% | 9% | 1.05 (0.72–1.53); P =0.814 | 0.89 (0.60–1.32); P =0.549 |
| RVEF 20–29% | 11% | 1.49 (1.03–2.16); P =0.036 | 1.03 (0.69–1.54); P =0.890 |
| RVEF <20% | 18% | 2.47 (1.70–3.58); P <0.0001 | 1.61 (1.03–2.52); P =0.037 |
| Sudden cardiac death | |||
| RVEF ≥40% | 11% | 1.00 (Reference) | 1.00 (Reference) |
| RVEF 30–39% | 15% | 1.35 (0.99–1.84); P =0.056 | 1.22 (0.89–1.67); P =0.221 |
| RVEF 20–29% | 15% | 1.55 (1.13–2.14); P =0.007 | 1.23 (0.88–1.72); P =0.228 |
| RVEF <20% | 20% | 2.01 (1.43–2.83); P <0.0001 | 1.29 (0.87–1.91); P =0.212 |
| All-cause hospitalization | |||
| RVEF ≥40% | 60% | 1.00 (Reference) | 1.00 (Reference) |
| RVEF 30–39% | 64% | 1.10 (0.96–1.90); P =0.168 | 1.03 (0.89–1.19); P =0.665 |
| RVEF 20–29% | 65% | 1.24 (1.07–1.44); P =0.005 | 1.09 (0.93–1.27); P =0.312 |
| RVEF <20% | 73% | 1.61 (1.36–1.90); P <0.0001 | 1.21 (1.00–1.47); P =0.056 |
| Heart failure hospitalization | |||
| RVEF ≥40% | 33% | 1.00 (Reference) | 1.00 (Reference) |
| RVEF 30–39% | 37% | 1.16 (0.96–1.39); P =0.126 | 1.00 (0.83–1.21); P =0.984 |
| RVEF 20–29% | 41% | 1.45 (1.20–1.75); P <0.0001 | 1.09 (0.89–1.34); P =0.395 |
| RVEF <20% | 55% | 2.25 (1.84–2.82); P <0.0001 | 1.39 (1.10–1.77); P =0.007 |
Multivariable model based on model 7 from Table 2
Association between RVEF and hospitalization
HF hospitalization occurred in 33%, 37%, 41% and 55% of patients with RVEF ≥40%, 30–39%, 20–29% and <20%, respectively (Table 3 and Figure 2b). Compared to patients with RVEF ≥40%, unadjusted HR for HF hospitalization for those with RVEF <20% was 2.25 (95% CI, 1.84–2.82; P<0.0001), which remained significant despite multivariable-adjustment (HR, 1.39; 95% CI, 1.10–1.77; P=0.007). Unadjusted and adjusted HR’s (95% CI’s) for all-cause hospitalization are displayed in Table 3.
Effect of LVEF on Outcomes by RVEF
All-cause mortality occurred in 40% and 29% of patients with LVEF <20% and ≥20%, respectively (unadjusted HR for LVEF <20%, 1.61; 95% CI, 1.38–1.87; P <0.0001). When adjusted for RVEF <20%, this association was attenuated but remained significant (HR, 1.48; 95% CI, 1.26–1.74; P <0.0001).
Effect of Bucindolol on Outcomes by RVEF
Among patients with RVEF ≥40%, all-cause mortality occurred in 24% and 30% of patients randomized to receive bucindolol and placebo respectively (HR for bucindolol, 0.73; 95% CI, 0.55–0.97; P=0.031). All-cause mortality for patients in the bucindolol versus placebo groups were 30% versus 33% for those with RVEF 30–39% (P=0.477), 32% versus 38% for those with RVEF 20–29%; (P=0.162) and 49% versus 43% for those in the RVEF <20% (P=0.314).
Discussion
The findings of the current comprehensive report, based on the largest study of RVEF in HF to date, confirm previous reports describing the importance of RV failure on outcomes in patients with chronic systolic HF and identify RVEF <20% as having an independent effect on mortality. While RVEF <40% was associated with progressive increase in the risk of mortality and hospitalization, only RVEF <20% had an intrinsic association with increased mortality and HF hospitalization that was independent of all measured baseline characteristics that included many potential confounders such as age, LVEF, and cardiovascular comorbidities. These findings suggest that RVEF may be useful both as a marker and mechanism of poor prognosis in systolic HF and that the estimation of RVEF should be considered as a part of a comprehensive assessment of these patients.
LVEF impairment is believed to be the most common cause of RVEF impairment.4, 6 An increase in RV afterload through the development of pulmonary arterial hypertension secondary to chronic pulmonary venous hypertension has long been considered the main underlying mechanism of RV failure.22 However, recent findings from animal models of pulmonary hypertension suggest that degree or duration of RV pressure overload may not fully explain RV failure and that complex heart-lung interactions at cellular and molecular levels resulting in angioproliferative pulmonary vascular disease and myocardial fibrosis underlie RV failure.23 RVEF may also be impaired due to other mechanisms including ventricular interdependence associated with septal dysfunction and limited pericardial flexibility, neurohormonal interactions and reduced RV coronary perfusion secondary to decreased systolic driving pressure.6, 7 Low RVEF, in turn, may further reduce left ventricular output by impairing adequate LV preload, thereby enhancing neurohormonal activation.7 This may precipitate end-organ hypoperfusion and progressive clinical deterioration leading to eventual poor outcomes. Therefore, low RVEF, while primarily a consequence of a low LVEF, may also be a cause of further LVEF impairment, disease progression and poor outcomes.
RVEF and LVEF may also be impaired simultaneously as in patients with idiopathic dilated cardiomyopathy. However, the prevalence of idiopathic dilated cardiomyopathy was low and was balanced across all four RVEF groups. These findings suggest that a reduced RVEF may not be a useful marker for idiopathic dilated cardiomyopathy in advanced chronic systolic HF. Low RVEF was also not associated with prior inferior-posterior myocardial infarction, which may involve the RV. Taken together, these data suggest that in patients with advanced chronic systolic HF, RVEF impairment may primarily be a consequence of LVEF impairment.
Interestingly, nearly 37% of patients in our study maintained a normal RVEF. This is intriguing and underlines the complex interdependent relationship between the LV and the RV. Patients with preserved RVEF in our study had higher mean LVEF and shorter duration of HF (Table 1), which may have played a role in the preservation of the RV systolic function. RV function may also be preserved due to a lower RV systolic load and unique RV hemodynamics that provide inherent protective mechanisms against permanent ischemic damage.24 There may also be individual susceptibilities to RVEF impairment as suggested by data from the pulmonary arterial hypertension literature.7, 25 Mechanisms by which some patients with pulmonary hypertension develop RV failure and others do not are not clearly understood. Altered gene expression and variations in neurohormonal activation have been proposed to partially account for these differences.5, 6, 23, 25 Further research is needed to understand the development of RVEF impairment in systolic HF.
The role of the RV in chronic HF was overlooked for many years, partly because it was merely considered to be a passive chamber.26 Several prior studies have reported an association between RVEF and poor outcomes in different HF populations using various methods of RVEF assessment.8–15 In one study of 34 patients with systolic HF, low RVEF (<35%) was associated with increased mortality.8 In the largest known study of RVEF to date, in 377 patients with systolic HF, RVEF <35% was similarly associated with increased mortality.14 Several echocardiographic indices of RV impairment have also been associated with poor outcomes.27–30 Compared to these previous studies, our study is distinguished by its large sample size, longer person-years of follow-up, adjustment for a large number of prognostically important covariates, and various cause-specific outcomes.
The overall prognosis for patients with advanced systolic HF is poor. RVEF may serve as a useful tool to identify patients at high risk for poor prognosis. Any decline in RVEF between 20% and 39% may be used as a marker of poor prognosis. However, RVEF <20% has a significant intrinsic association with an increased risk of death and HF hospitalization that appears to be independent of other risk factors. Unadjusted all-cause mortality rates in patients with RVEF ≥40% and <20% were 27% and 47%, respectively over a mean of 24 months of follow-up. This represents a 20% absolute difference in mortality rates between the highest and lowest category of RVEF, representing 20 excess deaths for every 100 patients followed over a 2-year period. The magnitude of absolute increased risk associated with low RVEF is thus substantial. Therefore, measurement of RVEF should be integrated into the global clinical evaluation of HF patients. Radionuclide imaging currently constitutes a well-validated quantitative estimate of RVEF and our data suggest that RVEF measured by this technique can be used to assess prognosis in these patients. However, echocardiographic assessment of the RV from multiple views including the apical four-chamber view can give overall qualitative assessments of RV size and function that may be prognostically useful. HF patients with RVEF <20% may benefit from referral to a specialized HF clinic.
Little is known about evidence-based therapy for HF with low RVEF as very few studies in chronic HF have evaluated the impact of therapies specifically on the RV or in patients with low RVEF.1, 4, 31 In one small study of 14 stable HF patients, captopril use was associated with reduction in both LV and RV end-diastolic volumes and improvement of LVEF and RVEF.32 Recently, sildenafil, a phosphodiesterase 5 inhibitor, has been shown to improve exercise capacity and quality of life in systolic HF patients (n=34) with a mean RVEF of 34% and secondary pulmonary hypertension.33 In that study, patients randomized to sildenafil (versus placebo) had improvement of both resting and exercise RVEF.33 In a small study of 22 patients with HF, therapy with carvedilol improved LVEF and RVEF.34 However, findings from the current study suggest that bucindolol had no effect on mortality in HF patients with low RVEF. Further data are needed to better understand the impact of existing and new HF therapies on HF patients with low RVEF.
Several limitations of our study need to be considered. RVEF in our study was estimated using gated-equilibrium radionuclide ventriculography, which has since been replaced by first-pass radionuclide ventriculography.21 However, it is has been validated extensively and has the advantage of being independent of geometric assumptions in contrast to conventional echocardiography.35 RVEF is highly dependent on loading conditions and may not adequately reflect intrinsic RV contractility.5 We also had no data on RV end-systolic and end-diastolic volumes. However, to the best of our knowledge, no study to date has examined the association of RV volume or dimension with outcomes in chronic systolic HF. Since a change in RV volume may be the first sign of RV dysfunction, future studies need to examine if it might be a better marker of prognosis than RVEF in these patients. Finally, BEST participants were not receiving beta-blockers approved for HF, which may limit generalizability of these findings.
In conclusion, in patients with advanced systolic HF, RVEF impairment is common and is associated poor outcomes. While RVEF <40% is a marker of increased risk of death and hospitalization, RVEF <20% has a significant intrinsic association with increased risk of death and HF hospitalization. RVEF may be used to risk-stratify advanced systolic HF patients during initial and subsequent evaluations. Future studies need to determine risk factors for RVEF impairment, and develop and test interventions to prevent RVEF impairment and improve outcomes in those with low RVEF.
Acknowledgments
Funding Sources: Dr. Ahmed is supported by grants (R01-HL085561 and R01-HL097047) from the National Heart, Lung, and Blood Institute (NHLBI), Bethesda, Maryland and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama
The Beta–Blocker Evaluation of Survival Trial (BEST) is conducted and supported by the NHLBI in collaboration with the BEST Study Investigators. This Manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the BEST or the NHLBI.
Footnotes
Conflict of Interest Disclosures: None
Clinical perspective
Right ventricular (RV) ejection fraction (EF) may often be low in patients with heart failure (HF) and reduced left ventricular (LV) EF. Studies of RVEF and outcomes in these patients are limited by small sample size and short follow-up. In the Beta-Blocker Evaluation of Survival Trial (BEST), 2708 HF patients with LVEF ≤35% were followed for a mean of 2 years and 2008 had data on baseline RVEF, estimated by gated-equilibrium radionuclide ventriculography. They had a mean RVEF of 35%, which was similar regardless of HF etiology. Of these patients, 37% had normal RVEF (≥40%) and 13% had RVEF <20%. Patients with reduced (versus normal) RVEF were more likely to be younger men with lower LVEF and longer duration of HF than those with higher RVEF. Compared with 27% death in those with RVEF ≥40%, 47% of those with RVEF <20% died. In contrast, death rates for those with RVEF 20–29% and 30–39% were 32% and 35%, respectively. When we adjusted for age, LVEF and other baseline characteristics, compared with RVEF ≥40%, only those with RVEF <20% had a significant increased risk for all-cause mortality and HF hospitalization. These findings suggest a decreasing RVEF is a marker of poor prognosis in HF patients with low LVEF. However, when RVEF is reduced below 20%, it may also have an intrinsic and independent effect on mortality. Future studies need to evaluate underlying mechanism of RVEF impairment in systolic HF and therapies that can improve prognosis in those with low RVEF.
References
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed A, Perry GJ, Fleg JL, Love TE, Goff DC, Jr, Kitzman DW. Outcomes in ambulatory chronic systolic and diastolic heart failure: a propensity score analysis. Am Heart J. 2006;152:956–966. doi: 10.1016/j.ahj.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez-Selles M, Garcia Robles JA, Prieto L, Dominguez Munoa M, Frades E, Diaz-Castro O, Almendral J. Systolic dysfunction is a predictor of long term mortality in men but not in women with heart failure. Eur Heart J. 2003;24:2046–2053. doi: 10.1016/j.ehj.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K, Vahanian A, Camm J, De Caterina R, Dean V, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29:2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 5.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right Ventricular Function in Cardiovascular Disease, Part I: Anatomy, Physiology, Aging, and Functional Assessment of the Right Ventricle. Circulation. 2008;117:1436–1448. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 6.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right Ventricular Function in Cardiovascular Disease, Part II: Pathophysiology, Clinical Importance, and Management of Right Ventricular Failure. Circulation. 2008;117:1717–1731. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 7.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. Right Ventricular Function and Failure: Report of a National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 8.Polak JF, Holman BL, Wynne J, Colucci WS. Right ventricular ejection fraction: an indicator of increased mortality in patients with congestive heart failure associated with coronary artery disease. J Am Coll Cardiol. 1983;2:217–224. doi: 10.1016/s0735-1097(83)80156-9. [DOI] [PubMed] [Google Scholar]
- 9.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–1153. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 10.Gavazzi A, Berzuini C, Campana C, Inserra C, Ponzetta M, Sebastiani R, Ghio S, Recusani F. Value of right ventricular ejection fraction in predicting short-term prognosis of patients with severe chronic heart failure. J Heart Lung Transplant. 1997;16:774–785. [PubMed] [Google Scholar]
- 11.Juilliere Y, Barbier G, Feldmann L, Grentzinger A, Danchin N, Cherrier F. Additional predictive value of both left and right ventricular ejection fractions on long-term survival in idiopathic dilated cardiomyopathy. Eur Heart J. 1997;18:276–280. doi: 10.1093/oxfordjournals.eurheartj.a015231. [DOI] [PubMed] [Google Scholar]
- 12.de Groote P, Millaire A, Foucher-Hossein C, Nugue O, Marchandise X, Ducloux G, Lablanche JM. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32:948–954. doi: 10.1016/s0735-1097(98)00337-4. [DOI] [PubMed] [Google Scholar]
- 13.La Vecchia L, Paccanaro M, Bonanno C, Varotto L, Ometto R, Vincenzi M. Left ventricular versus biventricular dysfunction in idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;83:120–122. A129. doi: 10.1016/s0002-9149(98)00795-4. [DOI] [PubMed] [Google Scholar]
- 14.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 15.Javaheri S, Shukla R, Zeigler H, Wexler L. Central sleep apnea, right ventricular dysfunction, and low diastolic blood pressure are predictors of mortality in systolic heart failure. J Am Coll Cardiol. 2007;49:2028–2034. doi: 10.1016/j.jacc.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 16.The BEST Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med. 2001;344:1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 17.National Heart Lung and Blood Institute. The Beta-Blocker Evaluation of Survival Trial Investigators (BEST) 2009 http://www.nhlbi.nih.gov/resources/deca/descriptions/best.htm.
- 18.The BEST Steering Committee. Design of the Beta-Blocker Evaluation Survival Trial (BEST) Am J Cardiol. 1995;75:1220–1223. doi: 10.1016/s0002-9149(99)80766-8. [DOI] [PubMed] [Google Scholar]
- 19.The BEST Investigators. Beta-blocker evaluation of survival trial (BEST) protocol: The National Heart, Lung and Blood Institute and the Department of Veterans Affairs Cooperative Studies Program. 1999 [Google Scholar]
- 20.Manno BV, Iskandrian AS, Hakki AH. Right ventricular function: methodologic and clinical considerations in noninvasive scintigraphic assessment. J Am Coll Cardiol. 1984;3:1072–1081. doi: 10.1016/s0735-1097(84)80368-x. [DOI] [PubMed] [Google Scholar]
- 21.Hesse B, Lindhardt TB, Acampa W, Anagnostopoulos C, Ballinger J, Bax JJ, Edenbrandt L, Flotats A, Germano G, Stopar TG, Franken P, Kelion A, Kjaer A, Le Guludec D, Ljungberg M, Maenhout AF, Marcassa C, Marving J, McKiddie F, Schaefer WM, Stegger L, Underwood R. EANM/ESC guidelines for radionuclide imaging of cardiac function. Eur J Nucl Med Mol Imaging. 2008;35:851–885. doi: 10.1007/s00259-007-0694-9. [DOI] [PubMed] [Google Scholar]
- 22.Setaro JF, Cleman MW, Remetz MS. The right ventricle in disorders causing pulmonary venous hypertension. Cardiol Clin. 1992;10:165–183. [PubMed] [Google Scholar]
- 23.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–1960. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 24.Dell’Italia LJ, Lembo NJ, Starling MR, Crawford MH, Simmons RS, Lasher JC, Blumhardt R, Lancaster J, O’Rourke RA. Hemodynamically important right ventricular infarction: follow-up evaluation of right ventricular systolic function at rest and during exercise with radionuclide ventriculography and respiratory gas exchange. Circulation. 1987;75:996–1003. doi: 10.1161/01.cir.75.5.996. [DOI] [PubMed] [Google Scholar]
- 25.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest. 2009;135:794–804. doi: 10.1378/chest.08-0492. [DOI] [PubMed] [Google Scholar]
- 26.Rigolin VH, Robiolio PA, Wilson JS, Harrison JK, Bashore TM. The forgotten chamber: the importance of the right ventricle. Cathet Cardiovasc Diagn. 1995;35:18–28. doi: 10.1002/ccd.1810350105. [DOI] [PubMed] [Google Scholar]
- 27.Gorcsan J, III, Murali S, Counihan PJ, Mandarino WA, Kormos RL. Right ventricular performance and contractile reserve in patients with severe heart failure: Assessment by pressure-area relations and association with outcome. Circulation. 1996;94:3190–3197. doi: 10.1161/01.cir.94.12.3190. [DOI] [PubMed] [Google Scholar]
- 28.Zornoff LA, Skali H, Pfeffer MA, St John Sutton M, Rouleau JL, Lamas GA, Plappert T, Rouleau JR, Moye LA, Lewis SJ, Braunwald E, Solomon SD. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol. 2002;39:1450–1455. doi: 10.1016/s0735-1097(02)01804-1. [DOI] [PubMed] [Google Scholar]
- 29.Dokainish H, Sengupta R, Patel R, Lakkis N. Usefulness of right ventricular tissue Doppler imaging to predict outcome in left ventricular heart failure independent of left ventricular diastolic function. Am J Cardiol. 2007;99:961–965. doi: 10.1016/j.amjcard.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 30.Anavekar NS, Skali H, Bourgoun M, Ghali JK, Kober L, Maggioni AP, McMurray JJ, Velazquez E, Califf R, Pfeffer MA, Solomon SD. Usefulness of right ventricular fractional area change to predict death, heart failure, and stroke following myocardial infarction (from the VALIANT ECHO Study) Am J Cardiol. 2008;101:607–612. doi: 10.1016/j.amjcard.2007.09.115. [DOI] [PubMed] [Google Scholar]
- 31.Bristow MR, Zisman LS, Lowes BD, Abraham WT, Badesch DB, Groves BM, Voelkel NF, Lynch DM, Quaife RA. The pressure-overloaded right ventricle in pulmonary hypertension. Chest. 1998;114:101S–106S. doi: 10.1378/chest.114.1_supplement.101s. [DOI] [PubMed] [Google Scholar]
- 32.Massie B, Kramer BL, Topic N, Henderson SG. Hemodynamic and radionuclide effects of acute captopril therapy for heart failure: changes in left and right ventricular volumes and function at rest and during exercise. Circulation. 1982;65:1374–1381. doi: 10.1161/01.cir.65.7.1374. [DOI] [PubMed] [Google Scholar]
- 33.Lewis GD, Shah R, Shahzad K, Camuso JM, Pappagianopoulos PP, Hung J, Tawakol A, Gerszten RE, Systrom DM, Bloch KD, Semigran MJ. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 34.Quaife RA, Christian PE, Gilbert EM, Datz FL, Volkman K, Bristow MR. Effects of carvedilol on right ventricular function in chronic heart failure. Am J Cardiol. 1998;81:247–250. doi: 10.1016/s0002-9149(97)00874-6. [DOI] [PubMed] [Google Scholar]
- 35.Jain D, Zaret BL. Assessment of right ventricular function. Role of nuclear imaging techniques. Cardiol Clin. 1992;10:23–39. [PubMed] [Google Scholar]