Abstract
Objective
To compare breath-hold 1H-MRS to respiratory-gated 1H-MRS and CT for quantification of hepatic lipid content.
Methods
Twenty-three premenopausal women underwent breath-hold point-resolved single voxel (PRESS) 1H-MRS of the liver followed by respiratory-gated 1H-MRS at 3Tesla and CT slice through the liver. Interscan variability for 1H-MRS was assessed in six volunteers. Pearson correlation coefficients, Bland-Altman 95% limit of agreement, and concordance correlation coefficients were calculated.
Results
There was a strong correlation between breath-hold and respiratory-gated 1H-MRS (r=0.94, p<0.0001, concordance correlation coefficient:0.75). Using Bland-Altman analysis, all but two data points were within the limits of agreement. Both 1H-MRS techniques had low inter-scan variability. There was an inverse correlation of both 1H-MRS techniques with CT attenuation values of the liver.
Conclusions
Breath-hold 1H-MRS is a reliable method to measure hepatic lipid content at 3Tesla. Breath-hold 1H-MRS of the liver provides data that closely correlates with that obtained from longer duration respiratory-gated technique.
Keywords: MR spectroscopy, liver, lipids
Introduction
Obesity is a major public health problem and is associated with increased metabolic and cardiovascular risk (1, 2). Obesity is the biggest risk factor for the development of nonalcoholic fatty liver disease. Seventy to eighty percent of obese subjects are estimated to have hepatic steatosis and 15–30% may have nonalcoholic steatohepatitis which carries a risk of cirrhosis and hepatocellular carcinoma (3–5). Liver biopsy is considered the gold standard for diagnosing fatty liver disease. However, biopsy is invasive, making it impractical for screening, disease monitoring, and for assessment of treatment response. Several studies using magnetic resonance imaging (MRI) and computed tomography (CT) have examined liver steatosis non-invasively (6–12). CT depicts fatty infiltration of the liver as a decrease in attenuation which relates to the degree of fatty infiltration by histology (10). Although CT is widely available and allows for easy quantification of hepatic lipid content, it involves radiation exposure. Hepatic lipid content can be quantified non-invasively with proton magnetic resonance spectroscopy (1H-MRS), which provides data that closely correlate with hepatic lipid content from biochemical and histologic analyses (13, 14). Therefore, 1H-MRS has been used as the standard of reference in several clinical studies (7, 8, 15–18). However, 1H-MRS image acquisition of the liver can be time consuming and is limited especially by patient motion leading to spectral degradation (15). Respiratory motion can lead to voxel misregistration and linewidth broadening of spectral resonances (15). This can be overcome by using motion correction sequences, respiratory-gated, or multiple breath-hold 1H-MRS techniques that require operator involvement and are also time consuming (8, 19–22).
The purpose of our study was to evaluate the feasibility and reproducibility of a single breath-hold 1H-MRS technique for non-invasive hepatic lipid quantification at 3 Tesla.
Methods
Subjects
This prospective study was approved by our institutional review board and complied with Health Insurance Portability and Accountability Act guidelines. Written informed consent was obtained from all subjects. The study group was comprised of 23 healthy pre-menopausal women (ages 21–45 years, mean: 34.0±7.7 years, mean BMI: 33.2±4.8 kg/m2) who were part of a clinical obesity trial. Seven women were overweight (BMI ≥ 25 kg/m2 and < 30 kg/m2) and 16 women were obese (BMI ≥ 30 kg/m2). Exclusion criteria for the study were pregnancy, presence of a pacemaker or metallic implant, claustrophobia, diabetes mellitus or other chronic illness, estrogen or glucocorticoid use. None of the patients had a history of liver disease, and all patients had a normal alanine aminotransferase. Breath-hold 1H-MRS data have been previously reported in 6 of the 23 subjects (23).
A separate reproducibility study was performed on 6 volunteers (5 males, 1 female, mean age: 33.7±5.1 years) who underwent breath-hold and respiratory-gated 1H-MRS before and after repositioning in the MRI scanner within 30 minutes. Two of the volunteers were overweight (mean BMI: 28.6±2.0 kg/m2) and 4 were of normal weight (mean BMI: 23.7±4.0 kg/m2).
1H-MR spectroscopy of liver
Study subjects (23 overweight/obese women) and subjects for the reproducibility component (6 healthy volunteers) were examined with the same 1H-MRS pulse sequences and equipment.1H-MRS was performed using a 3.0 Tesla (Siemens Trio; Siemens Medical Systems, Erlangen, Germany) MRI system. After an 8-hour overnight fast, each subject underwent 1H-MRS of the liver. Imaging was supervised by a radiologist who reviewed voxel placement and the quality of the spectra before the patient was discharged. Subjects were positioned supine and feet first in the magnet bore. A body matrix phased array coil was positioned over the abdomen. A tri-plane gradient echo localizer pulse sequence of the abdomen [repetition time (TR), 15 msec; echo time (TE), 5 msec; slice thickness, 3 mm] was obtained to localize the liver. A breath-hold True Fast Imaging with Steady Precession (True FISP) sequence of the liver (TR, 3.8 msec; TE, 1.9 ms; slice thickness, 10 mm; imaging time, 12 seconds) was obtained. A voxel measuring 20 × 20 × 20 mm (8 mL) was placed within the right hepatic lobe, avoiding vessels or artifact. For each voxel placement, automated optimization of gradient shimming was performed. The voxel placement was registered using screen captures to enable similar placement in the subsequent respiratory-gated 1H-MRS examination. Single breath-hold single-voxel 1H-MRS data were acquired in mid-expiration using point-resolved single voxel spectroscopy (PRESS) pulse sequence without water suppression with the following parameters: TR, 1,500 msec; TE, 30 msec; 8 averages; 1024 data points; and receiver bandwidth, 2000 Hz. The acquisition time was 18 seconds. Patients were instructed to inhale, exhale, then inhale and hold their breath.
Subsequently, a respiratory transducer was placed over the patient’s abdomen and used for gating of the 1H-MRS acquisition. A voxel measuring 20 × 20 × 20 mm (8 mL) was placed in the right hepatic lobe in a similar location as that of the breath-hold study. Similar voxel placement was ensured using screen capture from the breath-hold 1H-MRS. A PRESS pulse sequence with the following parameters was used: TR, 1,500 msec; TE, 33 msec; 64 averages; 1024 data points; and receiver bandwidth, 2000 Hz. Subjects were instructed to breathe regularly during signal acquisition. The acquisition time ranged from 4 to 10 minutes (mean 5 minutes).
For the separate reproducibility component of this study, each subject underwent breath-hold and respiratory-gated 1H-MRS of the liver using the above described protocol before and after repositioning. After initial examination using both 1H-MRS techniques, subjects were removed from the MRI scanner. Subjects were then repositioned in the scanner and both 1H-MRS techniques were repeated, including the localization pulse sequences described above.
1H MR Spectroscopic Data Analysis
Fitting of all 1H-MRS data was performed using LCModel (Stephen Provencher, Oakville, ON, Canada, version 6.1-4A) (24). Data were transferred from the MR scanner to a Linux workstation and metabolite quantification was performed using eddy current correction and water scaling. A customized fitting algorithm for liver analysis provided estimates for all lipid signals combined (0.9, 1.3, and 2.3 ppm). LCModel liver lipid estimates were automatically scaled to unsuppressed water peak (4.7 ppm). Values provided by 1H-MRS denote relative quantity of water to lipids in the volume of interest. The lipid to water ratio (%) is expressed.
Computed tomography
Subjects who were part of an obesity trial (n=23) underwent single slice CT of the upper abdomen (LightSpeed, General Electric, Milwaukee, WI) to determine liver density. Patients were placed supine and feet first in the CT scanner. Scan parameters for each image were standardized (144 table height, 80kV, 70 mA, 2 seconds gantry rotation, 1 cm slice thickness, 48 FOV). Single slice CT through the abdomen encompassing the liver and spleen was obtained. To assess hepatic fat content, the attenuation of the liver and spleen were determined within circular regions of interest placed in the dorsal aspect of each organ. Attempts were made to avoid vessels, artifacts, and other areas of inhomogeneity. Hepatic fat content was studied as the liver attenuation absolute values and as the liver-to-spleen attenuation ratio.
Statistical analysis
Statistical analysis was performed using JMP (version 5.0.1a, SAS Institute, Cary, NC) and MedCalc (version 9.2.1.0, MedCalc, Mariakerke, Belgium) software. All results are expressed as means ± standard deviation (SD). Linear regression analysis between breath-hold and respiratory-gated 1H-MRS of the liver and between liver density measurements was performed. Pearson correlation coefficients (r) are reported. In order to determine agreement, the 1H-MRS methods were compared using Bland-Altman analysis (25), in which the differences between the hepatic fat content measured with breath-hold and that with respiratory-gated 1H-MRS is plotted against their means with 95% confidence intervals. Bland Altman Analysis was also used to assess inter-scan variability of both 1H-MRS techniques. We also calculated the concordance correlation coefficient, which combines measures of precision and accuracy to determine whether the observed data deviate significantly from the line of perfect concordance (26).
Results
Respiratory-gated and breath-hold 1H-MRS measurements were of adequate quality for analysis in all patients (Figure 1). All patients were able to cooperate for the breath-hold 1H-MRS and no studies had to be repeated. Imaging time for breath-hold 1H-MRS was 18 seconds and mean imaging time for respiratory-gated 1H-MRS was 5 minutes.
Figure 1.
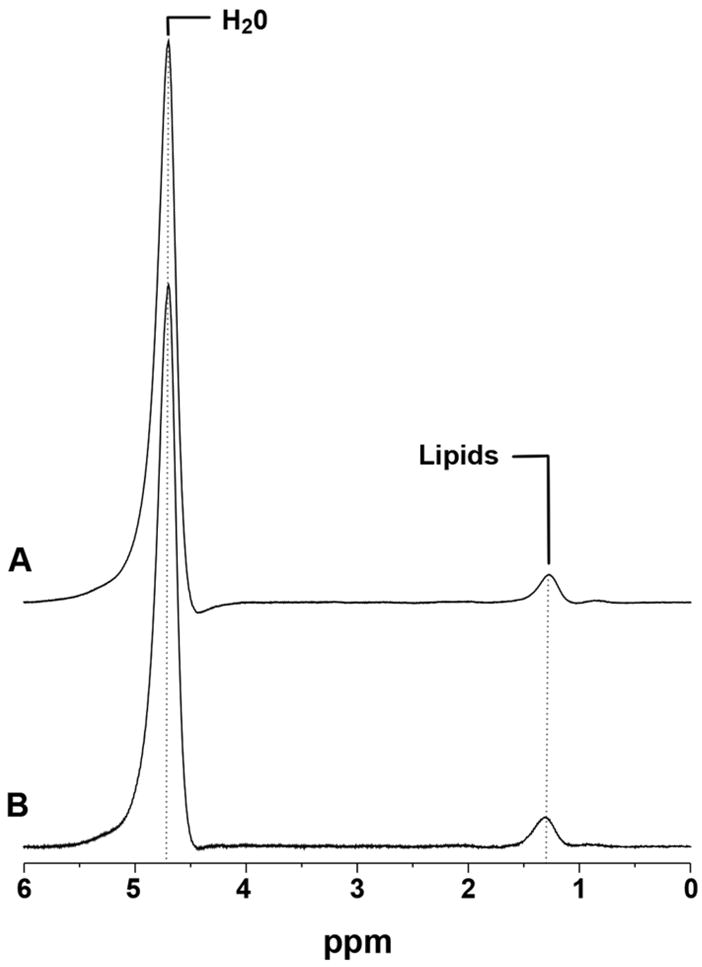
Qualitative assessment of respiratory-gated (A) and breath-hold (B) 1H-MRS of the liver at 3 Tesla from an obese subject (BMI: 30 kg/m2) showing comparable quality of lipid resonances at 1.3 ppm and water resonances at 4.7 ppm, respectively.
There was no significant difference between the mean hepatic lipid content in the group of premenopausal women with obesity (n=23) measured with breath-hold 1H-MRS (4.9±5.9%) and mean lipid content measured with respiratory-gated 1H-MRS (3.1±3.6%, p=0.2)
There was a strong correlation between breath-hold and respiratory-gated 1H-MRS for intrahepatic lipid quantification in overweight/obese premenopausal women (r=0.93, p<0.0001) (Figure 2). Likewise, breath-hold and respiratory-gated 1H-MRS showed good agreement using Bland Altman analysis (Figure 3). The values for hepatic lipid content obtained from breath-hold 1H-MRS were relatively higher when compared to respiratory-gated 1H-MRS. The mean difference between the techniques was 1.8% with a 95% confidence interval between −4.2 and 7.8%. The concordance correlation coefficient between breath-hold and respiratory-gated 1H-MRS was 0.75.
Figure 2.
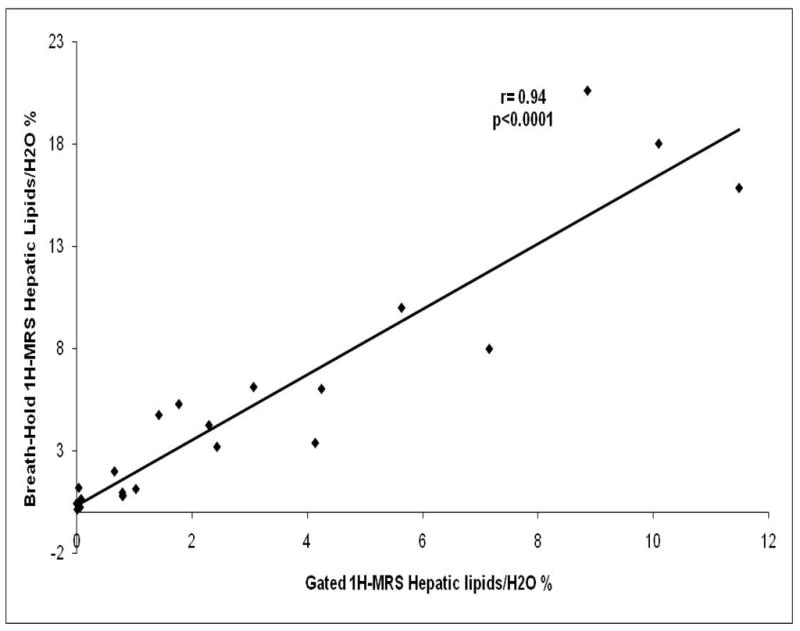
Correlation analysis between breath-hold and respiratory-gated 1H-MRS. There is a strong correlation between both techniques (r= 0.94, p<0.0001).
Figure 3.
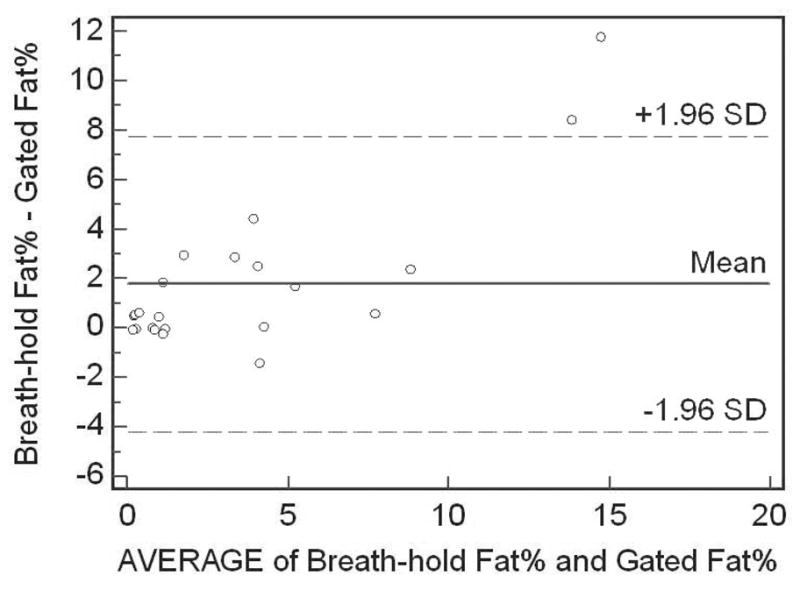
Bland Altman analysis of breath-hold and respiratory-gated 1H-MRS. All but two values are within limits of agreement (dotted lines), corresponding to ± 1.96 SDs from mean. Breath-hold 1H-MRS consistently provided relatively higher values of hepatic lipid content compared to respiratory 1H-MRS.
Both techniques showed an expected inverse correlation with liver density determined by CT. Pearson correlation coefficient for breath-hold 1H-MRS and liver density was r= -0.71, p<0.0002, and for respiratory-gated 1H-MRS was r= −0.67, p<0.0005, respectively. Pearson correlation coefficient for breath-hold 1H-MRS and liver-to-spleen attenuation ratio was r= −0.52, p<0.01, and for respiratory-gated 1H-MRS r= −0.57, p<0.004.
Reproducibility of both breath-hold and respiratory-gated 1H-MRS was high. Correlation analysis between the data before and after repositioning for breath-hold 1H-MRS was r= 0.99, p= 0.0003 and for respiratory-gated 1H-MRS was r= 0.93, p= 0.007. Using Bland Altman analysis the mean difference between same-day scans for the breath-hold technique was 0.29% with a 95% confidence interval between −1.46 and 2.05%. For respiratory-gated 1H-MRS the mean difference between same-day scans was −0.54% with a 95% confidence interval between −2.53 and 1.45%. The concordance correlation coefficient for breath-hold 1H-MRS was 0.97 and for respiratory-gated 1H-MRS was 0.91.
Discussion
We demonstrated the feasibility and reproducibility of a single breath-hold 1H-MRS technique for non-invasive intrahepatic lipid quantification at 3 Tesla. Single breath-hold 1H-MRS showed high correlation and agreement with respiratory-gated 1H-MRS and both techniques showed an inverse correlation with liver density determined by CT, which is considered a simple and widely available technique for determination of fatty liver disease. Both breath-hold and respiratory-gated 1H-MRS showed low inter-scan variability which is critical for longitudinal studies. However, breath-hold 1H-MRS consistently provided relatively higher values of hepatic lipid content compared to respiratory 1H-MRS with a mean difference of 1.8% hepatic lipid content.
Obesity has become the biggest risk factor for nonalcoholic fatty liver disease, affecting an increasing number of adults and children (5, 27). Several therapeutic interventions have been suggested to reduce the amount of hepatic lipids (7, 28, 29), requiring accurate, non-invasive methods for hepatic lipid quantification that can be performed longitudinally. Liver biopsy remains the standard of reference providing information about the severity of steatosis and disease activity. However, the procedure is invasive making it unsuitable for repeated examinations. Several imaging techniques have been described to evaluate intrahepatic lipid content. CT is a widely available technique that can be used for easy determination of liver attenuation. However, it requires radiation exposure. Moreover, liver attenuation is not only affected by fat infiltration but also by other metabolites such as iron and iron deposition within the liver that can mask decreased attenuation from fatty infiltration (30). 1H-MRS has been found to be a very sensitive imaging technique to quantify hepatic lipid content and has shown to correlate with liver biopsy results (7, 13, 14, 31, 32). However, several inherent factors can affect the accuracy of 1H-MRS, such as magnetic field inhomogeneity and voxel misregistration due to respiratory motion. Free-breathing ungated 1H-MRS would likely broaden the linewidth of lipid and water resonances leading to a greater measurement error and decreased reproducibility. In order to minimize artifact from respiratory motion, 1H-MRS has been performed with respiratory-gating, respiratory-motion correction, coordinated breath-holding or synchronization to the breathing pattern of the patient to minimize line broadening of the spectra (8, 19–22, 33). Those methods require operator involvement and are time consuming. The use of a respiratory transducer to monitor liver motion is limited by the variability in the subject’s breathing pattern that can lead to different tissues being sampled (20). We therefore instructed our patients to breathe in quiet, regular respiration during the respiratory-gated 1H-MRS acquisition.
In order to be feasible for routine clinical practice, 1H-MRS must be performed in a timeframe comparable with that of other MR imaging methods, preferably during a single breath-hold. The single breath-hold 1H-MRS technique described in our study required patients to hold their breath for 18 seconds and was well tolerated. We minimized operator involvement by using automated shimming procedures that makes it suitable for routine clinical practice. The post-processing steps performed offline were automated for speed and simplicity. The high field strength at 3 Tesla allowed the fast acquisition of good quality spectra in all patients using both techniques.
Cowin et al. (7) used a breath-hold 1H-MRS technique, in-and out-of phase MRI, and plus/minus fat saturation techniques to correlate MR findings with histology and monitor hepatic fat content at 1.5 Tesla in subjects with and without hepatic steatosis before and after weight loss. In their study, 1H-MRS showed a strong correlation with histological assessment of hepatic lipid content and MRI, and was able to monitor hepatic lipids before and after weight loss. Our study used a single-breath hold 1H-MRS technique with shorter TR at 3 Tesla and compared it to other imaging techniques that are frequently used to quantify hepatic fat content.
Our study had limitations. Although several non-invasive imaging techniques for hepatic lipid quantification were compared amongst each other, we did not obtain liver biopsies as a gold standard. None of our patients had a history of liver disease and all had normal liver enzymes, therefore it was not feasible to perform liver biopsies in our cohort. However, the focus of our study was to examine the performance of breath-hold 1H-MRS compared to other accepted imaging techniques for liver fat quantification. The hepatic lipid contents obtained from breath-hold 1H-MRS were relatively higher when compared to respiratory-gated 1H-MRS. Although the study was not designed to evaluate linewidth and SNR, a potential explanation for higher lipid content of breath-hold 1H-MRS could be that it yielded narrower linewidths and better SNR, which may have impacted lipid-to-water ratios. Our study group was comprised of healthy premenopausal women who were obese but had no history of fatty liver disease and had normal liver enzymes. As we did not study subjects with steatohepatitis, it is not possible to determine the performance of single breath-hold 1H-MRS in such cases. Also, the two subjects outside the confidence limit of the Bland-Altman Analysis were the ones with highest fat content. The cause of this discrepancy cannot be determined from our data. It is possible that these obese patients had difficulties holding their breath for the 1H-MRS sequence. Therefore, further testing in a population with a wider range of liver fat content and pathology is necessary. In our study, T2-corrections were not performed due to time constraints. While T2 relaxation effects may influence the quantification of hepatic lipid content (34, 35), such effects may have been limited considering the expected T1 and T2 liver relaxation times at 3 Tesla of 809 ms and 34 ms, respectively, and our use of breath-hold 1H-MRS with repetition time of 1,500 ms (36). Furthermore, Cowin et al. (7) showed high accuracy of a breath-hold 1H-MRS technique without T2 correction at 1.5 Tesla with liver biopsy samples. A standard limitation of single-voxel 1H-MRS is that sampling errors may occur if lipid accumulation is heterogeneous throughout the liver. In these cases multiple sample sites within different parts of the liver can be performed and the results averaged.
In conclusion, single breath-hold 1H-MRS is a reproducible and fast technique for non-invasive quantification of hepatic lipids. It has a low inter-scan variability that makes it suitable for longitudinal assessment and provides data that closely correlate to longer duration respiratory-gated 1H-MRS. Breath-hold 1H-MRS of the liver was well-tolerated by all subjects and is minimally operator dependent, making it feasible for routine clinical practice. Breath-hold 1H-MRS may represent a simple yet powerful extension of diagnostic liver MRI techniques.
Acknowledgments
This work was supported in part by National Institutes of Health Grants RO1 HL-077674, UL1 RR025758, and K23 RR-23090
Footnotes
Disclosure summary: The authors have nothing to disclose
References
- 1.Rexrode KM, Carey VJ, Hennekens CH, et al. Abdominal adiposity and coronary heart disease in women. Jama. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 2.Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117:1658–1667. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 3.Bugianesi E, Leone N, Vanni E, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 4.Falck-Ytter Y, Younossi ZM, Marchesini G, McCullough AJ. Clinical features and natural history of nonalcoholic steatosis syndromes. Semin Liver Dis. 2001;21:17–26. doi: 10.1055/s-2001-12926. [DOI] [PubMed] [Google Scholar]
- 5.Lall CG, Aisen AM, Bansal N, Sandrasegaran K. Nonalcoholic fatty liver disease. AJR Am J Roentgenol. 2008;190:993–1002. doi: 10.2214/AJR.07.2052. [DOI] [PubMed] [Google Scholar]
- 6.Cassidy FH, Yokoo T, Aganovic L, et al. Fatty liver disease: MR imaging techniques for the detection and quantification of liver steatosis. Radiographics. 2009;29:231–260. doi: 10.1148/rg.291075123. [DOI] [PubMed] [Google Scholar]
- 7.Cowin GJ, Jonsson JR, Bauer JD, et al. Magnetic resonance imaging and spectroscopy for monitoring liver steatosis. J Magn Reson Imaging. 2008;28:937–945. doi: 10.1002/jmri.21542. [DOI] [PubMed] [Google Scholar]
- 8.Guiu B, Loffroy R, Petit JM, et al. Mapping of liver fat with triple-echo gradient echo imaging: validation against 3.0-T proton MR spectroscopy. Eur Radiol. 2009;19:1786–1793. doi: 10.1007/s00330-009-1330-9. [DOI] [PubMed] [Google Scholar]
- 9.Guiu B, Petit JM, Loffroy R, et al. Quantification of liver fat content: comparison of triple-echo chemical shift gradient-echo imaging and in vivo proton MR spectroscopy. Radiology. 2009;250:95–102. doi: 10.1148/radiol.2493080217. [DOI] [PubMed] [Google Scholar]
- 10.Kodama Y, Ng CS, Wu TT, et al. Comparison of CT methods for determining the fat content of the liver. AJR Am J Roentgenol. 2007;188:1307–1312. doi: 10.2214/AJR.06.0992. [DOI] [PubMed] [Google Scholar]
- 11.Machann J, Thamer C, Schnoedt B, et al. Hepatic lipid accumulation in healthy subjects: a comparative study using spectral fat-selective MRI and volume-localized 1H-MR spectroscopy. Magn Reson Med. 2006;55:913–917. doi: 10.1002/mrm.20825. [DOI] [PubMed] [Google Scholar]
- 12.Ricci C, Longo R, Gioulis E, et al. Noninvasive in vivo quantitative assessment of fat content in human liver. J Hepatol. 1997;27:108–113. doi: 10.1016/s0168-8278(97)80288-7. [DOI] [PubMed] [Google Scholar]
- 13.Longo R, Pollesello P, Ricci C, et al. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging. 1995;5:281–285. doi: 10.1002/jmri.1880050311. [DOI] [PubMed] [Google Scholar]
- 14.Szczepaniak LS, Babcock EE, Schick F, et al. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol. 1999;276:E977–989. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 15.Fischbach F, Bruhn H. Assessment of in vivo 1H magnetic resonance spectroscopy in the liver: a review. Liver Int. 2008;28:297–307. doi: 10.1111/j.1478-3231.2007.01647.x. [DOI] [PubMed] [Google Scholar]
- 16.Hadigan C, Liebau J, Andersen R, Holalkere NS, Sahani DV. Magnetic resonance spectroscopy of hepatic lipid content and associated risk factors in HIV infection. J Acquir Immune Defic Syndr. 2007;46:312–317. doi: 10.1097/QAI.0b013e3181568cc2. [DOI] [PubMed] [Google Scholar]
- 17.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 18.van Werven JR, Hoogduin JM, Nederveen AJ, et al. Reproducibility of 3.0 Tesla magnetic resonance spectroscopy for measuring hepatic fat content. J Magn Reson Imaging. 2009;30:444–448. doi: 10.1002/jmri.21837. [DOI] [PubMed] [Google Scholar]
- 19.Kim H, Taksali SE, Dufour S, et al. Comparative MR study of hepatic fat quantification using single-voxel proton spectroscopy, two-point dixon and three-point IDEAL. Magn Reson Med. 2008;59:521–527. doi: 10.1002/mrm.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noworolski SM, Tien PC, Merriman R, Vigneron DB, Qayyum A. Respiratory motion-corrected proton magnetic resonance spectroscopy of the liver. Magn Reson Imaging. 2009;27:570–576. doi: 10.1016/j.mri.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pfleiderer B, Xu P, Ackerman JL, Garrido L. Study of aging of silicone rubber biomaterials with NMR. J Biomed Mater Res. 1995;29:1129–1140. doi: 10.1002/jbm.820290913. [DOI] [PubMed] [Google Scholar]
- 22.Star-Lack JM, Adalsteinsson E, Gold GE, Ikeda DM, Spielman DM. Motion correction and lipid suppression for 1H magnetic resonance spectroscopy. Magn Reson Med. 2000;43:325–330. doi: 10.1002/(sici)1522-2594(200003)43:3<325::aid-mrm1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Bredella MA, Torriani M, Thomas BJ, et al. Peak GHRH-arginine stimulated growth hormone is inversely associated with intramyocellular and intrahepatic lipid content in premenopausal women with obesity. J Clin Endocrinol Metab. 2009 doi: 10.1210/jc.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 25.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 26.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 27.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 28.Federico A, Niosi M, Vecchio Blanco CD, Loguercio C. Emerging drugs for non-alcoholic fatty liver disease. Expert Opin Emerg Drugs. 2008;13:145–158. doi: 10.1517/14728214.13.1.145. [DOI] [PubMed] [Google Scholar]
- 29.Huang MA, Greenson JK, Chao C, et al. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072–1081. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 30.Limanond P, Raman SS, Lassman C, et al. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology. 2004;230:276–280. doi: 10.1148/radiol.2301021176. [DOI] [PubMed] [Google Scholar]
- 31.Johnson NA, Walton DW, Sachinwalla T, et al. Noninvasive assessment of hepatic lipid composition: Advancing understanding and management of fatty liver disorders. Hepatology. 2008;47:1513–1523. doi: 10.1002/hep.22220. [DOI] [PubMed] [Google Scholar]
- 32.Thomas EL, Hamilton G, Patel N, et al. Hepatic triglyceride content and its relation to body adiposity: a magnetic resonance imaging and proton magnetic resonance spectroscopy study. Gut. 2005;54:122–127. doi: 10.1136/gut.2003.036566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyszka JM, Silverman JM. Navigated single-voxel proton spectroscopy of the human liver. Magn Reson Med. 1998;39:1–5. doi: 10.1002/mrm.1910390102. [DOI] [PubMed] [Google Scholar]
- 34.Pineda N, Sharma P, Xu Q, Hu X, Vos M, Martin DR. Measurement of Hepatic Lipid: High-Speed T2-Corrected Multiecho Acquisition at 1H MR Spectroscopy--A Rapid and Accurate Technique. Radiology. 2009 doi: 10.1148/radiol.2523082084. [DOI] [PubMed] [Google Scholar]
- 35.Sharma P, Martin DR, Pineda N, et al. Quantitative analysis of T2-correction in single-voxel magnetic resonance spectroscopy of hepatic lipid fraction. J Magn Reson Imaging. 2009;29:629–635. doi: 10.1002/jmri.21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Bazelaire CM, Duhamel GD, Rofsky NM, Alsop DC. MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology. 2004;230:652–659. doi: 10.1148/radiol.2303021331. [DOI] [PubMed] [Google Scholar]


