Abstract
The black-tailed prairie dog (Cynomys ludovicianus) is a member of the order Rodentia and the family Sciuridae. Ecologically, prairie dogs are a keystone species in prairie ecology. This species is used as an animal model for human gallbladder disease and diseases caused by infection with Clostridium difficile, Yersinia pestis, Francisella tularensis, and most recently, Orthopoxvirus. Despite increasing numbers of prairie dogs used in research and kept as pets, few data are available on their baseline physiology in animal facility housing conditions. To establish baseline physiologic reference ranges, we designed a study using 18 wild-caught black-tailed prairie dogs. Telemetry data were analyzed to establish circadian rhythms for activity and temperature. In addition, hematologic and serum chemistry analyses were performed. Baseline measurements were used to establish the mean for each animal, which then were compiled and analyzed to determine the reference ranges. Here we present physiologic data on serum chemistry and hematology profiles, as well as weight, core body temperature, and daily activity patterns for black-tailed prairie dogs. These results reflect the use of multiple measurements from species- and age-matched prairie dogs and likely will be useful to ecologists, scientists interested in using this animal model in research, and veterinarians caring for pet prairie dogs.
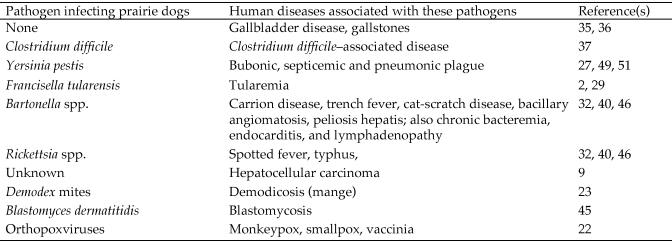
The black-tailed prairie dog (Cynomys ludovicianus) is a social rodent of the squirrel family (Sciuridae).17 The prairie dog is a keystone species of the grassland ecosystem of western North American because it substantially affects myriad other organisms. For example, prairie dogs maintain short vegetation by their grazing and by selective removal of tall plants and shrubs. In addition, their colony sites provide shelter, foraging grounds, and nesting habitats for diverse species, and black-tailed prairie dogs serve as prey for many predators.19 In addition to their natural ecologic role, black-tailed prairie dogs are susceptible to many pathogens and therefore used in research as animal models for multiple diseases (Figure 1).2,9,22,23,27,29,32,35-37,40,45,46,49,51
Figure 1.
A literature review was performed to identify pathogens that can infect prairie dogs. These pathogens, and the human diseases that can be modeled in the prairie dog, are listed with appropriate references.
In September 2008, the US Food and Drug Administration removed the portion of the November 4, 2003 Interim Final Ruling6 that banned interstate trade of black-tailed prairie dogs. Therefore, the black-tailed prairie dog is expected, again, to become an important sector of the exotic pet trade, making it important for veterinarians to be knowledgeable about the health and husbandry of these animals.20,24,38 Despite increasing numbers of black-tailed prairie dogs being used in scientific research, as well as being kept as pets, little information is available on the baseline physiology of prairie dogs kept in captivity. Reference ranges established from single blood samples for serum chemistry and hematology of wild-caught prairie dogs are published and recognized by the International Species Information System 5,16,33 but are statistically inconsistent with each other and with unpublished data from a study of pet prairie dogs.4 Inconsistencies may reflect differences in environment, species, diet, blood sampling technique, analysis used, age, sex, and geographic distribution, but available published data are too few to determine the precise cause of these differences.
Because the black-tailed prairie dog is the Cynomys species used most widely in research and kept most frequently as pets, we undertook a study in which venous blood collection and telemetry were used to measure physiologic parameters in wild-caught animals kept in captivity. This study establishes reference ranges based on multiple baseline measurements of temperature, activity, serum chemistry, and hematology in an age-matched manner for this species. We report reference ranges developed from 18 wild-caught black-tailed prairie dogs measured 9 times each over a 30-d period. In addition, extensive telemetry data were analyzed to provide insight into the circadian rhythms of body temperature and daily activity of black-tailed prairie dogs under these housing conditions. These data likely will be useful to laboratory animal scientists interested in the ecology of this species as well as those using this species as an animal model in disease research and to veterinarians caring for pet prairie dogs.
Materials and Methods
Animals.
Wild-caught black-tailed prairie dogs (11 female, 7 male) captured in late June in Boulder County, CO, by using live traps were used in this investigation. The animals were approximately 6 mo at the time that the reference range data reported here were gathered. This age is based on the timing of breeding (late February to early March), birth (late March to mid April), weaning of animals for 6 wk (late May to early June), and capture of approximately 2.5-mo-old prairie dogs at initial emergence from burrows (late June). The capture of the young animals in June allowed separation of young animals from older animals based on size. Prairie dogs were treated topically with permethrin prior to transport to our facility and upon arrival were communally housed for a 12-wk period, with PVC pipes and balls for enrichment. Prairie dogs (approximate age, 5.5 mo) were then moved to polycarbonate, static microisolation rat caging for a 1-wk acclimation period prior to implantation of telemetry transponders (Mini Mitter, Bend, OR) and acquisition of baseline measurements. Animals were kept in individual housing for the next 4 wk in rooms with constant temperature (70.4 ± 1.2 °F [21.3 ± 0.7 ºC]) and humidity (49.3% ± 9%) with a 12:12-h light:dark cycle (lights on, 0600 to 1800), which is the standard light cycle for both household pets and research animal facilities. PVC tubes were provided to the individually housed animals for enrichment purposes. Animals were cared for in accordance with and under a protocol approved by the Centers for Disease Control Institutional Animal Care and Use Committee. Animal handling and husbandry were performed by appropriately trained personnel, who wore Biosafety Level 2 personal protective equipment. At the conclusion of the current study, the prairie dogs were used in a viral infection study and then euthanized by isofluorane overdose with barbiturate overdose (1 mL/kg intracardiac), in accordance with IACUC policy 016.
Diet.
The prairie dogs were provided ad libitum with a commercially available diet (Exotic Nutrition, Newport News, VA) and water. The food is a high-fiber formula developed within Good Manufacturing Practices guidelines to replicate the prairie dogs’ natural high-fiber intake in the wild. The ingredients in this chow are soy hulls, calcium carbonate, manganese proteinate, wheat middlings, grinding oats, solvent extracted, soybean meal, zinc proteinate, colbolt proteinate, copper proteinate, mineral oil, and vitamin D3, A, and E supplements. The guaranteed analysis is: crude protein (minimum), 10.0%; calcium (minimum), 0.40%; crude protein (maximum), 12.0%; calcium (maximum), 0.50%; crude fat (minimum), 1.5%; phosphorus (minimum), 0.15%: crude fiber (maximum), 40.0%; and vitamin A (minimum), 1200 IU/lb. At least one of the following items was provided approximately 3 times a week for dietary enrichment: Sugar Beet Treats (Exotic Nutrition, Newport News, VA); Fruit Kabobs (Exotic Nutrition), fresh sweet potatoes, and alfalfa (Exotic Nutrition) or timothy (Exotic Nutrition) hay. In addition, the animals were provided with Monkey Biscuits (Exotic Nutrition). We used these biscuits as treats because we have found these are well liked by almost all prairie dogs. Therefore, the biscuits offer an easily measured indicator of inappetence if refused by the animals.
Pathogen screening.
Screening for Yersinia pestis, Francisella tularensis, Bartonella spp., and Rickettsia spp. was outsourced (Bacterial Diseases Branch, Division of Vector-Borne Infectious Diseases, Centers for Disease Control, and Rickettsial Zoonoses Branch, Division of Viral and Rickettsial Diseases), and samples of whole blood from prairie dogs were assayed by PCR as previously described.28,46,49 Briefly, these assays used primer sets to target the Bartonella and rickettsial citrate synthase gene (gltA), the F. tularensis pdpD gene, and the Y. pestis pla gene. In addition, serum from these animals taken prior to study start was tested by ELISA to detect IgG antibodies to Orthopoxvirus spp.25 Briefly, the IgG ELISA is an indirect assay using purified vaccinia virus as the coating antigen, 1:50 to 1:12150 dilution of prairie dog serum, and Protein A/G conjugate.
Biotelemetry.
After an acclimation period of at least 1 wk, each prairie dog underwent surgery for implantation of a biotelemetry transponder. To facilitate recovery from anesthesia, induction was performed by using a chamber with isoflurane at 5% in accordance with IACUC-approved protocol. Anesthesia was maintained by using isoflurane (2% to 3%) by nose cone. During surgery, prairie dogs were placed on a heating pad to maintain core body temperature between 36 and 37 °C. The ventral midline of the abdomen at the linea alba was clipped with a no. 40 blade. The clipped area had approximately 3 cm margins to the incision site. The area for surgical incision was scrubbed with 4% chlorhexidine gluconate and then 70% isopropyl alcohol. Chlorhexidine and alcohol solutions were alternated and repeated for a total of 3 times each, ending with alcohol. An incision (1.0 to 1.5 cm) was made at the linea alba, and a transponder was inserted. The incision was closed in 2 layers. The linea alba was closed with 4-0 polydioxonone monofilament in a continuous pattern, with 1 or 2 interrupted surgeon's knots medial of the incision for added closure security. The skin then was closed by using a subcuticular continuous pattern of 5-0 polyglactin 910 suture. Tissue glue (Nexaband, Abbott Animal Health, Abbott Park, IL) was applied to the entire length of the incision. Meloxicam (0.2 mg/kg SC; Metacam, Boehringer Ingelheim Vetmedica, St Joseph, MO) was administered to alleviate the discomfort of postoperative inflammation and was continued postoperatively daily for 3 d. This procedure has been shown to cause no lasting discomfort beyond a short recovery period in multiple species.8,11,14,34,47
The transmitter was programmed to record temperature and gross motor activity levels at 5-min intervals. This timing allows the monitoring of key vital signs without having to sedate or handle the animal, thus reducing handling-associated stress and minimizing potential human exposure to infection. The transponders implanted (model ER4000 E-Mitter, Mini Mitter) have no battery and obtain power from a radiofrequency field generated by the receiver, which is placed under the animals’ cage. Temperature and activity data from the transponder were analyzed by using VitalView (Mini Mitter) software. The animals were allowed 7 d to recover and clear any medications prior to being sampled for this study.
Blood sampling and analysis.
Anesthesia was induced in prairie dogs by using isoflurane in a chamber to effect, with maintenance of anesthesia by nose cone; animals were kept on heating block to maintain core temperature in accordance with IACUC-approved protocol. The hind limb and groin area was sprayed with 70% isopropanol. A 28-gauge needle was used to collect 1 mL blood from the saphenous vein; the femoral vein was used only when blood could not be obtained from the saphenous vein. Blood was transferred to a tube containing EDTA (Fisher Scientific, Pittsburgh, PA) for hematologic analysis or to a serum separation tube (Fisher Scientific) for chemistry analysis. EDTA-treated blood was analyzed for WBC, platelets, hematocrit, monocytes, granulocytes, lymphocytes, MCV, RBC, hemoglobin, MCH, and MCHC (CBC-Diff Veterinary Hematology System, Heska, Fort Collins, CO). An automated chemistry analyzer (Piccolo Serum Chemistry Analyzer, Abaxis, Union City, CA) was used to measure ALT, albumin, alkaline phosphatase, AST, calcium, chloride, creatinine, glucose, potassium, sodium, total bilirubin, total carbon dioxide, total protein, and BUN. Sampling occurred from midmorning to early afternoon every 3 to 4 d over a 30-d period.
Statistical analysis.
Prism 5.0 (GraphPad, San Diego, CA) was used to confirm the Gaussian distribution of our data and to calculate the mean and the 95th and fifth percentiles to establish reference ranges for blood chemistry, hematology, weight, temperature, and activity. Because the data passed the D'Agostino–Pearson omnibus test, which measures the deviation from a predicted Gaussian distribution by skewness (asymmetry) and kurtosis (shape), further statistics were performed after normal Gaussian distribution was assumed. The Grubbs test, which was developed for testing data sets for which the mean and standard deviation are unknown, was used to test for outliers from each data set. This methodology can only be used once per analysis so a maximum of a single outlier was removed per data set, but if no outliers were detected, the data were left unchanged. Outliers were removed to minimize the effects of partial hemolysis of sampled blood, mishandling of sample, or instrument malfunction. Data sets included each animal at a single time point for a single analyte. The column statistics option was used to determine means, medians, standard deviations, and percentiles. Additional statistics used in this paper included one-way ANOVA and 2-tailed t tests to determine differences in means between groups of animals, such as male and female, or all animals at different time points. The level of significance was set at a P value of less than 0.05. Unpaired t tests were performed by comparing our range data with previously reported ranges for both hematology and chemistry results; previously published data were insufficient to perform any other analysis.
Circadian rhythm software (Cosinor Periodogram; provided by Dr R Refinetti by means of the Internet [www.circadian.org/software.html]) was used to analyze temperature and activity data. This program provides the mesor, amplitude, acrophase, period, and statistical significance of the circadian rhythm for both temperature and activity by using the single cosinor method, which fits a 24-h cosine curve to the data. In addition, the mean period was calculated by using a minimum of 10 d of data (bin size, 5 min) for each animal by using the Periodogram software ([www.circadian.org/software.html]). This procedure gave us a period for each animal, and these values are reported as mean ± 1 SD. No statistically significant differences in chemistry, hematology, core body temperature, or activity values were found between male and female prairie dogs so the results from both sexes are combined in our analysis.
Results
Pathogen screening.
All 18 black-tailed prairie dogs used in this study were PCR-negative for Bartonella spp., Rickettsial spp., Y. pestis, and F. tularensis. Similarly, all animals used in this study were negative for antiorthopoxvirus IgG antibodies. These results, coupled with the same PCR-negative results for an additional 41 random animals from the same shipment that were purchased for various viral studies, demonstrate the overall health of our animals and ensure that the results we obtained are not confounded by these pathogens.
Chemistry profiles.
Table 1 includes the mean values, standard deviations, and reference ranges determined for serum chemistry parameters of our study population of black-tailed prairie dogs with relevant reference ranges for wild5 and pet4 prairie dogs. We obtained the blood samples for determining these values every 3 to 4 d over a 30-d period. The mean for each of the 18 animals was established by using 9 separate measurements; the individual means were compiled and analyzed to determine the 5th and 95th percentiles for use as reference ranges. In our study, alkaline phosphatase and ALT values were higher than values previously reported for wild prairie dogs (Table 1). AST and glucose values in our population were lower and formed more narrow ranges than did those of pet and wild prairie dogs (Table 1). All other serum chemistry parameter values (total protein, total bilirubin, albumin, BUN, creatinine, total CO2, calcium, sodium, potassium, and chloride) were similar to these previous reports and to the ranges currently accepted in the International Species Information System (ISIS) database.16
Table 1.
Blood chemistry reference ranges for prairie dogs
| Results of current study |
Previously published reference rangesa |
||||
| Parameter | Mean | Range | 1 SD | Pet prairie dogs4 | Wild prairie dogs5 |
| Alkaline phosphatase | 153 IU/L | 84–280 IU/L | 60 | 45–128 IU/L | |
| AST | 35 IU/L | 21–63 IU/L | 11 | 5–242 IU/L | 28–141 IU/L |
| ALT | 39 IU/L | 16–152 IU/L | 33 | 2.9–84 IU/L | |
| Total protein | 6 g/dL | 5–7 g/dL | 0.3 | ||
| Total bilirubin | 0.3 mg/dL | 0.2–0.6 mg/dL | 0.1 | ||
| Albumin | 2.4 g/dL | 2–3 g/dL | 0.3 | ||
| Glucose | 50 mg/dL | 28–84 mg/dL | 13 | 100–236 mg/dL | 138–510 mg/dL |
| BUN | 26 mg/dL | 22–31 mg/dL | 3 | ||
| Creatnine | 0.6 mg/dL | 0.4–0.8 mg/dL | 0.1 | ||
| Total CO2 | 28 mmol/L | 23–34 mmol/L | 2.4 | ||
| Calcium | 9 mg/dL | 8–11 mg/dL | 0.7 | ||
| Sodium | 142 mmol/L | 139–145 mmol/L | 1.6 | ||
| Potassium | 6 mmol/L | 5–8 mmol/L | 0.9 | ||
| Chloride | 84 mmol/L | 79–88 mmol/L | 2.2 | ||
Ranges presented are based on the 95th percentile of all values (n = 9 samples each of 18 animals). Significant outliers according to the Grubbs test were excluded from analysis.
For clarity, only previously published ranges that differed from our current results are shown.
Hematology profiles.
Hematology values for our studied prairie dogs are shown in Table 2 along with relevant reference ranges for wild5 and pet4 animals. These data were obtained from the same blood samples as used for the serum chemistry values. The WBC values of our animals were lower than those previously published for wild prairie dogs (Table 2). Platelet counts and WBC differential percentages were lower than previous reports for both pet and wild prairie dogs. Hematocrit, RBC, and hemoglobin values were lower for our animals than pet animals (Table 2). All other values (MCV, MCH, MCHC) were similar to previous reports and to the ranges currently accepted in the International Species Information System (ISIS).16
Table 2.
Hematology reference ranges for prairie dogs
| Results of current study |
Previously published reference rangesa |
||||
| Parameter | Mean | Range | 1 SD | Pet prairie dogs4 | Wild prairie dogs5 |
| WBC | 3 × 103/µL | 1–6 × 103/µL | 1.2 | 3.3–10.5 × 103/µL | |
| Platelets | 126 × 103/µL | 41–239 × 103/µL | 67 | 245 - 1190 103/µL | 138–783 × 103/µL |
| Hematocrit | 24% | 14%–36% | 6.7 | 33.5%–45.1% | |
| Monocytes | 0.5 × 103/µL | 0.2–0.9 × 103/µL | 0.2 | 0.03–0.58 103/µL | 0–0.2 × 103/µL |
| Granulocytes | 1.6 × 103/µL | 0.8–2.8 × 103/µL | 0.6 | 1–8 103/ µL | 0.8–8.4 × 103/µL |
| Lymphocytes | 1.2 × 103/µL | 0.5–3 × 103/µL | 0.5 | 0.2–3 103/µL | 1–5.6 × 103/µL |
| MCV | 62 fl | 57–66 fl | 2.5 | ||
| RBC | 4 × 106/µL | 2–5 × 106/µL | 1.1 | 5.9–8.1 106/µL | |
| Hemoglobin | 8 g/dL | 4–11 g/dL | 2.1 | 10.4–15.3 g/dL | |
| MCH | 20 pg | 19–22 pg | 0.8 | ||
| MCHC | 32 g/dL | 29–34 g/dL | 1.5 | ||
Ranges presented are based on the 95th percentile of all values (n = 9 samples each of 18 animals). Significant outliers according to the Grubbs test were excluded from analysis.
For clarity, only previously published ranges that differed from our current results are shown.
Body weight.
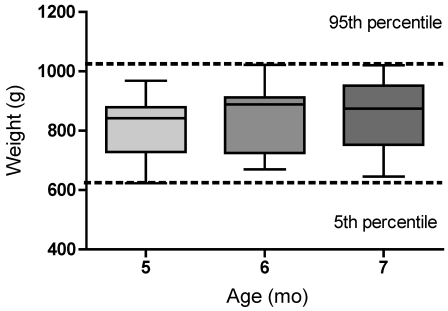
The reference range for body weight that we show here (Figure 2) is for 5- to 7-mo-old prairie dogs. Body weight (mean ± 1 SD) for 5-mo-old prairie dogs is 811 ± 97 g with a 95th percentile reference range of 623 g to 968 g. For 6-mo-old prairie dogs, the weight is 842 ± 106 g, with a reference range of 670 g to 1022 g. The weight of 7-mo-old prairie dogs is 852 ± 112 g, with a reference range of 646 to 1020 g. When these data were analyzed in the relation to the sex of the animal, male 6- to 7-mo-old prairie dogs had higher mean weights than the females but these differences are not statistically significant by ANOVA. These animals were fed a low-fat, high-fiber diet and were singly housed during this experiment, similar to the conditions under which both research and pet prairie dogs are kept. Therefore, these weights, although potentially higher than those of prairie dogs in the wild18 because of the presumed lower activity levels of captive animals, likely are accurate for use by scientists and veterinarians.
Figure 2.
Weight (g) ranges for 5- to 7-mo-old prairie dogs are shown. Each age group represents 4 measurements of each of 18 animals. The graph shows the median (line), 25th and 75th percentiles for each data set (box), and the 5th and 95th percentiles for each data set (whiskers). The dotted line represents the 5th and 95th percentile for all values.
Core body temperature.
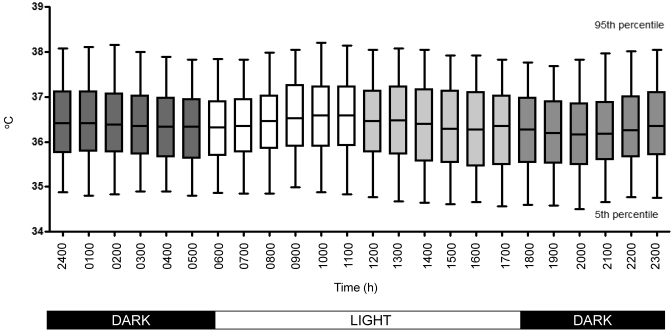
Figure 3 shows compiled data for core body temperatures measured 12 times per hour, 24 h per day for 30 d for all 18 prairie dogs in our population. The mean core body temperature for our prairie dogs (36.4 °C) differs significantly (P < 0.0001, two-tailed t test) from the mean temperatures for humans (37 °C), European ground squirrels (37.3 °C), and 13-lined ground squirrels (35.4 °C). The mean temperature over a 24-h period of all prairie dogs combined ranges from 36.2 to 36.6 °C (σ = 0.1140), indicating a small variance in the mean of the population over time. Conversely, the mean temperature between animals at any given hour of the day has a standard deviation of approximately 1, resulting in a 95th percentile reference range of 34.4 °C to 38.2 °C.
Figure 3.
Core body temperature (°C) was measured every 5 min for each prairie dog18 over a 30-d period (7640 readings per animal; 137,520 total readings). The mean values of all readings and the 5th and 95th percentiles were graphed on an hourly basis over a 24-h period. The fixed light:dark cycle under which the animals were housed is annotated. The graph shows the median (line), 25th and 75th percentiles for each data set (box), and 5th and 95th percentiles for each data set (whiskers).
To determine the circadian rhythm of body temperature, data were analyzed by using the single cosinor method, which fits a 24-h cosine curve to the data by the method of least squares. Circadian rhythm had a significant (P = 0.002) effect on our data for core body temperature. The characteristics of this rhythm include the mesor (36.4 ºC), which represents the 24-h average temperature, and the amplitude (0.1045), which represents half the peak–trough difference of the cosine. The acrophase—the time at which the peak of the rhythm occurs—of the temperature data occurs during the light cycle (0600 to 1800) beginning at 0700, with temperatures peaking from 1000 to 1100, as would be expected for a diurnal animal. A second, smaller, rise in core body temperature begins 3 h after the onset of the dark cycle (1800), with peak temperatures recorded from 2400 to 0100. The mean calculated period of this circadian rhythm is 21.13 ± 0.2156 h. This parameter is not the endogenous circadian period, given that the animals were kept singly housed on a prescribed light:dark cycle with controlled climate.
Daily activity patterns.
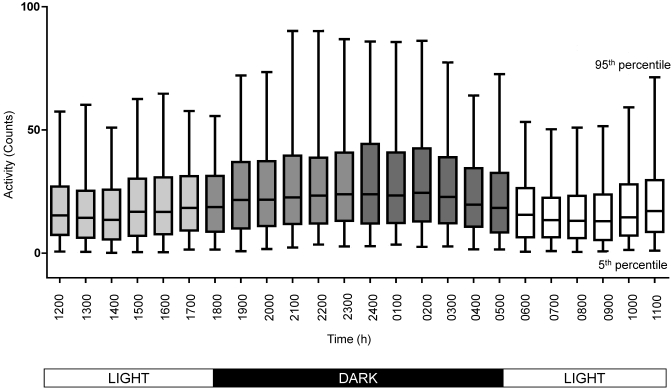
Activity counts (animal movements) were measured 12 times per hour, 24 h daily for 30 d on all 18 animals (Figure 4). The data indicate tremendous variability between prairie dogs, as shown by standard deviations that approach the value of the mean itself. Even on a population level, the variability is high (24.6 ± 4.9 counts). Activity began to increase approximately 3 h after the onset of the light cycle (0600), with peak activity from 1100 to 1200. The acrophase of the activity circadian rhythm occurred during the dark phase, with increasing activity immediately after the onset of the dark cycle (1800) peaking from 2300 to 2400, a pattern more similar to that for nocturnal than diurnal animals.41 The mean calculated period of this circadian rhythm is 25.96 ± 1.28 h. Again, this parameter is not the endogenous circadian period, given that the animals were singly housed on a prescribed light:dark cycle with controlled climate.
Figure 4.
Daily activity (gross motor activity; counts) was measured every 5 min for each animal18 over a 30-d period (7640 readings per animal, 137,520 total readings). The mean values of all readings and the 5th and 95th percentiles were graphed on an hourly basis over a 24-h period. The fixed light:dark cycle under which the animals were housed is annotated. The graph shows the median (line), 25th and 75th percentiles for each data set (box), and the 5th and 95th percentiles for each data set (whiskers).
Discussion
This study seeks to describe several physiologic reference ranges for wild-caught black-tailed prairie dogs. The relevance and novelty of these findings are based on this study being conducted under controlled conditions and specific to wild-caught prairie dogs housed in a research or domestic setting. Although these results cannot be extrapolated to the general prairie dog population, they should be useful to veterinarians and research scientists that care for young prairie dogs procured from the western United States and kept in a climate-controlled setting with a normal light cycle, such as an animal facility or household. Our animals were all approximately the same age, collected from the same location at the same time of year, and singly housed in climate-controlled conditions. Therefore, in our analysis we do not consider differences in physiologic parameters that may fluctuate with age, environment, season or climate, which one would consider in studies done in natural settings.30,31 In addition, because the black-tailed prairie dog is a facultative hibernator and because the conditions under which these prairie dogs were housed did not include food deprivation or cold stress, these data cannot be compared with those from studies on hibernating prairie dogs.12,13 The current animals were studied on arrival at our facility prior to being used in additional studies to obtain baseline reference ranges for later comparison and to aid in interpretation of planned virus studies. The specificity of these results to animals in a research or animal care facility coupled with the multiple measurements of baseline physiologic parameters provide useful data to both veterinarians and scientists caring for and using these animals under these conditions.
Some differences in the serum chemistry values measured in our study as compared with previous reports could be due in part to the use of different assays to measure chemistries. A previous report on pet prairie dogs4 outsourced the blood analysis to 1 of 2 labs, neither of which indicated the analysis method used. Another previous report on wild prairie dogs5 relied on an automated chemistry instrument (Ektachem 700XR, Eastman Kodak, Rochester, NY) that uses a different analysis technology than the system we used in the present study. The age of the animals could be a factor, in that both previous reports4,5 used animals older than 1 y, whereas our prairie dogs were 6 mo old at the time of these baseline studies. In addition, some differences could be accounted for by a variety of other factors whose influence cannot be ascertained, including time of year53 and location50 of collection. Some variations in parameters including alkaline phosphatase, AST, and ALT might reflect differences in the prairie dogs’ diets: the wild animals were on a high-protein, high-fat, rat chow diet; the pet animals received a strictly herbivorous diet; and our animals were fed a high-fiber, supplemented diet of prairie dog chow. In general, glucose values are difficult to compare, because the time of day, fasting, stress, and diet can all affect this parameter. Many of these factors can influence hematologic parameters as well.
Alternative explanations for differences in hematology between the wild and pet prairie dogs previously reported4,5 and our study may include the following factors. Improvements in animal husbandry techniques lower animal stress and the incidence of infection, in turn resulting in lower WBC counts. The choice of assay machine may account for the low platelet counts seen in our animals: as the system used in our studies (Heska CBC-Diff Veterinary Hematology System) is based on impedance measurements which, according to the manufacturer, are known to result in low platelet counts. Decreased RBC values, coupled with the low hematocrit and hemoglobin (mean, 8 g/dL; median, 7 g/dL) values, might indicate anemia in our animals, which when coupled with normal MCV and MCHC values might indicate that this is a chronic anemia of unknown etiology. Because anemia seems unlikely—we have no other indication that our prairie dogs were anemic—our proposed explanation is that inappropriate sample handling or hemolysis due to difficult blood draws caused the low RBC values, but the cause cannot be completely determined with the available data. Differences in differential counts might reflect subjects from geographically distinct populations1 or to the assay technology used, because impedance is well known to be a poor method for obtaining differential counts, and manual counts were done in the previous reports.4,5 In short, comparison of these studies serves to remind researchers that careful control of factors that influence blood chemistry and hematology is vital to using prairie dogs as an animal model. In addition, veterinarians should be familiar with the various conditions that can affect blood chemistry and hematology to identify these factors in the medical history and prescribe therapeutics appropriately.
The mean body temperature of prairie dogs in our study (36.4 °C) is within the previously reported range of 35.3 to 39.0 °C.16,30 Our established range (34.4 to 38.2 °C) is slightly lower than the published range, perhaps reflecting different measurement methods (telemetry versus rectal) but more likely is due to the specificity of the current study, in that our animals were age-matched and housed identically in a climate-controlled setting that eliminated climate changes and seasonality, which are known to be temperature-influencing factors.3,30 In addition, our study used multiple measurements for multiple animals housed under the same conditions to pinpoint the population temperature range. Although the mean body temperature of prairie dogs differs from that of its close relatives, the ground squirrels, the range of excursion of individual body temperatures in prairie dogs (3.8 °C) is similar to previous reports on this species,30 European ground squirrels,21 13-lined ground squirrels,41,43 and Anatolian ground squirrels.26 This finding is consistent with previous reports that the variance in mean body temperature is greater for individual animals than for the population at large.44 Clinically, this result indicates the importance of multiple measurements to determine ‘normal’ body temperatures for an individual animal before using core body temperatures as a disease marker or treating a pet for fever. Our data establish the normal body temperature of the black-tailed prairie dog species as 36.2 to 36.6 °C. This information is useful for evaluating fever as a disease marker in these animals.
In the current study, the acrophase of body temperature occurred during the light cycle, an unexpected result for a diurnal animal such as prairie dogs. In previous studies with 13-lined and Richardson ground squirrels,42 the acrophase peak occurred 9 to 12 h after lights-on, but it occurred at 4 h after lights-on among our prairie dogs. However, the 13-lined and Richardson ground squirrels were on a 14:10-h cycle, whereas our animals were on a 12:12-h photoperiod, thus perhaps affecting timing of the acrophase. For most mammals that have been studied, the circadian rhythms of activity and body temperature are highly synchronized.44 However body temperature in humans rises prior to the increase in activity levels.44 In 13-lined ground squirrels and Richardson ground squirrels, these rhythms are synchronized and peak during the day,43 with the mean phase reference points for these animals occurring at the transition between the dark and light phases. This pattern is consistent with what we observed in the prairie dogs relative to body temperature but not activity. In our current study, the acrophase of activity for the prairie dog occurred during the dark cycle, timing that is more consistent with that for nocturnal animals. Lack of activity during the day may explain in part the lower amplitude of the daily body temperature in prairie dogs increase as well as its earlier peak. Our prairie dogs were fed ad libitum and singly housed, thus unable to express all the species-typical behaviors of colony housing, and these conditions may partially explain the odd activity patterns and body temperature circadian rhythms. Similar plasticity in activity rhythms between diurnal and nocturnal animals has been observed in golden spiny mice,7 Octodon degus,52 chacma baboons,15 and Psammomys obesus.39 Recent data on golden hamsters indicates that, in terms of activity, they are diurnal in nature and nocturnal in the laboratory.10 In addition, although our prairie dogs were maintained at a relatively constant temperature (70.4 ± 1.18 °F) and humidity (49.3% ± 9.1%), the effect of small fluctuations in room temperature or humidity variability on either body temperature or activity patterns is unknown. This comment serves as a reminder for caution in using activity or temperature circadian-rhythm data as a marker for disease in pets or research animals, because changes in behavior and housing conditions can affect these parameters. The core body temperature and activity patterns of our study population are slightly different quantitatively but not relatively with those seen in previous studies of black-tailed prairie dogs.13,30,31 Therefore, note that these values are specific for black-tailed prairie dogs housed and tested under the particular conditions of the current study and should not be extrapolated to free-ranging or differently housed prairie dogs.
In summary, this study establishes the first blood chemistry and hematology reference ranges for black-tailed prairie dogs in these housing conditions. These ranges are based on multiple measurements under controlled laboratory housing conditions and likely will prove useful for veterinarians and research scientists.
References
- 1.Asadi F, Rostami A, Asadian P, Pourkabir M. 2007. Serum biochemistry and hematology values and hemoglobin electrophoresis in Persian squirrels (Sciurus anomalus). Vet Clin Pathol 36:188–191 [DOI] [PubMed] [Google Scholar]
- 2.Avashia SB, Petersen JM, Lindley CM, Schriefer ME, Gage KL, Cetron M, DeMarcus TA, Kim DK, Buck J, Montenieri JA, Lowell JL, Antolin MF, Kosoy MY, Carter LG, Chu MC, Hendricks KA, Dennis DT, Kool JL. 2004. First reported prairie dog-to-human tularemia transmission, Texas, 2002. Emerg Infect Dis 10:483–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakko EB, Brown LN. 1967. Breeding biology of the white-tailed prairie dog, Cynomys leucurus, in Wyoming. J Mammal 48:100–112 [PubMed] [Google Scholar]
- 4.Biggs CD. 2007. Establishing genetic and physiological baselines for the black-tailed prairie dog (Cynomys ludovicianus). [Dissertation]. Denton (TX): University of North Texas [Google Scholar]
- 5.Broughton G., 2nd 1992. Hematologic and blood chemistry data for the prairie dog (Cynomys ludovicianus). Comp Biochem Physiol Comp Physiol 101:807–812 [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention, Food and Drug Administration 2003. Control of communicable diseases; restrictions on African rodents, prairie dogs, and certain other animals. Interim final rule. Fed Regist 68:62353–62369 [PubMed] [Google Scholar]
- 7.Cohen R, Smale L, Kronfeld-Schor N. 2009. Plasticity of circadian activity and body temperature rhythms in golden spiny mice. Chronobiol Int 26:430–446 [DOI] [PubMed] [Google Scholar]
- 8.Dimarco NM, Dart L, Sanborn CB. 2007. Modified activity-stress paradigm in an animal model of the female athlete triad. J Appl Physiol 103:1469–1478 [DOI] [PubMed] [Google Scholar]
- 9.Garner MM, Raymond JT, Toshkov I, Tennant BC. 2004. Hepatocellular carcinoma in black-tailed prairie dogs (Cynomys ludivicianus): tumor morphology and immunohistochemistry for hepadnavirus core and surface antigens. Vet Pathol 41:353–361 [DOI] [PubMed] [Google Scholar]
- 10.Gattermann R, Johnston RE, Yigit N, Fritzsche P, Larimer S, Ozkurt S, Neumann K, Song Z, Colak E, Johnston J, McPhee ME. 2008. Golden hamsters are nocturnal in captivity but diurnal in nature. Biol Lett 4:253–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harkin A, O'Donnell JM, Kelly JP. 2002. A study of VitalView for behavioural and physiological monitoring in laboratory rats. Physiol Behav 77:65–77 [DOI] [PubMed] [Google Scholar]
- 12.Harlow HJ, Braun EJ. 1995. Kidney structure and function of obligate and facultative hibernators: the white-tailed prairie dog (Cynomys leucurus) and the black-tailed prairie dog (Cynomys ludovicianus). J Comp Physiol B 165:320–328 [DOI] [PubMed] [Google Scholar]
- 13.Harlow HJ, Frank CL. 2001. The role of dietary fatty acids in the evolution of spontaneous and facultative hibernation patterns in prairie dogs. J Comp Physiol B 171:77–84 [DOI] [PubMed] [Google Scholar]
- 14.Hebert J, Lust A, Fuller A, Maloney SK, Mitchell D, Mitchell G. 2008. Thermoregulation in pronghorn antelope (Antilocapra americana, Ord) in winter. J Exp Biol 211:749–756 [DOI] [PubMed] [Google Scholar]
- 15.Hill RA. 2006. Why be diurnal? Or why not be cathemeral? Folia Primatol 77:72–86 [DOI] [PubMed] [Google Scholar]
- 16.Hillyer EV, Quesenberry KE. 1997. Ferrets, rabbits, and rodents: clinical medicine and surgery. Philadelphia (PA): WB Saunders [Google Scholar]
- 17.Hoogland JL. 1995. The black-tailed prairie dog: social life of a burrowing mammal. Chicago (IL): University of Chicago Press [Google Scholar]
- 18.Hoogland JL. 2003. Sexual dimorphism of prairie dogs. J Mammal 84:1254–1266 [Google Scholar]
- 19.Hoogland JL. 2006. Conservation of the black-tailed prairie dog: saving North America's western grasslands. Washington (DC): Island Press [Google Scholar]
- 20.Hoogland JL, James DA, Watson L. 2009. Nutrition, care, and behavior of captive prairie dogs. Vet Clin North Am Exot Anim Pract 12:255–266, viii [DOI] [PubMed] [Google Scholar]
- 21.Hut RA, Barnes BM, Daan S. 2002. Body temperature patterns before, during, and after semi-natural hibernation in the European ground squirrel. J Comp Physiol B 172:47–58 [DOI] [PubMed] [Google Scholar]
- 22.Hutson CL, Olson VA, Carroll DS, Abel JA, Hughes CM, Braden ZH, Weiss S, Self J, Osorio JE, Hudson PN, Dillon M, Karem KL, Damon IK, Regnery RL. 2009. A prairie-dog animal model of systemic orthopoxvirus disease using West African and Congo Basin strains of monkeypox virus. J Gen Virol 90:323–333 [DOI] [PubMed] [Google Scholar]
- 23.Jekl V, Hauptman K, Jeklova E, Knotek Z. 2006. Demodicosis in 9 prairie dogs (Cynomys ludovicianus). Vet Dermatol 17:280–283 [DOI] [PubMed] [Google Scholar]
- 24.Johnson-Delaney CA. 2006. Common procedures in hedgehogs, prairie dogs, exotic rodents, and companion marsupials. Vet Clin North Am Exot Anim Pract 9:415–435, viii [DOI] [PubMed] [Google Scholar]
- 25.Karem KL, Reynolds M, Braden Z, Lou G, Bernard N, Patton J, Damon IK. 2005. characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab Immunol 12:867–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kart Gur M, Refinetti R, Gur H. 2009. Daily rhythmicity and hibernation in the Anatolian ground squirrel under natural and laboratory conditions. J Comp Physiol B 179:155–164 [DOI] [PubMed] [Google Scholar]
- 27.Kartman L, Quan SF, Lechleitner RR. 1962. Die-off of a Gunnison's prairie dog colony in central Colorado. II. Retrospective determination of plague infection in flea vectors, rodents, and man. Zoonoses Res 1:201–224 [PubMed] [Google Scholar]
- 28.Kugeler KJ, Pappert R, Zhou Y, Petersen JM. 2006. Real-time PCR for Francisella tularensis types A and B. Emerg Infect Dis 12:1799–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.La Regina M, Lonigro J, Wallace M. 1986. Francisella tularensis infection in captive, wild-caught prairie dogs. Lab Anim Sci 36:178–180 [PubMed] [Google Scholar]
- 30.Lehmer EM, Bossenbroek JM, Van Horne B. 2003. The influence of environment, sex, and innate timing mechanisms on body temperature patterns of free-ranging black-tailed prairie dogs (Cynomys ludovicianus). Physiol Biochem Zool 76:72–83 [DOI] [PubMed] [Google Scholar]
- 31.Lehmer EM, Savage LT, Antolin MF, Biggins DE. 2006. Extreme plasticity in thermoregulatory behaviors of free-ranging black-tailed prairie dogs. Physiol Biochem Zool 79:454–467 [DOI] [PubMed] [Google Scholar]
- 32.Leighton FA, Artsob HA, Chu MC, Olson JG. 2001. A serological survey of rural dogs and cats on the southwestern Canadian prairie for zoonotic pathogens. Can J Public Health 92:67–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lennox AM. 2007. Emergency and critical care procedures in sugar gliders (Petaurus breviceps), African hedgehogs (Atelerix albiventris), and prairie dogs (Cynomys spp). Vet Clin North Am Exot Anim Pract 10:533–555 [DOI] [PubMed] [Google Scholar]
- 34.Mertens A, Stiedl O, Steinlechner S, Meyer M. 2008. Cardiac dynamics during daily torpor in the Djungarian hamster (Phodopus sungorus). Am J Physiol 294:R639–R650 [DOI] [PubMed] [Google Scholar]
- 35.Meyer PD, Denbesten L. 1977. A comparison of methods for bile salt pool size measurements in the prairie-dog gallstone model. Proc Soc Exp Biol Med 156:452–456 [DOI] [PubMed] [Google Scholar]
- 36.Moser AJ, Gangopadhyay A, Bradbury NA, Peters KW, Frizzell RA, Bridges RJ. 2007. Electrogenic bicarbonate secretion by prairie dog gallbladder. Am J Physiol Gastrointest Liver Physiol 292:G1683–G1694 [DOI] [PubMed] [Google Scholar]
- 37.Muller EL, Pitt HA, George WL. 1987. Prairie dog model for antimicrobial agent-induced Clostridium difficile diarrhea. Infect Immun 55:198–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ness RD. 1999. Clinical pathology and sample collection of exotic small mammals. Vet Clin North Am Exot Anim Pract 2:591–620, vi [DOI] [PubMed] [Google Scholar]
- 39.Neuman A, Gothilf Y, Haim A, Ben-Aharon G, Zisapel N. 2005. Nocturnal patterns and upregulated excretion of the melatonin metabolite 6-sulfatoxymelatonin in the diurnal rodent Psammomys obesus postweaning under a short photoperiod. Comp Biochem Physiol A Mol Integr Physiol 142:297–307 [DOI] [PubMed] [Google Scholar]
- 40.Reeves WK, Rogers TE, Dasch GA. 2007. Bartonella and Rickettsia from fleas (Siphonaptera: Ceratophyllidae) of prairie dogs (Cynomys spp.) from the western United States. J Parasitol 93:953–955 [DOI] [PubMed] [Google Scholar]
- 41.Refinetti R. 1995. Body temperature and behaviour of golden hamsters (Mesocricetus auratus) and ground squirrels (Spermophilus tridecemlineatus) in a thermal gradient. J Comp Physiol [A] 177:701–705 [DOI] [PubMed] [Google Scholar]
- 42.Refinetti R. 1996. Comparison of the body temperature rhythms of diurnal and nocturnal rodents. J Exp Zool 275:67–70 [DOI] [PubMed] [Google Scholar]
- 43.Refinetti R. 1999. Relationship between the daily rhythms of locomotor activity and body temperature in 8 mammalian species. Am J Physiol 277:R1493–R1500 [DOI] [PubMed] [Google Scholar]
- 44.Refinetti R. 2006. Variability of diurnality in laboratory rodents. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 192:701–714 [DOI] [PubMed] [Google Scholar]
- 45.Roomiany PL, Axtell RC, Scalarone GM. 2002. Comparison of 7 Blastomyces dermatitidis antigens for the detection of antibodies in humans with occupationally acquired blastomycosis. Mycoses 45:282–286 [DOI] [PubMed] [Google Scholar]
- 46.Sackal C, Laudisoit A, Kosoy M, Massung R, Eremeeva ME, Karpathy SE, Van Wyk K, Gabitzsch E, Zeidner NS. 2008. Bartonella spp. and Rickettsia felis in fleas, Democratic Republic of Congo. Emerg Infect Dis 14:1972–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Serkova NJ, Rose JC, Epperson LE, Carey HV, Martin SL. 2007. Quantitative analysis of liver metabolites in 3 stages of the circannual hibernation cycle in 13-lined ground squirrels by NMR. Physiol Genomics 31:15–24 [DOI] [PubMed] [Google Scholar]
- 48.Snall T, O'Hara RB, Ray C, Collinge SK. 2008. Climate-driven spatial dynamics of plague among prairie dog colonies. Am Nat 171:238–248 [DOI] [PubMed] [Google Scholar]
- 49.Stevenson HL, Bai Y, Kosoy MY, Montenieri JA, Lowell JL, Chu MC, Gage KL. 2003. Detection of novel Bartonella strains and Yersinia pestis in prairie dogs and their fleas (Siphonaptera: Ceratophyllidae and Pulicidae) using multiplex polymerase chain reaction. J Med Entomol 40:329–337 [DOI] [PubMed] [Google Scholar]
- 50.Tryland M. 2006. ‘Normal’ serum chemistry values in wild animals. Vet Rec 158:211–212 [DOI] [PubMed] [Google Scholar]
- 51.Varela G, Vazquez A. 1954. [Finding of sylvatic plague in the Republic of Mexico; natural infection of Cynomys mexicanus (prairie dogs) with Pasteurella pestis]. Rev Inst Salubr Enferm Trop 14:219–223 [Article in Spanish] [PubMed] [Google Scholar]
- 52.Vivanco P, Rol MA, Madrid JA. 2009. Two steady-entrainment phases and graded masking effects by light generate different circadian chronotypes in Octodon degus. Chronobiol Int 26:219–241 [DOI] [PubMed] [Google Scholar]
- 53.Yokus B, Cakir DU, Kanay Z, Gulten T, Uysal E. 2006. Effects of seasonal and physiological variations on the serum chemistry, vitamins, and thyroid hormone concentrations in sheep. J Vet Med A Physiol Pathol Clin Med 53:271–276 [DOI] [PubMed] [Google Scholar]