Abstract
B6.Sle1b mice, which contain the Sle1b gene interval derived from lupus prone NZM2410 mice on a C57BL/6 background, present with gender-biased, highly penetrant anti-nuclear antibody (ANA) production. To obtain some insight into the possible induction mechanism of autoantibodies in these mice we compared antigen specific T dependent (TD) and T independent (TI-II) responses between B6.Sle1b and B6 mice before the development of high ANA titers. Our results show that B6.Sle1b mice mount enhanced responses to a TI-II antigen. Additionally, the memory T cell response generated by a TD antigen was also increased. This enhancement correlates with the greater ability of B cells from B6.Sle1b mice to present antigen to T cells. The SLAM Associated Protein (SAP) is critical for signaling of many of the molecules encoded by the SLAM/CD2 gene cluster, candidates for mediating the Sle1b phenotype; therefore, we also investigated the effect of sap deletion in these strains on the TD and TI-II responses as well as on ANA production. The results of these studies of responses to non-self antigens provide further insight for the mechanism by which responses to self-antigens might be initiated in the context of specific genetic alterations.
Keywords: ANA, antigen-specific responses, SAP, SLE
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized in both mice and humans by the production of antinuclear antibodies (ANA). The B6.Sle1 congenic strain carries the Sle1 susceptibility locus from NZM2410 on the C57/BL6 (B6) background (1). B and T cells from these mice possess a spontaneous activated phenotype and with age the mice produce ANA but do not develop kidney disease [2, 3). The Sle1 locus has been further dissected and four other strains have been generated containing smaller segments of the locus: B6.Sle1a, Sle1b, Sle1c and Sle1d. Among these subcongenic strains, B6.Sle1b has the highest penetrance in ANA production [4). Whereas B6.Sle1b T cells were found to exhibit greater Ca2+ flux response than B6 cells to stimulation through the TCR, similar assays did not reveal differences in B cells from the two strains [5). However, in this study, whole splenocytes, consisting of a heterogeneous population of activated and resting B cells were used; and the possibility remains that isolated B cell subpopulations from these strains may differ. We have therefore examined phenotypic as well as functional differences, which might be exhibited by B6.Sle1b mice that may be correlated with their propensity to produce ANA. In addition, genomic analyses of the Sle1b locus suggest that the SLAM/CD2 gene cluster, which encodes numerous cellular interaction molecules expressed by B, T, as well as NK cells, are the strongest candidates for mediating the Sle1b phenotype [5]. The SLAM receptors signal through SAP-related molecules [6]. Therefore, we crossed B6.sap-/- with B6.Sle1b mice to generate a B6.Sle1b.sap-/- strain in order to determine the effect of the absence of this signal transducer on antigen-specific antibody responses as well as ANA production. The results of our studies, performed in mice prior to the appearance of ANA, show that genes encoded within a restricted chromosomal region of a strain susceptible to SLE can alter responses to both T dependent and T independent antigens. Thus, given the appropriate genetic background, responses to these antigenic moieties present on infectious agents that may mimic self antigens could contribute towards autoantibody production.
METHODS
Mice
B6.Sle1b [4], B6.sap-/- [7], OT-II [8], PK136 transgenic [9] and C57BL/6 mice (B6) were bred and maintained under specific pathogen-free conditions at the UT Southwestern Animal Resources Center. To derive the B6.Sle1b.sap-/- strain [B6.Sle1b × B6.sap-/-] F1 mice were intercrossed and screened for homozygotes at both the Sle1b and the Sap loci.
Bone marrow chimeras
For NK cell depletion, bone marrow chimeras were made of B6.Sle1b or C57BL/6 control mice transferred into lethally irradiated PK136 [9] or B6 hosts [850 cGy 137Cs □-irradiation (Gamma Cell 40; Atomic Energy, Ottawa, Ontario, Canada)]. Each animal was injected intravenously with 1–2 × 107 bone marrow cells from 8-week-old B6.Sle1b or B6 control mice. The chimeras in PK136 mice were found to be depleted of NK cells for at least 3 months but NKT cells remained intact as determined by FACS analysis of stained liver and peripheral blood lymphocytes (PBLs) using anti-DX5, anti-NK1.1 and anti-CD3 (BD Bioscience) ([9] and data not shown).
Immunizations
2-month-old male mice were used. For TI-II responses mice were immunized intraperitoneally (i.p.) with 40 □gs NP-Ficoll in PBS. For primary antibody responses mice were immunized (i.p) with 100 □gs of FITC-KLH emulsified in RIBI adjuvant (Corixia, Seattle, WA), prepared as suggested by the manufacturer. After 30 days mice were challenged (i.p) with 100 □gs of TNP-KLH in PBS for assessment of the extent of help from memory T cells for a primary B cell response. Sera were obtained from tail bleeds before and at various times after immunizations. In one experiment NK cells were depleted from a group of mice by injection of anti-NK1.1 antibodies as previously described [10]. When given, Poly(I:C) was injected i.p 16 hours before NP-Ficoll administration.
Serum Antibody Titers
To determine serum antibody titers, subclass antigen specific immunoglobulin (Ig) ELISA was performed as previously described [10] using ELISA plates coated with saturating amounts of NP-BSA, FITC–BSA or TNP-BSA (Biosearch Technologies, Inc). Serial dilutions of serum samples were incubated overnight at 4°C. Bound Igs were detected by horseradish peroxidase (HRP)-conjugated, isotype-specific anti-mouse Ig antibodies (Southern Biotechnology, Birmingham, AL) and developed with the substrate, 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (Sigma, St Louis, MO). ELISA plates were read by an automated ELISA reader (Molecular Diagnostics) at OD 405 nm. Ig levels are shown as the difference in ODs before and after immunization. Results shown represent serum dilutions that allow the most sensitive detection of changes in antibody levels. ANA titers were determined on plates coated with double stranded calf thymus DNA and total histones as previously described [11] and detected by isotype-specific anti-mouse antibodies as above.
Cell Isolation
B cells from either B6.Sle1b or B6 mice were fractionated by Percoll gradient sedimentation as previously described [12]. High density cells were labeled with biotinylated anti-CD43 and further purified by by depletion of CD43+ cells using streptavidin (SA)-magnetic beads (BD Bioscience). T cells specific for the ova peptide323-339 in the context of MHC II were purified from OT-II mice by first passing splenocytes over a nylon wool column to remove the majority of B cells. The remaining cells were then negatively selected using biotinylated anti-MHCII (Ebioscience) and PE anti-DX5 followed by either SA or anti-PE conjugated magnetic beads. In all cases cell preparations were found to be at least 95% pure by FACS analysis.
Cell surface staining
Cells were preincubated with anti-Fc□RII (BD Bioscience) before staining with specific antibodies in PBS at 4°C. Labeled cells were collected on a FACScan flow cytometer and analyzed on CellQuest (BD Bioscience). All antibodies used for staining were obtained from BD Bioscience.
CD86 upregulation
Purified resting B cells (1×106 cells/ml) were cultured overnight in complete medium [10% FCS, glutamine, non-essential amino acids, penicillin-streptomycin at manufacturer's suggested concentrations (Invitrogen) and 50 □M □-mercaptoethanol in RPMI], in the presence or absence of 50 □g/ml LPS [12]. Cells were harvested and stained with indicated reagents for FACS.
T cell proliferation
2×104 B cells were cultured overnight in triplicate in 96-well plates, with or without OVA or OVA peptide323-339 in a final volume of 0.1ml complete medium. Appropriate antigen concentration was determined by titration so that the resulting T cell proliferation was within the linear range. In other experiments B cell numbers were titrated at a fixed antigen concentration. Following incubation B cells were irradiated at 1,300 rads (137Cs □-irradiation) after which 80% of the culture supernatant was removed and 0.1 ml of purified syngeneic T cells carrying the transgene with specificity for the OVA peptide323-339 (OT-II) at various concentrations were added to each well. After 2 days, 0.5 □Ci of 3H-thymidine was added per well and cells were harvested on glass fiber filters the following day. [3H]-TdR incorporation was measured in a Beckman scintillation counter (LS3801).
Statistical Analyses
Differences between groups were designated to be statistically significant when the two-tailed Student's t-test yielded a probability of ≤ 0.05 that the two groups were identical. Wilcoxon Rank-Sum (Mann-Whitney) Test was used to test differences between sample sets where applicable.
RESULTS
Activation status of high density B cells from B6.Sle1b mice
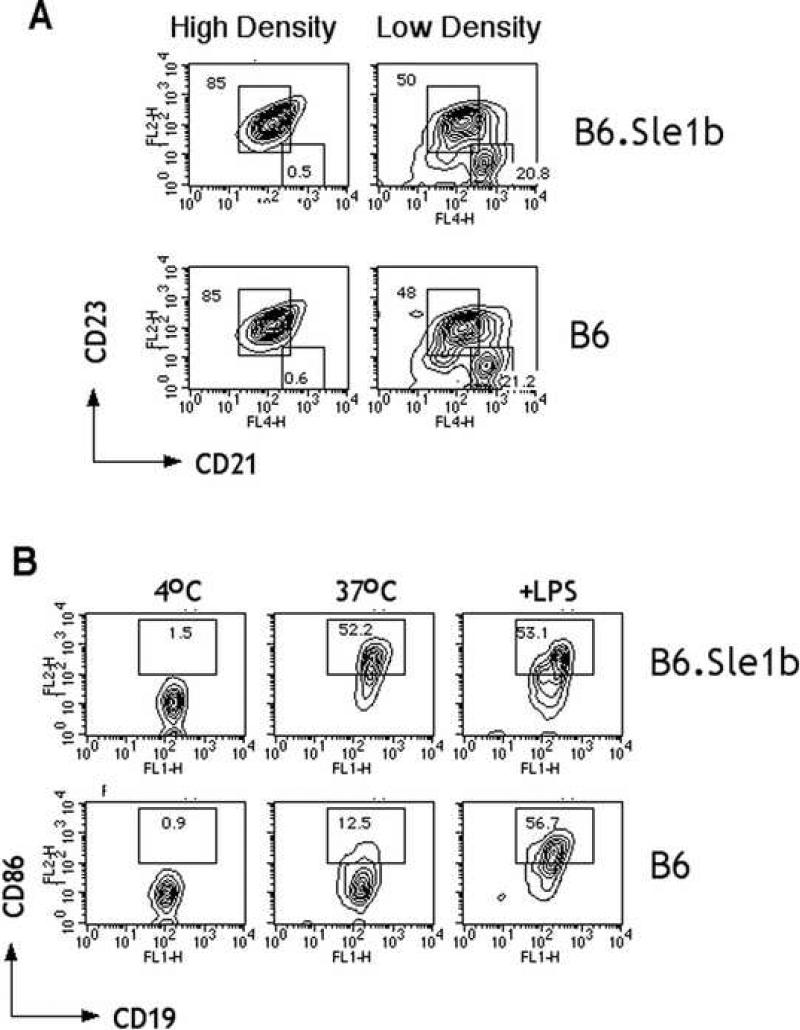
Whole splenocytes contain a mixed population of activated and resting B cells even though they may be housed under SPF conditions. The cause and extent of the apparently spontaneous activation are highly variable, which may account for the lack of difference in activation markers previously observed between B6.Sle1b and B6 mice [5]. To compare more homogenous populations, we isolated high and low density B cells from B6 and B6.Sle1b mice and examined the relative composition of the major B cell subsets by staining with anti-CD23 and anti-CD21. Fig. 1A shows that although high and low density B cells differed in the the representation of follicular (CD19+CD23hi, CD21intermediate ) and marginal zone (CD19+CD23lo, CD21high) subsets, the two strains did not differ significantly. Similarly, examination of the surface intensity of CD86, an inducible costimulatory molecule (Fig. 1B), on high density B cells showed that they were also comparable. Upon overnight incubation in culture medium a low level of spontaneous upregulation of CD86 was detectable for B cells from B6 mice, as has been shown by others [13]. Interestingly, however, the extent of upregulation was found to be much higher for B cells from B6.Sle1b mice. The extent of increase was so great, that addition of LPS did not cause further detectable increases (Fig.1B). Thus, it appears that all resting B cells from B6.Sle1b mice are more easily triggered compared to those from B6 mice.
Fig.1.
Resting B cells from 2-month old B6.Sle1b spontaneously upregulate CD86. The subset distribution of B cells from high and low density fractions of representative B6.Sle1b or B6 mice obtained by Percoll gradient centrifugation were compared by staining each fraction with CD19 followed by CD23 and CD21 and analyzed by FACS (A). The numbers shown indicate the percent of CD19+ cells contained within each gate. Purified high density B cells were incubated overnight in medium alone or with 50 □gs/ml LPS. Indicated in (B) are the percentages of cells in the defined region of representative FACS plots of live lymphocyte gated cells. Results shown are representative of three independent experiments.
Antigen specific responses of B6.Sle1b mice
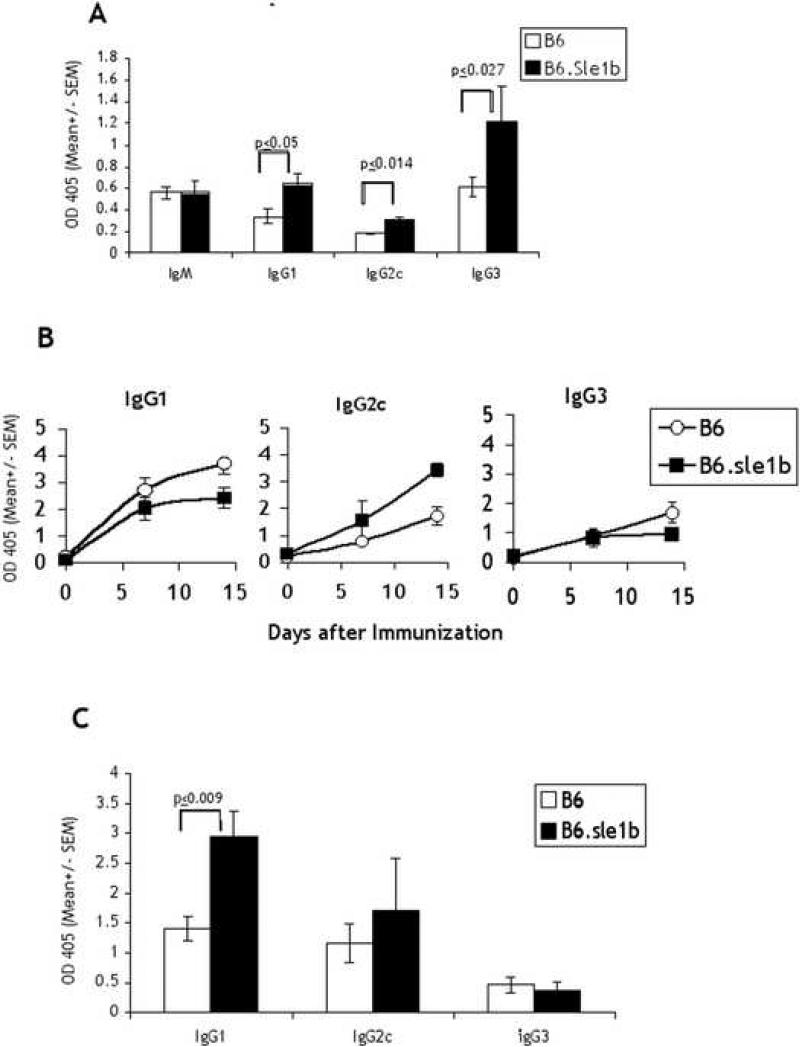
In order to determine whether B6.Sle1b differ from B6 mice in their reactivity to antigenic stimulation we examined their responses to a T independent (TI) as well as a T dependent (TD) antigen. 2-month-old male mice, which have not yet expressed increased levels of autoantibody production, and have lower penetrance compared to females [4] were tested. For the T independent response we chose to examine the response to a TI-II antigen, NP-Ficoll, because initial B cell activation by the former relies on crosslinking of the BCR rather than activation of TLRs, which adds another level of complexity to the response. Fig. 2A shows that as early as 7 days after immunization B6.Sle1b mice expressed significantly higher levels of NP specific IgG1 and IgG2c serum titers as compared to that from age matched B6 mice. The responses were incrementally higher on D14 (data not shown). However, the differences persisted. IgG2b titers were similar to IgG3 but the background binding to NP-BSA (d0) often registered high therefore responses were difficult to assess.
Fig. 2.
Antigen specific responses of B6.Sle1b vs. B6 mice. 2-month old mice (5/group) were immunized i.p with NP-Ficoll in PBS and sera collected by tail bleeds after 7 days were quantified by subclass specific ELISA on NP-BSA coated plates (A). After the last bleed for TI responses, mice were primed with FITC-KLH in RIBI i.p. Sera collected at 7 and 14 days later were quantified by ELISA on FITC-BSA-coated plates (B). 30 days after TD priming mice were challenged i.p with TNP-KLH in PBS and sera collected after 7 days were analyzed on TNP-BSA coated plates (C). Previous determinations showed that NP binding is not registered on TNP-coated plates. Prebleed values for each immunization were subtracted from values shown. Prebleed values for NP-BSA: IgM = 0.19-0.95; IgG = 0.1- 0.23; for FITC-BSA: IgM = 0.3-2.0 ; IgG = 0.08-0.16; for TNP-BSA: IgG = 0.09-0.2. P values are shown only for groups that differ significantly (P≤0.05). This experiment was performed only once. However, similar results were obtained after immunization with NP-Ficoll in the experiment shown in Fig. 6.
To examine the primary responses to a TD antigen, the mice were immunized with FITC-KLH in the presence of the adjuvant Ribi. The use of a different hapten ascertained that NP-specific antibodies made during the TI response which might persist would not confound the assessment of the TD response. Fig. 2B shows that, as expected, the level of IgG1 and IgG2a produced in response to the TD antigen was considerably higher than that obtained with the TI-II antigen. In this case, however, the titers of antibodies produced by B6.Sle1b mice did not differ significantly from that produced in B6 mice over the entire time course of the measured response as determined by the Wilcoxon Rank-Sum (Mann-Whitney) Test.
Since both T and B cells are primed it would be difficult to determine whether T or B cells differ in their ability to generate memory in the B6.Sle1b mice if we reimmunized them with the same antigen. We decided, therefore, to assess the secondary T cell response in a way that would distinguish it from the secondary B cell response. After a rest period of 30 days, we challenged the mice, previously immunized with FITC-KLH, with TNP-KLH given in PBS. Immunizing with a different hapten conjugated to the same carrier allowed us to use the primary B cell response to the hapten TNP, as an assessment of the helper activity of memory T cells generated during the primary immunization and to distinguish it from secondary B cell responses. Since TI responses do not generate memory B cells, it is unlikely that any cross-reactivity to TNP-KLH would be due to activation of NP-specific B cells generated by NP-Ficoll. Thus, it is interesting that by this assessment, IgG1, and only the IgG1 response, was found to be significantly increased in the B6.Sle1b strain (Fig. 2C) indicating an augmentation of the memory T cell response in these autoimmune prone mice. Thus even though we did not measure the secondary B cell response it is not unlikely that it would be enhanced as well.
Augmented antigen presentation by B6.Sle1b mice
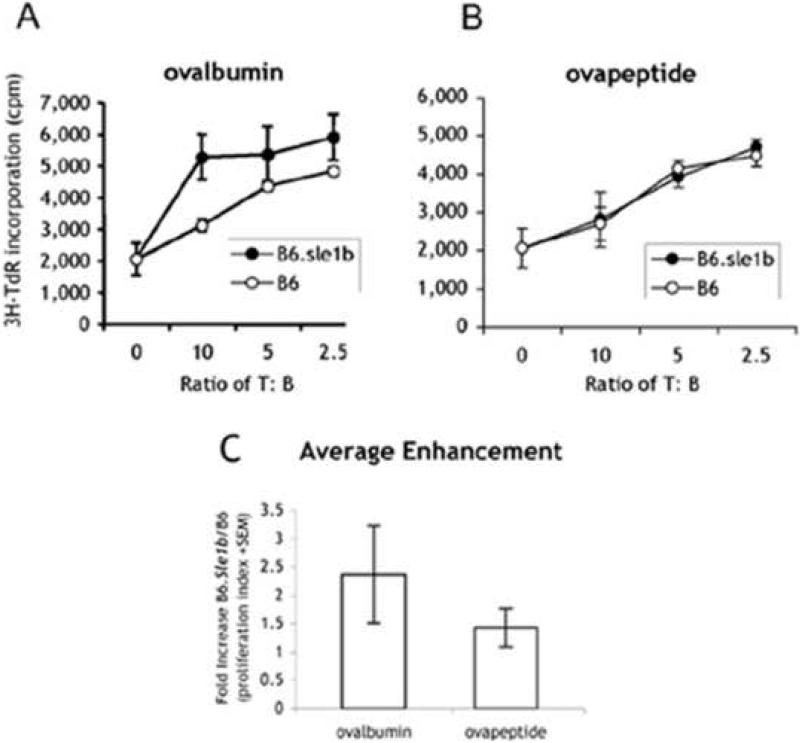
B cell antigen presentation during priming has been shown to be important for the development of memory T cells [14-16]. Therefore, it is possible that the activated phenotype of the B cells in B6.Sle1b mice may correspond to a greater ability to present antigen, which could lead to enhanced T cell help as revealed in the higher secondary responses. The increase in CD86 expression on B cells from B6.Sle1b mice upon culture without overt stimulation, further suggests that antigen presentation by B cells may be augmented. We therefore compared the antigen presentation ability between B cells from this strain with that from B6 mice. To decrease the effect of heterogeneity in the activation status of B cells from different strains of mice we purified high density B cells from each strain. The cells were incubated with either ovalbumin or OVA peptide323-339 for 16 hours. They were then irradiated and their ability to stimulate proliferation of T cells from OT-II transgenic mice, which express specificity for the OVA peptide323-339 on MHC II, was evaluated. Fig. 3A shows that T cell proliferation in response to B cells from B6.Sle1b B mice was increased to a low but significant extent in comparison to cultures with B cells from B6 mice. In this experiment, the increase was only apparent after incubation with ovalbumin, but not with peptide (Fig. 3B); however, in subsequent experiments (Fig. 3C) presentation of the OVA peptide can be also somewhat enhanced. Fig. 3C summarizes the results from 4 additional experiments comparing the maximum fold increase in stimulation index induced by B6.Sle1b over that induced by B6 B cells. No enhancement in proliferation was detected when either B6 or B6.Sle1b B cells were added to T cells in the absence of antigen (data not shown). In view of the increased expression of CD86 on resting B cells it is not surprising that presentation of peptide would be enhanced by B cells from B6.Sle1b mice. On the other hand, the enhancement of presentation of the intact protein by an average of 2.5 fold indicates that they also have a greater ability to process antigen. This increase is likely to be the reason underlying the greater memory T cell response to TD antigens observed in B6.Sle1b mice. This enhanced antigen presentation might not be easily detectable during the primary response when the role of B cells as APCs can be masked by the presence of other professional APCs.
Fig. 3.
Resting B cells from B6.Sle1b exhibit enhanced antigen presentation to T cells. Varying numbers of resting B cells in triplicates from 2-month old B6.Sle1b (closed) or B6 (open) mice were incubated overnight in medium with a previously defined non-saturating concentration of ovalbumin of OVA (A, 100 μgs/ml) or peptide (B, 120 ngs/ml). Cells were then irradiated at 1,300 rads followed by the addition of decreasing numbers of purified OT-II T cells in triplicate wells. After 2 days [3H]TdR was added and cells harvested the next day. The average stimulation index of all samples from B6.Sle1b mice in (A) differed significantly from those from B6 mice (p≤0.05). In (C) the average fold enhancement was determined by dividing the stimulation indices of all samples from B6.Sle1b cells in this plus 4 subsequent experiments (using 2-4 month old mice) by the stimulation indices of corresponding samples from B6 mice.
Since marginal zone B cells have been shown to have greater ability to present antigen [17] it is possible that increased representation of this subpopulation is responsible for the enhanced antigen presentation by B6.Sle1b B cells. However, as already shown in Fig. 1A, there is no detectable difference in the low percent of marginal zone B cells between the two strains.
Effect of SAP deletion on antigen-specific responses of B6.Sle1b mice
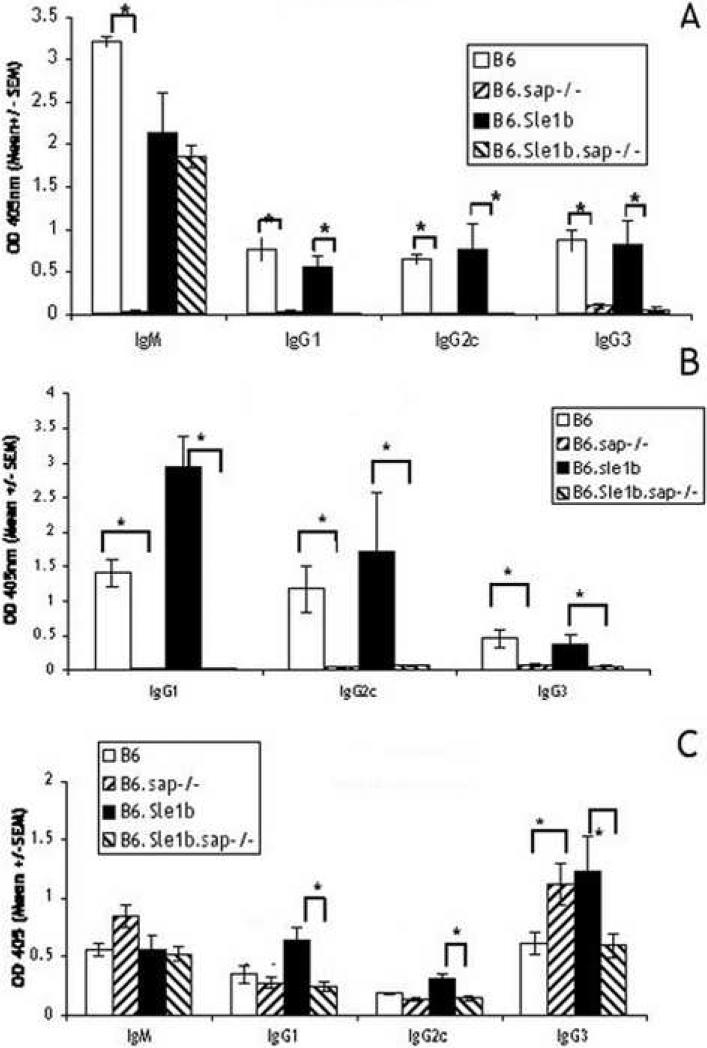
A putative candidate responsible for the activated phenotype in B6.Sle1b mice may reside within the SLAM/CD2 gene cluster. In order to determine whether interactions between some SLAM family members are involved, we examined the effect of disruption of the sap gene on these responses, because the adaptor molecule, SAP has been shown to be critically involved in signaling by SLAM family members (reviewed in [6]). B6.sap-/- and B6.Sle1b mice were crossed to generate B6.Sle1b.sap-/- mice. These mice were immunized as described for Fig. 2 and responses to both the TI-II and TD antigens were compared with that of B6 mice. Fig. 4A shows that, in agreement with previously published data [18-20], all mice with the sap disruption mounted barely detectable TD responses. Against the B6.Sle1b background, the sap disruption, with the exception of IgM, also reduced all other responses to background levels. Not unexpectedly, the memory T cell responses in both strains (Fig. 4B) were also eliminated in the absence of SAP. However, we found that, except for IgG3, disruption of sap did not affect the response to NP-Ficoll in B6 mice. Notably, however, in the B6.Sle1b strain the disruption resulted in a reduction of the increased IgG1 and IgG2c responses to NP-Ficoll to the levels found for B6 mice. Therefore, the increase in response to a TI-II antigen found in the B6.Sle1b mice also appear to require signaling via SAP.
Fig. 4.
Absence of SAP abolishes the response to a TD antigen and reduces the increased response to the TI-II antigen in B6.Sle1b mice. 2 month old B6.sap-/- and B6.Sle1b. sap-/- mice (groups of 5) were immunized as in Fig. 2. Shown are: primary TD response (A), memory T cell response measured in terms of help for primary B cell response (B), and TI-II responses (C) compared with B6 and B6.Sle1b mice. Sera from the two experiments (Fig. 2 and Fig. 4) were assayed at the same time. Brackets with *'s indicate values that differ by P≤0.05.
Responses to TI-II antigens require both cross-linking of BCR as well as a second signal derived from cytokines, produced by either activated NK or T cells; therefore, the reduction of the B6.Sle1b antibody response in the absence of SAP could be due either to a reduced ability of the mice to produce cytokines or to a disruption of the cellular interactions required for initiation of the cytokine circuit.
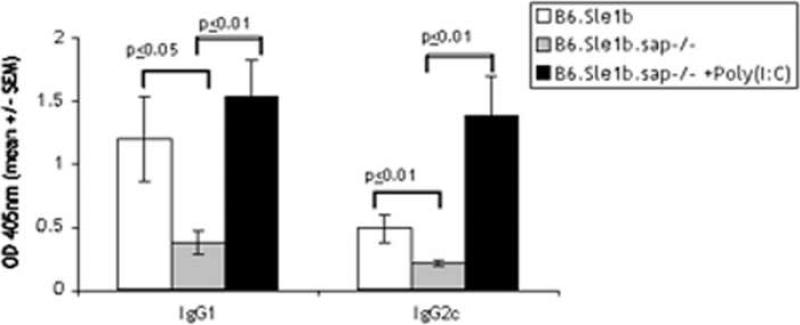
To test whether the absence of SAP affects production of cytokines, mice were injected with Poly(I:C), a double-stranded RNA analog, one day before immunization by NP-Ficoll. Poly(I:C) enhances IgG responses to both TI-I and TI-II antigens [10, 21]. This enhancement has been shown to be due to the amplification of a cytokine loop that is mainly initiated by cytokine production from APCs stimulated via TLR3 and the subsequent activation of NK and T cells by these cytokines [22, 23]. To determine whether the generation of this cytokine loop is compromised by the disruption of sap we evaluated the antibody response after Poly(I:C) stimulation of B6.Sle1b.sap-/- mice. Fig. 5 shows that the lower level of response to NP-Ficoll mounted by the B6.Sle1b.sap-/- mice can be restored by prior injection of Poly(I:C); therefore, in these mice the cytokine enhancement of the TI-II response is not disrupted. In conclusion, since SAP is not essential for the basal level of Ig production mounted by B cells upon stimulation by a TI-II antigen in the absence of overt cytokine stimulation, such as that induced by Poly(I:C), the increased response in B6.Sle1b mice that is reduced upon deletion of SAP implicates a role for direct cell-cell interactions via SLAM family members.
Fig. 5.
Poly(I:C) can enhance responses of B6.Sle1b. sap-/- mice to a TI-II antigen. B6.Sle1b and B6.Sle1b. sap-/- mice (groups of 5) were immunized with 40 μgs of NP-Ficoll in PBS. One group of B6.Sle1b. sap/-/- mice were injected i.p with 100 μg of Poly (I:C) per mouse 16 hours before NP-Ficoll immunization. Sera collected on D8 were analyzed by subclass specific ELISA as in Fig. 2.
Role of NK cells in the augmented antigen specific response of B6.Sle1b mice
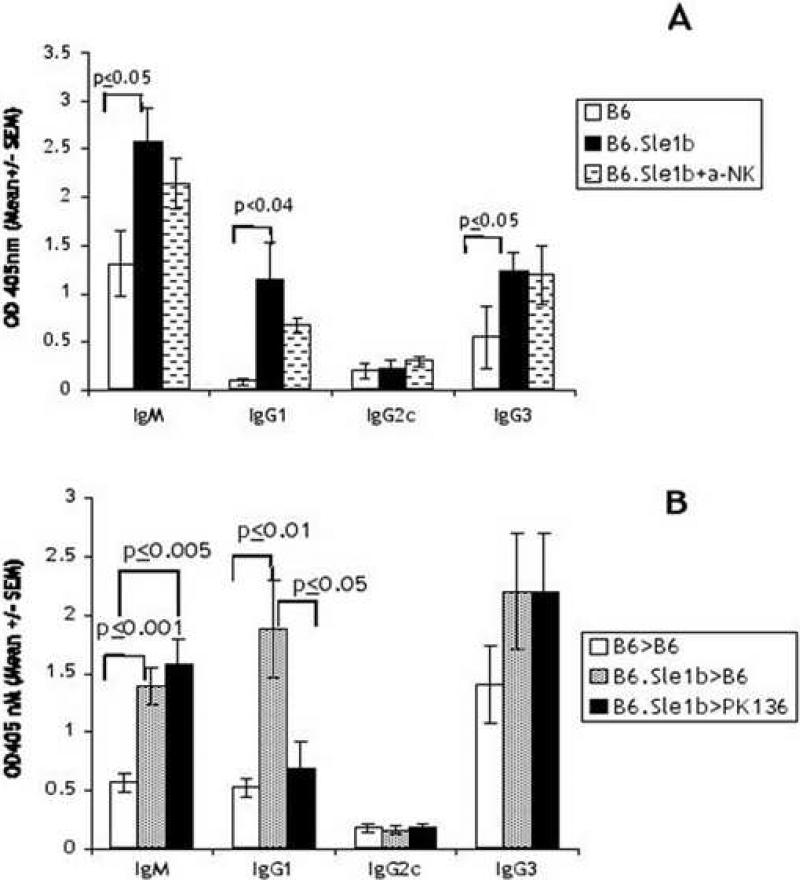
A candidate for direct cell-cell interaction is that between NK and B cells. We have previously shown that B cells, especially activated B cells, can activate IL-2 propagated NK cells [24] via direct cell-cell interactions that require SAP. In addition NK cells can activate B cells by direct cell-cell interaction [25]. Therefore, we investigated the role of NK cells in B6.Sle1b antibody responses by depleting NK cells. A group of B6.Sle1b mice were injected with anti-NK1.1 prior to immunization with NP-Ficoll and the levels of the responses were compared with non-treated mice. Fig. 6A shows that the depletion of NK cells prior to immunization decreased the enhanced response of the B6.Sle1b mice. However, the extent of decrease did not achieve statistical significance. It is possible that the activated phenotype of B6.Sle1b is attributed to continued long-term reciprocal interactions between activated NK and B cells therefore a transient depletion of NK cells may not be sufficient. In order to determine if chronic depletion of NK cells might have a greater effect we constructed radiation chimeras from bone marrow cells from B6.Sle1b mice in PK136 transgenic mice. These mice are chronically depleted of NK cells due to the continuous expression of anti-NK1.1 antibodies [9]. Control mice consisted of B6 bone marrow chimeras of cells from B6.Sle1b or from B6 mice. Fig. 6B shows that the antigen specific response to NP-Ficoll from B6.Sle1b chimeras is also higher than control B6 bone marrow chimeras, confirming our results shown in Fig. 2. In contrast, B6.Sle1b chimeras generated in PK136 transgenic mice showed a significant reduction in the IgG1 response to levels comparable to that found for control B6 chimeras. Thus, it is possible that NK cells, activated in the B6.Sle1b mice via SAP-mediated signaling, play a role in the increase in the TI-II response.
Fig. 6.
Depletion of NK cells alters the increased TI-II response observed in B6.Sle1b mice. 3 month old B6.Sle1b mice was depleted of NK cells by injection of anti-NK1.1 antibodies. Two days after the last injection the mice were challenged with NP-Ficoll along with control mice (5 animals per group). NP-specific antibodies were assessed on NP-BSA plates on D7 (A). Radiation chimeras (5 to 7 animals per group) were made from bone marrow cells of 2 month old B6 and B6.Sle1b mice in B6 or PK136 mice chronically expressing anti-NK1.1 antibodies. One month after transplant PBLs were collected and stained to confirm engraftment and NK cell depletion. Mice were then immunized with NP-Ficoll in PBS, and the sera collected on D10 were analyzed by ELISA as in Fig. 2 (B). Shown are values from one of two experiments with similar results.
Effect of SAP deletion on ANA production in B6.Sle1b mice
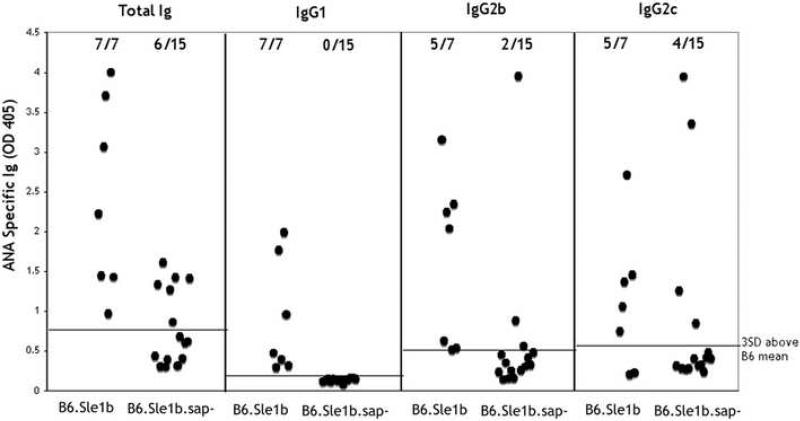
Finally, the role of SAP in the production of autoantibodies was also assessed by comparing the levels of anti-DNA/Histone antibodies between B6.Sle1b and B6.Sle1b.sap-/- mice at various ages. Fig. 7 shows that the sap disruption did not prevent the appearance of total anti-DNA/Histone antibodies as indicated by assessment with anti-Ig antibodies. Therefore the disruption of sap does not eliminate the breach of tolerance enabled by the Sle1b locus. Interestingly, however, the penetrance of autoantibody production, as measured by the percent of affected animals was significantly reduced by the sap disruption, (6/15 vs 7/7), indicating that the induction of ANA was affected in these mice. Furthermore, subclass distribution of the antibodies showed that none of the autoantibodies produced in the B6. Sle1b.sap-/- mice were of the IgG1 subclass, a switch which is most dependent on MHC II dependent T cell help, whereas all of the ANA produced in B6.Sle1b mice contained IgG1 antibodies. These results are consistent with the inability of the B6. Sle1b.sap-/- mice to make a T dependent response which becomes manifested in the subclass of ANA produced. On the other hand, most of the autoantibodies made in B6.Sle1b.sap-/- animals were of the IgG2c subclass which is more typical of T independent responses. Although antibodies of the IgG2b subclass were made in B6.Sle1b mice only a minority of antibodies made in the absence of SAP were of this subclass.
Fig. 7.
B6.Sle1b.sap-/- mice are able to produce ANA. B6, B6.sap-/-, B6.Sle1b and B6.Sle1b.sap-/- (all female) were bled at different ages and ANA levels were measured. Shown are Ig titers from 9 week old B6.Sle1b and from 12 week old B6.Sle1b.sap-/- mice analyzed by isotype specific ELISA. Dotted lines represent OD405 levels at 3×SD above the mean of 12 week old B6 animals for each isotype. Levels of IgG3 above background were not detectable in any of the sera tested.
DISCUSSION
It is clear that susceptibility to autoimmune diseases has multiple genetic components [26] and infection has been postulated to be a trigger for pathogenesis. Recent findings revealing the role of toll-like receptors in the development of autoimmunity have provided further evidence for this hypothesis [27-29]. Conversely, the increased representation of precursors to autoimmune cells may alter the immune response to infection [[30] and references therein]. Some SLE models with broad symptomatology have been shown to present increased antibody responses to model TI and TD antigens, which mimic antigenic determinants of pathogens ([31] and references therein). However, the role of each contributory cell type to the response was not clearly assigned. The autoimmune susceptibility locus Sle1b in the B6 background leads to a SLE phenotype which is restricted to autoantibody production and, although T cell help is required, they do not have to express the alleles contained within the locus [32]. Therefore, the results of our analysis of antibody responses to non-self antigens, should be mainly a reflection of B cell dysfunction. Although B6.Sle1b mice do not express high levels of spontaneous antibodies to non-self antigens [4], we found that they can mount increased responses upon immunization. It should be noted that the penetrance of the Sle1b phenotype is higher in females than in males and begins around 5 month of age but the differences we observed in the present study were already detected by 2–4 months of age and in male mice. Therefore the differences in responses are not dependent on factors directly associated with autoantibody production.
Despite the observation that T cells from 1 to 2 month old B6.Sle1b may be more easily activated by a polyclonal stimulus [5], the primary response to a TD antigen was not significantly different from B6 mice. However, the secondary T cell response was clearly increased in B6.Sle1b mice suggesting that the development of memory T cells is affected. Morel et al [4] did not find differences in secondary TD responses in the B6.Sle1 strain. However, in this study the mice were not immunized with a different hapten for the secondary challenge therefore the increased T cell response may have been obscured by the secondary B cell response. Our finding that the B cells are easily activated in these mice (Fig.1) suggests that they may alter the nature of antigen presentation in the course of the TD response. Indeed, we found that purified “resting” B cells from B6.Sle1b mice do display augmented ability to present antigen (Fig. 3A-C), which may be the basis for the enhanced secondary T cell responses (Fig. 2C). Crawford et al. [16] have suggested that B cell antigen presentation may affect secondary T cell responses to a greater extent than primary responses. It is interesting that in molecular mimicry studies, secondary T cells responses were found to exert more profound effects on the disease [33]. The increased ability of B6.Sle1b B cells to process antigen suggests that despite the high density phenotype, some of these B cells are intrinsically activated and in this aspect they share properties with low density B cells which also exhibit enhanced antigen presentation ability. Attempts to identify intracellular differences that can further characterize these cell populations are in progress.
There are several possible reasons for the increased response of B6.Sle1b mice to TI-II antigens. BAFF may be expressed in the mice even at this early age resulting in increased activation of B cells via the TACI receptor which is required for the TI-II response [34]. However, neither transduction of signals via the BCR or TACI has been shown to be mediated by SAP therefore the restoration to levels produced by B6 mice by the sap disruption argues against this possibility. On the other hand, we have previously shown that the response to TI antigen can be enhanced by NK cells if they are stimulated by agents such as poly(I:C) [10] or RIBI [9], This dependence of the TI-II response on cytokines suggests that another cause for the enhanced response in the B6.Sle1b mice is a general overall increased level of cytokines. However, we have shown in Fig. 5 that Poly(I:C) which augments the cytokine circuit, can also increase the response of the B6.Sle1b.sap-/- mice, therefore the decreased response to the antigen caused by the sap disruption indicates that altered cytokine levels cannot be solely responsible for the increased response in B6.Sle1b mice. In contrast, we have confirmed earlier findings [7] that disruption of sap in B6 mice does not affect responses to TI-II antigens. Thus, SAP may be required only to initialize the cytokine circuits that lead to the increased TI-II response in B6.Sle1b mice. Similar conclusions have been made from examinations of specific T cell dependent responses in sap-/- mice [20].
How then, is SAP involved in the initialization of the cytokine circuit? A possible mechanism is suggested by the decreased response found upon NK depletion (Fig. 6A and 6B). The presence of activated B cells may play a role in initiating the activation of NK cells. This activation may be mediated by a pathway that involves cellular interactions via SLAM family members. Indeed, the interaction between B and NK cells via CD48 and CD244, members of the SLAM family, have been shown by in vitro studies to play an important role in NK activation by B cells [24]. This pathway of direct NK-B cell interaction may also explain our results showing that chronic depletion of NK cells prior to antigenic challenge is more effective than acute depletion (Fig. 6). The absence of NK cells may decrease the contribution of partially activated B cells. It should be noted that although NKT cells are absent in sap-/- mice these cells are not likely to be involved because the regiment of NK depletion used in our studies does not deplete NKT cells [9].
Precursors of autoimmune B cells can arise by a number of mechanisms. First, they could be “ignorant B cells” which have arisen due to receptor editing of previously tolerized cells, or they could be anergic B cells that can nevertheless be triggered by strong T cell help [[35] and references within]. B cells in the B6.Sle1b strain have been shown to undergo increased receptor editing possibly due to dysregulated expression of Ly108 [36]. Thus, these cells may fit the criteria of “ignorant” B cells that can be more easily triggered by antigens. In addition, although T cells derived from the B6.Sle1b strain cannot, on their own, initiate autoimmunity, they are nonetheless more easily activated. Therefore they may function as helper T cells that can overcome the activation barrier presented by anergic B cells.
Whereas it is still not resolved whether the trigger for autoantibodies in the B6.Sle1b mice is initiated from a TI or TD response to antigens our analysis may provide some insight. We observed a reduced penetrance of ANA production in B6.Sle1b.sap-/- mice in comparison to B6.Sle1b mice (Fig. 7). The development of ANA in some of these mice despite the sap disruption, which dramatically decreases the response to TD antigens, suggests that if ANA production is initiated by antigenic stimulation then the antigen(s) can be T independent in nature. The absence of detectable ANA belonging to the IgG1 subclass confirms this contention. On the other hand, most of the ANA produced upon sap disruption are of the IgG2c subclass, which is more typical of T independent responses. The low, but detectable responses mounted by B6.Sle.sap-/- mice to a TI-II antigen suggest that such response to these antigenic determinants may serve to initiate the autoantibody production. Furthermore, we have not examined the response of B6.Sle1b mice to TI-I antigens, that require expression of TLRs on B cells, but SAP has not been implicated in signaling via these receptors; therefore the initiation event in these mice could be attributed to activation of B cells via TLRs. Moreover, increased levels of ANA in B6.Sle1b mice are only apparent in older animals. A similar time course was observed in the B6.Sle.sap-/- mice that break tolerance (data not shown). It is possible that the reduced contribution of SAP expression in these animals is due to the accumulation of immune complexes with age, which, together with activation of TLRs, can augment ANA production. Indeed, the effect of TLRs on ANA production is most pronounced in strains of mice that exhibit earlier onset of autoantibody production [37]. This mechanism may also explain why a mutation in the sap gene in the MRL-Fas lupus strain was found to not significantly affect the percent of animals that break tolerance but only the levels of antibodies produced [38]. In any case, our studies are the first to document that genetic differences targeted by the Sle1b locus can alter specific responses to non-self antigens even in the absence of stimulation by TLRs and thus may be involved in the initiation of the development of autoantibodies.
Acknowledgements
The study was supported by The National Institutes of Health grant: R01: AI69253
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Morel L, Yu Y, Blenman KR, Caldwell RA, Wakeland EK. Production of congenic mouse strains carrying genomic intervals containing SLE-susceptibility genes derived from the SLE-prone NZM2410 strain. Mamm Genome. 1996;7:335–339. doi: 10.1007/s003359900098. [DOI] [PubMed] [Google Scholar]
- 2.Sobel ES, Satoh M, Chen Y, Wakeland EK, Morel L. The major murine systemic lupus erythematosus susceptibility locus Sle1 results in abnormal functions of both B and T cells. J Immunol. 2002;169:2694–2700. doi: 10.4049/jimmunol.169.5.2694. [DOI] [PubMed] [Google Scholar]
- 3.Morel L, Mohan C, Yu Y, Croker BP, Tian N, Deng A, Wakeland EK. Functional dissection of systemic lupus erythematosus using congenic mouse strains. J Immunol. 1997;158:6019–6028. [PubMed] [Google Scholar]
- 4.Morel L, Blenman KR, Croker BP, Wakeland EK. The major murine systemic lupus erythematosus susceptibility locus, Sle1, is a cluster of functionally related genes. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:1787–1792. doi: 10.1073/pnas.031336098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wandstrat AE, Nguyen C, Limaye N, Chan AY, Subramanian S, Tian XH, Yim YS, Pertsemlidis A, Garner HR, Jr., Morel L, Wakeland EK. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity. 2004;21:769–780. doi: 10.1016/j.immuni.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Veillette A. Immune regulation by SLAM family receptors and SAP-related adaptors. Nature reviews. 2006;6:56–66. doi: 10.1038/nri1761. [DOI] [PubMed] [Google Scholar]
- 7.Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, Chen A, Sher A, Duckett CS, Ahmed R, Schwartzberg PL. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci U S A. 2001;98:7449–7454. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunology and cell biology. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 9.Yuan D, Bibi R, Dang T. The role of adjuvant on the regulatory effects of NK cells on B cell responses as revealed by a new model of NK cell deficiency. International immunology. 2004;16:707–716. doi: 10.1093/intimm/dxh071. [DOI] [PubMed] [Google Scholar]
- 10.Wilder JA, Koh CY, Yuan D. The role of NK cells during in vivo antigen-specific antibody responses. J Immunol. 1996;156:146–152. [PubMed] [Google Scholar]
- 11.Mohan C, Adams S, Stanik V, Datta SK. Nucleosome: a major immunogen for pathogenic autoantibody-inducing T cells of lupus. J Exp Med. 1993;177:1367–1381. doi: 10.1084/jem.177.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss EA, Michael A, Yuan D. Role of transcriptional termination in the regulation of mu mRNA expression in B lymphocytes. J Immunol. 1989;143:1046–1052. [PubMed] [Google Scholar]
- 13.Snyder CM, Zhang X, Wysocki LJ. Negligible class II MHC presentation of B cell receptor-derived peptides by high density resting B cells. J Immunol. 2002;168:3865–3873. doi: 10.4049/jimmunol.168.8.3865. [DOI] [PubMed] [Google Scholar]
- 14.Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165:5558–5565. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 15.Lund FE, Hollifield M, Schuer K, Lines JL, Randall TD, Garvy BA. B cells are required for generation of protective effector and memory CD4 cells in response to Pneumocystis lung infection. J Immunol. 2006;176:6147–6154. doi: 10.4049/jimmunol.176.10.6147. [DOI] [PubMed] [Google Scholar]
- 16.Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- 17.Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- 18.Hron JD, Caplan L, Gerth AJ, Schwartzberg PL, Peng SL. SH2D1A regulates T-dependent humoral autoimmunity. J Exp Med. 2004;200:261–266. doi: 10.1084/jem.20040526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Alem U, Li C, Forey N, Relouzat F, Fondaneche MC, Tavtigian SV, Wang ZQ, Latour S, Yin L. Impaired Ig class switch in mice deficient for the X-linked lymphoproliferative disease gene Sap. Blood. 2005;106:2069–2075. doi: 10.1182/blood-2004-07-2731. [DOI] [PubMed] [Google Scholar]
- 20.Cannons JL, Yu LJ, Jankovic D, Crotty S, Horai R, Kirby M, Anderson S, Cheever AW, Sher A, Schwartzberg PL. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2006;203:1551–1565. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koh CY, Yuan D. The functional relevance of NK-cell-mediated upregulation of antigen- specific IgG2a responses. Cell Immunol. 2000;204:135–142. doi: 10.1006/cimm.2000.1703. [DOI] [PubMed] [Google Scholar]
- 22.Biron CA, Gazzinelli RT. Effects of IL-12 on immune responses to microbial infections: a key mediator in regulating disease outcome. Current opinion in immunology. 1995;7:485–496. doi: 10.1016/0952-7915(95)80093-x. [DOI] [PubMed] [Google Scholar]
- 23.Trinchieri G, Gerosa F. Immunoregulation by interleukin-12. Journal of leukocyte biology. 1996;59:505–511. doi: 10.1002/jlb.59.4.505. [DOI] [PubMed] [Google Scholar]
- 24.Gao N, Schwartzberg P, Wilder JA, Blazar BR, Yuan D. B cell induction of IL-13 expression in NK cells: role of CD244 and SLAM-associated protein. J Immunol. 2006;176:2758–2764. doi: 10.4049/jimmunol.176.5.2758. [DOI] [PubMed] [Google Scholar]
- 25.Gao N, Dang T, Dunnick WA, Collins JT, Blazar BR, Yuan D. Receptors and Counterreceptors Involved in NK-B Cell Interactions. J Immunol. 2005;174:4113–4119. doi: 10.4049/jimmunol.174.7.4113. [DOI] [PubMed] [Google Scholar]
- 26.Vyse TJ, Todd JA. Genetic analysis of autoimmune disease. Cell. 1996;85:311–318. doi: 10.1016/s0092-8674(00)81110-1. [DOI] [PubMed] [Google Scholar]
- 27.Fields ML, Metzgar MH, Hondowicz BD, Kang SA, Alexander ST, Hazard KD, Hsu AC, Du YZ, Prak EL, Monestier M, Erikson J. Exogenous and endogenous TLR ligands activate anti-chromatin and polyreactive B cells. J Immunol. 2006;176:6491–6502. doi: 10.4049/jimmunol.176.11.6491. [DOI] [PubMed] [Google Scholar]
- 28.Rahman AH, Eisenberg RA. The role of toll-like receptors in systemic lupus erythematosus. Springer seminars in immunopathology. 2006;28:131–143. doi: 10.1007/s00281-006-0034-3. [DOI] [PubMed] [Google Scholar]
- 29.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nature reviews. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zandman-Goddard G, Shoenfeld Y. Infections and SLE. Autoimmunity. 2005;38:473–485. doi: 10.1080/08916930500285352. [DOI] [PubMed] [Google Scholar]
- 31.Park CL, Balderas RS, Fieser TM, Slack JH, Prud'Homme GJ, Dixon FJ, Theofilopoulos AN. Isotypic profiles and other fine characteristics of immune responses to exogenous thymus-dependent and - independent antigens by mice with lupus syndromes. J Immunol. 1983;130:2161–2167. [PubMed] [Google Scholar]
- 32.Sobel ES, Mohan C, Morel L, Schiffenbauer J, Wakeland EK. Genetic dissection of SLE pathogenesis: adoptive transfer of Sle1 mediates the loss of tolerance by bone marrow-derived B cells. J Immunol. 1999;162:2415–2421. [PubMed] [Google Scholar]
- 33.Olson JK, Ercolini AM, Miller SD. A virus-induced molecular mimicry model of multiple sclerosis. Curr Top Microbiol Immunol. 2005;296:39–53. doi: 10.1007/3-540-30791-5_3. [DOI] [PubMed] [Google Scholar]
- 34.Stohl W, Xu D, Kim KS, Koss MN, Jorgensen TN, Deocharan B, Metzger TE, Bixler SA, Hong YS, Ambrose CM, Mackay F, Morel L, Putterman C, Kotzin BL, Kalled SL. BAFF overexpression and accelerated glomerular disease in mice with an incomplete genetic predisposition to systemic lupus erythematosus. Arthritis Rheum. 2005;52:2080–2091. doi: 10.1002/art.21138. [DOI] [PubMed] [Google Scholar]
- 35.Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Kumar KR, Li L, Yan M, Bhaskarabhatla M, Mobley AB, Nguyen C, Mooney JM, Schatzle JD, Wakeland EK, Mohan C. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006;312:1665–1669. doi: 10.1126/science.1125893. [DOI] [PubMed] [Google Scholar]
- 37.Sadanaga A, Nakashima H, Akahoshi M, Masutani K, Miyake K, Igawa T, Sugiyama N, Niiro H, Harada M. Protection against autoimmune nephritis in MyD88-deficient MRL/lpr mice. Arthritis Rheum. 2007;56:1618–1628. doi: 10.1002/art.22571. [DOI] [PubMed] [Google Scholar]
- 38.Komori H, Furukawa H, Mori S, Ito MR, Terada M, Zhang MC, Ishii N, Sakuma N, Nose M, Ono M. A signal adaptor SLAM-associated protein regulates spontaneous autoimmunity and Fas-dependent lymphoproliferation in MRL-Faslpr lupus mice. J Immunol. 2006;176:395–400. doi: 10.4049/jimmunol.176.1.395. [DOI] [PubMed] [Google Scholar]