Abstract
Oral administration of drugs to laboratory rodents typically is achieved by using the gavage technique. Although highly effective, this method occasionally can cause esophageal injury as well as restraint-associated distress, particularly with repeated use. The aim of this study was to assess an alternative oral dosing method that could reduce the distress and morbidity associated with standard gavage techniques. The palatability and pharmacokinetic profile of 2 medicines approved for the treatment of Alzheimer disease, donepezil and galantamine, were investigated in male Lister hooded rats by using a syringe-feeding method and compared with results from traditional gavage administration. In addition, the stimulant nicotine was tested by using the syringe-feeding method in a separate series of experiments. Animals reliably learned to drink voluntarily from the syringe, and latency to drink decreased rapidly. The addition of donepezil, galantamine, or nicotine to sucrose had no apparent effect on the palatability of the solution, although nicotine produced aversive effects that inhibited subsequent voluntary intake. Oral bioavailability was improved by using syringe feeding with donepezil but not galantamine. Both drugs improved cognitive performance in the novel object recognition test, with similar behavioral profiles between the 2 methods of administration. Our results suggest that the syringe-feeding technique is an effective alternative oral dosing method in rats.
Abbreviation: NOR, novel object recognition
Oral administration of substances is a common procedure in scientific experiments using laboratory animals and typically is achieved in conscious animals by using the intragastric gavage technique. Gavage is the introduction of a solution into the stomach by means of a tube and is used clinically and for research. In laboratory animals, dosage by gavage involves removing the animal from its cage, manually restraining it, inserting a small-diameter tube into the esophagus, and delivering the drug directly into the stomach by means of a syringe. Although highly effective, care must be taken to ensure that the tube or needle does not enter the trachea or damage the esophagus or stomach. In one study,7 a 32% mortality rate was attributable to asphyxia caused by impacted food and bedding material in the oropharynx of gavaged rats; granulomatous inflammation caused by the gavaging procedure appeared to be the source of the impaction. To ensure competent administration of the test substance, the animal must be restrained manually, which can distress the animal. A study using implanted telemetry transponders to investigate the intensity and duration of the stress caused by oral gavage demonstrated that the acute effect of the procedure on laboratory rats can last for 30 to 60 min afterward.4
Over the years, the gavage procedure has been refined to reduce morbidity and mortality in laboratory animals through the use of flexible cannulas, but no procedure as yet has been found to effectively replace this method.13,17 Several investigators have investigated novel means of oral drug delivery in rats. For instance, one group developed a technique for oral drug administration to rats by using premixed drug–chocolate pellets.8 Results from this technique demonstrated appropriate levels of drug absorption as indicated by effects of the test drugs in an in vivo model. Although effective, this method has several limitations. First, the theobromine and caffeine contents in chocolate may proscribe its use for oral drug administration in drug discovery and efficacy studies. Second, the drug must be stable, mix easily, and remain active in chocolate and be adequately palatable in dry form mixed with chocolate.8 Other investigators introduced a novel method of oral drug administration in a study in which a mixture of 5% sucrose solution and a neuroleptic drug (clozapine, haloperidol, or diazepam) was given to the test animals by using a syringe.14 Behavioral effects associated with these drugs then were investigated. All animals adapted to daily drug administration without any difficulties and displayed minimal effects on wellbeing, as reflected by voluntary participation in both drug administration and behavioral testing. This alternative method of oral administration was sufficient to cause changes in behavioral parameters previously reported by using traditional methods of drug administration.6,14 However, the study did not compare the novel method with traditional methods of oral drug administration nor report on pharmokinetic exposure after syringe delivery.
The aim of the present study was to investigate the palatability and pharmacokinetic profile of donepezil and galantamine, which are approved for the treatment of Alzheimer disease, by dosing male Lister hooded rats with the syringe method and comparing subsequent results with those from the traditional administration method of orogastric gavage. In a separate series of experiments, we also evaluated the stimulant nicotine (frequently used as a positive control in many cognitive behavioral tests) by using both dosing methods. We then investigated the efficacy of syringe feeding of donepezil and galantamine in enhancing cognitive performance in the novel object recognition (NOR) test and compared the results with those after gavage dosing. The NOR assessment is a simple test of recognition memory that exploits the tendency or preference of rats to explore a novel rather than a familiar object in their environment.1,5 The assay is known to be particularly sensitive to both animal handling and drug administration that may induce distress, making this test an excellent cognitive model for evaluating the efficacy of the 2 methods of oral administration under study.
Materials and Methods
Animals.
Male Lister hooded rats (age, 3 mo; Harlan, Bicester,UK) were housed 4 per cage (Techiplast, Kettering, UK) with corncob bedding (Harlan, Indianapolis, IN) in a temperature- (18 ± 2 °C) and humidity- (40 ± 5%) controlled SPF environment on a 12:12-h light:dark cycle with lights on at 0730. Excluded pathogens included: Sendai virus, pneumonia virus of mice, sialodacryoadenitis virus, Kilham rat virus, Toolan H1 virus, rat parvovirus, reovirus 3, Theiler murine encephalomyelitis virus, lymphocytic choriomeningitis virus, Hantaan virus, mouse adenovirus types 1 and 2, Bordetella bronchiseptica, Corynebacterium kutscheri, Citrobacter rodentium, Helicobacter hepaticus, Helicobacter bilis, Helicobacter rodentium, Clostridium piliforme, Salmonella spp., Streptobacillus moniliformis, Streptococcus pneumoniae, Pasteurella pneumotropica, Pseudomonas aeruginosa, Streptococci β-haemolytica (groups A, B, and C), Klebsiella pneumoniae, Pasturella spp., Mycoplasma pulmonis, Encephalitozoon cuniculi, Tritrichomonas muris, Entamoeba muris, ectoparasites, and endoparasites. Food (diet 2918, Harlan Teklad) and water were available ad libitum. All experiments complied with guidelines from the Singapore National Advisory Committee for Laboratory Animal Research11 regarding the use and care of animals for scientific procedures and met institutional (GlaxoSmithKline, Singapore) standards for the ethical use of animals in research.
Drugs.
Donepezil hydrochloride and galantamine hydrobromide were synthesized by Manus Aktteva (Gujarat, India). Nicotine hydrogen tartrate was obtained from Sigma–Aldrich (Poole, UK).
All drugs (calculated as free base) were prepared fresh each experimental day in a 10% sucrose solution to disguise any aversive taste of the drugs, and the same solutions were used for both oral and gavage syringe feeding. The drug-containing solution or vehicle (10% sucrose) was administered at 2 mL/kg for both methods. A cohort of animals was designated for each study, and animals were assigned such that all treatment groups were represented within each cage.
Cannulation and pharmacokinetic profiling.
Rats in the time-course pharmacokinetics experiment each received analgesic (10 mg/kg SC; 0.1 mL/100 g body weight; Torbugesic, Fort Dodge Animal Health, Fort Dodge, IA) and antibiotic (1 mg/kg SC; 0.05 mL/100 g; Baytril, Bayer Animal Health, Shawnee Mission, KS) before surgery.
Isoflurane-anesthetized rats were cannulated through the jugular vein for blood sampling. The cannula was exteriorized at the back of the neck, and each rat was placed in a harness–tether counterbalance system and housed singly in a solid-bottom plastic cage. Animals were allowed to recover for at least 2 d prior to initiation of experiments. Each cannula was flushed daily with 100 μL heparinized saline to prevent clotting. The total volume of blood removed from each rat during the pharmacokinetics study did not exceed 10% of the circulating blood volume (that is, 65 mL/kg),12 with 120 µL removed at each time point.
Palatability test.
The aim of the palatability tests was to determine whether rats (n = 6 per group) would voluntarily drink a drug in 10% sucrose from a syringe. We also sought to determine whether the presence of specific drugs in the 10% sucrose solution affected the latency to drink. Training and measurement of drinking latency was conducted over 5 consecutive days.
Pharmacokinetic profiling.
The aim of the pharmacokinetic experiments was to compare the drug exposure levels achieved in blood and brain between the syringe-dosing and intragastric gavage methods. In an acute study, rats were trained to drink a 10% sucrose solution (vehicle) from a syringe and on the fifth day received either donepezil hydrocholoride or galantamine hydrobromide (0.5 mg/kg; n = 3 per group) dissolved in the vehicle. Rats were euthanized 30 min after dosing, and drug concentrations in blood and brain were analyzed. For the time-course study, catheter-bearing rats were trained to drink the 10% sucrose solution; they then received either donepezil hydrocholoride or galantamine hydrobromide (0.5 mg/kg; n = 3 per group) in 10% sucrose on the final day by syringe. Control rats received both solutions by traditional orogastric gavage. Blood samples obtained at various times after dosing were analyzed for drug concentration.
Pharmacokinetic analysis.
Rats were euthanized by decapitation, trunk blood samples were collected into tubes containing EDTA, the tube contents were diluted 1:1 with deionized water, and 100-µL aliquots of each sample were transferred to tubes (Micronic, Quintech Scientific, Lelystad, Netherlands) in duplicate. The brain was removed as the entire organ and washed free of all blood by using sterile saline. Sample and control brains were homogenized with deionized water (1 mL/g tissue) by means of ultrasonication (TomTec Autogiser, Receptor Technologies, Adderby, Oxon, UK); 50-µL aliquots of control and sample brain homogenates were transferred to microfuge tubes for analysis.
Stock solutions (100 µg/mL) of galantamine and donepezil were prepared in ethanol. The compounds were cassetted and further diluted in ethanol to give spiking solutions in the range of 0.01 to 10 µg/mL. Standard curves were prepared in microfuge tubes by adding known amounts of the diluted stock solutions to 50 μL control rat blood diluted with 50 µL water or to 50 µL control brain homogenate to yield a calibration range of 5 to 5000 ng/mL or 0.5 to 500 ng/mL, depending on study requirements.
Samples were extracted by using protein precipitation; 350 µL precipitation buffer (80% acetonitrile, 20% 10 mM ammonium acetate [native pH]) and 100 ng/mL internal standard (a GlaxoSmithKlein proprietary compound of similar structure) were added to all standards and samples. The extracts were mixed on a plate shaker for approximately 20 min, followed by centrifugation at approximately 320 × g for 15 min.
HPLC was accomplished by using a pumping system (model 1100, Agilent, Santa Clara, CA), autosampler (CTC HTS PAL, Presearch, Hampshire, UK), vacuum degasser, and column (5 cm × 4.6 mm; particle size, 5 µm, Discovery Cyano column, Supelco, Sigma–Aldrich). Mass spectrometric analysis was performed in positive-ion mode by using a triple-quadrupole system (Applied Biosystems, Foster City, CA) instrument equipped with a ion-spray (Turbo Ion Spray, Applied Biosystems) interface. The separation column was stabilized at 40 °C. Acetonitrile (from 1% to 90% in 1.2 min and held at 90% for 0.4 min before returning to 1% initial conditions in 0.1 min) was the solvent; the total flow rate was 1 mL/min. Injection volume was 3 µL with a run-time of 2 min for both blood and brain homogenate analysis. The supernatant was injected directly from the microfuge tubes. The column effluent was split so that 250 µL/min was directed into the ion-spray source; the heater temperature was set at 690 °C. The compounds were screened and quantitated by using multiple-reaction monitoring. The mass spectrometer was programmed to scan the transitions m/z 288.04 to 213.30 for galantamine and m/z 380.10 to 91.00 for donepezil, with dwell times of 150 ms. The transitions and voltages used were selected by using an automated optimization procedure (Quantitative Optimization, Applied Biosystems).
Calibration lines were generated by using a linear 1/x2 regression with an acceptance criterion of ±20% for each standard, and at least 12 of 16 standards had to pass the acceptance criteria for the assay results to be accepted.
The lower limits of quantification for donepezil and galantamine in blood were 0.5 ng/mL (0.001 µM) and 5 ng/mL (0.017 µM) in blood, respectively. In brain, the lower limit of quantification for both compounds was 10 ng/mg, which equates to 0.026 µM and 0.035 µM for donepezil and galantamine, respectively. Any sample that was below the lowest limit of quantification was reported as nonquantifiable.
NOR: 24-h temporal deficit model.
NOR analysis was carried out as described previously.1 Briefly, animals were prehandled and sham-dosed with 10% sucrose (syringe feeding or intragastric gavage method) before and after a 1-h habituation session to the test caging (Tecniplast) for 2 d before the initial presentation of the objects (object exploration trial). The objects used in these studies were custom-fabricated black acrylic cubes (5 cm3) and cylinders (diameter and height: 5 cm; Labman Design, Singapore). Magnets were recessed into the base of the objects and the test cage positioned upon a magnetic plate which prevented the animals from moving the objects during the exploration trials. Based on the findings from our PK studies, donepezil hydrocholoride, galantamine hydrobromide (0.5 mg/kg; n = 8 per group) or vehicle (n = 8 per group) was administered 30 min prior to the novel object exploration and object recognition trials.
For the novel object exploration trial, the animals were habituated to the test cage without objects for 3 min. The animals were then briefly moved to an adjacent cage for approximately 10 s, while 2 identical objects were placed into the test cage. The animals were then placed back for a further 3 min, and the time spent exploring each object recorded by an experienced observer. For the object recognition trial, animals were placed back into the test cage for a further 3 min habituation period, 24 h after the novel object exploration trial and then presented with 1 familiar and 1 novel object for a total of 3 min, and object exploration recorded.
Objects were assigned randomly to ensure that treatment groups were fully balanced for both the novel object and its position within the test cage (either left or right). Object exploration was recorded only when the rat's nose or mouth was in close contact with the object. Climbing or resting on the objects was not scored as exploration. The discrimination index was calculated as time spent exploring novel object – time spent exploring familiar object / total exploration time.
Statistical analysis.
All graphs were prepared using GraphPad Prism (version 4, GraphPad Software, San Diego, CA). Pharmacokinetic data are expressed as mean ± 1 SD; data from the novel object recognition tests are expressed as mean ± SEM. Statistical analysis was performed by using StatSoft Statistica (version 6, StatSoft, Tulsa, OK), and all data were checked for normality prior to analysis. A P value of less than 0.05 was used to define significance.
Drinking latencies over time and data from the time-course experiment were analyzed by using repeated-measures one-way ANOVA followed by a least significant difference test. Pharmacokinetic data were analyzed by ANOVA followed by the post hoc Dunnett t test.
For the novel object recognition, one-way ANOVA followed by planned comparisons was used to compare treatment groups in the novel object presentation and discrimination index data. For the novel object recognition data, repeated-measures ANOVA followed by planned comparisons was used to compare novel with familiar object exploration.
Results
Overall animal behavior.
All animals voluntarily and readily learned to drink from the syringes containing the sucrose and donepezil and galantamine. After 3 d of training, all of the rats would line up at the cage edge (Figure 1) to receive their respective treatments once the cage lid was pulled back. Therefore, no physical restraint was necessary during dosing, and administration could be carried out in the animal's homecage. Rats were not water-deprived during any experiments. Preliminary studies indicated that the volume associated with 5-mL/kg dosing was too large for a single administration, whereas a 2-mL/kg volume was manageable.
Figure 1.
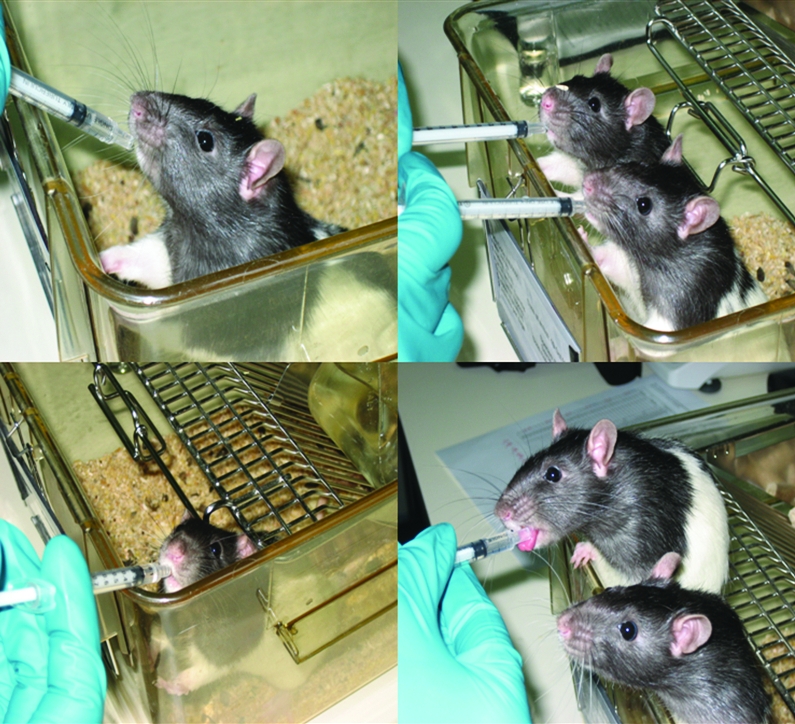
Rats readily drinking galantamine (0.5 mg/kg) by the novel syringe-dosing method on day 3 of training.
For the nicotine group, all animals voluntarily drank from the syringes containing the sucrose solution on day 1. However by day 2, all rats lined up at the front of the cage but as soon as they tasted the nicotine solution, they rapidly moved away from the syringe and returned to the back of the cage, refusing to cooperate to drink voluntarily.
Palatability test.
Total latency to drinking did not differ between sucrose solution containing donepezil (Figure 2 A) or galantamine (Figure 2 B) over the 5-d training period. The average time needed to administer a 2-mL/kg dose dramatically decreased from around 60 s on day 1 to less than 30 s by day 5. No animal refused to drink from the syringe, and all animals appeared normal during and after drug administration. The rats’ voluntary participation and lack of difference between the 2 treatment groups suggested to us that any potential aversive taste of the drug solutions was masked effectively by the sucrose solution. Therefore, we decreased the training period to 3 d for all subsequent experiments.
Figure 2.
Latency to drinking for (A) donepezil (0.5 mg/kg; syringe-dosed) and (B) galantamine (0.5 mg/kg; syringe-dosed). Total drinking latency did not differ between vehicle only (10% sucrose), donepezil, and galantamine over the 5-d training period. Data are expressed as mean ± SEM (n = 5).
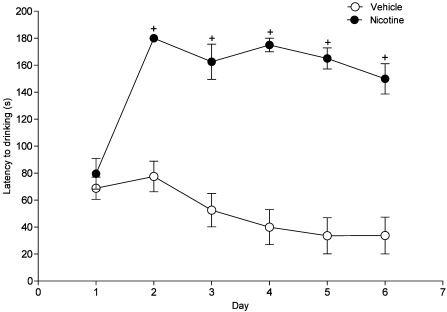
Drinking latency on day 1 did not differ between the sucrose control and nicotine experimental groups. However, rats refused to drink the nicotine solution on subsequent days (Figure 3). On day 1, rats displayed various side effects, including labored breathing, increased heart rate, and decreased locomotor activity immediately after ingestion of the nicotine solution.
Figure 3.
Drinking latency of nicotine (0.5 mg/kg; syringe-dosed). No differences in total drinking latencies between the vehicle (10% sucrose solution) and nicotine were present during the first training day (day1). However by day 2, total drinking latency differed significantly (+, P < 0.001) between vehicle- and nicotine-treated rats. All animals subsequently failed to drink the nicotine solution over the remaining training days. Data are expressed as mean ± SEM (n = 6).
Acute pharmacokinetic profiling of donepezil and galantamine.
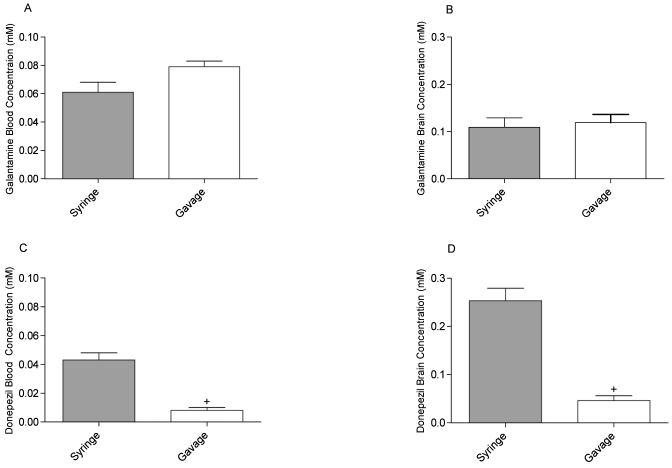
Blood and brain galantamine concentrations were not different in syringe- and gavage-dosed rats when sampled 30 min after a single dose (Figure 4 A, B). However, syringe-dosed animals showed a 5.4-fold increase in donepezil concentration in the blood (F [2, 6] = 139.50, P < 0.001, Figure 4 C) and brain (F [2, 6] = 92.43, P < 0.001, Figure 4 D) concentrations when compared with gavage-dosed animals.
Figure 4.
(A, C) Blood and (B, D) brain concentrations of (A, B) galantamine and (C, D) donepezil in syringe- and gavage-dosed rats. Donepezil syringe-dosed animals showed 5.4-fold (+, P < 0.001) increases in donepezil concentration in blood and brain compared with gavage-dosed animals. Data are expressed as mean ± SEM (n = 3).
Time-course study of donepezil and galantamine pharmacokinetics.
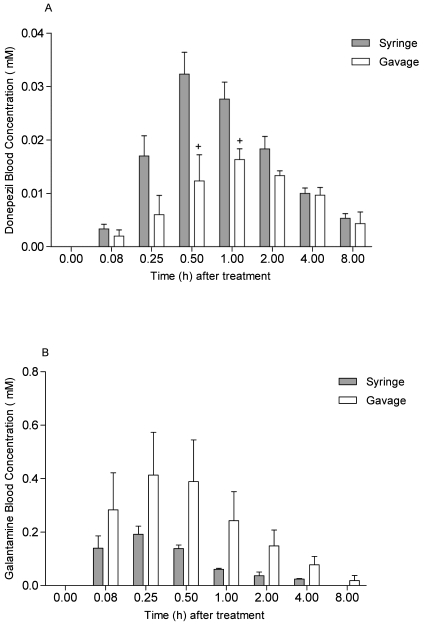
After a single dose of donepezil, both syringe- and gavage-dosed rats reached maximal blood concentration (0.032 ± 0.007 nM to 0.028 ± 0.006 nM and 0.012 ± 0.009 nM to 0.016 ± 0.004 nM, respectively; Figure 5 A) within 30 to 60 min after treatment. Peak concentrations steadily declined, and by the 8-h time point, blood concentration was reduced to 0.005 ± 0.002 nM (syringe-dosed) and 0.0054 ± 0.004 nM (gavage-dosed) rats. Syringe-dosed animals showed significantly (F [6, 24] = 6.48, P < 0.001) higher blood concentrations at the 30- and 60-min postdose time points when compared with gavage-dosed animals.
Figure 5.
Blood pharmacokinetics profiles of (A) donepezil and (B) galantamine. Blood samples were collected over an 8-h period after administration of a single dose (0.5 mg/kg). Whereas blood concentrations of galantamine did not differ over time between syringe and gavage-dosed rats, donepezil syringe-dosed animals showed significantly (+, P < 0.05) higher blood concentrations at 30 and 60 min after dosage. Data are expressed as mean ± SEM (n = 6).
Similarly, a single dose of galantamine reached maximal blood concentration (syringe-dosed rats, 0.138 ± 0.023 nM; gavage-dosed rats, 0.389 ± 0.270 nM; Figure 5 B) by 30 min after treatment. This peak steadily declined, and by the 8-h time point, blood galantamine was below the limit of detection in syringe-dosed rats and 0.019 ± 0.032 nM in gavage-dosed animals. Overall galantamine blood exposure levels did not differ over time between routes of administrations (F [1, 6] =0.93, P = 0.49). Detailed pharmacokinetics data are summarized in Table 1. Because rats could not be trained to drink a nicotine solution, no PK samples were taken from this experiment.
Table 1.
Summary of galantamine and donepezil pharmacokinetic data
| Donepezil |
Galantamine |
|||
| Syringe | Gavage | Syringe | Gavage | |
| Maximal concentration (µM) | 0.032 ± 0.004a | 0.018 ± 0.020 | 0.196 ± 0.030 | 0.414 ± 0.160 |
| Tmax (h; median [range])# | 0.500 (0.5–0.5) | 1.0 (0.5-2.0) | 0.3 (0.1-0.3) | 0.3 (0.3-0.5) |
| AUC(0-t) (µM • h) | 0.104 ± 0.020 | 0.074 ± 0.005 | 0.282 ± 0.006 | 0.942 ± 0.405 |
| AUCt/Dose (min • kg / L) | 5.070 ± 0.540 | 3.900 ± 0.370 | 9.924 ± 0.141 | 31.283 ± 13.540 |
AUC, area under the time–concentration curve; t, time of the last measured blood sample
Data are expressed as mean ± SEM (n = 3).
Significantly (P < 0.05) different from value for gavage-dosed donepezil.
NOR: 24-h deficit model.
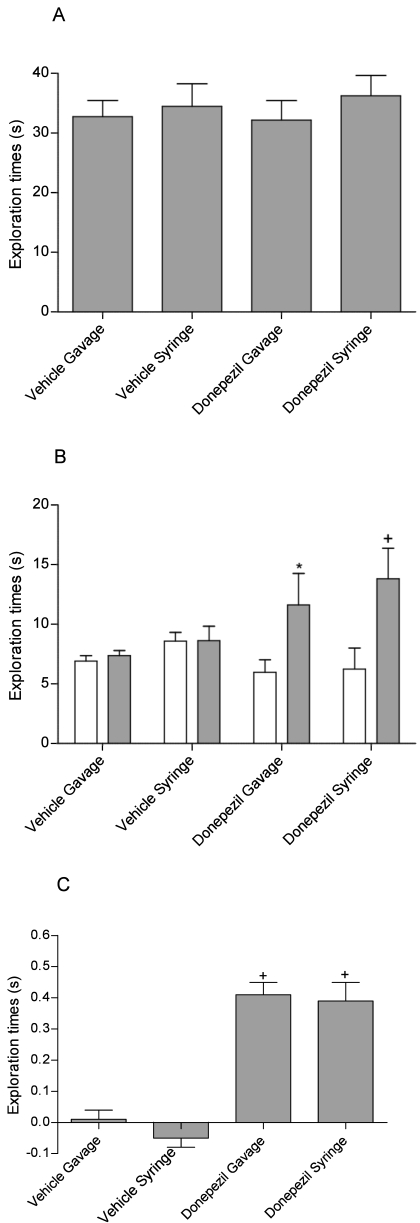
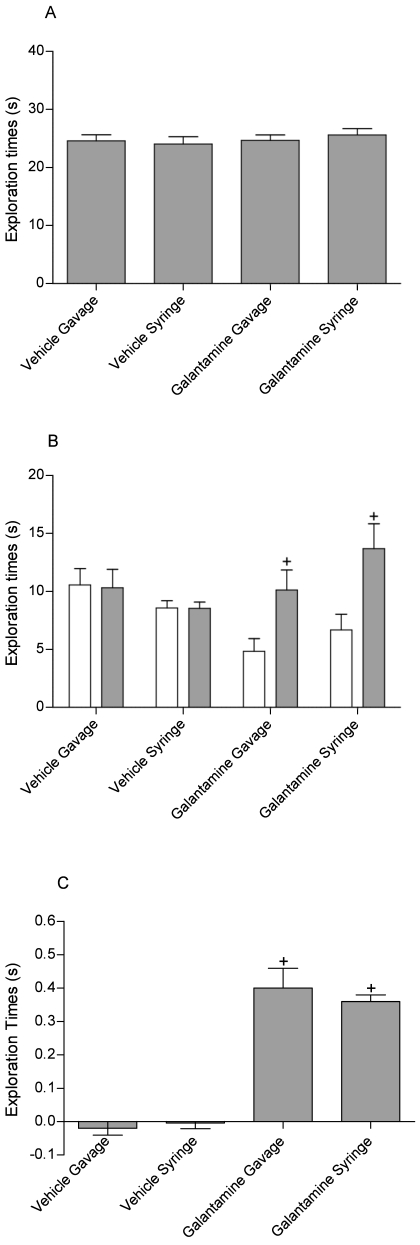
As expected in this 24-h temporal deficit model,1,5 vehicle-treated rats explored the novel and familiar objects at similar levels during both studies (Figures 6 A and 7 A). Donepezil treatment had no effect on overall object exploration during the T1 trial (F [3, 28] = 0.37, P = 0.77, Figure 6 A). Analysis of data from the T2 donepezil study revealed a significant effect of treatment on novel versus familiar exploration (F [1, 28] = 15.02, P < 0.001), and posthoc analysis revealed that both gavage- and syringe-dosing of donepezil significantly (syringe, P < 0.001; gavage, P < 0.01; Figure 6 B) enhanced exploration. Furthermore, recognition index values revealed a significant (F [3, 28] = 29.00, P < 0.001) effect of treatment, and planned comparison analysis showed that both dose groups were significantly (syringe, P < 0.001; gavage, P < 0.001; Figure 6 C) different from their vehicle controls.
Figure 6.
Effect of donepezil (0.5 mg/kg) administered 30 min prior to testing on (A) total object exploration, (B) novel versus familiar object exploration, and (C) the recognition index (Exploration of novel object [s] – exploration of familiar object [s] / total exploration [s]). Donepezil enhanced recognition memory, as evident by a significant (+, P < 0.001) increase in novel versus familiar exploration, which translated to a significant (*, P < 0.01, +, P < 0.001) increase in the recognition index in both syringe- and gavaged-dosed animals. Overall recognition memory did not differ between syringe and gavaged-dosed animals. Data are expressed as mean ± SEM (n = 8).
Figure 7.
Effect of galantamine (0.5 mg/kg) administered 30 min prior to testing on (A) total object exploration, (B) novel versus familiar object exploration, and (C) the recognition index (Exploration of novel object [s] – exploration of familiar object [s] / total exploration [s]). Galantamine enhanced recognition memory, as evident by a significant (P < 0.001) increase in novel versus familiar exploration, which translated to a significant (P < 0.001) increase in the recognition index in both syringe- and gavaged-dosed animals. Overall recognition memory did not differ between syringe- and gavaged-dosed animals. Data are expressed as mean ± SEM (n = 8).
The galantamine study similarly showed a significant effect of treatment on novel versus familiar exploration (F [3, 28] = 103.41, P < 0.001, Figure 7 B), and posthoc analysis revealed a significant (syringe, P < 0.001; gavage, P < 0.001; Figure 7 B) effect in both dose groups. Analysis of the recognition index values also revealed a significant effect of treatment (F [3, 28] = 0.30, P = 0.82, Figure 7 C), and planned comparison analysis showed that both dose groups were significantly (syringe, P < 0.001; gavage, P < 0.001; Figure 7 C) different from their vehicle controls.
Discussion
The aim of this study was to assess a syringe-dosing technique that may reduce the adverse effects, distress due to physical restraint, and morbidity associated with the gavage procedure in rats, thereby providing an alternative to gavage.
The syringe-dosing technique initially involved a 5-d training period during which the rats learned to drink a vehicle solution (10% sucrose) from the syringe. All rats successfully drank the vehicle solution (10% sucrose) from a syringe by day 3, and all approached this task voluntarily. Furthermore, all rats cooperated with the daily drug administration of both donepezil and galantamine. In contrast, rats did not cooperate with daily syringe administration of nicotine. All rats voluntarily drank the nicotine solution on day 1 of the 5-d training period; therefore palatability was not a problem. On day 1 we observed that animals displayed various side-effects immediately after nicotine administration, including labored breathing, increased heart rate, and decreased locomotor activity. By day 2, all rats refused to drink the nicotine solution voluntarily and moved away from the syringe when they tasted the solution. We consider that the animals clearly displayed a conditioned taste aversion to the adverse drug effects.
We then initiated pharmacokinetic profiling to compare the blood and brain drug concentrations achieved by the 2 oral-dosing techniques. Blood and brain galantamine concentrations did not differ between syringe- and gavage-dosed animals. The pharmacokinetics time-course study showed no differences in measured parameters between galantamine syringe- and gavage-dosed rats (Table 1). In contrast, donepezil showed a 5.4-fold increase in blood and brain concentrations in syringe-dosed rats compared with gavage-dosed animals. In addition, syringe-dosed rats had higher blood concentrations of donepezil at the 30- and 60-min time points than did gavage-dosed animals (Figure 5). Furthermore, the peak concentration of donepezil was greater (P < 0.05, Table 1), in syringe-dosed compared with gavaged-dosed animals.
Donepezil undergoes extensive first-pass hepatic metabolism after oral administration.9 The 5-fold increase in blood and brain concentrations of donepezil in the syringe-dosed animals may reflect lingual or buccal absorption of part of the dose, involving rapid absorption through the capillaries of the mouth.3,15,16 In contrast, the entire donepezil dose in the gavage-dosed animals would have been subjected to first-pass hepatic metabolism; therefore, more of the dose would have been exposed to first-pass metabolism before being distributed throughout the body.9
Both donepezil and galantamine significantly improved rodent object recognition memory. Both syringe- and gavage-dosed animals demonstrated increased cognitive performance at the 0.5-mg/kg dose. Memory retention did not differ between the 2 oral dosing procedures in both the donepezil and galantamine studies, suggesting that the syringe-dosing method offers a useful alternative route of oral administration. However, the increased levels of donepezil due to the syringe-dosing technique did not lead to an improvement in cognitive enhancement at the dose level evaluated. The novel object recognition test is known to be particularly sensitive to both animal handling and drug administration methods that may induce distress. In such situations, a distressed animal can become afraid of the objects and will spend very little time exploring them (or sometimes not even approach the object), resulting in a failed study. The fact that we observed no difference in memory retention between the 2 oral dosing procedures used in both drug studies provides good confidence that our animals were not unduly distressed when treated by using the traditional gavage method (that is, the operator was competent). This feature is important because physiologic changes due to pain and distress may increase variability in experimental data, masking real results. Furthermore, techniques that reduce variability have the potential to decrease the number of animals required for a given experiment.
Oral gavage is a precise technique that a skilled individual can perform in only a few seconds. However, gavage can be difficult if the operator is not fully competent or confident in his or her animal handling skills. In such situations, animal morbidity may increase due to repeated incorrect intubations during which the gavage tube can pass accidentally into the trachea instead of the esophagus, causing fluid to be deposited in the lungs.2 Reflux of fluid also can occur if gavage dosing is performed too rapidly. Furthermore, gavage dosing requires an animal to be restrained through scruffing.2 The degree of scruffing is important, because if the animal is not scruffed sufficiently, it can struggle and move its head during gavage dosing, which can result in an incomplete or missed dose. In contrast, if the animal is scruffed too tightly, it will display various signs of distress and aggression, such as biting, scratching, and vocalization and can lead to esophageal damage.2 These potential complications can be avoided with the syringe-feeding method. Furthermore, the syringe-feeding method described here allows accurate drug administration to individual animals by operators with minimal handling experience. Another advantage of the syringe method is that animals need not be individually housed, as is the case when drug is mixed with food or drinking water and serum screening is avoided.2
Limitations of the syringe-dosing method include a learning period of approximately 3 d to ensure consistent and reliable drug administration. This lag may be suboptimal if a study involves dosing an animal only once. In such a scenario, a learning period of 3 d may be too time-consuming and gavage dosing might be more appropriate. In contrast, the syringe-dosing method may be more appropriate for studies involving subchronic dosing over several days or multiple doses daily; in these situations, syringe dosing would minimize animal restraint and handling, thus decreasing distress, over gavage dosing. However, syringe feeding is more time-consuming, requiring more time to dose each animal, than is gavage dosing. Some studies, especially those that involve a large number of animals, may not be able to accommodate the increased time needed for syringe dosing. In such cases, assessing technical competency in terms of manual animal restraint versus required dosing time becomes an important consideration in experimental design.
For the syringe-dosing method to be successful, the drug must be sufficiently palatable in sucrose solution for voluntary consumption and must be free from aversive side-effects. Results obtained from the nicotine experiment illustrated the importance of conditioned aversion in an animal's learning. Our study showed that all animals experienced adverse effects immediately after voluntarily drinking nicotine solution from a syringe, such that all animals learned to refuse the solution offered by syringe feeding on all subsequent days. Another limitation of syringe dosing is the limited dose volume compared with that for gavage dosing.
We found that a dose volume of 5 mL/kg was too large for syringe dosing and that animals were unable to drink this volume voluntarily in a single attempt. Reducing the dose volume to 2 mL/kg was more appropriate for syringe dosing, because animals could drink this entire volume easily in a single attempt. Dose volume should be taken into account when designing studies, because some drugs do not dissolve readily into solution and may have to be administered in suspension, which typically requires a higher dose volume. In these situations the gavage dosing method would be more suitable. Finally, masking drugs in sucrose solution may not be appropriate for studies involving diabetic models, particularly diabetic transgenic animals (such as the nonobese diabetic mouse or the db/db diabetic mouse), in which excess glucose can exacerbate the condition. In such cases, saccharin may be an alternative way to mask the taste of drugs making it solution more palatable for the animals.10
In conclusion, we describe a syringe-dosing technique that may offer an alternative form of oral drug administration in rats. Future studies will need to identify whether this syringe-dosing technique will be a useful method for long-term dosing. Additional studies also will need to investigate whether rats are still capable of voluntarily and readily drinking the sucrose solution over a few weeks, rather than a few days, and whether latency to drinking remains consistent. The effects of alterations in hepatic structure and function during long-term (that is, weeks rather than days) and increased (that is, to mask the strong adverse taste of a compound) use of sucrose also warrant further investigation, because overnight feeding of sucrose (sugar cubes) can alter hepatic structure and function.18
Acknowledgments
The authors thank Paul F Chapman (Centre for Cognition and Neurodegeneration, GlaxoSmithKline Research and Development China, Singapore) for his advice regarding the manuscript and Simon Bate and Andrew Lloyd from the GlaxoSmithKline Discovery Statistics Department (Harlow, UK) for their advice regarding the statistical analysis of these data.
References
- 1.Atcha Z, Chen WS, Ong AB, Wong FK, Neo A, Browne ER, Witherington J, Pemberton DJ. 2009. Cognitive enhancing effects of ghrelin receptor agonists. Psychopharmacology (Berl) 206:415–427 [DOI] [PubMed] [Google Scholar]
- 2.Barnett SW. 2007. Manual of animal technology, 1st ed Hoboken (NJ): Wiley–Blackwell [Google Scholar]
- 3.Beckett AH, Triggs EJ. 1967. Buccal absorption of basic drugs and its application as an in vivo model of passive drug transfer through lipid membranes. J Pharm Pharmacol 19Suppl:31S–41S [PubMed] [Google Scholar]
- 4.Bonnichsen M, Dragsted N, Hansen AK. 2005. The welfare impact of gavaging laboratory rats. Anim Welf 14:223–227 [Google Scholar]
- 5.Ennaceur A, Delacour J. 1988. A new one-trial test for neurobiological studies of memory in rats I: behavioral data. Behav Brain Res 31:47–59 [DOI] [PubMed] [Google Scholar]
- 6.Gao XM, Hashimoto T, Cooper TB, Tamminga CA. 1997. The dose-response characteristics of rat oral dyskinesias with chronic haloperidol or clozapine administration. J Neural Transm 104:97–104 [DOI] [PubMed] [Google Scholar]
- 7.Germann PG, Ockert D. 1994. Granulomatous inflammation of the oropharyngeal cavity as a possible cause for unexpected high mortality in a Fischer 344 rat carcinogenicity study. Lab Anim Sci 44:338–343 [PubMed] [Google Scholar]
- 8.Huang-Brown KM, Guhad FA. 2002. Chocolate, an effective means of oral drug delivery in rats. Lab Anim (NY) 31:34–36 [DOI] [PubMed] [Google Scholar]
- 9.Matsui K, Mishima M, Nagai Y, Yuzuriha T, Yoshimura T. 1999. Absorption, distribution, metabolism, and excretion of donepezil (Aricept) after a single oral administration to rat. Drug Metab Dispos 27:1406–1414 [PubMed] [Google Scholar]
- 10.Messier C, White NM. 1984. Contingent and noncontingent actions of sucrose and saccharin reinforcers: effects of taste preference and memory. Physiol Behav 32:195–203 [DOI] [PubMed] [Google Scholar]
- 11.National Advisory Committee for Laboratory Animal Research. [Internet]. 2004. Guidelines on the care and use of animals for scientific purposes. [Cited Jul 2009]. Available at http://www.ava.gov.sg/NR/rdonlyres/C64255C0-3933-4EBC-B869-84621A9BF682/8338/Attach3_AnimalsforScientificPurposes.PDF.
- 12.Office of Animal Care and Use. [Internet]. 2008. Guidelines for survival bleeding of mice and rats. [Cited Jul 2009]. Available at http://oacu.od.nih.gov/ARAC/survival.pdf.
- 13.Õkva K, Tamoševiciute E, Cižiute A, Pokk P, Rukšenas O, Nevalainen T. 2006. Refinements for intragastric gavage in rats. Scandinavian J Lab Anim Sci 33:243–252 [Google Scholar]
- 14.Schleimer SB, Johnston GAR, Henderson JM. 2005. Novel oral drug administration in an animal model of neuroleptic therapy. J Neurosci Methods 146:159–164 [DOI] [PubMed] [Google Scholar]
- 15.Shojaei AH. 1998. Buccal mucosa as a route for systemic drug delivery: a review. J Pharm Pharm Sci 1:15–30 [PubMed] [Google Scholar]
- 16.Smart JD. 2005. Buccal drug delivery. Expert Opin Drug Deliv 2:507–517 [DOI] [PubMed] [Google Scholar]
- 17.Wheatley JL. 2002. A gavage dosing apparatus with flexible catheter provides a less stressful gavage technique in the rat. Lab Anim (NY) 31:53–56 [DOI] [PubMed] [Google Scholar]
- 18.Turner PV, Albassam M, Walker RM. 2001. The effects of overnight fasting, feeding, or sucrose supplementation prior to necropsy in rats. Contemp Top Lab Anim Sci 40:36–40 [PubMed] [Google Scholar]