Abstract
Humans exhibit considerable variance in cognitive decline with age, with some exhibiting little disruption while others become significantly impaired. In aged rodents, individual differences in spatial memory have been used to identify putative compensatory mechanisms underlying successful hippocampal aging. However, there are few parallel rodent models of cognitive decline in frontal cortex-mediated functions. We tested the hypothesis that, like aged humans, aged mice would exhibit greater variance in executive function measures compared to young mice. We examined the performance of young and aged C57BL/6N mice in the attentional set-shifting task. While young and old mice did not differ on trials to criterion performance, aged mice exhibited significantly greater variance in mean correct latency – selective to the extradimensional shifting stage – compared to their younger counterparts. Thus this task may be used to identify mechanisms underlying individual differences in decline of frontal-mediated performances with age.
Keywords: Aging, Mice, Set-Shifting, Cognition, Odor, Attention, Executive Function, Memory
Introduction
The number of elderly Americans is rapidly growing, with estimates of a 400% increase in the number of adults over 60 between 2000 and 2050 (Division, 2001). In view of a concomitant increase in health risks with aging, research has tended to focus on ameliorating the likely impact of this trend on healthcare services. Just as important as treating age-related declines in health and cognitive functioning, however, is the need to understand why some people do not exhibit an age-related decline (Glatt, Chayavichitsilp, Depp, Schork, & Jeste, 2007; Rowe & Kahn, 1987), a phenotype sometimes termed “successful aging”. While the phenotype of successful aging has been debated in the literature, there is a growing consensus that understanding the biological mechanisms of healthy aging, as well as developing early identification and putative therapies for, those whose cognitive performance deteriorates with age (Glatt, et al., 2007; Hendrie, et al., 2006; Rowe & Kahn, 1987; Silverman, et al., 2008; Zubenko, Stiffler, Hughes, Fatigati, & Zubenko, 2002).
Examining cognitive aging in rodents offers greater experimental control than in humans to identify genetic, environmental, and epigenetic factors that may contribute to successful cognitive aging (those whose cognitive performance does not deteriorate with age). Numerous rodent tasks exist that are analogous to human cognitive tasks and show validity for selective cognitive domains (Young, Powell, Risbrough, Marston, & Geyer, 2009). Zhang et al, (2007) demonstrated that, similar to humans, rodents exhibit variance in spatial cognitive decline with age. Age-unimpaired (AU) rats could be identified using the Morris water maze, which assessed spatial memory. Similar to young rats, the AU rats exhibited higher muscarinic-mediated GTP-Eu binding in the hippocampus and prefrontal cortex compared to age-impaired (AI) rats (Zhang, et al., 2007). Cognitive reserve is one theory that proposes how people retain cognitive performance at levels comparable to that of young subjects (Whalley, Deary, Appleton, & Starr, 2004). Data suggest, however, that maintenance of cognitive performance with age may also be supported by adaptive behavioral strategies and/or recruitment of additional mechanisms or networks when compared to younger subjects (Velanova, Lustig, Jacoby, & Buckner, 2007). Compensatory mechanisms have been identified in aged rats which may support intact spatial memory in the water maze task. For example, young and AI rats exhibit NMDA receptor dependent long-term depression (LTD) in the hippocampus, whereas AU rats (similar spatial memory to young) exhibited non-NMDA receptor dependent hippocampal LTD (Lee, Min, Gallagher, & Kirkwood, 2005). These data suggest that adaptive mechanisms may contribute to maintaining normal cognitive performance with age. Overall, these data support the use of rodents for investigating mechanisms of successful cognitive aging.
Age-associated impairment in cognitive functioning is not observed across all cognitive domains, however (Anstey & Low, 2004; Gazzaley & D’Esposito, 2007). Hence, when investigating successful cognitive aging in rodents, it is important to focus on those cognitive domains that are most sensitive to age-induced impairment. One such domain with high sensitivity to age-related cognitive decline in humans is executive functioning (Gazzaley & D’Esposito, 2007). One of the most common tests of executive function in humans is the Wisconsin Card Sorting Task (Eling, Derckx, & Maes, 2008), with numerous reports on age-related decline in performance (Ashendorf & McCaffrey, 2008; Rhodes, 2004). Performance in the WCST requires contribution from working memory, reversal learning, attentional set-shifting and sustained attention, however, without separate measures for each of these domains (Eling, et al., 2008). The complexity and multi-faceted aspect of the task has led to questions about the ability to interpret results from this task as being relevant to any one cognitive domain (Kremen, Eisen, Tsuang, & Lyons, 2007). In-depth analyses of age-related deficits in WCST implicate poor shifting of an attentional set as the core deficiency in performance (Ashendorf & McCaffrey, 2008; Rhodes, 2004). Modifications to the WCST have been made to specifically examine attentional set-shifting and reversal learning, referred to as the intradimensional (ID)/extradimensional (ED) task (Owen, Roberts, Polkey, Sahakian, & Robbins, 1991). Age-related decline in ED set-shifting in this task has been reported (Owen, et al., 1991). An animal analogue of the ID/ED task exists (Attentional Set Shifting Task, ASST; (Birrell & Brown, 2000)) which exhibits high face, predictive, and construct validity for attentional set shifting in humans (Young, et al., 2009). The ASST has been performed in rats, and more recently been validated in mice (Bissonette, et al., 2008), and may provide a tool to assess successful cognitive aging. Barense et al. (2002) reported that aged rats had poorer performance in the ASST compared to young rats. Upon further examination, Nicolle and Baxter (Nicolle & Baxter, 2003) demonstrated that AI set-shifting rats exhibited lower levels of [3H] kainate binding in the cingulate cortex and higher levels of NMDA binding in the dorsomedial striatum compared to young and AU rats. Few studies have examined the genetic or epigenetic contribution to performance in this task, which may, in part, be due to the difficulty of genetic manipulation in rats compared to mice. One study examined ASST performance in mice over-expressing the Swedish amyloid precursor protein (APP) mutation, Tg(HuAPP695.K70N-M671L)2576, used as a mouse model of Alzheimer’s disease, and their wildtype littermates aged 6 vs. 14 months (Zhuo, et al., 2007). Because no within-group effects of reversals or ED shifts were observed, however (Zhuo, et al., 2007), it remains unclear whether the task was assessing executive functioning accurately (Birrell & Brown, 2000). Moreover, since individual differences in performance within the 14 month-old mice were not reported (Zhuo, et al., 2007), it is unknown whether successful cognitive aging can be measured using this task.
We sought to examine the effects of aging (4 vs. 24 mo) on ASST performance in C57BL/6 mice, an inbred strain that is commonly used in behavioral testing and is often the strain of choice when backcrossing mutants onto a particular inbred line. We hypothesized that, consistent with humans (Owen, et al., 1991) and rats (Barense, et al., 2002), aged C57BL/6 mice would exhibit a significant deficit in ASST performance compared to young mice specific to ED shifting. Moreover, we expected that there would be a separation of performance levels within the aging group so that successful and unsuccessful cognitive aging performers could be identified.
Methods and design
Aged (24 months) male C57BL/6N mice (n=20) were obtained from the NIA aged colony (Charles, River). Young (5 months) C57BL/6N mice (n=20) were purchased from Charles River laboratories. Training began with mice weighing 20–40 g. Mice were housed in pairs in a vivarium on a reversed day-night cycle (lights on at 8.00 PM, off at 8.00 AM), and were maintained at 85% of free-feeding weight with water available ad libitum. Prior to training and testing, mice were brought to the laboratory for 60 minutes between 9.00 AM and 6.00 PM. All procedures were approved by the UCSD Institutional Animal Care and Use Committee. The UCSD animal facility meets all federal and state requirements for animal care, and has been approved by the American Association for Accreditation of Laboratory Animal Care.
2.1. Apparatus
Mice readily dig in bedding placed in small bowls to retrieve a food reward, using olfactory cues to solve tasks (Young, Crawford, et al., 2007; Young, Kerr, et al., 2007; Young, Sharkey, & Finlayson, 2008). Ceramic pots (4.5×2.5 cm) were used for digging bowls, which were placed on platforms (11×5 cm). Specific odors and platforms were utilized as cues to guide the selection of mice. Odors were derived from commercially available powdered spices including; ground ginger, nutmeg, garlic, coriander, thyme, and cinnamon (Albertsons ®). Platforms included sandpaper, wood, neoprene, metal wire, tile, and a scrubber (Homebase ®). Platforms were used as opposed to digging medium because 1) mice do not readily manipulate their environment (Caine, Negus, & Mello, 1999) and 2) in initial pilot studies it proved difficult to identify whether mice were manipulating the medium in order to sample the medium, or to identify the correct stimulus (i.e. digging for a reward). We chose to use platforms as the second dimension as it had previously been demonstrated that rodents can utilize platform textures to solve tasks (Stefani, Groth, & Moghaddam, 2003; Stefani & Moghaddam, 2003). The food reward was a single 25 mg food pellet for each correct trial (Noyes Precision). The test apparatus was an adapted perspex home cage (30×18×12 cm) with clear plastic panels used to separate half of the cage into two equal sections. The two digging bowls were placed in each quarter section, with access to these sections limited by removable dividers. The removable dividers were used to deny the mice access to the other bowl after they had made a selection.
2.2. Shaping
Day 1: mice were introduced to the testing chamber and room and trained to dig in unscented bedding for food reward. Day 2: the mice were required to dig selectively in bowls containing each of the odors they would encounter in the main task, with platforms varied so that they encountered each platform prior to testing. The criterion for a dig was defined as when the nose or paws of the mouse broke the surface of the digging medium. This criterion was used throughout testing.
2.3. ASST paradigm
Each trial was initiated by raising the divider allowing access to two digging bowls, one of which was baited. For the first 4 trials mice were permitted to dig in the unbaited bowl without consequence, although an error was recorded, so that if one bowl was investigated in error, the mouse could move to the second baited bowl and learn the cue contingency. Errors on subsequent trials resulted in the mouse being denied entry to the other area, an error recorded, and the trial restarted. Trials would continue until 6 consecutive correct responses were made. Throughout the test session, the mice were required to perform a series of discriminations whereby they must select a bowl dependent upon a stimulus in a particular dimension, either odor or platform. Examples of the discriminations required to be made are found in Table 1. For example, in the simple discrimination (SD), the only relevant dimension present in the example provided would be odor. Compound discrimination (CD) would introduce the second dimension, platform, but the relevant stimulus (odor) in the SD would still identify the correct bowl. The first reversal (CDR) would require the mouse to respond in the bowl with the previously irrelevant odor. Once acquired, the mice would have to perform an ID shift wherein two novel odors were presented and only one would identify the baited bowl. The second reversal (IDR) would require the mouse to respond in the previously irrelevant odor-filled bowl. The mice would then be required to perform an ED shift, wherein the previously irrelevant dimension (platform) would then be relevant, and one stimulus would identify the baited bowl. The third and final reversal (EDR) would require the mice to attend to the previously irrelevant stimuli in the platform dimension. At each stage, the mice were required to make 6 consecutive correct responses prior to moving to the next stage. Mice were counter-balanced so that the initial SD would be either odor or platform. Stimuli combinations and locations were selected in a varied order. Trials to criterion and errors were recorded for each stage. Given the speed/accuracy trade-off observed with increasing task difficulty in mice (Abraham, et al., 2004; Rinberg, Koulakov, & Gelperin, 2006), we also recorded mean correct latency by stage. Latencies to respond were measured using stopwatches, initiated as the doors were raised and stopped when the mouse dug in one of the choices available. The total correct latency was divided by the total correct to produce the mean correct latency.
Table 1.
Example of the Testing Stages and Possible Stimuli Combinations in the Attentional Set-Shifting Task for Mice.
| Dimensions | Exemplar Combinations | |||
|---|---|---|---|---|
| Discriminations | Relevant | Irrelevant | Correct | Incorrect |
| Simple Discrimination (SD) | Odor | O1 | O2 | |
| Compound Discrimination (CD) | Odor | Platform | O1/P1 | O2/P1 |
| O1/P2 | O2/P2 | |||
| CD Reversal (CDR) | Odor | Platform | O2/P1 | O1/P1 |
| O2/P2 | O1/P2 | |||
| Intradimensional (ID) Shift | Odor | Platform | O3/P3 | O4/P3 |
| O3/P4 | O4/P4 | |||
| ID Reversal (IDR) | Odor | Platform | O4/P3 | O3/P3 |
| O4/P4 | O4/P4 | |||
| Extradimensional (ED) Shift | Platform | Odor | P5/O5 | P6/O5 |
| P5/O6 | P6/O6 | |||
| ED Reversal (EDR) | Platform | Odor | P6/O5 | P5/O5 |
| P6/O6 | P5/O6 | |||
Note: Mice were counter-balanced so that one half received odor as the initial relevant dimension, while the other half received platform. Thus, the correct exemplar is provided in bold.
2.4. Statistical analyses
Trials to criterion, errors to criterion, and correct and incorrect response times were recorded for every mouse at each stage. Data were initially analyzed using a repeated measures 3-factor ANOVA with stage (SD, CD, CDR, ID, IDR, ED, and EDR) as a within subjects factor, with group (age) and dimension change (odor to platform, platform to odor) as between subject factors. As age and stage did not significantly interact with dimension change (F<1, ns), dimension change data were not analyzed further. Different stages were also analyzed separately, because these stages are believed to be mediated by different cognitive constructs (Birrell & Brown, 2000) and different underlying neural substrates. Thus, 2-factor ANOVAs compared SD, CD, and ID as discrimination learning, and CDR, IDR, and EDR as tests of reversal learning (Birrell & Brown, 2000). Post-hoc analyses for significant effects or interactions were performed using Tukey’s test, with α set p<0.05. Planned t-test comparisons were conducted for trials to criteria and mean correct latencies on ED shifting by age, given the different cognitive constructs measured at this stage, as well as ID shifting for comparative purposes. Where significant effects of age were observed, equality of variance was also assessed using Levene’s test of equality of error variances. The relationship between ED shifting as measured by trials to criterion and correct response latencies were assessed via continuous (regression) and categorical (quartile split – top and bottom 25%) analyses as described previously (Swerdlow et al, 2006). Data were analyzed using SPSS (Chicago, U.S.A.).
Results
3.1. Overall performance
The performances of young and old C57BL/6N mice were compared in the ASST. Given the novelty of the parameters used, the data were analyzed for conceptual validity, i.e. the performance in the ED shift was significantly worse than that in the ID shift and that this effect was apparent in young and old mice irrespective of starting stimulus dimension. A significant main effect of stage was observed on trials to criterion in the ASST (F(6,198)=5.6, p<0.001; Figure 1). Aged mice exhibited no significant difference in trials to criteria, with no effect of starting dimension, nor a stage ×age interaction observed for this measure. A main effect of stage was noted on mean correct latency (F(6,198)=4.8, p<0.01), while aged mice did not differ significantly from young mice in overall mean correct latency. No stage ×age interaction was observed for mean correct latency. Given the differences in performance at the various stages, performance was analyzed according to the cognitive constructs represented by the stages (Birrell & Brown, 2000).
Figure 1.

Trials to criterion for each stage of the Attentional Set-Shifting Task (ASST) in young and old mice.
Mice could perform the ASST when using odors and platforms as the perceptual dimensions. The mice were required to perform six consecutively correct responses in each stage before moving onto the next stage, from simple discrimination (SD), to compound discrimination (CD), CD reversal (CDR), intradimensional (ID) shift, ID reversal (IDR), extradimensional (ED) shift, and finally ED reversal (EDR). Data presented as mean + s.e.m., * indicates p<0.05.
3.2. Discrimination learning
We examined the performance of animals in discrimination learning - stages SD, CD, and ID because they each required no rule shift. There were no differences in the number of trials to criteria based on age or stages, nor was there an age × stage interaction. No mean correct latency differences were observed between young and old mice or in an age × stage interaction. A main effect of stage was observed on mean correct latency however (F(2,111)=5.7, p<0.005); post-hoc analyses revealed that performance at CD was significantly faster in all groups compared to the SD or ID stages (p<0.05).
3.3. Reversal learning
After the discrimination stages CD, ID, and ED, the correct exemplars were reversed for CDR, IDR, and EDR assessing reversal learning. No differences in trials to criteria were observed between young and old mice, nor an age × stage interaction, or a main effect of stage. Young and old mice also did not differ in mean correct latency during reversal learning, nor was there an age × stage interaction.
3.4. ID/ED task validation
The ASST has been designed so that internal validation of attentional set formation can be assessed by comparing the performance of animals in the ID vs. ED stages. Poorer ED vs. ID performance would suggest that the animals had formed an attentional set to the initial stimulus dimension. Hence, we compared the ID/ED performance of mice. When comparing overall trials to criterion, a significant ID/ED difference was observed (F(1,70)=20.1, p<0.0001; Figure 1). Tukey’s post-hoc analyses revealed that mice took significantly more trials to perform an ED shift compared to an ID shift (p<0.05). A significant main effect of stage was also observed for mean correct latency (F(1,70)=15.0, p<0.0001). Post-hoc analyses revealed that mean correct latencies were longer for ED trials compared to ID trials (p<0.05; Figure 2).
Figure 2.

Mean correct latency performances of mice during intradimensional (ID) and extradimensional (ED) shifts by starting dimension
The mean correct latency differences of mice were measured when switching from odor (O) to platform (P) or P to O between ID and ED shifts, respectively. Irrespective of initial starting dimension (O or P), mice took significantly longer to choose the correct bowls when switching to the novel stimulus dimension. Data presented as mean + s.e.m.,* denotes p<0.05 when compared to ID shift in corresponding starting dimension.
3.5. Aging effects on ED shifting
The performances of young and old mice were compared in ID and ED shifting. These comparisons were made to determine whether there was a general aging effect on rule learning in novel stimuli (ID shifting), or if aging selectively affected prefrontally mediated (Bissonette, et al., 2008) attentional set-shifting (ED shift) (Birrell & Brown 2000). As ED was the only stage which specifically examined attentional set-shifting, planned t-test comparisons were performed on the effect of age on ED shift performance. No effect of age on total trials to criterion was observed on ID or ED shifting. Old mice took significantly longer to make a correct response in ED shifting compared to young mice, however, as measured by mean correct latency (t=−2.2, p<0.05; Fig. 3). No difference in the mean correct latency of ID shifting was observed between old and young mice (t<1 N.S.). Furthermore, there was no difference in the variability between young and old mice in latency to respond during ID shifting (Figure 4A). Aged mice however, exhibited a significantly greater increase in the variability of mean correct latency during the ED shift compared to young mice (Levene’s test of sphericity: F(1,35)=5.0, p<0.05; Figure 4B). These data are best represented by a scatter plot depicting the greater individual variance in mean correct latency in aged animals in the ED but not ID phase of the task (Figure 4B).
Figure 3.
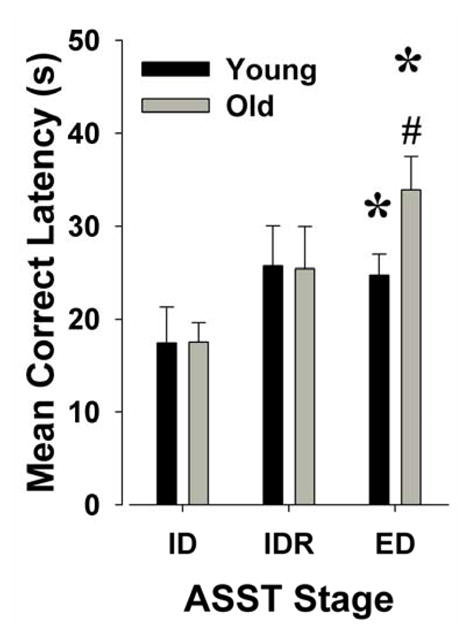
Age-related differences in mean correct latency of mice moving through attentional set-shifting task (ASST) stages
The mean correct latencies of young and old mice were compared as they performed the intradimensional (ID) shift, ID reversal (IDR), and the extradimensional (ED) shift. Age did not affect ID or IDR correct latencies, but negatively impacted the latency for mice to complete a correct ED shift. Data presented as mean + s.e.m., * denotes p<0.05 when compared to performance at ID shift, # denotes p<0.05 when compared to young mice.
Figure 4.
Individual mean correct latency of mice at ID and ED shifts
The mean correct latency of individual mice at the intradimensional (ID) and extradimensional (ED; B) shift stages were plotted and compared. Latencies to perform ID shifts did not differ by age (A). Latencies to perform ED shifts did however separate by age with some old mice (age unimpaired) exhibiting performance at comparable levels to young mice, while others mice exhibited an age-related decline in performance (age impaired) as measured by increased mean correct latency (B). * denotes p<0.05 for test of equality of error variance compared to young mice.
Because latency changes can be interpreted as either changes in speed/accuracy trade off or simply alterations in locomotor activity in aged mice, we examined the relationship between trials to criterion and choice latency. If mice with high latencies show low trials to criterion, this result may indicate a speed/accuracy trade off strategy. Although not significant, mean correct latency exhibited a negative correlation to trials to criterion on ED shift performance (r=−0.25, p=0.15). The relationship between ED trials to criterion and mean correct latencies was also examined using only the mice in the highest and lowest quartiles of trials to criterion in the ED phase as has been described previously for other measures (Swerdlow et al, 2006). The mean correct latencies of young mice with high vs. low ED trials to criterion did not differ (F(1,8)=3.0, p=0.21; Figure 5A). Old mice with low ED trials to criteria were significantly slower than old mice with high ED trials to criterion (F(1,8)=6.4, p<0.05; Figure 5B).
Figure 5.
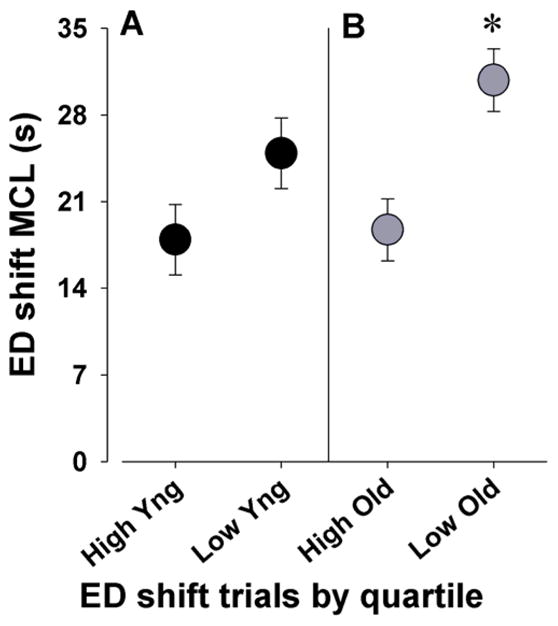
Examination of the relationship between ED shift trials to criteria and mean correct latency in young and old mice
The ED shift performance of old and young (Yng) mice as measured by trials to criterion were split within the two age groups into high (poor performers) and low (good performers) quartiles (i.e. top and bottom 25%). The mean correct latencies (MCL) of these mice were then compared between quartiles within each age group. Top performing young mice as measured by ED trials to criteria did not differ significantly in speed from poorer performing mice (A). Older mice that performed well in ED shift trials to criteria were significantly slower than poorly performing older mice (B). Data presented as mean ± s.e.m. * denotes p<0.05 when compared to poor performing old mice.
Discussion
In the present studies we demonstrated that mice can readily be trained to discriminate between odors or platforms in performance of the ASST. Surprisingly however, aged (24 mo) mice did not exhibit a deficit in ASST performance compared to young (4 mo) mice as measured by trials to criterion. Aged mice did, however, exhibit significantly slower correct latencies compared to young mice specifically in the more difficult stages of the task, i.e. the ED shift stage, with no differences observed at any other stages. This slowing of performance in some mice may reflect a speed/accuracy trade-off. This interpretation is supported by our findings that the aged mice with the lowest trials to criterion exhibited the slowest choice latencies, which was not observed in the young mice. These data support the conclusion that aged mice may use a different strategy (speed to accuracy trade off) in the ASST to maintain accurate performance.
The validity of the ASST to probe executive functions is thought to be dependent upon observing a significant increase in trials to criterion for the ED stage compared to the ID stage. Here we observed a clear ID/ED difference irrespective of initial perceptual dimension, suggesting that ED shift performance was assessing attentional set-shifting. This ID/ED shift is not always observed in mice in this task, likely due to methodological differences. To our knowledge only two (DeSteno & Schmauss, 2008; Papaleo, et al., 2008) of eight (Brigman, Bussey, Saksida, & Rothblat, 2005; Colacicco, Welzl, Lipp, & Wurbel, 2002; Garner, Thogerson, Wurbel, Murray, & Mench, 2006; Laurent & Podhorna, 2004; Levi, Kofman, Schwebel, & Shaldubina, 2008; Zhuo, et al., 2007) mouse studies have demonstrated an ID/ED shift in parameters consistent with rats and humans (reviewed in (Young, et al., 2009)). Bisonette et al, (Bissonette, et al., 2008), utilized four ID shift stages to ensure mice showed an attentional set when moved to the ED stage. The use of multiple ID stages to demonstrate an ID/ED effect is in contrast to the present studies, which utilized only one ID shift, consistent with the original report in the task in rats (Birrell & Brown, 2000). The data presented here support the conclusion that mice can form an attentional set with ID/ED differences observed in trials to criterion and mean correct latency. The present data also extend the dimensions from which stimuli can be selected when assessing ASST, since mice readily utilized platform cues to discriminate between stimuli. These findings are consistent with the utility of platforms as cues for rats in the cross-maze set-shifting paradigm (Stefani, et al., 2003; Stefani & Moghaddam, 2003).
Only one aging study in mice in the ASST has been described previously. This longitudinal study assessed ASST performance in mice with the amyloid precursor protein mutation (TG2576). While an effect of aging was observed for every stage in the task, neither an ID/ED shift nor performance differences from simple to reversal learning were observed (Zhuo, et al., 2007). Thus while an effect of aging was observed, the interpretation of the data remain ambiguous as there was not a clear attentional set formed in these mice. These ambiguities also make it more difficult to compare to the lack of effect of age on trials to criterion in the present studies. The present data do not fully support the idea that mice exhibit age-related deficits in performance of a set-shifting task. This finding is in contrast with previous human (Ashendorf & McCaffrey, 2008; Owen, et al., 1991; Rhodes, 2004), and rat studies (Barense, et al., 2002), and may reflect the fact that mice in the present study were younger than required for an observed deficit in trials to criterion. The data do support the conclusion, however, that some aged mice did begin to exhibit altered performance in the task, because we found much higher variability in latency to respond in aged mice compared to young mice. While humans and rats exhibit poorer trials to criterion in ED shifting (Barense, et al., 2002; Owen, et al., 1991), aged mice in the present studies exhibited slower mean correct latencies in ED shifting. The strategy for maintenance of accuracy at the expense of speed is consistently observed in psychological testing in humans including the elderly, and is referred to as a speed/accuracy trade-off (Busemeyer & Townsend, 1993). The observation that the aged mice that performed most accurately in this test were significantly slower than poorer performing older mice support the hypothesis that they adopt a speed/accuracy trade-off strategy. Younger mice, however, did not show a relationship between mean correct latency and accuracy, suggesting that this strategy was adopted only in the aged group.
Previous studies suggest that mice demonstrate a speed/accuracy trade-off in other cognitive domains, especially when using olfactory cues. In the odor discrimination choice reaction time task, increasing odor similarity, which presumably increases discrimination difficulty, resulted in longer reaction times in mice that maintained high accuracy (Abraham, et al., 2004; Rinberg, et al., 2006). Interestingly, when challenged similarly, rats did not slow their reaction times but committed more errors, reducing their accuracy in responding (Uchida & Mainen, 2003). Therefore, consistent with these findings, increased task difficulty with ED rule shifting (Fig 2) and age in mice resulted in slower latencies (Figs. 3 & 5), whereas rats were reported to exhibit an increase in errors (Barense, et al., 2002). When performing a sustained attention task, aged rats exhibited both reduced accuracy and slower response latencies when compared to younger rats (Muir, Fischer, & Bjorklund, 1999). These results were interpreted as indicative of age-induced inattention although processing speed deficiencies may have contributed as performance improved to levels comparable of younger rats with a small increase in stimulus duration (Muir, et al., 1999). Thus caution must be taken when interpreting data which may have been confounded by processing speed differences in rodents. These data highlight the utility of the speed/accuracy trade-off as a strategy in older animals to maintain accuracy of performance. These speed/accuracy trade-off data in olfactory discrimination therefore support the hypothesis that older mice may therefore have had greater difficulty in performing the ED shift compared to younger mice. It is certainly possible that latency increases were due to non-selective slowing of locomotor activity in aged mice; however the selectivity of these findings to stages that were most difficult (i.e. latency reductions were observed only in ED and not ID stages) would argue against this interpretation. Additionally, the aged mice in these studies did not show a significant change in locomotor activity/exploration as measured in the open field via distance traveled or average velocity (data not shown), suggesting that the slow response rate was not due simply to non specific reductions in motor activation.
In the present study, young and old mice did not differ in simple discrimination learning (SD, CD, and ID shifting) as measured by trials to criterion or mean correct latency. While there was an effect of stage, this effect was only observed as mean correct latency for CD being faster than SD and ID, likely due to the familiarity of the stimuli. Reversal learning performance of mice (CDR, IDR, and EDR shifts) were significantly poorer as measured by trials to criterion compared to simple learning stages (CD, ID, ED shifts), however. These data indicate that mice had acquired the discrimination rules, and were not simply using an unknown strategy (e.g. bait scent) to perform the task. No effect of age on reversal learning performance as measured by either errors or response latency was observed, findings that are consistent with human studies of aging in similar tasks (Owen, et al., 1991). Therefore, in the present studies the only age-related effect on mouse performance of the ASST appeared to be selective for latencies to respond in ED shifting. The selectivity for aging affects on this stage exhibits some consistency with human and rat studies (Barense, et al., 2002; Owen, et al., 1991). Another practical consideration of the use of the ASST in modeling cognitive decline with age is whether or not there is enough power to detect individual differences across aged animals for future mechanistic or treatment studies. Although power to detect age-impaired and un-impaired groups will vary with criterion used (e.g. median split, quartile extremes, or two-standard deviations from mean of young performers) and methods (e.g. age at testing), in the present studies, aged animals showed the highest variability in ED latency performance (Figure 4B). Thus this measure may allow for the strongest detection of differences in individual task performance compared to other phases of the task or in trials to criterion measures.
While the relationship between speed and accuracy is not always clear, there is evidence to support a speed/accuracy effect in ED shifting in mice performing the ASST. Bisonette et al, (Bissonette, et al., 2008), demonstrated that consistent with human and rat neuroanatomical studies (Birrell & Brown, 2000; McAlonan & Brown, 2003; Owen, et al., 1991), medial prefrontal cortex lesioned mice exhibit impaired ED shifts, while orbitofrontal lesioned mice exhibited impaired reversal learning. Interestingly, mice with mPFC lesions exhibited a poorer accuracy in the ED shifting stage only, while the response latency of these mice were faster than sham lesioned controls in ED shifting only (Bissonette, et al., 2008), suggesting that faster responses may be linked to poor performance in impaired animals. The present findings suggest that the converse may also be observed, with age impaired animals slowing response time to maintain accuracy. In conclusion, mice can demonstrate an attentional set shift. Moreover, platforms can be used as a perceptual dimension which may alleviate the ambiguity of differentiating between digging and sampling when using digging media. The present studies add to the cross-species translational validity of using the ASST in mice, and highlight the importance of assessing latency to respond, because this measure may unmask subtle cognitive differences or strategies. Finally, the ASST in mice may be useful to examine compensatory changes or resilience factors (Mhyre, et al., 2005), that may contribute to successful cognitive aging (Glatt, et al., 2007) in the executive function domain.
Acknowledgments
We would like to thank Dr. Verity Brown and her laboratory for advice on statistical analyses and setup of the attentional set-shifting task. We would also like to thank Christine Scott and Mahalah Buell for their assistance. These studies were supported, in part, by a Sam and Rose Stein Institute for Research in Aging Fellowship (JWY) and junior faculty pilot award (VBR), as well as the Veterans Affairs Center of Excellence for Stress and Mental Health, and National Institute of Mental Health grants P30 MH080002-01 and R01 MH071916. Drs. Young, Powell, Geyer, Jeste, and Risbrough report no conflict of interest.
References
- Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44(5):865–876. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Low LF. Normal cognitive changes in aging. Aust Fam Physician. 2004;33(10):783–787. [PubMed] [Google Scholar]
- Ashendorf L, McCaffrey RJ. Exploring age-related decline on the Wisconsin Card Sorting Test. Clin Neuropsychol. 2008;22(2):262–272. doi: 10.1080/13854040701218436. [DOI] [PubMed] [Google Scholar]
- Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Mem. 2002;9(4):191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20(11):4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Martins GJ, Franz TM, Harper ES, Schoenbaum G, Powell EM. Double dissociation of the effects of medial and orbital prefrontal cortical lesions on attentional and affective shifts in mice. J Neurosci. 2008;28(44):11124–11130. doi: 10.1523/JNEUROSCI.2820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigman JL, Bussey TJ, Saksida LM, Rothblat LA. Discrimination of multidimensional visual stimuli by mice: intra- and extradimensional shifts. Behav Neurosci. 2005;119(3):839–842. doi: 10.1037/0735-7044.119.3.839. [DOI] [PubMed] [Google Scholar]
- Busemeyer JR, Townsend JT. Decision field theory: a dynamic-cognitive approach to decision making in an uncertain environment. Psychol Rev. 1993;100(3):432–459. doi: 10.1037/0033-295x.100.3.432. [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK. Method for training operant responding and evaluating cocaine self-administration behavior in mutant mice. Psychopharmacology (Berl) 1999;147(1):22–24. doi: 10.1007/s002130051134. [DOI] [PubMed] [Google Scholar]
- Colacicco G, Welzl H, Lipp HP, Wurbel H. Attentional set-shifting in mice: modification of a rat paradigm, and evidence for strain-dependent variation. Behav Brain Res. 2002;132(1):95–102. doi: 10.1016/s0166-4328(01)00391-6. [DOI] [PubMed] [Google Scholar]
- DeSteno DA, Schmauss C. Induction of early growth response gene 2 expression in the forebrain of mice performing an attention-set-shifting task. Neuroscience. 2008;152(2):417–428. doi: 10.1016/j.neuroscience.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Division, U. S. C. B. P. D. a. H. a. H. E. S. (2001). National Population Projections.
- Eling P, Derckx K, Maes R. On the historical and conceptual background of the Wisconsin Card Sorting Test. Brain Cogn. 2008;67(3):247–253. doi: 10.1016/j.bandc.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Garner JP, Thogerson CM, Wurbel H, Murray JD, Mench JA. Animal neuropsychology: validation of the Intra-Dimensional Extra-Dimensional set shifting task for mice. Behav Brain Res. 2006;173(1):53–61. doi: 10.1016/j.bbr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, D’Esposito M. Top-down modulation and normal aging. Ann N Y Acad Sci. 2007;1097:67–83. doi: 10.1196/annals.1379.010. [DOI] [PubMed] [Google Scholar]
- Glatt SJ, Chayavichitsilp P, Depp C, Schork NJ, Jeste DV. Successful aging: from phenotype to genotype. Biol Psychiatry. 2007;62(4):282–293. doi: 10.1016/j.biopsych.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Hendrie H, Albert M, Butters M, Gao S, Knopman DS, Launer L, et al. The NIH cognitive and emotional health project report of the critical evaluation study committee. Alzheimer’s & Dementia. 2006;2:12–32. doi: 10.1016/j.jalz.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Kremen WS, Eisen SA, Tsuang MT, Lyons MJ. Is the Wisconsin Card Sorting Test a useful neurocognitive endophenotype? Am J Med Genet B Neuropsychiatr Genet. 2007;144B(4):403–406. doi: 10.1002/ajmg.b.30527. [DOI] [PubMed] [Google Scholar]
- Laurent V, Podhorna J. Subchronic phencyclidine treatment impairs performance of C57BL/6 mice in the attentional set-shifting task. Behav Pharmacol. 2004;15(2):141–148. doi: 10.1097/00008877-200403000-00006. [DOI] [PubMed] [Google Scholar]
- Lee HK, Min SS, Gallagher M, Kirkwood A. NMDA receptor-independent long-term depression correlates with successful aging in rats. Nat Neurosci. 2005;8(12):1657–1659. doi: 10.1038/nn1586. [DOI] [PubMed] [Google Scholar]
- Levi Y, Kofman O, Schwebel M, Shaldubina A. Discrimination and avoidance learning in adult mice following developmental exposure to diisopropylfluorophosphate. Pharmacol Biochem Behav. 2008;88(4):438–445. doi: 10.1016/j.pbb.2007.09.017. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146(1–2):97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Mhyre TR, Chesler EJ, Thiruchelvam M, Lungu C, Cory-Slechta DA, Fry JD, et al. Heritability, correlations and in silico mapping of locomotor behavior and neurochemistry in inbred strains of mice. Genes Brain Behav. 2005;4(4):209–228. doi: 10.1111/j.1601-183X.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Baxter MG. Glutamate receptor binding in the frontal cortex and dorsal striatum of aged rats with impaired attentional set-shifting. Eur J Neurosci. 2003;18(12):3335–3342. doi: 10.1111/j.1460-9568.2003.03077.x. [DOI] [PubMed] [Google Scholar]
- Owen AM, Roberts AC, Polkey CE, Sahakian BJ, Robbins TW. Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29(10):993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, et al. Genetic dissection of the role of catechol-O-methyltransferase in cognition and stress reactivity in mice. J Neurosci. 2008;28(35):8709–8723. doi: 10.1523/JNEUROSCI.2077-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes MG. Age-related differences in performance on the Wisconsin card sorting test: a meta-analytic review. Psychol Aging. 2004;19(3):482–494. doi: 10.1037/0882-7974.19.3.482. [DOI] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Speed-accuracy tradeoff in olfaction. Neuron. 2006;51(3):351–358. doi: 10.1016/j.neuron.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL. Human aging: usual and successful. Science. 1987;237(4811):143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- Silverman JM, Schnaider-Beeri M, Grossman HT, Schmeidler J, Wang JY, Lally RC. A phenotype for genetic studies of successful cognitive aging. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(2):167–173. doi: 10.1002/ajmg.b.30483. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Groth K, Moghaddam B. Glutamate receptors in the rat medial prefrontal cortex regulate set-shifting ability. Behav Neurosci. 2003;117(4):728–737. doi: 10.1037/0735-7044.117.4.728. [DOI] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Distinct contributions of glutamate receptor subtypes to cognitive set-shifting abilities in the rat. Ann N Y Acad Sci. 2003;1003:464–467. doi: 10.1196/annals.1300.064. [DOI] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci. 2003;6(11):1224–1229. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- Velanova K, Lustig C, Jacoby LL, Buckner RL. Evidence for frontally mediated controlled processing differences in older adults. Cereb Cortex. 2007;17(5):1033–1046. doi: 10.1093/cercor/bhl013. [DOI] [PubMed] [Google Scholar]
- Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing Res Rev. 2004;3(4):369–382. doi: 10.1016/j.arr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Young JW, Crawford N, Kelly JS, Kerr LE, Marston HM, Spratt C, et al. Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. Eur Neuropsychopharmacol. 2007;17(2):145–155. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Young JW, Kerr LE, Kelly JS, Marston HM, Spratt C, Finlayson K, et al. The odour span task: a novel paradigm for assessing working memory in mice. Neuropharmacology. 2007;52(2):634–645. doi: 10.1016/j.neuropharm.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Young JW, Powell SB, Risbrough V, Marston HM, Geyer MA. Using the MATRICS to guide development of a preclinical cognitive test battery for research in schizophrenia. Pharmacol Ther. 2009 doi: 10.1016/j.pharmthera.2009.02.004. S0163–7258(09)00035–7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, Sharkey J, Finlayson K. Progressive impairment in olfactory working memory in a mouse model of Mild Cognitive Impairment. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Watson ML, Gallagher M, Nicolle MM. Muscarinic receptor-mediated GTP-Eu binding in the hippocampus and prefrontal cortex is correlated with spatial memory impairment in aged rats. Neurobiol Aging. 2007;28(4):619–626. doi: 10.1016/j.neurobiolaging.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Zhuo JM, Prescott SL, Murray ME, Zhang HY, Baxter MG, Nicolle MM. Early discrimination reversal learning impairment and preserved spatial learning in a longitudinal study of Tg2576 APPsw mice. Neurobiol Aging. 2007;28(8):1248–1257. doi: 10.1016/j.neurobiolaging.2006.05.034. [DOI] [PubMed] [Google Scholar]
- Zubenko GS, Stiffler JS, Hughes HB, 3rd, Fatigati MJ, Zubenko WN. Genome survey for loci that influence successful aging: sample characterization, method validation, and initial results for the Y chromosome. Am J Geriatr Psychiatry. 2002;10(5):619–630. [PubMed] [Google Scholar]



