Abstract
Sarcolemmal ATP sensitive potassium Channels (KATP) act as metabolic sensors that facilitate adaptation of the left ventricle (LV) to changes in energy requirements. This study examined the mechanism by which KATP dysfunction impairs the LV response to stress using transgenic mouse strains with cardiac specific disruption of KATP activity (SUR1-tg mice) or Kir6.2 gene deficiency (Kir6.2 KO). Both SUR1-tg and Kir6.2 KO mice had normal LV mass and function under unstressed conditions. Following chronic transverse aortic constriction (TAC), both SUR1-tg and Kir6.2 KO mice developed more severe LV hypertrophy and dysfunction as compared with their corresponding wild type controls. Both SUR1-tg and Kir6.2 KO mice had significantly decreased expression of PGC-1α̣ and a group of energy metabolism related genes at both protein and mRNA levels̃. Furthermore, disruption of KATP repressed expression and promoter activity of PGC-1α in cultured rat neonatal cardiac myocytes in response to hypoxia, indicating that KATP activity is required to maintain PGC-1α expression under stress conditions. PGC1α gene deficiency also exacerbated chronic TAC-induced ventricular hypertrophy and dysfunction, suggesting that depletion of PGC1α can worsen systolic overload induced ventricular dysfunction. Both SUR1-tg and Kir6.2 KO mice had decreased FOXO1 after TAC, in agreement with the reports that a decrease of FOXO1 can repress PGC-1α expression. Furthermore, inhibition of KATP caused a decrease of FOXO1 associated with PGC-1α promoter. These data indicate that KATP channels facilitate the cardiac response to stress by regulating PGC-1α and its target genes, at least partially through the FOXO1 pathway.
Keywords: ATP sensitive potassium channels, cardiac hypertrophy, PGC-1α
Introduction
ATP-sensitive potassium channels (KATP) act as metabolic sensors that can regulate cellular activity to meet energetic demands 1. In the cardiac myocyte KATP channels are composed of the pore forming subunit Kir6.2 and the regulatory subunit SUR2A. Patients with missense or frameshift mutations in genes encoding the cardiac KATP channel are predisposed to cardiomyopathy or sudden death 2, 3. Moreover, in genetically modified mouse models, global Kir6.2 gene knockout (Kir6.2 KO) resulted in abolition of ischemic preconditioning 4, reduced exercise capacity 5, impaired the response to adrenergic challenge 6, compromised the tolerance to hypertension 7, and impaired the ability to tolerate hemodynamic overload produced by transverse aortic constriction (TAC) 8. Two recent studies using Kir6.2 KO mice reported that disruption of KATP channel activity led to activation of calcium-dependent calcineurin pathways, which in turn increased nuclear accumulation of the pro-hypertrophic transcription factors MEF2 and NF-AT 7, 8. However, the molecular mechanisms by which KATP channels regulate cardiac function, particularly during adaptation of the heart to the increased metabolic requirements produced by hemodynamic overload, are largely unknown. Most importantly, as a well defined metabolic stress sensor, the role of KATP channels in regulation of genes related to myocardial metabolism has not been studied.
At the transcriptional level, several families of transcription factors have been identified that regulate energy production processes, including peroxisome proliferator-activated receptor (PPAR) 9, estrogen-related receptor (ERR) 10, 11, nuclear respiratory factor (NRF) 12, mitochondrial transcription factor A (Tfam) 13, PPARγ coactivator-1α (PGC-1α), and PGC-1β 14. PGC-1α and PGC-1β bind to both nuclear receptors and non-nuclear receptors and control cellular energy metabolic pathways. In transgenic mice overexpression of PGC-1α resulted in massive proliferation of enlarged mitochondria in the heart 15, while PGC-1α deficient mice showed decreased myocardial mitochondrial enzymes and diminished cardiac function in response to an increased work load 16, 17. PGC-1α expression is repressed by insulin in HepG2 cells and skeletal muscle through the three insulin response sequences (IRS) in the PGC-1α promoter 18, 19. Insulin exerts its function through activation of Akt, which in turn phosphorylates FOXO1, one of the positive regulators of PGC-1α transcription. Phosphorylated FOXO1 is exported out of the nuclei and targeted for degradation 20.
Here using both kir6.2 KO mice 21 and a transgenic mouse strain in which ventricular KATP channel activity was disrupted by cardiac specific overexpression of the KATP channel regulatory subunit SUR1 (SUR1-tg) 22, we demonstrate that loss of KATP channel activity attenuates the expression of PGC-1α and mitochondrial energy metabolism-related enzymes in the heart exposed to pressure overload, indicating a role for KATP channels in the chronic response to increased cardiac work. We provide further evidence that the protective effect of KATP occurs at least partially by regulating the activity of FOXO1, which in turn influences the expression of PGC-1α and its downstream target genes.
Materials and Methods
SUR1-tg mice 22, Kir6.2 KO mice 21and PGC-1α KO mice (Gifts from Dr. Daniel Kelly) 23 were used in this study according to a protocol approved by the University of Minnesota Institutional Animal Care and Use Committee. Transverse aortic constriction (TAC) was used to crease systolic pressure overload as previously described 24, 25. An expanded Methods section is available in the online data supplement at http://circres.ahajournals.org.
Results
Disruption of KATP channels exacerbated LV hypertrophy and dysfunction produced by TAC
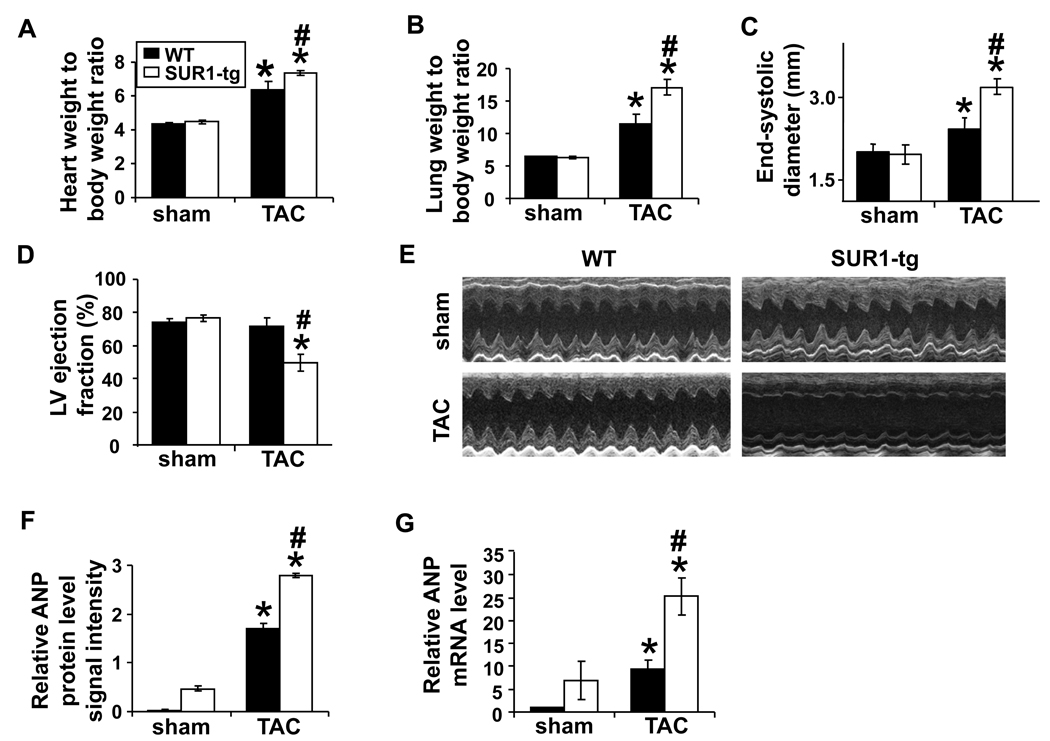
Similar to previous reports 22, mice with disrupted cardiac KATP channel activity secondary to cardiac specific SUR1 overexpression develop and grow similarly to their wild type littermates (WT) under normal conditions, and had normal cardiac function under unstressed conditions (Online Table I). LV dimensions and ejection fraction of the Kir6.2 KO were also similar to their wild type control mice under unstressed conditions, consistent with previous reports 7, 8. The sustained TAC will increase myocardial ATP demands and may compromise coronary perfusion to result in activation of KATP channels; consequently, we performed TAC to stress the hearts of SUR1-tg and WT. As expected, 4 weeks of severe TAC produced significant ventricular hypertrophy in both WT and SUR1-tg mice; however, the degree of hypertrophy assessed by heart weight-to-body weight ratio was ~15% greater in the SUR1-tg mice (Figure 1A). In addition, the cardiomyocyte cross-sectional area following TAC was significantly larger in SUR1-tg than in WT littermates (427±26µm2 vs. 359.3±14.4 µm2, respectively, p<0.05). Both lung weight and lung weight-to-body weight ratio were significantly greater in the SUR1-tg mice as compared with WT following TAC (Figure 1B), indicating that SUR1-tg mice had more pulmonary congestion. Moreover, there was more LV dilation and greater impairment of LV function in SUR1-tg mice, with a greater decrease of LV ejection fraction than in the WT (Figure 1C-E, Online Table I).
Figure 1.
After 4 weeks of TAC, SUR1-tg mice had significantly more ventricular hypertrophy (A) and pulmonary congestion (B), a greater increase of LV end-systolic diameter (C), a marked decrease of LV ejection fraction (D), and significantly higher mRNA and protein levels of ANP (F and G) as compared with WT mice. E, representative echocardiograms. *p<0.05 as compared with sham; #, p<0.05 as compared with WT.
Consistent with a greater degree of LV dysfunction, myocardial atrial natriuretic peptide (ANP), was significantly higher at both protein and mRNA levels in the SUR1-tg mice as compared with WT littermates after TAC (Figure 1F, 1G and Online Figure I). The expression level of Kir6.2 was not altered in response to TAC or ectopic expression of SUR1 (Online Figure I).
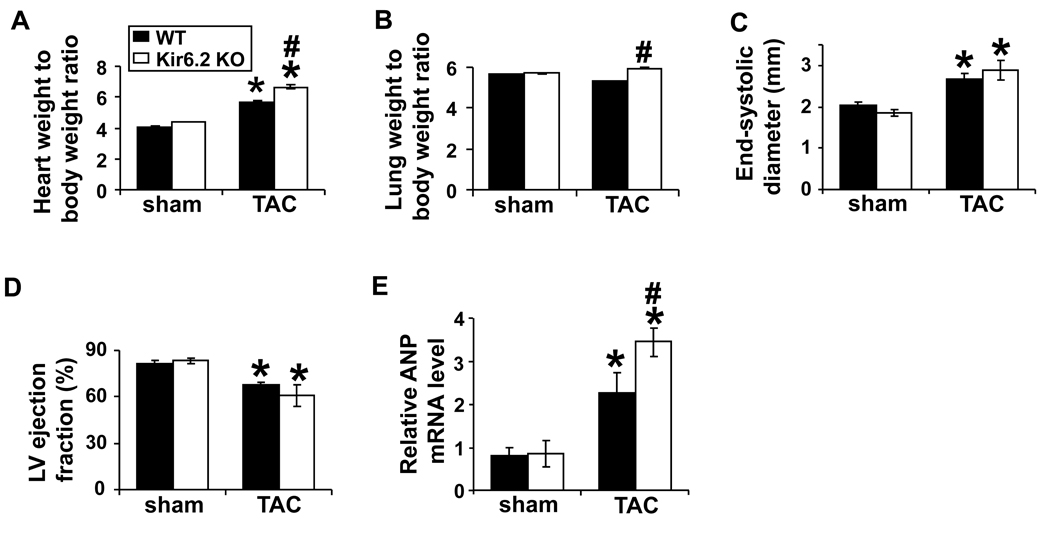
In order to confirm that the greater ventricular hypertrophy and dysfunction in SUR1-tg mice was indeed due to loss of KATP channel activity, we subsequently studied Kir6.2 KO mice. After 6 weeks of moderate TAC, Kir6.2 KO mice also developed more severe cardiac hypertrophy as compared with the wild type mice (Figure 2A), and this was associated with a small but significant increase of the ratio of lung weight-to-body weight in Kir6.2 KO mice (Figure 2B). There was also a trend toward a greater decrease of LV ejection fraction in the Kir6.2 KO mice, but this difference was not significant (Figure 2C and 2D). Kir6.2 KO also exacerbated the increase of myocardial ANP produced by TAC (Figure 2E). The mortality after TAC was not different between Kir6.2 KO and wild type mice.
Figure 2.
TAC induced more severe cardiac hypertrophy (A) and pulmonary congestion (B) in the Kir6.2 KO mice. There was a trend toward deterioration of cardiac function (C and D) and a significant increase of ANP mRNA level (E) in the Kir6.2 null mice as compared with their wild type littermates.
Taken together, these data demonstrate that disruption of KATP channels exacerbated TAC-induced LV dysfunction, and indicate that KATP channel activity is important for the compensatory responses that allow the heart to adapt to chronic systolic overload.
Disruption of KATP channels attenuated myocardial PGC-1α expression in response to chronic pressure overload
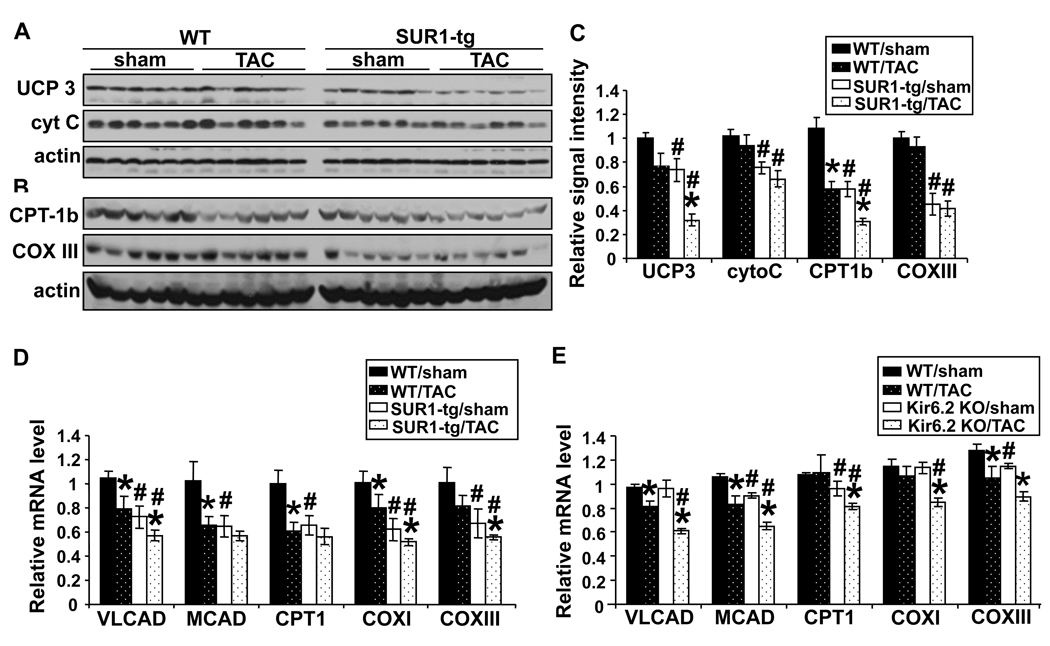
Studies of the Kir6.2 KO mice indicate that the presence, and presumably activation, of KATP channels during metabolic stress helps to preserve cellular ATP and maintain energy homeostasis. To determine if disruption of KATP channels in the SUR1-tg mice altered components of energy producing systems, we examined the expression of enzymes related to myocardial ATP production. We found that uncoupling protein-3 (UCP3), cytochrome C, cytochrome C oxidase subunit-III (COX-III), and carnitine palmitoyltransferase-1 muscle isoform (CPT-1b) were each significantly decreased in the SUR1-tg mice both under control conditions and after TAC (Figure 3A-C and Online Figure II), indicating that abnormal mitochondrial function might contribute to the LV dysfunction that we observed in the SUR1-tg mice after TAC. Furthermore, the mRNA content of very long chain acetyl-CoA dehydrogenase (VLCAD), medium chain acetyl-CoA dehydrogenase (MCAD), CPT-1b, COX-I, and COX-III were all significantly decreased in the SUR1-tg mice as compared to wild type littermates under both control conditions and after TAC (Figure 3D and Online Figure III). Similarly, TAC caused significantly decreased expression of these enzymes in the Kir6.2 KO mice as compared with the wild type mice (Figure 3E). These results indicate that disrupting KATP channel activity attenuated the expression of mitochondrial energy metabolism related enzymes. However, there was no significant difference in myocardial mitochondrial volume density between the wild type and SUR1-tg mice before or after TAC, suggesting that the quality, but not the quantity, of mitochondria was changed in the SUR1-tg hearts (Online Figure IV).
Figure 3.
Following TAC the expression of myocardial energy metabolism related enzymes was significantly decreased at both protein (A–C) and mRNA levels (D) in SUR1-tg mice as compared with WT. The mRNA levels of these enzymes were also significantly decreased in the Kir6.2 KO mice (E). *p<0.05 as compared to the corresponding sham group; # p<0.05 as compared to WT.
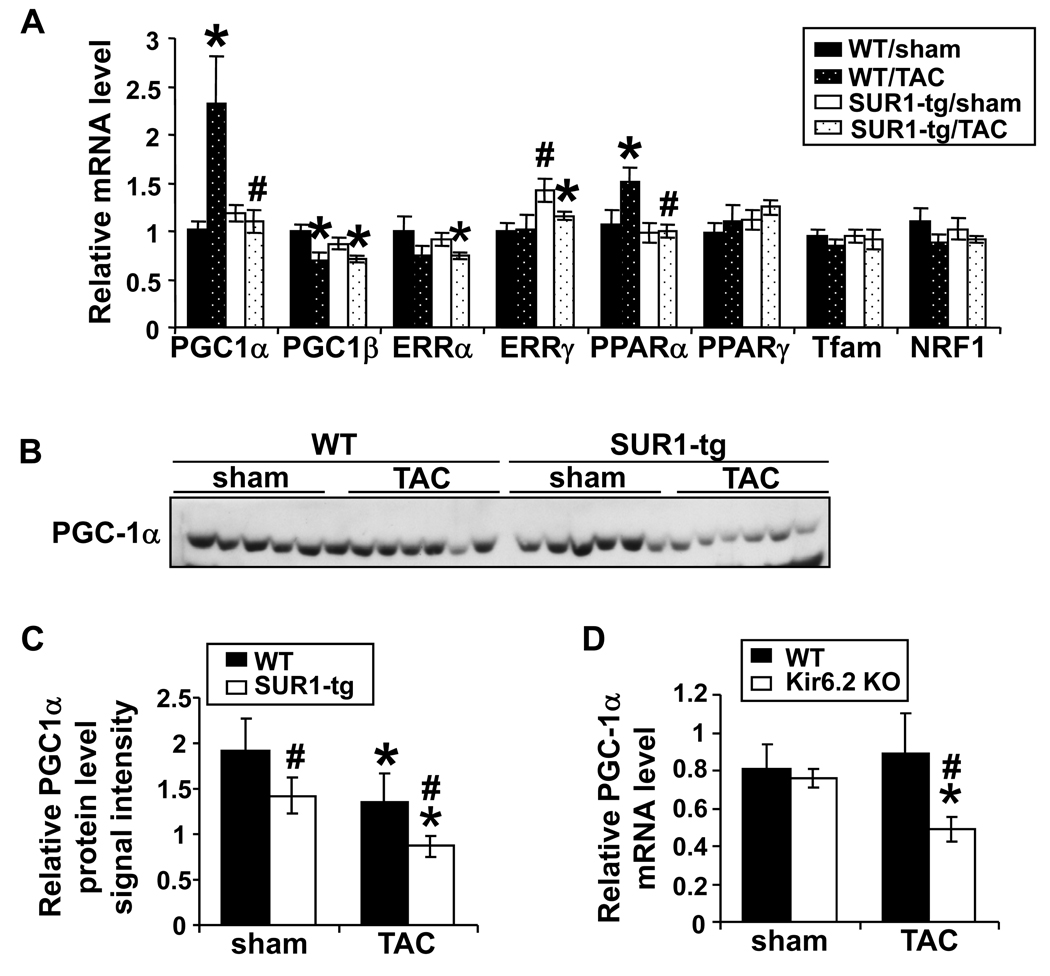
We also examined upstream transcriptional factors that have been shown to regulate energy metabolism related genes, including PGC-1α, PGC-1β, ERRα, ERRγ, PPARα, PPARγ, Tfam and NRF1. TAC caused significant up regulation of PGC-1α and PPARα in WT, while this induction was abolished by ablation of KATP activity in the SUR1-tg mice (Figure 4A). Interestingly, myocardial PGC-1α protein content was significantly decreased in SUR1-tg mice compared to wild type mice under both control conditions and after TAC (Figure 4B and 4C), In addition, TAC caused significantly greater decreases of myocardial PGC-1α mRNA in Kir6.2 KO mice as compared with their wild type littermates (Figure 4D). These data indicate that KATP channel dysfunction leads to deregulation of PGC-1α expression and its downstream target genes in response to systolic overload.
Figure 4.
TAC caused significant increases in PGC-1α and PPARα mRNA in WT mice, which were absent in the SUR1-tg mice (A). The PGC-1α protein level was decreased in SUR1-tg mice at basal conditions, and further decreased following TAC (B and C). The mRNA level of PGC-1α was also significantly decreased after TAC in the Kir6.2 KO mice (D). * p<0.05 as compared to sham; #, p<0.05 as compared to wild type.
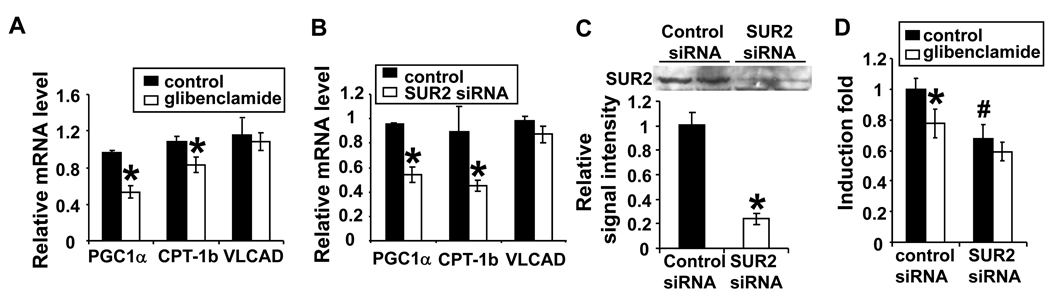
Blocking KATP channel activity in rat neonatal cardiomyocytes decreased the promoter activity and mRNA level of PGC-1α
A decrease in myocardial PGC-1α content has been reported in several heart failure models 17, 26. However, changes of PGC-1α expression in vivo could be the result of altered neurohormonal signaling in the setting of heart failure, rather than a direct effect of KATP activity. To determine whether inhibition of KATP channel activity can directly affect PGC-1α expression in cardiac myocytes, we studied the effect of inhibiting KATP channels on PGC-1α expression in rat neonatal cardiac myocytes. As cardiac KATP channels are likely to be closed in cultured cardiac myocytes under basal conditions, we challenged the cells with hypoxia/reoxygenation (H/R) to activate KATP channels (24hrs of 1% oxygen followed by 7–8 hrs of reoxygenation). KATP channel activity was suppressed either pharmacologically with glibenclamide or by selective gene silencing of cardiac KATP regulatory subunit, SUR2A. Both pharmacological and genetic suppression significantly repressed the expression of PGC-1α at the mRNA level (Figure 5A and B). Expression of the two downstream targets of PGC-1α genes, CPT-1b and VLCAD, was also determined. The mRNA level of CPT-1b was significantly reduced by glibenclamide treatment or SUR2 gene silencing. The expression of VLCAD also tended to decrease after glibenclamide treatment (Figure 5A and B). In a subsequent study, rat neonatal cardiomyocytes were transfected with a luciferase reporter driven by a 3.1 kb mouse PGC-1α promoter 27. Either glibenclamide treatment or knocking down SUR2 expression significantly reduced reporter activity by ~ 22% and 32%, respectively (Figure 5C), suggesting that blocking KATP channel activity can repress expression of PGC-1α at the transcriptional level.
Figure 5.
The mRNA levels of PGC-1α and its target gene CPT-1b were significantly when KATP channels were pharmacologically blocked with glibenclamide (A) or genetically inhibited by SUR2 specific siRNA (B). Glibenclamide treatment and SUR2 gene silencing reduced the luciferase activity of the reporter gene driven by the PGC-1α promoter (C).
PGC-1α KO exacerbated left ventricular hypertrophy and dysfunction produced by moderate TAC
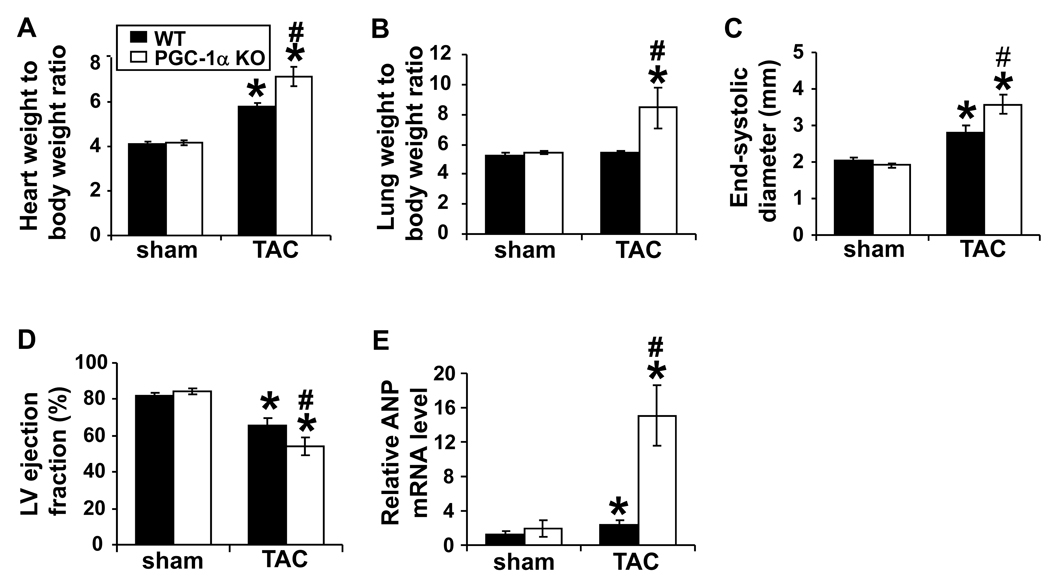
To determine whether a decrease of PGC-1α can contribute to pressure overload induced myocardial hypertrophy and dysfunction, we determined ventricular structure and function of PGC-1α KO and wild type mice under control conditions and after 6 weeks of moderate TAC. Disruption of PGC-1α had no effect on cardiac functions during basal conditions (Figure 6). However, 6 weeks of TAC caused significantly more hypertrophy in PGC-1α KO mice (Figure 6A). In addition, TAC caused a significantly greater increases of the ratio of lung weight-to-body weight and LV end systolic diameter, a greater decrease of LV ejection fraction (Figure 6B-D), and a greater increase of ANP expression (Figure 6E), indicating that diminished PGC-1α exacerbated TAC-induced ventricular hypertrophy and dysfunction.
Figure 6.
TAC induced more severe hypertrophy (A), pulmonary congestion (B), LV dilation (C) and dysfunction (D), and ANP expression (E) in the PGC-1α null mice. * p<0.05 as compared to sham; #, p<0.05 as compared to wild type.
Disruption of KATP activity in the SUR1-tg mice reduced total FOXO1 after TAC
To this end, our findings indicate that cardiac KATP channel dysfunction contributes to the repressed expression of PGC-1α during stress conditions. An important remaining question is which signaling pathway(s) provide the link(s) between KATP activity and PGC-1α expression. It has been reported in HepG2 cells and in skeletal muscle that FOXO1 activates PGC-1α promoter through IRS. Phosphorylation of FOXO1 at Thr24 by Akt decreases PGC-1α promoter activity by decreasing the nuclear FOXO1 associated with the PGC-1α promoter 18, 19.
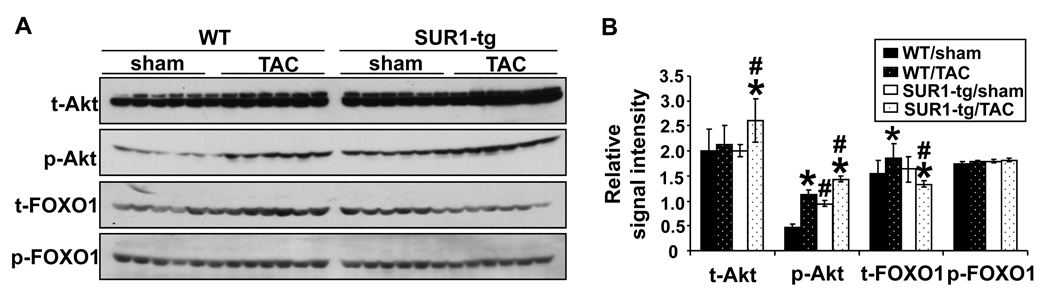
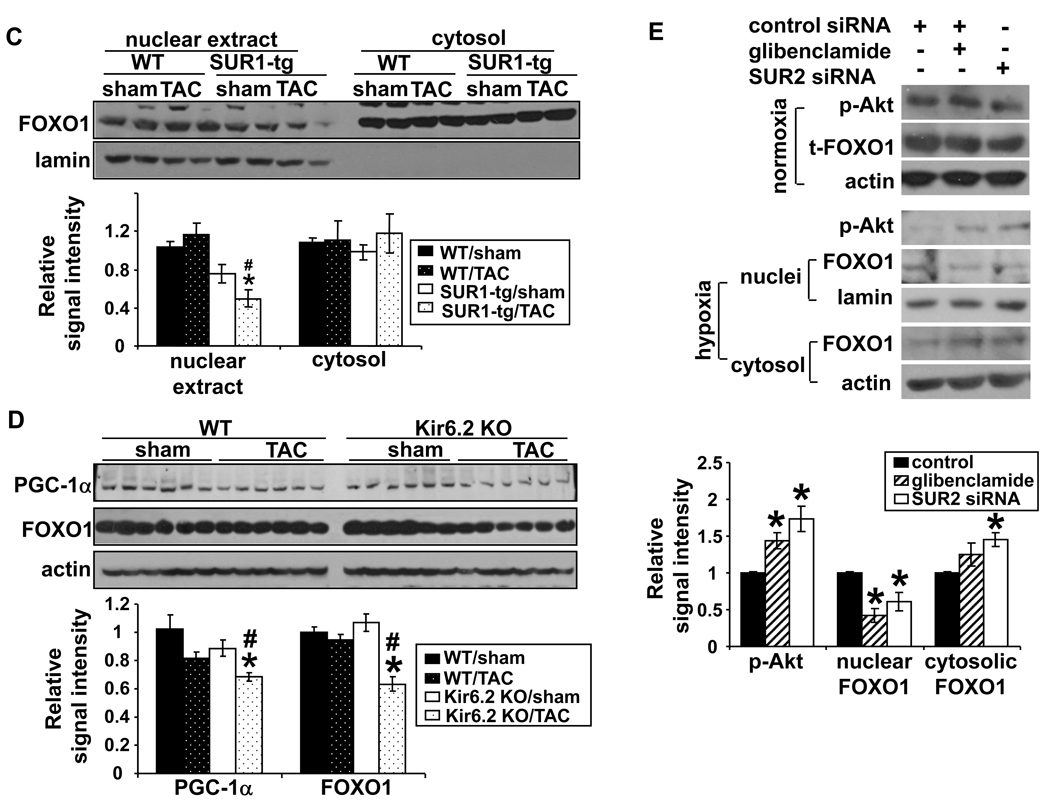
To examine whether differences in the Akt–FOXO1 signaling pathway might be responsible for the down regulation of PGC-1α in the SUR1-tg mice, we compared the levels of total- and phos-Akt Ser473 and total- and phos-FOXO1Thr24 in WT and SUR1-tg hearts by Western blot. As shown in Figure 7A and 7B, after TAC total Akt was significantly increased in the SUR1-tg mice but not in the WT. TAC also caused an increase of phos-Akt Ser473 in both WT and SUR1-tg mice, but the amount of phos-AktSer473 was significantly higher in the SUR1-tg mice under both control conditions and after TAC. Moreover, though TAC had no effect on phos-FOXO1Thr24 in either the SUR1-tg or the WT mice, it did cause a significant increase of total FOXO1 in the WT mice, while this induction was abolished in the SUR1-tg mice (Figure 7A and 7B). The findings of unchanged phos-FOXO1Thr24 but decreased total FOXO1 indicates that nuclear FOXO1 may be decreased in the SUR1-tg mice after TAC. To confirm this, we prepared nuclear extract from flash frozen heart samples. The SUR1-tg mice had a significantly decreased nuclear FOXO1 as compared with the sham group and WT banded mice. Interestingly, nuclear FOXO1 was slightly increased in the WT mouse heart following TAC, but decreased in the SUR1-tg heart under basal conditions; although neither was statistically significant. The amount of cytoplasmic fraction of FOXO1 was not different among the four groups (Figure 7C). Similarly, TAC resulted in significantly decreased expression of both PGC-1α and FOXO1 in the Kir6.2 KO mice, but not in wild type mice (Figure 7D). Taken together, these data suggest that decreased FOXO1 might partially account for the repressed expression of PGC-1α when KATP channel activity is disrupted.
Figure 7.
Following TAC both total and phos-Akt levels were increased in SUR1-tg mice as compared with WT (A and B). Total FOXO1 levels were significantly less in both SUR1-tg mice (A and B) and Kir6.2 KO mice (D) as compared with their corresponding controls. Nuclear FOXO1 was significantly decreased in the banded SUR1-tg hearts (C). *p<0.05 as compared to sham; #, p<0.05 as compared to WT. In rat neonatal cardiomyocytes, blocking KATP activity enhanced Akt phosphorylation and reduced the nuclear fraction of FOXO1 (E). * p<0.05 as compared with cells transfected with non-specific siRNA and treated with vehicle (DMSO).
We further examined the levels of Akt and FOXO1 and the subcellular distribution of FOXO1 in neonatal cardiomyocytes subjected to H/R, with or without disruption of KATP. Under normoxic conditions glibenclamide or silencing SUR2 gene expression did not activate Akt or change FOXO1 expression. Under hypoxic conditions the amount of phos-Akt Ser473 was increased when KATP channels were pharmacologically blocked with glibenclamide or silencing SUR2 gene expression (Figure 7E). Furthermore, KATP channel blockade significantly decreased the amount of FOXO1 in nuclei, while cytosolic FOXO1 was significantly increased. These data indicate that blocking KATP channel activity under stress conditions leads to decreased nuclear FOXO1, and increased phos-Akt Ser473 may be one of the factors that induces nuclear exclusion of FOXO1 when KATP is disrupted.
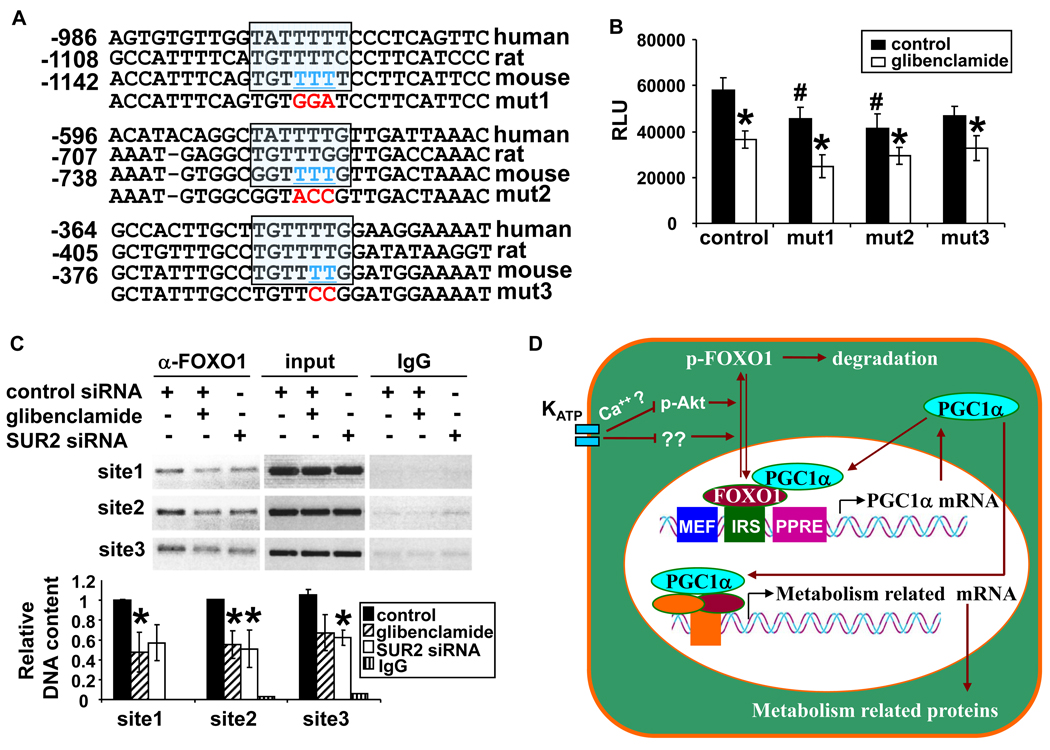
Mutations of potential FOXO1 binding sites on PGC-1α promoter abolished H/R induced PGC-1α promoter activity
To determine whether reduced nuclear FOXO1 leads to repression of PGC-1α promoter activity, point mutations were introduced into each IRS in the luciferase reporter construct and reporter activities were determined (Figure 8A and 8B). After H/R, the promoter activity of PGC-1α was significantly decreased when the IRS were mutated, indicating that these IRS are important in maintaining PGC-1α promoter activity. Glibenclamide treatment further decreased the luciferase activity of the mutated reporters, suggesting that KATP channels might influence the PGC-1α promoter through other pathway(s) in addition to FOXO1. Finally, ChIP assay was performed using anti-FOXO1 antibody (Figure 8C). Treatment with glibenclamide or knock-down of SUR2 subunit expression decreased the amount of FOXO1 associated with the IRS. Taken together, these data indicate that inactivation of KATP activity under stress conditions represses PGC-1α expression, at least partially through dissociation of the positive regulator FOXO1 from its promoter.
Figure 8.
Three IRS were identified by sequence alignment and underlined bases (in blue) were mutated (in red) (A). Reporter activity was reduced when IRSs were mutated (B). Disruption of KATP activity disassociated FOXO1 from PGC1-1α promoter (C). A diagram of regulation of PGC-1α expression by KATP channels (D). RLU, relative luciferase unit.
Discussion
The major finding of this study is that KATP channels can regulate energy metabolism related gene expression through PGC-1α. To our knowledge, this provides the first evidence that functional KATP channels influence the expression of myocardial PGC-1α and the expression of a group of proteins related to ATP production. Thus, KATP channels play a critical role in augmenting the myocardial energy supply during chronic hemodynamic overload by regulating PGC-1α expression at the transcriptional level.
In this study, we used cardiac specific SUR1-tg mice in which ventricular KATP channel activity is essentially abolished as the result of ectopic expression of the SUR1 regulatory subunit 22. Using cardiac specific disruption of KATP channels avoids potential unwanted systemic effects of global gene deletion such as insulin secretion defects 28 or coronary spasm 29 that have been described in other global KATP gene deficient mouse strains. To confirm that our observations were caused by KATP ablation, we also studied the effects of global depletion of the pore forming subunit in Kir6.2 KO mice. Our findings that disruption of KATP exacerbated the TAC induced cardiac hypertrophy and dysfunction in both the SUR1-tg and the Kir6.2 KO mice are consistent with a previous report that disruption of KATP impaired the tolerance of Kir6.2 KO mice to TAC-induced systolic overload. Those investigators reported that in response to TAC, action potential duration shortened in wild type mice but was prolonged in Kir6.2-KO mice, a response that resulted in calcium overload in the Kir6.2-KO myocytes 8. Interestingly, we now show that suppression of KATP channel activity during TAC also disrupts the expression of PGC1α via the FOXO1 signaling pathway. The link between KATP channels and regulation of this master metabolic regulatory pathway remains to be determined, but several studies have demonstrated that [Ca2+]I is able to regulate Akt activity 30, 31. It is conceivable that disruption of KATP channel activity led to Ca2+ overload in the cardiomyocytes 32 which in turn contributed to activation of Akt. The results of the present study demonstrate that KATP channel activity affects the expression of metabolism related enzymes important for ATP production. In a recent study, Jilkina et al observed that the baseline ATP level in Kir6.2 KO hearts was decreased by 30%, and this difference was even more evident when the heart was stressed by metabolic inhibition or isoproterenol infusion 33. We demonstrate here that some myocardial metabolic enzymes were decreased in unstressed SUR1-tg and Kir6.2 KO mice, suggesting that KATP activity controls the expression of metabolic enzymes during control conditions. The depression of PGC1α signaling as a result of KATP suppression is likely to reduce the myocardial metabolic reserve available for energy production during increases of cardiac work 34 and may thereby provide a mechanistic basis for the link between KATP activity and ATP production reported in Kir6.2 KO mice.
Previous studies have shown that defects of mitochondrial respiratory chain complexes can lead to LV dysfunction or cardiomyopathy 35. Similarly, two PGC-1α knockout mouse models exhibited differing severities of cardiac phenotype, but both showed impairment of the cardiac response to stress (TAC, dobutamine challenge or exhaustive exercise) 16, 17, 23, suggesting that PGC-1α becomes more critical during stress conditions, consistent with our findings of greater LV dysfunction and pulmonary congestion in PGC1α null mice following TAC. It should be noted that in the SUR1-tg study, PGC-1α mRNA was increased while the PGC-1α protein was decreased in the WT mice after TAC. This disparity between mRNA and protein expression suggests that the expression of PGC-1α is regulated at the translational and/or post-translational level in response to pressure overload. Our finding that PGC-1α mRNA was increased in wild type mice after TAC is contrary to reports that TAC caused a decrease of myocardial PGC-1α 17, 26. This discrepancy may be the result of differences in the degree of LV dysfunction or the duration of TAC. However, TAC caused more ventricular hypertrophy and a greater decrease of PGC-1α in both SUR1-tg and Kir6.2 KO mice as compared with their corresponding WT littermates, indicating that intact KATP activity is important for maintaining myocardial PGC-1α expression and ventricular function when the heart is stressed.
Our previous studies consistently show that TAC induces myocardial Akt activation 24, 25. Several studies using overexpression of activated Akt demonstrate that short-term Akt activation results in “physiological” hypertrophy, but chronic, unregulated Akt activation in the heart can be detrimental 36, 37. Thus, both the extent and duration of Akt signaling are important in regulating cardiac function. Cardiac specific overexpression of a constitutively active mutant of Akt (myr-Akt), leads to ~2 fold reduction in expression of PPARα and PGC-1α mRNA, suggesting that Akt may regulate the expression of these two genes, presumably through the phosphorylation and nuclear exclusion of phos-FOXO1 38. In the present study, we observed increased phos-Akt in the SUR1-tg mice relative to WT in both unstressed and stressed conditions. In addition, disruption of KATP channel activity in neonatal cardiac myocytes enhanced phosphorylation of Akt (Figure 7E). However, the activation of Akt did not correlate very well with FOXO1 protein levels in the SUR1-tg study. One possible explanation for this is that other signaling pathways that induce the nuclear retention of FOXO1 39 might overcome the Akt signaling that induces cytoplasmic localization of FOXO1. To this end, our results support the concept that disruption of KATP channels enhanced TAC-induced Akt activation. However, whether the increased Akt activation contributed to the decreased PGC1α is uncertain. The combined insult of TAC and KATP suppression in the SUR1-tg and Kir6.2 KO mice led to a decrease in total FOXO1 suggesting synergistic effect of metabolic stress and KATP channel activity on FOXO1 protein level.
We used rat neonatal cardiomyocytes to further characterize the effect of KATP channels on PGC-1α. Since the aim was to determine whether inhibition of KATP channel activity would depress PGC-1α expression, we exposed the cells with hypoxia/reoxygenation to activate the KATP channels. Suppression of KATP channel activity either with glibenclamide or by selective gene silencing of SUR2 significantly repressed expression of PGC-1α and its downstream target gene CPT-1b at mRNA level. Furthermore, both glibenclamide and knocking down SUR2 expression significantly reduced reporter activity driven by PGC-1α promoter, indicating that KATP channels are able to influence PGC-1α expression at the transcriptional level. The studies in isolated cardiomyocytes were designed to examine whether the regulation of PGC-1α expression by KATP channels might be a general response of heart under stress conditions. Although challenging cultured cells with H/R can effectively manipulate cardiomyocyte KATP activity in the absence of changes in neurohormonal agents that occur in vivo, it does not cause myocyte hypertrophy. Therefore, the H/R study is not a specific or ideal model to mimic the in vivo pressure overload induced ventricular hypertrophy, which is a limitation of this study. However, it should be noted that Sano et al. demonstrated that TAC does cause myocardial hypoxia in vivo, likely due to decreased vascular density in conjunction with increased metabolic demands secondary to increased cardiac work 40.
Taken together, using two mouse strains in which ventricular KATP channel activity was disrupted, we demonstrate that loss of KATP channel activity attenuated the expression of PGC-1α and mitochondrial energy metabolism-related enzymes in the heart, and exacerbated TAC-induced ventricular hypertrophy and dysfunction, indicating a role for KATP channels in the chronic response to increased cardiac work. We provide further evidence that the protective effect of KATP occurs at least partially by regulating the activity of FOXO1, which in turn influences the expression of PGC-1α and its downstream target genes. Our findings support an important role for KATP channels in regulating the myocardial expression of metabolism related genes in the cardiac response to hemodynamic overload (Figure 8D).
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported by NHLBI Grants HL71790 (YC) and HL21872 (RJB) from the National Institutes of Health.
Footnotes
Disclosures: none.
References
- 1.Miki T, Seino S. Roles of KATP channels as metabolic sensors in acute metabolic changes. Journal of Molecular and Cellular Cardiology. 2005;38:917–925. doi: 10.1016/j.yjmcc.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Bienengraeber M, Olson TM, Selivanov VA, Kathmann EC, O'Cochlain F, Gao F, Karger AB, Ballew JD, Hodgson DM, Zingman LV, Pang Y-P, Alekseev AE, Terzic A. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet. 2004;36:382–387. doi: 10.1038/ng1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui N, Li L, Wang X, Shi Y, Shi W, Jiang C. Elimination of allosteric modulation of myocardial KATP channels by ATP and protons in two Kir6.2 polymorphisms found in sudden cardiac death. Physiol Genomics. 2006;25:105–115. doi: 10.1152/physiolgenomics.00106.2005. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Seino S, Marban E, Nakaya H. Role of sarcolemmal KATP channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kane GC, Behfar A, Yamada S, Perez-Terzic C, O'Cochlain F, Reyes S, Dzeja PP, Miki T, Seino S, Terzic A. ATP-Sensitive K+ Channel Knockout Compromises the Metabolic Benefit of Exercise Training, Resulting in Cardiac Deficits. Diabetes. 2004;53:S169–S175. doi: 10.2337/diabetes.53.suppl_3.s169. [DOI] [PubMed] [Google Scholar]
- 6.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. PNAS. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kane GC, Behfar A, Dyer RB, O'Cochlain DF, Liu X-K, Hodgson DM, Reyes S, Miki T, Seino S, Terzic A. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet. 2006;15:2285–2297. doi: 10.1093/hmg/ddl154. [DOI] [PubMed] [Google Scholar]
- 8.Yamada S, Kane GC, Behfar A, Liu X-K, Dyer RB, Faustino RS, Miki T, Seino S, Terzic A. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J Physiol. 2006;577:1053–1065. doi: 10.1113/jphysiol.2006.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finck BN. The PPAR regulatory system in cardiac physiology and disease. Cardiovascular Research. 2007;73:269–277. doi: 10.1016/j.cardiores.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 10.Huss JM, Imahashi K-i, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, Giguère V, Murphy E, Kelly DP. The Nuclear Receptor ERR[alpha] Is Required for the Bioenergetic and Functional Adaptation to Cardiac Pressure Overload. Cell Metabolism. 2007;6:25–37. doi: 10.1016/j.cmet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker Johan W, Giles W, Naviaux RK, Giguère V, Evans RM. ERR[gamma] Directs and Maintains the Transition to Oxidative Metabolism in the Postnatal Heart. Cell Metabolism. 2007;6:13–24. doi: 10.1016/j.cmet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Xia Y, Buja LM, McMillin JB. Activation of the Cytochrome c Gene by Electrical Stimulation in Neonatal Rat Cardiac Myocytes. ROLE OF NRF-1 AND c-Jun. J Biol Chem. 1998;273:12593–12598. doi: 10.1074/jbc.273.20.12593. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Wilhelmsson H, Graff C, Li H, Oldfors A, Rustin P, Bruning JC, Kahn CR, Clayton DA, Barsh GS, Thoren P, Larsson N-G. Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nat Genet. 1999;21:133–137. doi: 10.1038/5089. [DOI] [PubMed] [Google Scholar]
- 14.Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor {gamma} coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu P-H, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, Spiegelman BM. Transcriptional coactivator PGC-1[alpha] controls the energy state and contractile function of cardiac muscle. Cell Metabolism. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-{gamma} coactivator 1 {alpha} PNAS. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daitoku H, Yamagata K, Matsuzaki H, Hatta M, Fukamizu A. Regulation of PGC-1 Promoter Activity by Protein Kinase B and the Forkhead Transcription Factor FKHR. Diabetes. 2003;52:642–649. doi: 10.2337/diabetes.52.3.642. [DOI] [PubMed] [Google Scholar]
- 19.Southgate RJ, Bruce CR, Carey AL, Steinberg GR, Walder K, Monks R, Watt MJ, Hawley JA, Birnbaum MJ, Febbraio MA. PGC-1α gene expression is down-regulated by Akt-mediated phosphorylation and nuclear exclusion of FoxO1 in insulin-stimulated skeletal muscle. FASEB J. 2005 doi: 10.1096/fj.05-3993fje. 05-3993fje. [DOI] [PubMed] [Google Scholar]
- 20.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt Promotes Cell Survival by Phosphorylating and Inhibiting a Forkhead Transcription Factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 21.Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J-i, Seino S. Defective insulin secretion and enhanced insulin action in K ATP channel-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flagg TP, Remedi MS, Masia R, Gomes J, McLerie M, Lopatin AN, Nichols CG. Transgenic overexpression of SUR1 in the heart suppresses sarcolemmal KATP. Journal of Molecular and Cellular Cardiology. 2005;39:647–656. doi: 10.1016/j.yjmcc.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 23.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, O. Holloszy J, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. PGC-1α Deficiency Causes Multi-System Energy Metabolic Derangements: Muscle Dysfunction, Abnormal Weight Control and Hepatic Steatosis. PLoS Biology. 2005;3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, Fassett J, Hu X, Zhu G, Lu Z, Li Y, Schnermann J, Bache RJ, Chen Y. Ecto-5'-Nucleotidase Deficiency Exacerbates Pressure-Overload-Induced Left Ventricular Hypertrophy and Dysfunction. Hypertension. 2008;51:1557–1564. doi: 10.1161/HYPERTENSIONAHA.108.110833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang P, Xu X, Hu X, van Deel ED, Zhu G, Chen Y. Inducible Nitric Oxide Synthase Deficiency Protects the Heart From Systolic Overload-Induced Ventricular Hypertrophy and Congestive Heart Failure. Circ Res. 2007;100:1089–1098. doi: 10.1161/01.RES.0000264081.78659.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehman JJ, Kelly DP. Transcriptional Activation Of Energy Metabolic Switches In The Developing And Hypertrophied Heart. Clinical and Experimental Pharmacology and Physiology. 2002;29:339–345. doi: 10.1046/j.1440-1681.2002.03655.x. [DOI] [PubMed] [Google Scholar]
- 27.Czubryt MP, McAnally J, Fishman GI, Olson EN. Regulation of peroxisome proliferator-activated receptor gamma coactivator 1alpha (PGC-1alpha ) and mitochondrial function by MEF2 and HDAC5. Proceedings of the National Academy of Sciences. 2003;100:1711–1716. doi: 10.1073/pnas.0337639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Remedi M, Rocheleau J, Tong A, Patton B, McDaniel M, Piston D, Koster J, Nichols C. Hyperinsulinism in mice with heterozygous loss of KATP channels. Diabetologia. 2006;49:2368–2378. doi: 10.1007/s00125-006-0367-4. [DOI] [PubMed] [Google Scholar]
- 29.Chutkow WAPJ, Wheeler MT, Wada T, Makielski JC, Burant CF, McNally EM. Episodic coronary artery vasospasm and hypertension develop in the absence of Sur2 K(ATP) channels. J Clin Invest. 2002;110:203–208. doi: 10.1172/JCI15672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deb TB, Coticchia CM, Dickson RB. Calmodulin-mediated Activation of Akt Regulates Survival of c-Myc-overexpressing Mouse Mammary Carcinoma Cells. J Biol Chem. 2004;279:38903–38911. doi: 10.1074/jbc.M405314200. [DOI] [PubMed] [Google Scholar]
- 31.Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396:584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- 32.Gumina RJ, O'Cochlain DF, Kurtz CE, Bast P, Pucar D, Mishra P, Miki T, Seino S, Macura S, Terzic A. KATP channel knockout worsens myocardial calcium stress load in vivo and impairs recovery in stunned heart. Am J Physiol Heart Circ Physiol. 2007;292:H1706–H1713. doi: 10.1152/ajpheart.01305.2006. [DOI] [PubMed] [Google Scholar]
- 33.Jilkina O, Kuzio B, Rendell J, Xiang B, Kupriyanov VV. K+ transport and energetics in Kir6.2-/- mouse hearts assessed by 87Rb and 31P magnetic resonance and optical spectroscopy. Journal of Molecular and Cellular Cardiology. 2006;41:893–901. doi: 10.1016/j.yjmcc.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 34.Marcil M, Ascah A, Matas J, Belanger S, Deschepper CF, Burelle Y. Compensated volume overload increases the vulnerability of heart mitochondria without affecting their functions in the absence of stress. Journal of Molecular and Cellular Cardiology. 2006;41:998–1009. doi: 10.1016/j.yjmcc.2006.08.117. [DOI] [PubMed] [Google Scholar]
- 35.Figarella-Branger D, Pellissier JF, Scheiner C, Wernert F, Desnuelle C. Defects of the mitochondrial respiratory chain complexes in three pediatric cases with hypotonia and cardiac involvement. Journal of the Neurological Sciences. 1992;108:105–113. doi: 10.1016/0022-510x(92)90195-q. [DOI] [PubMed] [Google Scholar]
- 36.Schiekofer S, Shiojima I, Sato K, Galasso G, Oshima Y, Walsh K. Microarray analysis of Akt1 activation in transgenic mouse hearts reveals transcript expression profiles associated with compensatory hypertrophy and failure. Physiol Genomics. 2006;27:156–170. doi: 10.1152/physiolgenomics.00234.2005. [DOI] [PubMed] [Google Scholar]
- 37.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest. 2005;115:2108–2118. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook SA, Matsui T, Li L, Rosenzweig A. Transcriptional Effects of Chronic Akt Activation in the Heart. J Biol Chem. 2002;277:22528–22533. doi: 10.1074/jbc.M201462200. [DOI] [PubMed] [Google Scholar]
- 39.Kawamori D, Kaneto H, Nakatani Y, Matsuoka T-a, Matsuhisa M, Hori M, Yamasaki Y. The Forkhead Transcription Factor Foxo1 Bridges the JNK Pathway and the Transcription Factor PDX-1 through Its Intracellular Translocation. J Biol Chem. 2006;281:1091–1098. doi: 10.1074/jbc.M508510200. [DOI] [PubMed] [Google Scholar]
- 40.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.