Abstract
Oral gavage is a common route of precise oral dosing for studies in rodents. Complications including tracheal administration, esophageal trauma, and aspiration are common and usually related to animal resistance to the procedure, and the stress induced by oral gavage can be a confounding variable in many studies. The taste of sucrose conveys a pacifying and analgesic effect in newborns, whereas sour solutions can induce the swallow reflex in humans that are dysphagic. We hypothesized that precoating a gavage needle with sucrose or citrate (or both) would pacify mice and induce them to swallow, reducing the stress and complications associated with the technique. To validate this hypothesis, we quantitated time to passage, stress-related behavioral reactions to the procedure, and plasma corticosterone levels in mice after precoating gavage needles with water, sucrose, citrate, sucrose and citrate, or sodium chloride prior to oral gavage. Precoating needles with sucrose reduced the time to passage, decreased observable stress-related reactions to the procedure, and maintained plasma corticosterone levels similar to those in ungavaged control mice. Coating needles with water, sucrose and citrate, or citrate had no beneficial effects on these parameters. Our findings describe a novel, validated technique that measurably decreases signs of stress and thereby improves animal welfare during oral gavage. Furthermore, the use of sucrose may be a valuable tool to refine other minor or nonsurgical procedures in the field of laboratory animal research.
Despite continued innovation in new drug delivery systems for research animals, oral gavage remains the most widely used and preferred technique for oral dosing in experimental, toxicokinetic and pharmacokinetic studies. For rodents, oral gavage allows for more precise volume and dose delivery and faster peak absorption of unstable or unpalatable compounds, as compared with delivery in feed.20,26 Gastric or intestinal catheterization represents an alternative method of precise dosing that can approximate oral dosing but presents the disadvantage of surgical implementation.32
Although oral gavage is used widely, the procedure undoubtedly introduces scientific variability, mainly attributed to increased animal stress,19 which can be compounded by relative inexperience of the performing technician. The oral gavage technique requires stiff restraint of alert rodents to prevent technical complications,20 and this type of restraint has been shown to increase plasma corticosterone levels in rats and mice.10,12 The oral gavage substance itself can also affect the stress response and substances with high viscosity, such as methyl cellulose, have been shown to increase the stress response in rats.10 Complications associated with oral gavage include inadvertent tracheal administration, aspiration pneumonia, esophageal perforation, esophageal impaction, and gastric rupture, which all can increase morbidity and mortality.14,20,25 One study assessing daily oral gavage in alert rats for 10 d reported mortality of 22% that mostly was attributed to esophageal trauma from resistance of gavage needle passage.25 Similarly, in a long-term (2 y) gavage study in Fischer 344 rats,14 a mortality rate of 53% was observed, most likely due to strain predisposition and chronic irritation from the gavage needle. Perfecting the method of oral gavage in rodents, specifically by reducing stress and associated complications should be investigated to decrease scientific variability and improve animal welfare.
Oral gavage involves passage of a gavage needle into the esophagus, and this technique often involves the animal swallowing the gavage needle as it approaches the pharynx.1 The process of swallowing can be divided into 3 phases—oral, pharyngeal, and esophageal13—of which the pharyngeal phase is most relevant, as it relates to the involuntary phase during which the gavage needle is allowed to pass through the pharynx and into the esophagus. The pharyngeal phase is a complex reflex initiated by afferents of the superior laryngeal and glossopharyngeal nerves.15 Application of water to the laryngeal regions of cats, rabbits, and humans is effective at inducing the swallow reflex through stimulation of water receptors innervated by the superior laryngeal nerve.36 Chemical stimulation by acidic solutions, such as acetic or citric acid, induces the swallow reflex in humans by increased sensory input through the superior laryngeal and glossopharyngeal nerves stimulating the swallowing center of the brain.28 The intense sour taste of purely acidic solutions is unpleasant to consume, although sweetening can improve their palatability without complete aberration of the gain in swallow reflex.28
In addition to its sweetening capability, sucrose is used as an effective analgesic for minor procedures in newborns21,24 and has been shown to have analgesic properties in rodents.3,7,9,11,27,33,35 Analgesic effects attributed to sucrose thus far are believed to be mediated through indirect mechanisms, involving the release of endogenous opioids that subsequently act at their receptors,31 and are not due to direct action of sucrose polysaccharides on opioid receptors.23 The beneficial opioid effects of analgesia and euphoria are incurred instantaneously with sweet taste rather than with carbohydrate absorption.4,7,21
Moistening the tip of a gavage needle with water or oil is one technique to ease the process of oral gavage by decreasing the friction of the needle as it slides past the pharynx and into the esophagus.26 We hypothesized that moistening the tip of a gavage needle with a sucrose solution would improve the speed of the procedure and refine the technique by eliciting analgesic or calming effects (or both). In addition, we hypothesized that the addition of citrate would further improve the oral gavage technique by inducing the swallow reflex through direct stimulation of chemoreceptors.22 Herein, we demonstrate that sucrose precoating of gavage needles significantly decreased the time to passage and decreased observable stress and plasma corticosterone levels in mice as a result of oral gavage, whereas precoating with other tastants resulted in no benefit.
Materials and Methods
Animals.
Female, 8-wk-old (average weight, 18 g), specific pathogen-free C57Bl/6J mice (Mus musculus) were purchased from Jackson Laboratories (Bar Harbor, ME). Prior to shipping, all mice were verified free of contagious ectoparasites, helminth endoparasites, and antibodies to 17 murine viruses. Mice were group-housed (5 per cage) and maintained according to normal barrier rodent husbandry procedures at the AAALAC-accredited Indiana University School of Medicine animal facility. Specifically, animals were housed in ventilated microisolator cages (Super Mouse 750, Lab Products, Seaford, DE) on pelletted paper bedding (Teklad 7084, Harlan, Indianapolis, IN) and a 12:12-h light:dark cycle. Animals were provided a commercial rodent chow (2018SC, Harlan) and acidified water ad libitum. Animals were acclimated for 14 d before enrollment onto study. All procedures performed in this study were approved by the Animal Care and Use Committee of Indiana University School of Medicine and were performed in parallel with associated research projects. Different groups of mice were used for each oral gavage experiment as outlined in following sections.
Oral gavage.
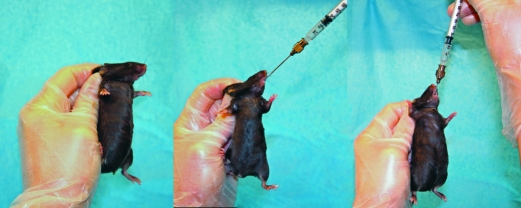
Animals were restrained by tightly scruffing with the nongavage hand (Figure 1). Oral gavage was performed by using 1.5-in., curved, 20-gauge, stainless steel feeding needles with a 2.25-mm ball (Braintree Scientific, Braintree, MA). Each gavage treatment was given in a 0.2-mL bolus (10 mL/kg) of 0.5% methyl cellulose (Methyl Cellulose M0512, Sigma–Aldrich, St Louis, MO) used as vehicle with or without treatment compounds. Twice-daily gavage treatments were performed between the hours of 0800 to 1000 and 1600 to 1900.
Figure 1.
Example of mouse restraint and oral gavage technique. Left, Demonstration of handling technique to immobilize subject for oral gavage by tightly scruffing with nongavage hand. Center, Introduction of the gavage needle into oral cavity and start of the time to passage measurement. Right, Passage of the gavage needle to stopping point in the esophagus and stop of the time to passage measurement.
Animal reactions to oral gavage were documented by the same veterinarian performing the procedure, who was blinded to the tastant coating the gavage needle. Reactions considered to indicate stress were defined as excessive struggling during gavage or abnormal behavior after gavage (for example, labored breathing, frozen stance, exaggerated jumping, shuddering, and hunched posture). If mice demonstrated any of these signs during gavage itself or the 10 s immediately after the procedure, the procedure was scored as a stressful event. The most common stressful events observed during oral gavage trials were excessive struggling during the procedure and exaggerated jumping or frozen stance with a brief period (1 to 2 s) of labored breathing immediately after procedure. The mice were monitored for prolonged labored breathing, hunched posture, and shuddering, but such events did not occur.
Time to passage was measured by a secondary, nonblinded observer. Time was reported to the nearest second, and the time interval began when the gavage needle first touched the animal's mouth (Figure 1, middle) and ended with complete passage of the gavage needle prior to dose administration (Figure 1, right). Instances when the oral gavage procedure could not be performed in the first attempt and resulted in release of the mouse occurred rarely and were not included in any of the accompanying data sets. Instantaneous passages were defined by passage of the oral gavage needle in a single fluid motion, resulting in a time to passage of 2 s or less.
Oral gavage trials comparing time to passage and stressful events by using water- or sucrose-coated gavage needles were performed on 36 C57Bl/6J mice being dosed for a parallel study. Mice were gavaged twice daily by a single trained veterinarian, with limited experience, for 7 trials. Mice were assigned randomly to water- or sucrose-coated gavage needle independently for each trial, to reduce possible cumulative effects of repeated administration of a single compound. Individual mice were not tracked across gavage trials.
Oral gavage trials comparing time to passage by using gavage needles coated in various tastant solutions were similarly performed on 40 (n = 8 per group) C57Bl/6J mice, but the solution coated on the gavage needle remained consistent throughout all trials. The ball tip of the gavage needle was dipped into 1 of 5 different solutions: ultrapure water (purified with a Milli-Q Biocel system, Millipore, Billerica, MA), 1 g/mL sucrose (Sucrose, S7903, Sigma), 27 mg/mL citrate (Citric Acid Monohydrate C1909, Sigma), 1 g/mL sucrose plus 27 mg/mL citrate, or 0.5 M NaCl (Sodium Chloride S9888, Sigma) immediately prior to each gavage in a single-blind fashion by a second researcher.
Plasma corticosterone.
Physiologic stress response for mice gavaged with needles coated with various tastants was performed by using animals naïve to both handling and oral gavage. The mice were gavaged once with 0.2 mL 0.5% methyl cellulose by using gavage needles precoated with various tastants (n = 5 per group) and compared with nongavaged controls (n = 5). A second group of naïve animals were used to compare physiologic stress response to oral gavage using needles coated in water or sucrose, keeping procedure time constant between both groups (n = 8 per group). At 1 h after gavage,10 mice were euthanized by carbon dioxide asphyxiation; blood (0.5 mL) was collected by cardiocentesis and placed into EDTA-coated tubes (BD Microtainer, RF 365974, Becton Dickinson, Franklin Lakes, NJ) and plasma collected for analysis of corticosterone levels. Plasma corticosterone was analyzed by an enzyme-linked immunoassay by using a commercially available kit (Corticosterone EIA kit 500651, Cayman Chemical, Ann Arbor, MI) according to the manufacturer's protocol. Briefly, corticosteroid was extracted from the plasma by the addition of methylene chloride (Sigma) and extraction of the bottom organic layer, in triplicate. Methylene chloride was evaporated under nitrogen at 30 °C, and residues reconstituted in EIA buffer included in the commercial kit. Samples were run in duplicate on a 96-well plate alongside standards. The absorbance was read on an automated plate reader (Microplate Manager 4.0 software and model 550, BioRad Laboratories, Hercules, CA) at 405 nm, and the final plasma corticosterone concentrations were determined by using logit transformation.
Statistical analysis.
Values are reported as mean ± SEM. Trials involving time to passage measurements between sucrose- and water-treated gavage needles and those coated with various tastants underwent ANCOVA by using fixed variables of treatment and trial number with Bonferroni post hoc comparisons of treatment groups to control (water) when appropriate. Initially the interaction between treatment and trial was included in the model but was found to be nonsignificant (P = 0.2309) and thus was removed from the model. Statistical significance of stressful to nonstressful events and instantaneous passages to non-instantaneous passages in mice gavaged with water- or sucrose-coated needles was determined by using χ2 analysis. Statistical analysis for plasma corticosterone levels after gavaging with needles coated in various tastants was determined by using ANOVA with Tukey Studentized range tests for post hoc analysis comparing treatment groups to nongavaged animals (control) and to those gavaged with water-coated needles when appropriate. Significance for plasma corticosterone levels comparing a 10-s gavage with water or sucrose coated needles was done using a 2-tailed t test. In all cases, analyses were performed by using SAS software (SAS Institute, Cary, NC), and statistical significance was set at a P value less than 0.05.
Results
To evaluate the effectiveness of sucrose coating of gavage needles as a means to reduce stress and complications associated with the technique, 36 mice receiving gavage treatments with a vehicle of 0.5% methyl cellulose in a parallel study were gavaged randomly with water- (control) or sucrose-coated needles twice daily for a total of 7 trials, and stress-related reactions were counted. Mice treated with water-coated gavage needles demonstrated significantly (P < 0.05) more stressful reactions than did those treated by using sucrose-coated needles (Table 1). Habituation to the oral gavage procedure was evident as the frequency of stress responses decreased with increasing trial exposure. Although behavioral reactions to gavage were evident, severe complications related to oral gavage, including dyspnea, prolonged hunched posture, lethargy, and emaciation, were not observed.
Table 1.
Frequency of stressful events and instantaneous passage of gavage needle in mice
| Water-coated gavage needles |
Sucrose-coated gavage needles |
|||
| Trial | No. of stressful events (%) | No. of instantaneous passages (%) | No. of stressful events (%) | No. of instantaneous passages (%) |
| 1 | 13 of 18 (72.2) | 0 of 18 (0) | 5 of 18 (27.7) | 1 of 18 (5.6) |
| 2 | 7 of 17 (41.1) | 0 of 17 (0) | 6 of 19 (31.6) | 3 of 19 (15.8) |
| 3 | 6 of 20 (30.0) | 0 of 20 (0) | 2 of 16 (12.5) | 4 of 16 (25.0) |
| 4 | 6 of 17 (35.3) | 0 of 17 (0) | 1 of 19 (5.3) | 4 of 19 (21.1) |
| 5 | 4 of 18 (22.2) | 0 of 18 (0) | 1 of 18 (5.6) | 4 of 18 (22.2) |
| 6 | 2 of 17 (11.8) | 2 of 17 (11.8) | 1 of 19 (5.3) | 8 of 19 (42.1) |
| 7 | 2 of 16 (12.5) | 0 of 16 (0) | 1 of 20 (5.0) | 10 of 20 (50.0) |
| Total | 40 stressful events 84 nonstressful events | 2 instantaneous passages 122 non-instantaneous passages | 17 stressful events 111 nonstressful eventsa | 34 instantaneous passages 94 non-instantaneous passagesb |
Number of stressful events significantly fewer (P < 0.01, χ2 analysis) compared with value for water-coated gavage needles.
Number of instantaneous gavage passages significantly increased (P < 0.01, χ2 analysis) compared with value for water-coated gavage needles.
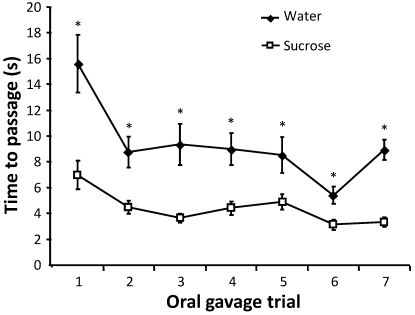
The time to passage of the gavage needle was measured to determine whether water or sucrose coating decreased procedure time, thereby improving technique efficiency and decreasing necessary restraint time. The average time to passage was significantly (P < 0.001) lower in mice gavaged with sucrose-coated needles compared with those coated with water throughout the entire experimental period (Figure 2). Use of sucrose-coated needles decreased the average time to passage by approximately 5 s compared with water-coated needles (P < 0.001). Time to passage of the gavage needle also decreased significantly (P < 0.001) with increasing trial number in both groups, demonstrating the effect of habituation on oral gavage. Mice that received sucrose-coated gavage needles also had a significantly (P < 0.05) higher frequency of instantaneous passages, where the gavage needle passed in a single fluid motion in 2 s or less, compared with that of those coated with water (Table 1). The effect of habituation on the frequency of instantaneous passages was apparent with increasing numbers of instantaneous passages occurring in later trials.
Figure 2.
Time to needle passage during oral gavage. The time to passage from the introduction of the gavage needle to needle stopping point was recorded for 36 mice gavaged with water- or sucrose-coated needles for each gavage trial (n = 16 to 20 per group). Mice were assigned randomly to treatment groups before each trial. Values are expressed as mean ± SEM. *, P < 0.001 by ANCOVA with Bonferroni post hoc analysis.
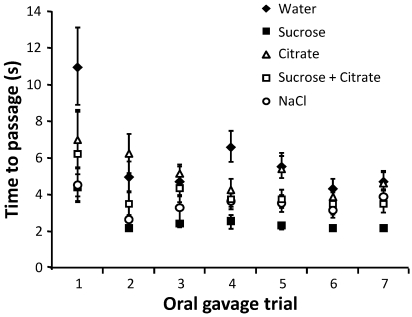
To determine whether more rapid gavage needle passage during the procedure was related to direct stimulation of chemoreceptors, we analyzed time to passage after coating the gavage needle with several solutions thought to either increase or decrease latency to swallow. Groups of 8 mice were gavaged twice daily for a total of 7 trials in a parallel study, by using needles coated with the same solution throughout all trials, to verify whether habituation was specific to a certain solution or an intrinsic effect of repeated gavage. Citrate has been suggested to decrease latency to swallow;28 however, time to passage did not differ between needles coated with a citrate solution compared with water (Figure 3). Compared with water, sodium chloride, hypothesized to increase latency to swallow,36 actually decreased (P < 0.001) time to passage in the context of oral gavage. Gavage needles coated with sucrose resulted in the fastest (P < 0.001) average time to passage throughout all trials. The effect of habituation was evident as time to passage of the gavage needle decreased significantly (P < 0.001) with increasing trial number in all groups.
Figure 3.
Average time to passage for repeated treatments by using gavage needles coated with various tastants. Average time to passage for each group of mice gavaged with needles coated with water, sucrose, citrate, sucrose plus citrate, or NaCl (n = 8 per group) is shown. Mice were gavaged with the same treatment throughout all trials.
Physiologic alteration in the stress response caused by different gavage techniques was evaluated by measuring plasma corticosterone levels. Naïve mice were gavaged with needles precoated with water, sucrose, citrate, sucrose and citrate, or NaCl; mice were euthanized 1 h after the procedure. Mice that experienced gavage with sucrose-coated needles had significantly (P < 0.001) lower plasma corticosterone levels after gavage compared with those that had gavage with water-coated needles (Figure 4 A); corticosterone levels in all other treatment groups did not differ from those of the water-treated group. Only sucrose-coated gavage needles resulted in plasma corticosterone levels that did not differ significantly from those of nongavaged control mice. To evaluate duration of procedure, rather than a tastant effect, as a factor in reducing corticosterone elevation, we measured corticosterone levels after oral gavage with needles coated in water or sucrose but kept the time to complete oral gavage constant (10 s, from the time at which the gavage needle entered the mouse's mouth through complete dose delivery and needle withdrawal). Plasma corticosterone levels of mice gavaged with sucrose-coated needles were significantly (P < 0.05) lower than those of mice gavaged with water-coated gavage needles (Figure 4 B), suggesting that the decrease in plasma corticosterone associated with sucrose-coated gavage needles is unrelated to procedural time.
Figure 4.
Plasma corticosterone levels after oral gavage by using gavage needles coated with various tastants. Naïve mice were gavaged with needles coated with water, sucrose, citrate, sucrose plus citrate, or NaCl solutions, and blood was collected 1 h after dosing by cardiocentesis. (A) Plasma corticosterone levels of tastant treatment groups were compared with those of control mice that did not undergo oral gavage procedure and of mice gavaged with water-coated needles. Values are expressed as mean ± SEM (n = 5 per group; *, P < 0.001 (ANOVA with Tukey post hoc analysis) compared with nongavaged controls; #, P < 0.001 (ANOVA with Tukey post hoc analysis) compared with group that received water-coated gavage needles. (B) Plasma corticosterone levels after oral gavage procedure in groups of mice by using water- or sucrose-coated needles with a total procedure time of 10 s. Values are expressed as mean ± SEM (n = 8 per group). +, P < 0.05 (2-tailed Student t test).
Discussion
Use of a sucrose solution as a mild analgesic and pacifier is widespread in hospital neonatal units.8,16,21 The administration of sucrose to newborns prior to a minimally painful procedure effectively reduces crying time4,5,17 and has lasting effects. Although sucrose has been shown to have analgesic properties in rodents,3,7,9,11,27,33,35 the use of sucrose or sweet solutions to refine animal procedures is underused in the field of laboratory animal medicine. The object of the current study was to determine whether precoating gavage needles with sucrose would facilitate oral gavage in mice, as measured by decreases in procedural time, observable animal stress, and animal glucocorticoid stress response. Improvements in these parameters likely will result in less variability in research studies and enhance animal welfare, both of which can result in technique refinement and increased data validity.34
Sucrose consistently proved to be an effective enhancer of oral gavage in mice. In both random and repeated gavage trials, time to passage was decreased when the gavage needle was coated with sucrose. In addition, observable stress was decreased with the use of sucrose-coated needles compared with water-coated, especially in naïve mice. The most compelling observation was that the addition of sucrose coating to the oral gavage procedure effectively eliminated the increase in plasma corticosterone that occurred in mice gavaged by using water-coated needles. Furthermore, the lower plasma corticosterone levels associated with sucrose coating were not merely due to shorter procedure time because when procedure time was held constant, plasma corticosterone levels were lower when sucrose-coated needles were used, compared to water-coated needles.
We hypothesized that citrate coating would induce reflex swallowing by stimulation of the superior laryngeal and glossopharyngeal nerves,22,28 however this treatment was not an effective enhancer of oral gavage. In addition, coating with citrate increased the corticosterone response and decreased the effectiveness of sucrose, possibly by reducing or eliminating the sweet taste. We expected a NaCl solution to similarly act as a negative control to water because salt increases latency to swallow due to interference with water receptors in the distal pharynx;36 however, we saw the opposite effect. Our results likely indicate that the swallow reflex, as mediated by chemoreceptors, has little influence on enhancement of the gavage procedure in mice. Recent work by others has begun to identify the brain regions associated with opioid-dependent analgesia and has established links between tastant responses from sucrose and ammonium chloride and the activation of brain regions in the brainstem and spinal cord believed to elicit analgesia.2 The beneficial effects that we report here of sucrose and NaCl on oral gavage may involve these same pathways, but further exploration is needed.
Animals exposed repeatedly to a specific stressor demonstrate either increased responsiveness (sensitization) or decreased responsiveness (habituation) to the stimulus.30 Factors such as stressor intensity, duration, frequency, and animal or strain predispositions influence habituation rate. Our results demonstrated that mice habituate to the oral gavage procedure after only one exposure and continue to habituate as trials progress. This habituation response was evident in terms of both time to passage and behavioral response across trials regardless of the solution used to precoat gavage needles.
We have validated the use of sucrose to improve the oral gavage technique in adult mice. This result is in contrast to earlier reports in humans29 and rats,3,35 which suggest that analgesic effects of sucrose are only present in newborns or young children, and decline with age, although conflicting reports in humans suggest adults are also responsive to sucrose.6 Our results clearly demonstrate that 8-wk-old mice are responsive to sucrose solutions, and suggest that the exploration of intraoral sucrose for painful or stressful procedures should be performed in groups other than neonates.
The use of sucrose to facilitate oral gavage offers several benefits over other published refinements including the use of gaseous anesthesia, which introduces additional risk and may increase the incidence of incomplete vehicle retention due to relaxation of the lower esophageal sphincter.25 In addition, the use of gaseous anesthetic agents introduces variables that may confound research, including cardiovascular and respiratory depression, transient immunosuppression, and sequestering of blood cell populations.18 Arguably, the use of sucrose may introduce confounding variables as well, due to increased blood glucose levels; however, the amount of sucrose ingested due to gavage needle coating is negligible, and any increases in blood glucose elevation likely are insignificant. Furthermore, the immediate pacification due to sucrose is not related to direct interaction between sucrose and opioid receptors23 but rather to release of endogenous opioids in response to a sweet taste sensation,4 suggesting that solutions using artificial sweeteners are likely effective. Intriguingly, the calming effects of sugars are due to their relative sweetness,9 and a noncarbohydrate solution of aspartame, with the same relative sweetness as sucrose, results in equivalent efficacy in infants.4 However, we cannot rule out possible nutritive effects of sucrose that may facilitate the beneficial reduction in stress during oral gavage.11 Further research needs to be conducted to evaluate the effectiveness of sweet solutions other than sucrose that will not affect blood glucose levels or perhaps will have increased efficacy.
Our results suggest that investigators using oral gavage in mouse studies should explore sucrose precoating of gavage needles to improve animal welfare and reduce confounding variables associated with procedure stress. Trained but relatively inexperienced operators likely will benefit more from using sucrose to facilitate oral gavage than will those who are highly experienced. Furthermore, the use of sucrose coating should be considered in the context of training, given that sucrose coating of gavage needles likely would decrease subject morbidity and improve the training experience for instructors and trainees. The use of sucrose to refine laboratory animal techniques need not only be confined to oral gavage in rodents. Investigation into ways the sensation of sweet taste can be used to improve common techniques for various laboratory species in turn will improve the field of laboratory animal science overall.
Acknowledgments
We thank Dr Jeff Fortman for critical review of the manuscript and for helping AH to conduct this research in parallel with research studies ongoing at IUSM. These studies were supported by grant HL069669 and HL096305 (to LMP) from the National Institutes of Health. JH is supported by NIH Training Grant DK07519.
References
- 1.American Association of Laboratory Animal Science [Internet]. Working with the laboratory mouse, chapter 12: oral gavage, 2005update [cited 21 Nov 2009]. Available at https://www.aalaslearninglibrary.org/demo/course2.asp?strKeyID=DCEBB092-A443-4C89-8A36-2511AFA1DF1C-0&Library=10&Track=8&Series=1243&Course=2451&Lesson=26654
- 2.Anseloni VC, Ren K, Dubner R, Ennis M. 2005. A brainstem substrate for analgesia elicited by intraoral sucrose. Neuroscience 133:231–243 [DOI] [PubMed] [Google Scholar]
- 3.Anseloni VC, Weng HR, Terayama R, Letizia D, Davis BJ, Ren K, Dubner R, Ennis M. 2002. Age-dependency of analgesia elicited by intraoral sucrose in acute and persistent pain models. Pain 97:93–103 [DOI] [PubMed] [Google Scholar]
- 4.Barr RG, Pantel MS, Young SN, Wright JH, Hendricks LA, Gravel R. 1999. The response of crying newborns to sucrose: is it a ‘sweetness’ effect? Physiol Behav 66:409–417 [DOI] [PubMed] [Google Scholar]
- 5.Barr RG, Quek VS, Cousineau D, Oberlander TF, Brian JA, Young SN. 1994. Effects of intraoral sucrose on crying, mouthing, and hand–mouth contact in newborn and 6-wk-old infants. Dev Med Child Neurol 36:608–618 [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharjee M, Bhatia R, Mathur R. 2007. Gender specificity of sucrose-induced analgesia in human adults. Indian J Physiol Pharmacol 51:410–414 [PubMed] [Google Scholar]
- 7.Blass EM, Fitzgerald E, Kehoe P. 1987. Interaction between sucrose, pain, and isolation distress. Pharmacol Biochem Behav 26:483–489 [DOI] [PubMed] [Google Scholar]
- 8.Blass EM, Hoffmeyer LB. 1991. Sucrose as an analgesic for newborn infants. Pediatrics 87:215–218 [PubMed] [Google Scholar]
- 9.Blass EM, Shide DJ. 1994. Some comparisons among the calming and pain-relieving effects of sucrose, glucose, fructose, and lactose in infant rats. Chem Senses 19:239–249 [DOI] [PubMed] [Google Scholar]
- 10.Brown AP, Dinger N, Levine BS. 2000. Stress produced by gavage administration in the rat. Contemp Top Lab Anim Sci 39:17–21 [PubMed] [Google Scholar]
- 11.D'Anci KE, Kanarek RB, Marks-Kaufman R. 1997. Beyond sweet taste: saccharin, sucrose, and polycose differ in their effects upon morphine-induced analgesia. Pharmacol Biochem Behav 56:341–345 [DOI] [PubMed] [Google Scholar]
- 12.Dobrakovova M, Jurcovicova J. 1984. Corticosterone and prolactin responses to repeated handling and transfer of male rats. Exp Clin Endocrinol 83:21–27 [DOI] [PubMed] [Google Scholar]
- 13.Dodds WJ. 1989. Physiology of swallowing. Dysphagia 3:171–178 [DOI] [PubMed] [Google Scholar]
- 14.Germann PG, Ockert D. 1994. Granulomatous inflammation of the oropharyngeal cavity as a possible cause for unexpected high mortality in a Fischer 344 rat carcinogenicity study. Lab Anim Sci 44:338–343 [PubMed] [Google Scholar]
- 15.Goyal RK, Padmanabhan R, Sang Q. 2001. Neural circuits in swallowing and abdominal vagal afferent-mediated lower esophageal sphincter relaxation. Am J Med 111Suppl 8A:95S–105S [DOI] [PubMed] [Google Scholar]
- 16.Gradin M, Finnstrom O, Schollin J. 2004. Feeding and oral glucose—additive effects on pain reduction in newborns. Early Hum Dev 77:57–65 [DOI] [PubMed] [Google Scholar]
- 17.Graillon A, Barr RG, Young SN, Wright JH, Hendricks LA. 1997. Differential response to intraoral sucrose, quinine, and corn oil in crying human newborns. Physiol Behav 62:317–325 [DOI] [PubMed] [Google Scholar]
- 18.Hanusch C, Hoeger S, Beck GC. 2007. Anaesthesia of small rodents during magnetic resonance imaging. Methods 43:68–78 [DOI] [PubMed] [Google Scholar]
- 19.Jacoby RO, Fox JG, Davisson M. 2002. Biology and diseases of mice, p 35–133. : Fox JG, Anderson LC, Lowe FM, Quimby FW. Laboratory animal medicine. New York (NY): Academic Press [Google Scholar]
- 20.Johnson MD. 2007. The rat, p 150–193. : Gad SC. Animal models in toxicology. Boca Raton (FL): CRC Press [Google Scholar]
- 21.Johnston CC, Filion F, Snider L, Majnemer A, Limperopoulos C, Walker CD, Veilleux A, Pelausa E, Cake H, Stone S, Sherrard A, Boyer K. 2002. Routine sucrose analgesia during the first week of life in neonates younger than 31 wk postconceptional age. Pediatrics 110:523–528 [DOI] [PubMed] [Google Scholar]
- 22.Kajii Y, Shingai T, Kitagawa J, Takahashi Y, Taguchi Y, Noda T, Yamada Y. 2002. Sour taste stimulation facilitates reflex swallowing from the pharynx and larynx in the rat. Physiol Behav 77:321–325 [DOI] [PubMed] [Google Scholar]
- 23.Kracke GR, Uthoff KA, Tobias JD. 2005. Sugar solution analgesia: the effects of glucose on expressed µ opioid receptors. Anesth Analg 101:64–68, table of contents [DOI] [PubMed] [Google Scholar]
- 24.Marceau JR, Murray H, Nanan RK. 2010. Efficacy of oral sucrose in infants of methadone-maintained mothers. Neonatology 97:67–70 [DOI] [PubMed] [Google Scholar]
- 25.Murphy SJ, Smith P, Shaivitz AB, Rossberg MI, Hurn PD. 2001. The effect of brief halothane anesthesia during daily gavage on complications and body weight in rats. Contemp Top Lab Anim Sci 40:9–12 [PubMed] [Google Scholar]
- 26.Nebendhal C. 2000. Routes of administration, p 463–483 : Krinke GJ. The laboratory rat. London (UK): Academic Press [Google Scholar]
- 27.Nikfar S, Abdollahi M, Etemad F, Sharifzadeh M. 1997. Effects of sweetening agents on morphine-induced analgesia in mice by formalin test. Gen Pharmacol 29:583–586 [DOI] [PubMed] [Google Scholar]
- 28.Pelletier CA, Lawless HT. 2003. Effect of citric acid and citric acid–sucrose mixtures on swallowing in neurogenic oropharyngeal dysphagia. Dysphagia 18:231–241 [DOI] [PubMed] [Google Scholar]
- 29.Pepino MY, Mennella JA. 2005. Sucrose-induced analgesia is related to sweet preferences in children but not adults. Pain 119:210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitman DL, Ottenweller JE, Natelson BH. 1988. Plasma corticosterone levels during repeated presentation of 2 intensities of restraint stress: chronic stress and habituation. Physiol Behav 43:47–55 [DOI] [PubMed] [Google Scholar]
- 31.Pomonis JD, Jewett DC, Kotz CM, Briggs JE, Billington CJ, Levine AS. 2000. Sucrose consumption increases naloxone-induced c-Fos immunoreactivity in limbic forebrain. Am J Physiol Regul Integr Comp Physiol 278:R712–R719 [DOI] [PubMed] [Google Scholar]
- 32.Qu WM, Huang ZL, Matsumoto N, Xu XH, Urade Y. 2008. Drug delivery through a chronically implanted stomach catheter improves efficiency of evaluating wake-promoting components. J Neurosci Methods 175:58–63 [DOI] [PubMed] [Google Scholar]
- 33.Roane DS, Martin RJ. 1990. Continuous sucrose feeding decreases pain threshold and increases morphine potency. Pharmacol Biochem Behav 35:225–229 [DOI] [PubMed] [Google Scholar]
- 34.Rowan AN. 1990. Refinement of animal research technique and validity of research data. Fundam Appl Toxicol 15:25–32 [DOI] [PubMed] [Google Scholar]
- 35.Segato FN, Castro-Souza C, Segato EN, Morato S, Coimbra NC. 1997. Sucrose ingestion causes opioid analgesia. Braz J Med Biol Res 30:981–984 [DOI] [PubMed] [Google Scholar]
- 36.Shingai T, Miyaoka Y, Ikarashi R, Shimada K. 1989. Swallowing reflex elicited by water and taste solutions in humans. Am J Physiol 256:R822–R826 [DOI] [PubMed] [Google Scholar]