Abstract
Audiogenic stress is a well-documented phenomenon in laboratory rodents. Despite the recommendation in the Guide for the Care and Use of Laboratory Animals to consider noise a concern in the animal facility, only a small body of literature empirically addresses the effects of facility noise on laboratory rodents, particularly mice. The objective of this study was to determine whether facility noise generated by a vacuum cleaner induces an acute stress response in a commonly used strain of laboratory mouse under common housing conditions. In each of 2 experiments, 10 young adult, female C57BL/6Cr mice were exposed for 1 h to noise produced by a vacuum cleaner, and 10 control mice were not. In the first experiment, fecal samples were collected to measure concentrations of fecal corticosterone metabolites just before and 2, 4, 6, 8, 10, 14, 24, and 32 h after noise exposure. In the second experiment, stress-sensitive behavioral tests were performed 2 d before, immediately after, and 24 h after noise exposure. Physiologic and behavioral measurements indicated that vacuum cleaner noise did not cause an acute stress response in the noise-exposed mice but may have affected the diurnal variation of their corticosterone levels. These findings could contribute to the development of best practices in noise-control protocols for animal facilities.
Abbreviation: ACTH, adrenocorticotropic hormone; EIA, enzyme immunoassay; FCM, fecal corticosterone metabolites; HPA, hypothalamic–pituitary–adrenal
Laboratory rodents are exposed to a number of environmental factors that originate from both inside and outside of their cages, including cage mates, odors, light, relative humidity, and temperature. Noise in the animal facility is one such factor—it can vary from day to day, and at some level is unavoidable.26,43,53 Audiogenic stress is a recognized phenomenon.52 Experimental noise exposure has been used extensively as a psychogenic stressor in rodent models.52 Effects of audiogenic stress include unfavorable reproductive outcomes,9,20,36,38 changes in cardiovascular and immune parameters,2,7,54,58 and enhanced disease development.1,27 In addition, noise has been documented to increase plasma corticosterone levels in laboratory rodents.3,10,24,28,53 The noise produced in many of these experiments is usually specifically targeted, very loud, and prolonged. Studies examining the effects of less-intense noise characteristic of that produced daily in an animal facility are considerably scarcer.41,52
Animal facility personnel make great efforts to control environmental factors. Food, lighting, temperature, relative humidity, and water supplies are often strictly controlled, but less consideration is given to the acoustic environment.53 Despite this lack of attention, members of the laboratory animal science community have been concerned about the potential effect of noise in animal facilities for decades.43,47 Anecdotal evidence and a small body of empirical research indicate that many noises in and around animal facilities are likely to cause measurable stress in laboratory rodents.5,16,37,41 For example, nearby construction and maintenance have been implicated in facilities experiencing poor breeding performance41 and the precipitous and confounding drop in appetite and weight in a growing rat.16 The noise produced by vacuuming was shown to exceed the hearing threshold for young mice of 2 common strains, suggesting that this activity could be a source of stress to these mice;37 but the behavioral and biologic significance of this finding was not explored. Blood vessels supplying the intestines of rats chronically exposed to noise similar to that produced by poorly maintained machinery were significantly more permeable than those of control rats.5 Several authors have recognized the paucity of research addressing the effects of nonexperimental noise on laboratory animals.46,52,56 Laboratory mice are particularly poorly represented given their prevalence as research animals, and measures taken to control facility noise in their environment might be based on assumptions rather than evidence.
Measuring physiologic stress responses in laboratory rodents, particularly mice, is challenging. Standard methods of blood collection to quantify serum or plasma glucocorticoids are limited in these animals, because the collection methods themselves are significant stressors, and the animals’ small size does not allow for frequent or long-term sampling.50 Using a sample that can be collected noninvasively solves this problem, and assays to quantify fecal corticosterone metabolites (FCM) have been validated as a measure of stress-related hormone changes in laboratory mice.21,49-51
Behavioral testing to evaluate stress and anxiety is well-established in mice.3,4,13-15,25,30,40,48 Open-field and light–dark box tests are 2 unconditioned response tests, which measure spontaneous, naturalistic behaviors within the innate repertoire of the animal. Specifically, open-field testing analyzes general locomotion and exploratory behavior. Light–dark box testing measures the conflict between the natural tendencies of mice to explore a novel environment but avoid the aversive properties of an open field.8,13,17
The Guide for the Care and Use of Laboratory Animals recommends the consideration of sounds and their effects on laboratory animals.26 However, without more research into this issue, reasonable, evidence-based recommendations are difficult to formulate with regard to which noises should be controlled most rigorously and in which species or strain. The objective of this study was to determine whether noise generated by a vacuum cleaner induces an acute stress response in a commonly used strain of laboratory mouse under standard housing conditions. An initial adrenocorticotropic hormone (ACTH) stimulation test was performed to determine whether responses of the hypothalamic–pituitary–adrenal (HPA) axis changed. Subsequently, both physiologic (FCM concentrations) and behavioral (open-field and light–dark box testing paradigms) criteria were evaluated for effects of noise exposure.
Materials and Methods
Animals and housing.
Female C57BL/6Cr mice arrived at 6 wk of age, were housed in groups of 5, and were allowed a 2-wk acclimation period before experiments began. A total of 60 mice were used; 20 mice were used for the ACTH stimulation test and for each of 2 noise-exposure experiments. Mice were housed in the same room on the same rack shelf, within a suite and remote from loud air vents and cage washing areas. Mice were housed in static, polycarbonate microisolation caging (18.4 × 29.2 × 12.7 cm; Alternative Design, Siloam Springs, AR) as they had been at the breeding facility. The mice were housed on recycled paper bedding (Tek-Fresh, Harlan Teklad, Indianapolis, IN) that was changed once weekly. Mice were fed (Teklad Irradiated Diet 7912, Harlan Teklad) and provided with bottled tap water ad libitum. Environmental temperature and relative humidity were maintained at 18 to 26 °C and 30% to 70%, respectively. A 12:12-h light:dark cycle, with lights on at 0700 and off at 1900, was used. Paired sentinel female CD1 mice housed in a single cage per rack were tested and found negative for mouse hepatitis virus, Sendai virus, pneumonia virus of mice, Mycoplasma pulmonis, mouse encephalomyelitis virus, reovirus 3, mouse parvovirus, rotavirus, lymphocytic choriomeningitis virus, ectromelia virus, and parasites. The Institutional Animal Care and Use Committee at the University of California–Berkeley approved the experimental protocol. Mice were euthanized with CO2 inhalation followed by cervical dislocation at the end of the experiments.
Experimental protocol.
Three experiments were conducted, each on a different cohort of mice: 1) an ACTH hormone stimulation test assessed concentrations of FCM before and after administration of synthetic ACTH; 2) noise-exposure experiment 1 assessed FCM levels before and after exposure to vacuum cleaner noise; and 3) noise-exposure experiment 2 assessed stress-sensitive behaviors before and after exposure to vacuum cleaner noise. All groups of mice were carried in their home cages to a nearby quiet room and allowed to acclimate for 1 h prior to all experiments. For the ACTH test, all mice were carried to the same room. For the noise-exposure experiments, mice in the control and noise-exposure groups were placed in adjacent rooms, one of which was the room used for the ACTH stimulation test. In the noise room, the vacuum cleaner was placed on a stand adjacent to but not touching the cage platform, to avoid vibration. Experiments were not performed on the day after cage changes. A vacuum cleaner was not used in the living quarters during the animals’ stay. All experiments began between 0800 and 0900.
ACTH stimulation test.
Because our objective included studying mice housed under standard conditions (that is, groups of 5 to a cage), sample collection, although noninvasive, did require minimal handling of the animals. To confirm that FCM measurements reflected changes in the HPA axis under our experimental protocol, an ACTH stimulation test was performed on mice housed and handled in an identical fashion to those in noise-exposure experiment 1. Mice in the treatment group (n = 10) received 60 µg/100 g of synthetic ACTH50 (Cortrosyn, Amphastan Pharmaceuticals, Rancho Cucamonga, CA) in 1 mL 0.9% saline solution intraperitoneally. Mice in the control group (n = 10) received 1 mL of 0.9% saline solution intraperitoneally.
Noise exposure.
In the 2 noise-exposure experiments, mice in the noise groups (n = 10) were exposed to 1 h of noise produced by a vacuum cleaner (model 71, type A, Eureka, Bloomington, IL), whereas mice in the control groups (n = 10) were not. The vacuum-cleaner noise was measured prior to the experiments to ensure that it exceeded the hearing threshold of young adult C57BL/6 mice. Average sound pressure levels were measured at cage level by placing the instrument (Noise Dosimeter model Q300, Quest Technologies, Oconomowoc, WI) inside a closed microisolation cage that contained the bedding used to house the mice. Instrument configuration prevented placement of the wire bar lid. As for noise experiments 1 and 2, the vacuum cleaner was placed on a stand adjacent to but not touching the cage platform and was 15 cm from the cage during sound measurement. Further characterization of the noise produced by the vacuum cleaner was performed by using a calibrated electronet microphone (ACO, Belmont, CA) placed inside the cage. The signal was produced by using analog-to-digital hardware (TDT System III, Alachua, FL), and a power spectrum displayed in ¼-octave bandwidths (Matlab software, MathWorks, Natick, MA).
Fecal sample collection.
Mice were housed in groups of 5, necessitating their separation for individual fecal sample collection. At each time point, feces were collected by gently placing mice into clean collection cages that were lined with clean paper towels and divided into sections by custom-made acrylic dividers. One mouse was placed into each section for a maximum of 10 min before being placed back into the home cage. Homecage groups were kept constant during transfer to collection cages, and home cages were left untouched during collection so that mice were immediately returned to their familiar environment. Fecal pellets then were placed into microcentrifuge tubes and frozen at −80 °C until extraction. Dividers were cleaned and disinfected with 0.4% quaternary ammonium solution (Steris, Mentor, OH) and 70% isopropol alcohol and allowed to dry completely between uses. To habituate the mice to this minimal handling procedure, all animals were placed in collection cages once or twice daily at anticipated sample collection times for 4 d prior to the experiments. Fecal samples were collected at 0, 2, 8, 10, and 14 h after treatment with ACTH or saline. Fecal samples were collected at 0, 2, 4, 6, 8, 10, 14, 24, and 32 h after initiation of vacuum cleaner noise. Collection times that occurred during the dark phase were performed by using a low-light headlamp to minimize disruption.
Fecal sample extraction.
Fecal samples were dried in an oven at 60 °C for 4 h, crushed with a mortar and pestle to form a powder, and weighed. For the ACTH stimulation test, the powder was suspended in 10 volumes of 100% ethanol (0.05 g feces was suspended in 0.5 mL ethanol). The samples were shaken 3 times for 30 s each on a hand vortexer and centrifuged for 10 min at 2500 × g, and the supernatant was transferred to a clean microcentrifuge tube and stored at −20 °C until assayed. For the noise-exposure experiment, the powder was suspended in 20 volumes of 80% methanol (0.05 g feces in 1.0 mL methanol). The samples were shaken 3 times for 30 s each on a hand vortexer and centrifuged for 10 min at 2500 × g, and the supernatant was transferred to a clean microcentrifuge tube and dried under nitrogen before being stored at −80 °C until shipment for assay. Both ethanol and methanol have been used to extract steroids from the feces of many species34,35 and appear comparable in their extraction capabilities.29,55 We used 80% methanol for samples from the noise experiment study because it was the solvent for extraction in previous studies using the 5α-pregnane-3β,11β,21-triol-20-one enzyme immunoassay (EIA) in mice.50,51
Fecal corticosterone metabolite assays.
For the ACTH stimulation test, immunoreactive metabolites were measured in duplicate by using a commercially available corticosterone EIA kit (Cayman Chemical, Ann Arbor, MI). Duplicates exhibiting coefficients of variation greater than 20% were reassayed. Extracted samples were diluted with EIA buffer to bring the metabolite concentration within the range most reliably measured by the assay, and parallelism was demonstrated between dilutions of pooled fecal extracts and the standard curve. For the noise-exposure experiment, dried fecal extracts were shipped to the Institute of Biochemistry at the University of Veterinary Medicine (Vienna, Austria) for analysis, and FCM were determined by using an extensively validated50,51 5α-pregnane-3β,11β,21-triol-20-one EIA. This group-specific EIA was chosen for the noise experiment because it is better suited for assessing FCM levels in mice than are commercially available kits.49,50
Behavior testing.
Animals were filmed in 2 consecutive 5-min tests designed to assess anxiety levels, which was reflected in more time spent along the walls of the open field apparatus17,25,30,44,48 and in the dark chamber of the light-dark box apparatus18,25,30 as well as longer latency to leave the dark box.22,33 These tests were administered 3 times: 2 d before noise exposure (basal), within 2 h after noise exposure (at exposure), and 24 h after noise exposure (recovery). To acclimate the mice to handling before running the behavior tests, mice were handled gently daily for 2 d prior to the basal behavioral trials and on the day between the basal and at-exposure testing.
The open-field testing apparatus consisted of a square acrylic arena (58.4 × 58.4 × 45.7 cm).44 Mice were placed individually at the same corner of a marked inner zone to begin. The total amount of time spent in the aversive inner zone (10.2 cm from any wall) was recorded.44 The light–dark box testing apparatus18 consisted of 2 attached, equal-sized acrylic chambers (38.1 × 38.1 × 20.3 cm), one of which was clear and the other of which was black and opaque. An opening (10.2 × 10.2 cm) allowed mice to move freely from one chamber to the other. Immediately after the open-field test, mice were placed individually at the light–dark box opening heading into the dark chamber. Latency to exit the dark chamber and total time spent in the exposed chamber were recorded.18,22,33 To control for diurnal activity variability, all testing was performed between the hours of 0800 and 1000. Testing was achieved under fluorescent light, and experimenters left the room during testing. Testing apparatuses were cleaned with 0.4% quaternary ammonium solution (Steris, Mentor, OH) and dried completely between mice. All behavior tests were scored under blinded conditions, where video tapes were labeled by using a numbering system to disguise the group identification from the scorer.
Statistical analysis.
We tested all data for normality by using the Shapiro–Wilk test. FCM data were normally distributed and met requirements for parametric testing. Therefore, data for each of the experiments (ACTH test and noise experiment 1) were analyzed by 2-way ANOVA (treatment or noise exposure × time) by using GraphPad Prism software (GraphPad, La Jolla, CA). Data for noise experiment 1 were analyzed by 2-way ANOVA (cage × time) to evaluate differences by cage within the noise exposure and control groups separately. Post hoc pairwise comparisons of main effects were performed by using Bonferroni-corrected t tests. Behavioral testing data were not normally distributed. Therefore, we analyzed the behavioral data by using the Kruskal–Wallis equality of populations rank test (Stata, StataCorp, College Station, TX). We tested for differences between groups at each time point and for differences over time within the noise and control groups. Statistical significance was defined as P < 0.05.
Results
Sound measurement.
Ambient noise inside the cage in the quiet room had an average sound pressure level of 53 dB. This level did not change with the vacuum cleaner on in the adjacent room. In the room containing the vacuum cleaner, ambient noise inside the cage had an average sound pressure level of 49 dB. Turning on the vacuum cleaner resulted in an average sound pressure level of 85.6 dB. Real-time powers for frequencies around 4000, 8000, and 25,000 Hz were calculated to be 74, 70, and 75 dB, respectively. Given the hearing threshold of young adult C57BL/6 established in previous studies (55 dB at 8000 Hz) and the high-frequency hearing of mice in general (best around 20,000 Hz),23,37 we concluded that the noise produced by the vacuum cleaner was audible to the mice.
ACTH stimulation test.
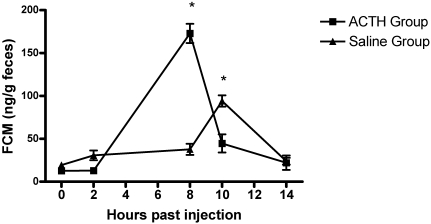
The FCM concentrations of ACTH-treated and saline control mice are presented in Figure 1. At 8 h after injection, FCM were 3 times higher (t = 14.81, P < 0.001) in the treatment group compared with the control group. The 8-h peak reflects expected HPA axis activity in response to pharmaceutical stimulation after metabolism of plasma corticosterone and gut-transit of metabolites in female mice.51 Significant sources of variation included time (F = 62.34, df = 4, P < 0.0001), treatment (F = 6.35, df = 1, P < 0.0001), and the treatment × time interaction (F = 55.38, df = 4, P < 0.0001), indicating that FCM concentrations changed differently over the course of our experiment between groups. Results confirmed that FCM measurements reflected significant changes in the HPA axis activity under our experimental protocol.
Figure 1.
Mean fecal corticosterone metabolite concentrations in mice during the ACTH stimulation test and in control mice injected with saline. Significant (P < 0.001) differences between groups are marked with asterisks. Error bars represent standard error. Significant sources of variation include time (P < 0.0001), treatment (P < 0.0001), and treatment × time (P < 0.0001).
Noise exposure experiment 1.
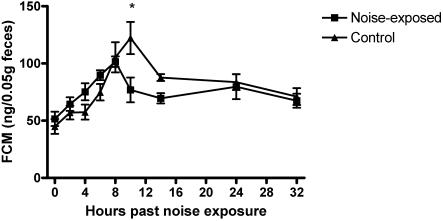
The FCM concentrations of noise-exposed and control mice are presented in Figure 2. Mean FCM concentrations changed over time for both groups (F = 9.37, df = 8, P < 0.0001), with a significant noise exposure × time interaction (F = 2.60, df = 8, P < 0.0114). Peak FCM concentrations occurred at the 8-h time point for noise-exposed mice and at the 10-h time point for the control group. At the 10-h time point, mean concentrations of FCM were significantly (P < 0.01) lower in noise-exposed compared with control mice. Significant differences were not detected for FCM levels or patterns between cages within groups (P > 0.5).
Figure 2.
Mean fecal corticosterone metabolite concentrations in mice exposed to vacuum-cleaner noise and in control mice. Significant (P < 0.01) differences between groups are marked with asterisks. Error bars represent standard error. Significant sources of variation include time (P < 0.0001) and noise exposure × time (P = 0.0114).
Noise exposure experiment 2.
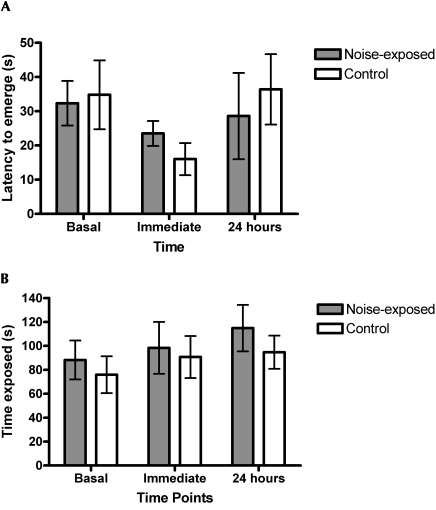
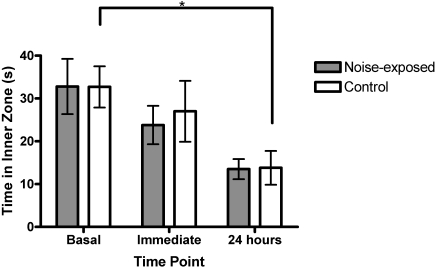
Noise-exposed and control groups did not differ significantly in the open-field or light–dark box testing before, at, or 24 h after noise exposure (Figures 3 and 4; Kruskal–Wallis P values ranged from 0.14 to 0.94). Latency to emerge in the light–dark box tended to be shorter at the time of noise exposure for the control group (Kruskal–Wallis x2 = 4.658, df = 2, P = 0.0974), but no such tendency was defined in the noise-exposed group (Figure 3 A). Time had a significant effect on open-field behavior among controls (Figure 4), with mice spending less time in the inner zone in later trials (Kruskal–Wallis x2 = 7.47, df = 2, P = 0.024); noise-exposed mice showed a trend in the same direction (Kruskal–Wallis x2 = 5.59, df = 2, P = 0.061)
Figure 3.
Light–dark box test scores in mice before, immediately after, and 24 h after exposure to vacuum-cleaner noise. Comparisons between noise-exposed and control mice included: (A) latency to exit dark box in light–dark box apparatus, and (B) time spent in exposed side of light–dark box apparatus. Error bars represent standard error.
Figure 4.
Open-field test scores in mice before, immediately after, and 24 h after vacuum noise exposure. The control group spent significantly (*, P = 0.024) less time in the inner zone of the apparatus during subsequent trials. Error bars represent standard error.
Discussion
In this study, we hypothesized that acute exposure to moderately loud noise (85.6 dB) produced by a vacuum cleaner would induce measurable responses in the exposed mice. However, results indicated that FCM concentrations were lower in noise-exposed versus control mice at one time point, whereas behavioral testing scores did not differ between noise-exposed and mice.
Concentrations of FCM reflect plasma corticosterone levels after a time delay that incorporates metabolism of the plasma hormone and intestinal transit of the hormone metabolite before it is excreted in the feces. A prominent peak in FCM concentrations occurred in the noise-exposed mice 8 h after administration of ACTH. These results are consistent with previous studies in female C57BL/6 mice50 and substantiate our experimental model. Mice were studied under standard housing conditions. Potential confounding variables included housing mice in groups of 5, minimal handling for individual fecal sample collection, and moving mice to a quiet room prior to noise exposure. Experimental and control groups were handled identically. Documenting that predictable changes in the HPA axis could be measured was important to show that a noise-induced HPA response could be detected under these conditions.
In noise-exposure experiment 1, FCM concentrations in the mice exposed to vacuum-cleaner noise differed significantly from those in control mice at one time point. Contrary to expectations, concentrations of FCM of noise-exposed mice were significantly lower than those of control mice 10 h after exposure to vacuum cleaner noise. Although noise exposure can improve some functions, such as maze navigation in rats45 and protection from age-related hearing loss in mice,56 it seems unlikely that the acute exposure to moderate noise reported in this study would reduce HPA activation. In addition, FCM concentrations at the 8-h point, when levels would likely reflect serum hormone levels at the time of noise exposure, did not differ between the noise-exposed and control groups.
Further examination of the data revealed that another explanation for this unexpected result is possible. Over the testing period, both groups exhibited a significant change in FCM concentrations, with the levels increasing and then decreasing over time, but their peaks differed. The control group exhibited peak concentrations of FCM at the 10-h time point, similar to the control group in the ACTH stimulation test (Figures 1 and 2). FCM concentrations in mice have been shown to reflect the diurnal variation of glucocorticoids in both male and female C57BL/6 mice,50 and the change in FCM concentrations exhibited by both control groups could reflect the influence of diurnal rhythms on corticosterone levels in these mice. In contrast, after peaking at the 8-h time point, FCM concentrations in the noise-exposed group fell below those of the control group at the 10-h time point. One interpretation of this difference is that noise exposure affected diurnal rhythms. The timing of noise exposure has been found to effect cyclic changes in serum corticosterone levels. In rats, serum corticosterone levels were found to pulse hourly; loud noise exposure during the rise of this peak caused an even greater increase whereas noise exposure during the fall of this peak did not change basal levels.57 Serum corticosterone levels in BALB/c mice increased immediately after exposure to loud noise (120 dB); this effect was even more pronounced at the start of the dark phase when diurnal corticosterone levels were peaking.28 Rather than exaggerating diurnal variation, attenuation or a shift of the diurnal peak might be a response to moderate noise in the C57BL/6 strain of this study.
To confirm and compare the physiologic measurement of stress responses reflected by FCM concentrations, behavioral measurements of stress were evaluated in noise-exposure experiment 2. As discussed previously, FCM measurements reflect serum corticosterone levels after a lag time that allows for metabolism and gut transit. Because an effect on behavioral testing would be expected to correspond with an effect on serum corticosterone levels, testing was performed immediately after noise exposure. Basal (prior to any noise exposure) and recovery (24 h after noise exposure) testing were performed at the same time of day to serve as a comparison.4,14 We chose to evaluate the mice by using 2 ethologically relevant testing paradigms: an open-field task and a light–dark box task. We administered these tests in sequence at the 3 time points described earlier. Although repeated testing on a given behavioral task might lead to acclimation or habituation, results of the open-field testing did not support habituation.12 Female C57BL/6 mice do not demonstrate this phenomenon when tested repeatedly on the open-field task or the light–dark box task.42 In C57BL/6 mice, the order of testing for these 2 particular tasks does not appear to result in differences in performance.31 Test results did not differ significantly between noise-exposed and control groups. Control mice spent significantly less time in the inner zone of the open-field test in later trials while noise-exposed mice exhibited a trend in the same direction. In comparison, control mice tended to emerge from the dark box sooner at the at-exposure time point than at other time points, but noise-exposed mice did not. Although these tendencies might reflect qualitative differences between the 2 groups, overall, in our study, acute exposure to moderate noise produced by a vacuum cleaner did not appear to increase anxiety-related behavior in exposed mice.
We predicted that exposure to noise produced by a vacuum cleaner would cause acute stress responses in the mice of our study. The results did not support our hypothesis but did suggest a possible attenuation or shifting effect on the diurnal rhythm of FCM concentrations of noise-exposed mice. Although the results were surprising, their reliability was supported in several ways. First, FCM were shown to be an indicator of circulating corticosterone levels in response to an ACTH stimulation test, which produced predictable, significant changes in the HPA axis. Second, the 5α-pregnane-3β,11β,21-triol-20-one EIA used to measure FCM concentrations for the noise experiment has been extensively validated and successfully applied in laboratory mice,19,48,50,51 including female C57BL/6 mice. In addition, this EIA has proven its sensitivity in detecting changes in adrenocortical activity to address welfare-based questions.6,39 Finally, behavioral testing with a separate cohort supported the physiologic evaluation.
These results contribute to the greater body of knowledge regarding the effects of facility noise on laboratory mice. The development of an achievable noise-control plan requires empirical evidence demonstrating not only which facility noises cause stress in strains of laboratory mice but also which ones might not. C57BL/6 mice were chosen as study subjects not only because they are the most commonly used strain at the study facility but also because they are the strain studied in the validation of the group-specific EIA used to measure FCM concentrations.50 In addition, the hearing threshold of C57BL/6 mice has been well-characterized,37 making it possible to predict whether certain facility noise is likely to be audible to the mice exposed to it.
Female mice were used in this project because the effect of stress on breeding success is an important concern in many facilities. Circulating corticosterone levels might vary significantly throughout the estrous cycle,50 but estrous cycles were not specifically determined in the mice for this study. However, we have several reasons to believe that our data are robust. First, due to the nature of the testing, individual mice are likely to represented a random distribution of estrus stages.40,50 Next, group-housing tends to suppress the estrous cycle in mice,11 reducing the likelihood that differences in sex hormones are significantly affecting outcome variables. Finally, in one report, stage of estrus had no significant effect on behavioral tests (including open-field, rotarod, acoustic startle, and tail-flick tests) in female C57BL/6 mice.32
These results apply to young adult, female C57BL/6 mice. Comparisons across sexes and across strains might reveal different responses to this particular source of facility noise, particularly among more stress-susceptible strains such as BALB/c and CBA.15 In addition, many other common facility noises could be examined for their effects on mice, including those at higher frequencies, which have the potential to interfere with mouse vocalization.47 Vibrations associated with noise might be a significant stressor. In this study, care was taken to avoid any contact between the source of the noise and the table on which the cages sat. This step was a purposeful attempt to avoid vibration as a variable, but future studies are planned comparing the response of young adult female C57BL/6 mice exposed to the same noise with its concurrent vibrations. Finally, results indicating a possible effect of facility noise on the diurnal rhythm of corticosterone in mice warrant further study, particularly given that certain facility noises occur once or even multiple times daily.
In summary, the current study contributes to the body of literature addressing the effects of common research animal facility noise on laboratory mice. Results indicated that exposure to the moderate noise of a vacuum cleaner did not cause increased concentrations of fecal corticosterone metabolites or anxiety-related behavior in young adult female C57BL/6 mice compared with control mice. This and further research could contribute to the development of best practices in noise-control protocols for animal facilities.
Acknowledgments
We thank Dr Helen Diggs for providing animals, Professor Tyrone Hayes and his laboratory for use of their equipment, Mrs Edith Klobetz-Rassam for EIA analysis, the OLAC animal health technicians (Mr Jose Castillo, Ms Lindsey Jennings, and Ms Maura Lane) and animal husbandry staff (particularly Ms Ailene Basila, Mr Erik Newman, and Ms Annette Webb), Mr Tim Sheiner for construction of cage dividers, Professor Frederic Theunissen for help with noise measurement, and Ms Julie Woodruff for technical support. This work was funded by a grant awarded to DDF by the UC Berkeley Committee on Research.
References
- 1.Aguas AP, Esaguy N, Grande NR, Castro AP, Castelo Branco NA. 1999. Acceleration of lupus erythematosus-like processes by low-frequency noise in the hybrid NZB/W mouse model. Aviat Space Environ Med 70:A132–A136 [PubMed] [Google Scholar]
- 2.Aguas AP, Esaguy N, Grande NR, Castro AP, Castelo Branco NA. 1999. Effect of low-frequency noise exposure on BALB/c mice splenic lymphocytes. Aviat Space Environ Med 70:A128–A131 [PubMed] [Google Scholar]
- 3.Anisman H, Hayley S, Kelly O, Borowski T, Merali Z. 2001. Psychogenic, neurogenic, and systemic stressor effects on plasma corticosterone and behavior: mouse strain-dependent outcomes. Behav Neurosci 115:443–454 [PubMed] [Google Scholar]
- 4.Anisman H, Zacharko R. 1986. Behavioral and neurochemical consequences associated with stressors. Ann N Y Acad Sci 467:205–225 [DOI] [PubMed] [Google Scholar]
- 5.Baldwin AL, Bell IR. 2007. Effect of noise on microvasculature integrity in laboratory rats. J Am Assoc Lab Anim Sci 46:58–65 [PubMed] [Google Scholar]
- 6.Blottner D, Serradj N, Salanova M, Touma C, Palme R, Silva M, Aerts JM, Berckmans D, Vico L, Liu Y, Giuliani A, Rustichelli F, Cancedda R, Jamon M. 2009. Morphological, physiological, and behavioural evaluation of a ‘mice in space’ housing system. J Comp Physiol [B] 179:519–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borg E. 1977. Tail artery response to sound in the unanesthetized rat. Acta Physiol Scand 100:129–138 [DOI] [PubMed] [Google Scholar]
- 8.Bourin M, Hascoet M. 2003. The mouse light–dark box test. Eur J Pharmacol 463:55–65 [DOI] [PubMed] [Google Scholar]
- 9.Briese V, Fanghänel J, Gasow H. 1984. [Effect of pure sound and vibration on the embryonic development of the mouse]. Zentralbl Gynakol 106:379–388[Article in German] [PubMed] [Google Scholar]
- 10.Burow A, Day HE, Campean S. 2005. A detailed characterization of loud noise stress: intensity analysis of hypothalamo–pituitary–adrenocortical axis and brain activation. Brain Res 1062:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Champlin AK. 1971. Suppression of oestrus in grouped mice: the effects of various densities and the possible nature of the stimulus. J Reprod Fertil 27:233–241 [DOI] [PubMed] [Google Scholar]
- 12.Crawley JN. 1999. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res 835:18–26 [DOI] [PubMed] [Google Scholar]
- 13.Crawley JN. 2000. Emotional behaviors: animal models of psychiatric disease, p 183–189 : What's wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. New York (NY): Wiley–Liss [Google Scholar]
- 14.Crawley JN. 2003. Behavioral phenotyping of rodents. Comp Med 53:140–146 [PubMed] [Google Scholar]
- 15.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Lisva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. 1997. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 132:107–124 [DOI] [PubMed] [Google Scholar]
- 16.Dallman MF, Akana SF, Bell ME, Bhatnagar S, Choi S, Chu A, Gomez F, Laugero K, Soriano L, Viau V. 1999. Warning! Nearby construction can profoundly affect your experiments. Endocrine 11:111–113 [DOI] [PubMed] [Google Scholar]
- 17.Francis DD, Szegda K, Campbell G, Martin WD, Insel TR. 2003. Epigenetic sources of behavior differences in mice. Nat Neurosci 6:445–446 [DOI] [PubMed] [Google Scholar]
- 18.Frye CA, Sumida K, Dudek BC, Harney JP, Lydon JP, O'Malley BW, Pfaff DW, Rhodes ME. 2006. Progesterone's effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology (Berl) 186:312–322 [DOI] [PubMed] [Google Scholar]
- 19.Godfrey D, Silverman J. 2009. Effects of a fire-alarm strobe light on fecal corticosterone metabolite concentrations in mice. Lab Anim (NY) 38:61–68 [DOI] [PubMed] [Google Scholar]
- 20.Haque SF, Izumi S, Aikawa H, Suzuki T, Matsubayashi H, Murano T, Kika G, Ikeda M, Goya K, Makino T. 2004. Anesthesia and acoustic stress-induced intrauterine growth retardation in mice. J Reprod Dev 50:185–190 [DOI] [PubMed] [Google Scholar]
- 21.Harper JM, Austad SN. 2000. Fecal glucocorticoids: a noninvasive method of measuring adrenal activity in wild and captive rodents. Physiol Biochem Zool 73:12–22 [DOI] [PubMed] [Google Scholar]
- 22.Harris RBS, Zhou J, Shi M, Redmann S, Mynatt RL, Ryan DH. 2001. Overexpression of agouti protein and stress responsiveness in mice. Physiol Behav 73:599–608 [DOI] [PubMed] [Google Scholar]
- 23.Heffner HE, Heffner R. 2007. Hearing ranges of laboratory animals. J Am Assoc Lab Anim Sci 46:20–22 [PubMed] [Google Scholar]
- 24.Heinrichs SC, Koob GF. 2005. Application of experimental stressors in laboratory rodents, p 8.4.1–8.4.17 : Crawley J, Gerfen C, McKay R, Rogawski M, Sibley D, Skolnick P. Current protocols in neuroscience. Hoboken (NJ): John Wiley and Sons; [DOI] [PubMed] [Google Scholar]
- 25.Holmes A, Yang RJ, Crawley JN. 2002. Evaluation of an anxiety-related phenotype in galanin-overexpressing transgenic mice. J Mol Neurosci 18:151–165 [DOI] [PubMed] [Google Scholar]
- 26.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals, p 36 Washington (DC): National Academies Press [Google Scholar]
- 27.Joachim RA, Quarcoo D, Arck P, Herz U, Renz H, Klapp B. 2003. Stress enhances airway reactivity and airway inflammation in an animal model of allergic bronchial asthma. Psychosom Med 65:811–815 [DOI] [PubMed] [Google Scholar]
- 28.Kim JY, Kang H, Agn J, Chung J. 2008. Circadian changes in serum corticosterone levels affect hearing in mice exposed to noise. Neuroreport 19:1373–1376 [DOI] [PubMed] [Google Scholar]
- 29.Mateo JM, Cavigelli SA. 2005. A validation of extraction methods for noninvasive sampling of glucocorticoids in free-living ground squirrels. Physiol Biochem Zool 78:1069–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mathis C, Paul SM, Crawley JN. 1994. Characterization of benzodiazepine-sensitive behaviors in the A/J and C57BL/6J inbred strains of mice. Behav Genet 24:171–180 [DOI] [PubMed] [Google Scholar]
- 31.Mcllwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. 2001. The use of behavioral test batteries: effects of training history. Physiol Behav 73:705–717 [DOI] [PubMed] [Google Scholar]
- 32.Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W. 2007. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav 6:192–200 [DOI] [PubMed] [Google Scholar]
- 33.Morgan MA, Pfaff DW. 2002. Estrogen's effects on activity, anxiety, and fear in 2 mouse strains. Behav Brain Res 132:85–93 [DOI] [PubMed] [Google Scholar]
- 34.Möstl E, Palme R. 2002. Hormones as indicators of stress. Domest Anim Endocrinol 23:67–74 [DOI] [PubMed] [Google Scholar]
- 35.Möstl E, Rettenbacher S, Palme R. 2005. Measurement of corticosterone metabolites in birds’ droppings: an analytical approach. Ann N Y Acad Sci 1046:17–34 [DOI] [PubMed] [Google Scholar]
- 36.Murata M, Takigawa H, Sakamoto H. 1993. Teratogenic effects of noise and cadmium in mice: does noise have teratogenic potential? J Toxicol Environ Health 39:237–245 [DOI] [PubMed] [Google Scholar]
- 37.Naff KA, Riva CM, Craig SL, Gray KN. 2007. Noise produced by vacuuming exceeds the hearing thresholds of C57BL/6 and CD1 mice. J Am Assoc Lab Anim Sci 46:52–57 [PubMed] [Google Scholar]
- 38.Nawrot PS, Cook RO, Staples RE. 1980. Embryotoxicity of various noise stimuli in the mouse. Teratology 22:279–289 [DOI] [PubMed] [Google Scholar]
- 39.Nicholson A, Malcolm RD, Russ PL, Cough K, Touma C, Palme R, Wiles MV. 2009. The response of C57BL/6J and BALB/cJ mice to increased housing density. J Am Assoc Lab Anim Sci 48:740–753 [PMC free article] [PubMed] [Google Scholar]
- 40.Painsipp E, Sperk G, Herzog H, Holzer P. 2009. Delayed stress-induced differences in locomotor and depression-related behaviour in female neuropeptide–Y Y1 receptor knockout mice. J Psychopharmacol Apr 7. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patterson-Kane EG, Farnworth MJ. 2006. Noise exposure, music, and animals in the laboratory: a commentary base on laboratory animal refinement and enrichment forum (LAREF) discussions. J Appl Anim Welf Sci 9:327–332 [DOI] [PubMed] [Google Scholar]
- 42.Paylor R, Spencer C, Yuva-Paylor L, Pieke-Dahl S. 2006. The use of behavioral test batteries, II: effect of test interval. Physiol Behav 87:95–102 [DOI] [PubMed] [Google Scholar]
- 43.Pfaff J. 1974. Noise as an environmental problem in the animal house. Lab Anim 8:347–354 [DOI] [PubMed] [Google Scholar]
- 44.Priebe K, Brake WG, Romeo RD, Sisti HM, Mueller A, McEwen BS, Francis DD. 2005. Maternal influences on adult stress and anxiety-like behavior in C57BL/6J and BALB/cJ mice: a cross-fostering study. Dev Psychobiol 47:398–407 [DOI] [PubMed] [Google Scholar]
- 45.Prior H. 2006. Effects of the acoustic environment on learning in rats. Physiol Behav 87:162–165 [DOI] [PubMed] [Google Scholar]
- 46.Rabat A. 2007. Extra-auditory effects of noise in laboratory animals: the relationship between noise and sleep. J Am Assoc Lab Anim Sci 46:35–41 [PubMed] [Google Scholar]
- 47.Sales GD, Wilson KJ, Spencer KEV, Milligan SR. 1988. Environmental ultrasound in laboratories and animal houses: a possible cause for concern in the welfare and use of laboratory animals. Lab Anim 22:369–375 [DOI] [PubMed] [Google Scholar]
- 48.Touma C, Bunck M, Glasl L, Nussbaumer M, Palme R, Stein H, Wolferstätter M, Zeh R, Zimbelmann M, Holsboer F, Landgraf R. 2008. Mice selected for high versus low stress reactivity: a new animal model for affective disorders. Psychoneuroendocrinology 33:839–862 [DOI] [PubMed] [Google Scholar]
- 49.Touma C, Palme R. 2005. Measuring fecal glucocorticoid metabolites in mammals and birds: the importance of validation. Ann N Y Acad Sci 1046:54–74 [DOI] [PubMed] [Google Scholar]
- 50.Touma C, Palme R, Sachser N. 2004. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm Behav 45:10–22 [DOI] [PubMed] [Google Scholar]
- 51.Touma C, Sachser N, Mostl E, Palme R. 2003. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol 130:267–278 [DOI] [PubMed] [Google Scholar]
- 52.Turner JG, Bauer CA, Rybak LP. 2007. Noise in animal facilities: why it matters. J Am Assoc Lab Anim Sci 46:10–13 [PubMed] [Google Scholar]
- 53.Turner JG, Parrish JL, Hughes LF, Toth LA, Caspary DM. 2005. Hearing in laboratory animals: strain differences and nonauditory effects of noise. Comp Med 55:12–23 [PMC free article] [PubMed] [Google Scholar]
- 54.Van Raaij M, Oortgiesen M, Timmerman H, Dobbe C, Van Loveren H. 1996. Time-dependent differential changes of immune function in rats exposed to chronic intermittent noise. Physiol Behav 60:1527–1533 [DOI] [PubMed] [Google Scholar]
- 55.Wasser SK, Hunt KE, Brown JL, Cooper K, Crockett CM, Bechert U, Millspaugh JJ, Larson S, Monfort SL. 2000. A generalized fecal glucocorticoid assay for use in a diverse array of nondomestic mammalian and avian species. Gen Comp Endocrinol 120:260–275 [DOI] [PubMed] [Google Scholar]
- 56.Willott JF. 2007. Factors affecting hearing in mice, rats, and other laboratory animals. J Am Assoc Lab Anim Sci 46:23–27 [PubMed] [Google Scholar]
- 57.Windle RJ, Wood SA, Shanks N, Lightman SL, Ingram CD. 1998. Ultradian rhythm of basal corticosterone release in the female rat: dynamic interaction with the response to acute stress. Endocrinology 139:443–450 [DOI] [PubMed] [Google Scholar]
- 58.Zheng K-C, Ariizumi M. 2007. Modulations of immune functions and oxidative status induced by noise stress. J Occup Health 49:32–38 [DOI] [PubMed] [Google Scholar]