Abstract
Seasonal changes in menstrual cycle patterns and internal reproductive organs were studied in female rhesus macaques (n = 16) in indoor–outdoor housing in Chongqing, China. Uterine size and shape and endometrial thickening were evaluated during the early-secretory phase by using ultrasonography and MRI. From October to February, the macaques’ menstrual cycles were short and regular, and the endometrial lining were easily visible by ultrasonography and MRI. However, from March through to September, menstrual cycles became irregular, endometrial lining were unclear, and the endometrium did not change markedly during the early-secretory phase. We conclude that the reproductive season for a female rhesus macaque in Chongqing, China is from October through February, whereas the nonreproductive season is from March through September. The menstrual cycle patterns and reproductive organs of the macaques showed marked seasonal variation throughout the 12-mo observation period. In addition, uterine size, volume, and imaging characteristics varied dramatically between reproductive and nonreproductive seasons.
Rhesus macaques (Macaca mulatta) share so many biologic characteristics with humans that these animals are excellent models for studying human health and disease.9,13 In particular, the reproductive anatomy and menstrual cycles of female rhesus macaques are similar to those of women. As research in the areas of reproductive physiology and fertility expands, the study of female rhesus macaques will increase over time.4,5 Therefore, background information regarding the reproductive physiology of female rhesus macaques needs to be established.
Ovarian growth and maturity, ovulation, and the formation and recession of corpora lutea cause periodic changes in the endometrium of women and macaques. Seasonal features of the reproductive physiology of rhesus macaques are closely related to climate and environmental factors.15 Chongqing Municipality in Western China experiences marked climate changes. During summer (June through September), the average temperature is 36 to 40 °C, which causes the unique menstrual physiology of our study rhesus monkeys during the nonreproductive season. Characterizing these changes is one of the purposes of the current research.
Here we use MRI and ultrasonography to describe the seasonal effects on menstrual cycle patterns and internal reproductive organs of female rhesus macaques in indoor–outdoor captive housing.
Materials and Methods
Animals.
We obtained 16 female rhesus macaques from the Laboratory Monkey Base (Medical Ultrasound Engineering Institute, Chongqing Medical University, China). All rhesus were healthy, of childbearing age (6.2 ± 1.7 y), and weighed 7.1 ± 1.2 kg. Each primate enclosure consists of an 8-m2 indoor section and a 10-m2 outdoor section with perches and an activity area to which the primates had continuous access year round. Each enclosure housed 3 macaques, in accordance with the Monkey Feeding Standards of Chongqing Zoo. The protocol was approved by The Ethics Committee at Chongqing Medical University.
Animal records were completed for each of the 16 macaques. Signs of menstruation were monitored for 12 consecutive months and included detailed observations of the rhesus macaque's vulvar appearance, the presence of menstrual blood, and any skin changes during the cycles. Twice-daily (morning and evening) observation for vaginal bleeding in the perineum began 5 d prior to the anticipated next menstruation, based on the individual menstrual cycle history. In addition urine samples were collected every 2 d and examined under the microscope for the presence of red blood cells. The menstrual cycle of the rhesus monkeys was defined from the first day of menses to 1 d before menses of the next menstrual period.2,10
Equipment and pharmaceuticals.
For ultrasonography, we used an HP Sonos 5500 (Philips, Surrey, UK) machine with a 3.5-MHz (model L5040, Philips) or 3.7-MHz (model T5012, Philips) probe. For MRI, a 1.5-T Signa Excite HiSpeed Plus (General Electric, Waukesha, WI) was used. The MRI contrast agent (0.5 mmol/mL; batch number, 10116527; Omniscan) was produced by Nycomed (Dublin, Ireland). Ketamine (100 mg/mL; batch number, 011013) was obtained from Lianyungang Pharmaceutical Company (Jiangsu, China).
Ultrasound examination.
For ultrasound examination, each animal was sedated with ketamine hydrochloride (6 mg/kg) once during the 15th to 20th day of each rhesus macaque's menstrual cycle. The abdominal skin was prepared routinely by clipping the hair and degreasing with 75% ethanol. Each animal was restrained in the supine position, exposing the entire abdomen, and a urinary catheter is inserted through the urethral opening. The bladder either was filled with approximately 20 mL saline or urine was released to maintain sufficient fluid to displace the intestines, which can hinder ultrasound examination of the uterus. Ultrasonography was conducted to assess the uterus, endometrial lining, myometrium, and uterine length (overall and uterine body), width, and thickness. The uterine volume was calculated according to the formula for ellipsoids:
where A is uterine length, B is uterine width, and C is uterine thickness.
Examination with MRI.
After the ultrasound examination, MRI scanning followed by contrast-enhanced imaging after intravascular injection of 5 mL Omniscan was performed. During each menstrual cycle, if no menses were observed in the perineum at the same time point as the previous menstruation, MRI was conducted every 7 d until menses was observed. MR signals from the uterus of rhesus macaques were observed in axial and sagittal views, from which the uterine volume was calculated.
Statistical analysis.
Measurements from ultrasound images and MRI were analyzed for linear correlation before a t test was used to assess between-group differences. Differences in uterine diameter obtained by MRI and ultrasonography were calculated by using statistical analysis software (SPSS version 10.0, SPSS, Chicago, IL). Statistical significance was defined as a P value less than 0.05.
Results
Physical observation.
We defined the menstrual cycle of rhesus monkeys as the first day of menses until 1 d before menses of the next menstrual period. The day on which vaginal bleeding was first discovered was considered the first day of the menstrual cycle. The menstrual period comprised the days on which vaginal bleeding occurred.
The observations we recorded for each animal revealed that seasonal sexual skin changes of female rhesus monkey were most obvious from October through February. During the proliferative phase, 80% of female rhesus monkeys began to show skin redness, which gradually spread in area and intensified in color through the early-secretory phase. During the secretory phase, 90% of rhesus monkeys showed skin redness accompanied with marked sexual skin edema. From March through September, no cyclic skin changes were observed.
During the year of observation and data collection, only 52 menstrual cycles were observed, due to polymenorrhea and oligomenorrhea in some of the animals. The observed menstrual cycles were divided into 4 time periods for statistical analysis (Table 1). The 18 cycles during March through September accounted for 34.62% of the 52 observed menstrual cycles, whereas the 34 cycles that occurred during October through February accounted for 65.38%. From October through February, the menstrual cycle was short and regular, whereas it was irregular during March through September, when 13 of the 18 cycles that occurred during this period exceeded 51 d.
Table 1.
Characteristics of 52 menstrual cycles and periods
| Duration of menstrual cycle (d) | Mar through May |
Jun through Sep |
Oct through Dec |
Jan through Feb |
||||||||
| Cycles (n) | Menstrual period (d) | % | Cycles (n) | Menstrual period (d) | % | Cycles (n) | Menstrual period (d) | % | Cycles (n) | Menstrual period (d) | % | |
| 19–23 | 0 | 0 | 0 | 0 | 4 | 3–4 | 7.69 | 2 | 1–3 | 3.85 | ||
| 24–31 | 0 | 0 | 0 | 0 | 12 | 3–7 | 23.08 | 7 | 1–6 | 13.46 | ||
| 32–40 | 1 | 4 | 1.92 | 0 | 0 | 3 | 2–4 | 5.77 | 3 | 1–2 | 5.77 | |
| 41–50 | 3 | 5–6 | 5.77 | 1 | 8 | 1.92 | 0 | 0 | 3 | 5–7 | 5.77 | |
| 51–60 | 1 | 6 | 1.92 | 3 | 7–9 | 5.77 | 0 | 0 | 0 | 0 | ||
| >60 | 3 | 9–10 | 5.77 | 6 | 11.54 | 0 | 0 | 0 | 0 | |||
| Total | 8 | 4–10 | 15.38 | 10 | 7–9 | 19.23 | 19 | 2–7 | 36.54 | 15 | 1–7 | 28.85 |
Calculation of the 95% confidence interval (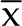 ± 2 SD) of 34 menstrual cycles of rhesus monkeys in indoor–outdoor housing from October to February indicated that their normal menstrual cycle is 28 ± 8.26 d. Correspondingly, their menstrual period lasted 1 to 7 d. We further analyzed the features of the subgroup of 19 menstrual cycles (36.54% of the total) that were 24 to 31 d long during October through February (Table 2).
± 2 SD) of 34 menstrual cycles of rhesus monkeys in indoor–outdoor housing from October to February indicated that their normal menstrual cycle is 28 ± 8.26 d. Correspondingly, their menstrual period lasted 1 to 7 d. We further analyzed the features of the subgroup of 19 menstrual cycles (36.54% of the total) that were 24 to 31 d long during October through February (Table 2).
Table 2.
Characteristics of a subset of 19 menstrual cycles and periods
| Menstrual cycle |
Menstrual period |
|||||||||
| Duration of menstrual cycle (d) | 1–2 d |
3–4 d |
5–6 d |
7 d |
||||||
| Cycles (n) | % | n | % | n | % | n | % | n | % | |
| 24 | 3 | 15.79 | 2 | 10.53 | 1 | 5.26 | ||||
| 25 | 1 | 5.26 | 1 | 5.26 | ||||||
| 27 | 1 | 5.26 | 1 | 5.26 | ||||||
| 28 | 5 | 26.32 | 2 | 10.53 | 2 | 0.53 | 1 | 5.26 | ||
| 29 | 3 | 15.79 | 1 | 5.26 | 2 | 10.53 | ||||
| 30 | 5 | 26.32 | 2 | 10.53 | 3 | 15.79 | ||||
| 31 | 1 | 5.26 | 1 | 5.26 | ||||||
| Total | 19 | 100.00 | 3 | 5.79 | 9 | 47.38 | 6 | 1.57 | 1 | 5.26 |
Our observations revealed that our study population of rhesus macaques had menses year round, and features of both the menstrual cycle and period varied markedly by season. Specifically, the menstrual cycle was short and regular from October through February and irregular from March through September. The shortest period was 19 d, and the longest was greater than 60 d. These observations indicated that the reproductive season for female rhesus monkeys held in indoor–outdoor housing is October through February, whereas March through September is the nonreproductive season.
Ultrasonography.
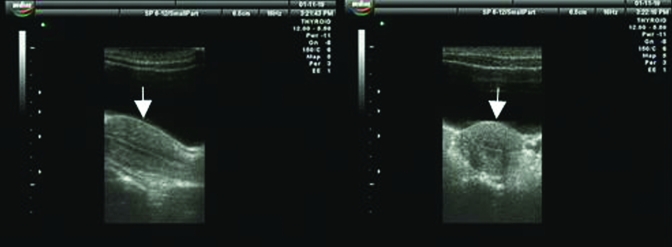
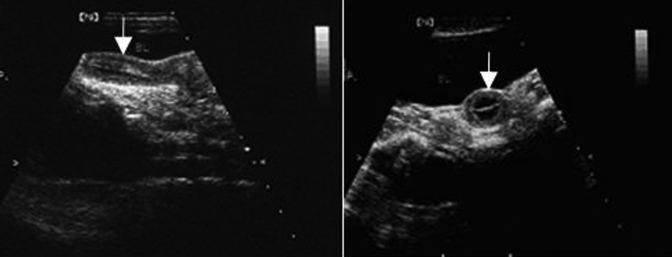
From March through September, the uterus and cervix of our female rhesus macaques demonstrated the characteristic ‘dumbbell’ ultrasound profile of a simplex uterus. In the sagittal plane, the cervix appeared long and thick, and the endometrium was thin, with a visible endometrial lining. Ultrasonography of our macaques from October through February showed that despite a marked increase in the volume of the uterus, the cervix was relatively unchanged when compared with images collected from March through September. In longitudinal section, the uterus was shaped like an inverted pear (Figure 1), whereas in transverse section, it was triangular, with a well-defined border. The endometrium showed various periodic changes, including hyperplasia and increased secretion. During the secretory period, the endometrium became thickest, at approximately 5.5 mm. Ovulation was estimated to occur between days 15 through 20 of the menstrual cycle, and ultrasound images taken at this stage during both reproductive and nonreproductive seasons are shown in Figure 2. Ultrasonographically, the average dimensions of the uterus were 4.75 cm × 2.53 cm × 1.78 cm during the reproductive season and 2.76 cm × 1.52 cm × 1.28 cm during the nonreproductive season (Table 3).
Figure 1.
Ultrasonography of the rhesus macaque uterus during the reproductive season. Left, longitudinal section; right, transverse section; uterine body indicated by white arrow. Figure 1A adapted and reprinted with permission from reference 6.
Figure 2.
Ultrasonography of the rhesus macaque uterus during the nonreproductive season. Left, longitudinal section; right, transverse section; uterine body indicated by white arrow.
Table 3.
Uterine measurements (mean ± SEM) during reproductive and nonreproductive seasons
| Ultrasonography (n = 16) |
MRI (n = 16) |
|||
| Reproductive | Nonreproductive | Reproductive | Nonreproductive | |
| Diameter, cm | 4.75 ± 0.15 | 2.76 ± 0.08 | 4.9 ± 0.05 | 3.0 ± 0.06 |
| Length, cm | 3.50 ± 0.02 | 1.76 ± 0.02 | 3.3 ± 0.04 | 1.8 ± 0.03 |
| Width, cm | 2.53 ± 0.05 | 1.52 ± 0.04 | 2.6 ± 0.05 | 1.76 ± 0.02 |
| Thickness, cm | 1.78 ± 0.02 | 1.28 ± 0.03 | 1.9 ± 0.01 | 1.32 ± 0.01 |
| Volume, cm3 | 11.93 ± 0.50 | 2.81 ± 0.02 | 12.66 ± 0.02 | 3.64 ± 0.02 |
Paired comparison of values from ultrasonography and MRI revealed no differences (P > 0.05)
MRI.
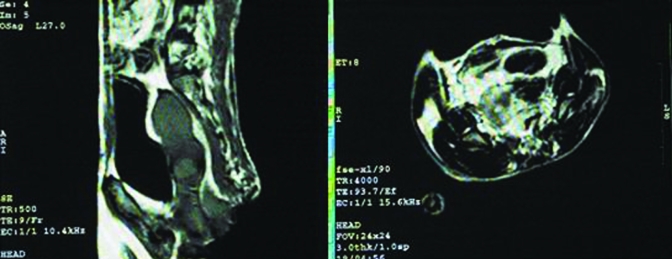
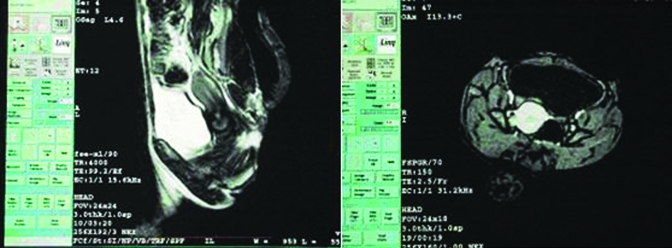
Routine MRI scanning and contrast-enhanced images revealed uterine changes during the reproductive and nonreproductive seasons that were consistent with those observed through ultrasound examination. Specifically, a hollow was present between the body of the uterus and cervix. In sagittal T2-weighted imaging, signals from the myometrium were higher than those of the cervix, and the transition zone between them represented their junction. In T1-weighted imaging, the signals from every structural layer in the body of uterus were uniform and similar to those of striated muscle in the pelvic cavity. In contrast, T2-weighted imaging revealed 2 or 3 layers of different signal intensity in the body of the uterus, whereas the signal intensity of the uterine cavity was lower, and the myometrium and endometrium were isointense. MRI at ovulation (day 15 to 20) during the reproductive (Figure 3) and nonreproductive (Figure 4) seasons revealed that the average dimensions of the uterus were 4.9 cm × 2.6 cm × 1.9 cm and 3.0 cm × 1.76 cm × 1.32 cm, respectively (Table 3). Uterine dimensions measured by ultrasonography and MRI did not differ significantly (P > 0.05) but showed a linear correlation (r = 0.866) with uterine volume.
Figure 3.
MRI of the rhesus macaque uterus during the reproductive season. SE sequence, T1-weighted imaging. Left, sagittal view; right, axial view; uterine body indicated by white arrow. Figure 3A adapted and reprinted with permission from reference 6.
Figure 4.
MRI of the rhesus macaque uterus during the nonreproductive season. SE sequence, T2-weighted imaging. Left, sagittal view; right, axial view; uterine body indicated by white arrow. Intravenous contrast agents were used in to better visualize changes in uterine morphology and size.
Discussion
The phases of the menstrual cycle of rhesus macaques reflect the periods of ovarian growth and maturity, ovulation, and formation and recession of corpora lutea and their associated cyclic changes in the endometrium.3,17 These physiologic activities occur repeatedly throughout the life of rhesus macaques until menopause. In the wild, the seasonal nature of the menstrual cycle is subject to the environmental climate as well as the internal hormone levels of the individual animal. Cessation of menstruation can be caused by poor nutrition, hormonal changes, and other factors.16
Data from the present study indicated that the normal menstrual cycle of rhesus macaque in indoor–outdoor housing in the Chongqing area of China is about 28 d. This duration is in accord with previously published data on macaques in Japan.12
Fluctuation of the estrogen level in rhesus macaque lead to marked periodic swelling in the perineum and hip area.1 Observation of individual animals revealed that that the seasonal sexual skin changes of female rhesus macaques were most obvious during October through February; discernable cyclic skin changes did not occur from March through September. If synchronized with the seasonal changes in their menstrual cycles, these skin changes may play an important role during courtship in rhesus macaques.
The anatomy, physiology, biochemistry, and metabolism of rhesus macaques are quite similar to those of humans, making these nonhuman primates excellent animal models for medical and biologic research.8 Here we used MRI and ultrasonography to study well-known characteristics of the uterus of rhesus macaques during various seasons to provide baseline information for future research applications of this animal model.6 We confirmed that the uterus rhesus macaques is shaped like an inverted pear on MRI and sonograms during the reproductive season but like a dumbbell during the nonreproductive season. Uterine dimensions did not differ according to imaging modality but were linearly correlated (r = 0.866) with uterine volume. The increase in the uterus volume during the reproductive season is related to fluctuation of hormone levels.11,14 Also during the reproductive season, female rhesus macaques show sexual dermatologic reactions, including blushing and swelling of the vulva and abdominal skin, and animals that manifested these signs were more sexually active, shown by their increased frequency of mating, than were macaques that did not.17 The combination of these signs implies a high level of sex hormones. Ultrasonographically, the body of the uterus yielded a homogeneous echo whereas the cervical echo was uneven; this difference may reflect the different imaging characteristics of the various tissues and structures comprising the uterine body and cervix.
The cervix and body of the uterus could be distinguished clearly by MRI scanning in the sagittal view. T2-weighted imaging identified 2 of 3 bands of different signal intensity in the body of uterus. The high-intensity signals primarily were associated with endometrium and secretory mucus, and the thickness of these signal bands changed throughout the menstrual cycle. The middle of the uterine wall yielded a low-intensity band, representing the endometrial–myometrial junction. The outermost band consisted of moderate-intensity MR signal, corresponding to the exterior two-thirds of the myometrium. These findings are consistent with MRI imaging characteristics of normal human uteri.8
Our results indicate that the reproductive season of female rhesus macaques in indoor–outdoor captive housing in Chongqing occurs from October through February. Uterine size, volume, and imaging characteristics in individual animals varied dramatically between the reproductive and nonreproductive seasons. However, uterine size and volume did not differ depending on imaging modality, and either ultrasonography or MRI could be used to monitor the uterus of rhesus macaques.
Acknowledgments
This research was supported by the Natural Science Foundation Project of CQ CSTC (grant no. 2009BB5256) and the Natural Science Foundation of China (grant no. 30770816, 30470654).
References
- 1.Baulu J. 1976. Seasonal sex skin coloration and hormonal fluctuations in free-ranging and captive monkeys. Horm Behav 7:481–494 [DOI] [PubMed] [Google Scholar]
- 2.Cao W, Mah K, Carroll RS, Slayden OD, Brenner RM. 2007. Progesterone withdrawal upregulates fibronectin and integrins during menstruation and repair in the rhesus macaque endometrium. Hum Reprod 22:3223–3231 [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Zhou D, Liu Y, Peng J, Li C, Chen W, Wang Z. 2008. A comparison between ultrasound therapy and laser therapy for symptomatic cervical ectopy. Ultrasound Med Biol 34:1770–1774 [DOI] [PubMed] [Google Scholar]
- 4.D'Hooghe TM, Kyama CM, Chai D, Fassbender A, Vodolazkaia A, Bokor A, Mwenda JM. 2009. Nonhuman primate models for translational research in endometriosis. Reprod Sci 16:152–161 [DOI] [PubMed] [Google Scholar]
- 5.Downs JL, Urbanski HF. 2006. Neuroendocrine changes in the aging reproductive axis of female rhesus macaques (Macaca mulatta). Biol Reprod 75:539–546 [DOI] [PubMed] [Google Scholar]
- 6.Du YH, Xiong ZA, Tan Y, Wang ZB. 2004. Influence of early pregnancy termination by focused ultrasound beams on menstrual recovery of macaques. J Reprod Contracept 15:87–94 [Google Scholar]
- 7.Fong YF, Singh K. 1999. Effect of the levonorgestrel-releasing intrauterine system on uterine myomas in a renal transplant patient. Contraception 60:51–53 [DOI] [PubMed] [Google Scholar]
- 8.Hu HP, Chen SL. 1997. MRI diagnosis of the pelvis, p 33–35 Beijing (China): Military Medical Science Press [Google Scholar]
- 9.Liu ZH, Wang SH, Chen Y, Chen BJ. 1996. [Development and identification of monoclonal antibodies for antihuman trophoblast cells]. Cell Mol Immunol Mag 12:59–60 Article in Chinese [Google Scholar]
- 10.Maginnis G, Wilk J, Carroll R, Slayden OD. 2008. Assessment of progestin-only therapy for endometriosis in macaques. J Med Primatol 37 Suppl 1:52–55 [DOI] [PubMed] [Google Scholar]
- 11.Morgan PM, Hutz RJ, Kraus EM, Bavister BD. 1987. Ultrasonographic assessment of the endometrium in rhesus monkeys during the normal menstrual cycle. Biol Reprod 36:463–469 [DOI] [PubMed] [Google Scholar]
- 12.Nigi H. 1975. Menstrual cycle and some other related aspects of Japanese monkeys (Macaca fuscata). Primates 16:207–216 [Google Scholar]
- 13.Otto LN, Slayden OD, Clark AL, Brenner RM. 2002. The rhesus macaque as an animal model for pelvic organ prolapse. Am J Obstet Gynecol 186:416–421 [DOI] [PubMed] [Google Scholar]
- 14.Piiroinen O, Kaihola HL. 1975. Uterine size measured by ultrasound during the menstrual cycle. Acta Obstet Gynecol Scand 54:247–250 [DOI] [PubMed] [Google Scholar]
- 15.Walker ML, Gordon TP, Wilson ME. 1983. Menstrual cycle characteristics of seasonally breeding rhesus monkeys. Biol Reprod 29:841–848 [DOI] [PubMed] [Google Scholar]
- 16.Whitney RA, Wickings EJ. 1987. Macaques and other old world simians, p 599–600 : Poole T. The UFAW handbook on the care and management of laboratory animals, 6th ed. Harlow (UK): Longman Scientific and Technical [Google Scholar]
- 17.Zehr JL, Van Meter PE, Wallen K. 2005. Factors regulating the timing of puberty onset in female rhesus monkeys (Macaca mulatta): role of prenatal androgens, social rank, and adolescent body weight. Biol Reprod 72:1087–1094 [DOI] [PubMed] [Google Scholar]