Abstract
We report on the reversible association of anionic liposomes induced by an antimicrobial peptide (LAH4). The process has been characterized for mixed membranes of POPC and POPS at molar ratios of 1:1, 3:1, and 9:1. Although the vesicles remain in suspension in the presence of excess amounts of peptide, the addition of more lipids results in surface charge neutralization, aggregation of the liposomes, and formation of micrometer-sized structures that coexist in equilibrium with vesicles in suspension. At low ratios of anionic lipids, vesicle aggregation is a reversible process, and vesicle disassembly is observed upon inversion of the surface charge by further supplementation with anionic vesicles. In contrast, a different process, membrane fusion, occurs in the presence of high phosphatidylserine concentrations. Upon binding to membranes containing low POPS concentrations, the peptide adopts an in-plane α-helical structure, a secondary structure that is conserved during vesicle association and dissociation. Our finding that peptides are essential for vesicle aggregation contributes to a better understanding of the activity of antimicrobial peptides, and suggests an additional layer of complexity in membrane-protein lipid interactions.
Abbreviations used: ATR, attenuated total reflectance; CD, circular dichroism; DH, hydrodynamic diameter; DLS, dynamic light scattering; SLS, static light scattering; FTIR spectroscopy, Fourier transformed infrared spectroscopy; LUV, large unilamellar vesicle; SUV, small unilamellar vesicle; PDI, polydispersity index; L/P, phospholipid-to-peptide ratio; POPC, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine; POPS, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoserine; NBD-PC, 1-Oleoyl-2-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]lauroyl]-sn-glycero-3-phosphocholine; Rhodamine-DHPE, 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(Lissamine rhodamine B sulfonyl); RET, resonance energy transfer
Introduction
Linear cationic amphipathic peptides serve many important biological functions and are of considerable biomedical and biotechnical interest (1–3). Hundreds of such compounds have been isolated from natural sources, including fungi, protista, eubacteria, plants, and animals (4,5), and many more have been created by design (6–9). For example, numerous organisms produce and store linear cationic peptides, and many these peptides exhibit fungicidal or bactericidal activities (1,2). Furthermore, cell-penetrating peptides have been identified that are cationic and often amphipathic, and are able to transport nucleic acids and polypeptides across cellular membranes (3,10,11).
The amphipathic distribution of polar and hydrophobic residues results in pronounced interactions of these peptides with phospholipid membranes (12,13). In many cases, membrane-active compounds have been shown to disturb the integrity of lipid bilayers, even at low concentrations, by the creation of defects, disruption, or pore formation (12,14). Such changes result in the collapse of transmembrane electrochemical gradients and therefore provide an explanation for the antimicrobial activities of some of these peptides (12,14). Alternatively, depending on the sequence and the environment, a number of peptides have been identified that interact and pass the cytoplasmic membrane without causing cell death (3,10,11,15–18). Before we can rationally design cheaper and/or more efficient analogs of antimicrobial and/or cell-penetrating peptides, we must achieve a better understanding of how they work and how they interact with membranes (6–9).
By using magainins, cecropins, and other linear amphipathic peptides as templates, researchers have synthesized a number of peptide sequences with a high propensity to form amphipathic α-helical structures in membrane environments. These include several LAH4 sequences that possess a central core of leucines, alanines, and four histidines (19). With the latter residues, one can manipulate the polarity and hydrophobic moment of the helix by merely changing the pH, and thus gain deeper insights into the energetic determinants of membrane topology (19–21). Indeed, many of these sequences align parallel to the membrane surface at acidic pH, when the histidines carry positive charges, but adopt transmembrane alignments at neutral pH (19–21). Of interest, the peptides exhibit lower antimicrobial activities in neutral environments than in acidic ones (6–9), concomitant with the formation of self-aggregated structures in solution when the histidines are deprotonated (19). The pH-dependent transitions in charge and amphipathic properties play another important role in another known biological activity of this peptide family, namely, the transfection of nucleic acids into eukaryotic cells (22–24,57).
In this work, we report that LAH4 induces the reversible association of liposomes in a manner that is strongly dependent on the phospholipid-to-peptide (L/P) ratio. This process is different from the irreversible fusion of membranes that has been observed for a number of membrane-active peptides (25–31), and, to our knowledge, it has not yet been described in detail. To that end, we analyzed the aggregation/dissociation phenomena in a quantitative manner. Our data indicate that aggregation occurs when the peptide bound to the membranes neutralizes the negative charges of the phospholipid headgroups. The process is reversible when the lipid concentration is increased by supplementing the sample with additional vesicles. To ascertain the role of the peptide in the clustering of the vesicle, we performed a set of experiments in which the membrane surface charge density was varied with the use of several different mixtures of POPC and POPS. Furthermore, by using different peptide concentrations, we were able to analyze the role of the peptide in the displacement of the equilibrium established between aggregated and nonaggregated vesicles. We found that the α-helical structure of the peptide did not change during the process of vesicle aggregation and dissociation. The results are interpreted in terms of interactions between in-plane oriented peptides and/or between peptides and phospholipids belonging to adjacent bilayers.
Materials and Methods
Peptide and lipids
The LAH4 peptide (sequence: KKALLALALHHLAHLALHLALALKKA) was synthesized using standard FMOC solid-state chemistry on a Millipore 9050 synthesizer and purified by high-performance liquid chromatography on a ProntoSIL-C4 column with an acetonitrile/water gradient (purity > 90%). Its identity was confirmed by matrix assisted laser desorption ionization mass spectrometry (m/z = 2779.4). The lipids POPC and POPS were purchased from Avanti Polar Lipids (Alabaster, AL) and used without further purification. Two dye-labeled lipids (NBD-PC and Rhodamine-DHPE) were obtained from Avanti Polar Lipids and Invitrogen (Carlsbad, CA), respectively.
Liposome preparation
Calibrated LUVs for light-scattering experiments, and SUVs for CD, FTIR, and RET spectroscopy were prepared according to well-established procedures (32). Chloroform was used to dissolve the mixtures of lipids. After evaporation under nitrogen gas flow, the homogeneous films deposited onto the walls of test tubes were dried overnight under high vacuum. Subsequently, they were hydrated in buffers (200 mM or 10 mM acetate, pH = 5) and subjected to several freeze-thaw cycles. LUVs were produced by 21 cycles of mechanical extrusion through a membrane with pores having a diameter of 100 nm (Avestin, Ottawa, Canada). SUVs were obtained by 2 min of tip sonication of the phospholipid suspensions.
Dynamic and static light-scattering spectroscopy
Measurements were performed on a Zetasizer Nano-S system (Malvern Instruments, Malvern, UK) equipped with a 4 mW He-Ne laser (λ0 = 633 nm). Samples were placed in quartz cells maintained at 25°C, and the light scattered backward was collected at an angle of θ = 173°.
In dynamic light scattering, the time autocorrelation function of the scattered light is used to extract the size distribution of dissolved particles. According to theory (33), in the most general case the normalized time autocorrelation function of the scattered light intensity g(2)(τ) for a given delay time τ follows the Siegert relation:
where B stands for the baseline of the measurement, β is a factor that depends on the geometry of the experimental system, and g(1)(τ) is the electric field/field time autocorrelation function. The latter takes several forms depending on the size, shape, and composition of the scattering particles.
In the peculiar case of a monodisperse population of particles, the field correlation function decays exponentially. The particle's hydrodynamic radius RH is proportional to the inverse of the time relaxation of the decay. In this ideal case, g(1)(τ) = exp(−Γτ) and the decay rate is Γ = Dq2, where D is the diffusion coefficient of the particles and q is the magnitude of the scattering wave vector that depends on λ0, θ, and the refractive index of the solvent. The Stokes-Einstein relation, , where kB is Boltzmann's constant and η is the dynamic viscosity, then relates the diffusion coefficient to RH.
In most cases, the sample is polydisperse and the problem becomes more complex. g(1)(τ) is no longer a single exponential and must be represented as an integral over a distribution of normalized decay rates G(Γ) by:
If G(Γ) is monomodal, a simplification can be made using the method of cumulants (34), or G(Γ) can be analyzed in terms of moments about the mean of the distribution function (33). If G(Γ) is polymodal, data inversion may lead to severe numerical artifact and misinterpretation. Since the solutions analyzed here are polydisperse, we prefer to consider only the autocorrelation function, and not the inverted data (i.e., a distribution of particle size).
SLS was used to monitor the formation of liposome aggregates. In titration experiments, an increasing amount of liposomes was added to the peptide solution and the intensity I of the scattered light was recorded. For monodisperse samples, I is proportional to the number of vesicles in suspension. Deviation from linearity indicates that larger objects are forming in solution.
CD spectroscopy
Spectra from 250 to 190 nm (spectral resolution: 1 nm; data pitch: 1 nm; scan speed: 200 nm/min) were recorded with a J-810 spectropolarimeter (Jasco, Tokyo, Japan). Samples containing 23 μM of peptide in 10 mM sodium acetate buffer were transferred into 1 mm long quartz cells and maintained at 25°C. Increasing amounts of SUVs (POPC/POPS, 3:1) were titrated in a stepwise fashion into the peptide solution. The fraction F of the peptide bound to the liposomes was estimated assuming that the peptide generates a maximum helicity signal when attached to the bilayers. The fraction of random coil versus α-helix contribution in the CD spectrum was calculated using a standard least-square fit procedure implemented in the Igor Pro software package (Wavemetrics, Lake Oswego, OR). It was assumed that the peptide could adopt only two configurations: a high degree of α-helical structure when attached to the SUVs, and a random coil conformation when dissolved in solution.
FTIR spectroscopy
FTIR measurements were performed to monitor the secondary structure of the peptide upon its association with liposomes and during the vesicle aggregation process. Spectra were recorded on a Nicolet 6700 FT-IR spectrometer (Thermo Scientific, Waltham, MA) equipped with an ATR germanium crystal and a heated Globar light source. The spectrometer has a KBr beam splitter, and the signal was collected on a liquid nitrogen-cooled mercury-cadmium-telluride detector.
Measurements were done at room temperature after a few microliters of the sample solution were deposited on the germanium crystal. A stock solution of LAH4 at 7.5 mg/mL in 10 mM sodium acetate buffer was prepared with D2O (pD = 5.0) to eliminate the intense absorption band of water overlapping with the amide I absorption structure. A 100 mg/mL stock solution of SUVs with a POPC/POPS ratio of 3:1 was prepared in the same buffer. A total of 64 scans at a resolution of 4 cm−1 between 1100 and 2000 cm−1 were accumulated per spectrum. The blank spectrum of the solvent recorded under otherwise identical experimental conditions was subtracted. No automatic data treatment, such as smoothing, water suppression line algorithm, or baseline correction, was performed digitally on the spectra. Data were exported and manually treated using the Igor Pro software package. Spectra of samples containing vesicles were corrected for the two intense phospholipid absorbance structures near 1625.5 and 1733.5 cm−1. To facilitate comparison, they were normalized to the maximum absorption of the amide I structure near 1650 cm−1 and smoothed by means of a binomial smoothing filter (one pass) numerical procedure (35). For the purpose of band-narrowing, second derivatives spectra were obtained by applying twice the derivative function to the absorption spectra.
RET emission spectroscopy
The fusion of vesicles was monitored in an experiment of fluorescence induced by RET (36). Emissions spectra were recorded on a Fluorolog 3-22 spectrometer (Horiba Jobin-Yvon, Longjumeau, France). Then, 40 μL of LAH4 solution (CLAH4 = 230 μM) were transferred to a low-volume quartz cuvette, equilibrated at room temperature for a few minutes, and then titrated to an increasing amount of a suspension of SUVs: 95% of unlabeled vesicles (POPC/POPS, 1:1) supplemented with 5% of labeled vesicles (POPC/POPS/Rhodamine-DHPE/NBD-PC, 49.5:49.5:0.5:0.5). The sample was excited at λexc. = 455 nm while the fluorescence intensity was recorded from λfluo. = 465–650 nm. A limited bandwidth of Δλ = 1 nm was set for both excitation and emission. To estimate the efficiency of the RET process, a standard least-square analysis, implemented numerically, was applied to the spectra. For this, it was assumed that the emission spectra encompassed only two contributions: one with maximum transfer of energy, when the NBD and the Rhodamine molecules are in close vicinity, and one with negligible transfer when the two fluorophores do not interact. The latter spectra were recorded on a suspension of pure labeled LUVs and after solvation of the vesicles with Triton X-100 at 1% of the total volume.
Results
Association and dissociation of LUVs induced by LAH4
Fig. 1 A shows the variation in the intensity of backscattered light during the titration of a 230 μM solution of LAH4 with a suspension of anionic LUVs (composed of a 3:1 mixture of POPC and POPS, diameter: ∼100 nm) so that L/P ratios ranged from 10 to 40 (round symbols). At ratios below 20, the data superimpose on those of the control (square symbols), indicating that the suspension of LUVs is monodisperse. At ratios between 20 and 29, the turbidity of the sample increases and becomes visible to the unaided eye. In addition, the SLS measurements strongly deviate from those of pure LUVs. The important intensity decrease is due to Mie scattering and means that the liposomes associate into larger structures. This association reaches its maximum at L/P ≈ 23.7 (arrow b). Above this ratio and up to 40, the turbidity of the solution decreases and the intensity of the light scattered increases. Both traces in Fig. 1 A converge when the aggregates are completely dissociated.
Figure 1.
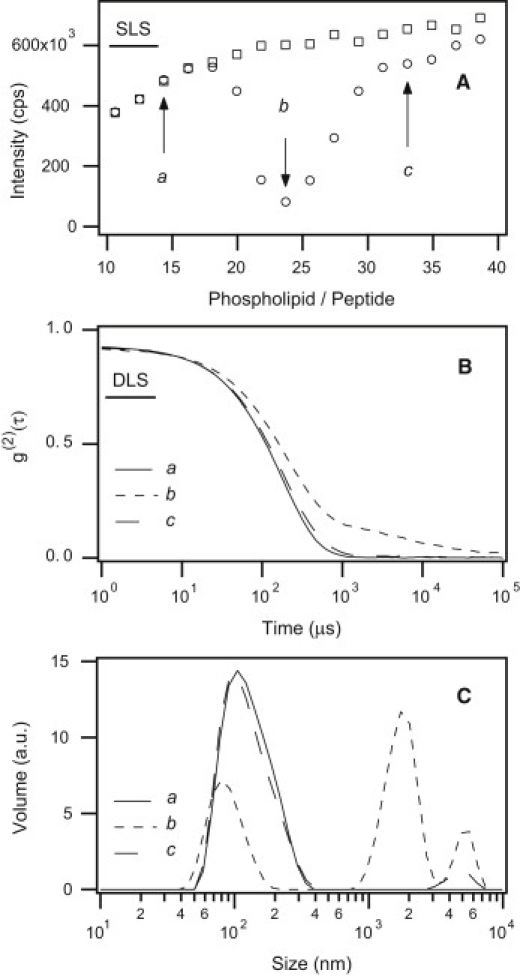
(A) Variation of the intensity of light scattered in static mode by a mixture of LAH4 and an increasing amount of LUVs (POPC/POPS: 3:1) in 200 mM acetate buffer pH = 5. The phospholipid-to-peptide ratio ranges from 10 to 40 (round symbols) and the lipid (LUV) concentration from CL = 2.32 to 5.66 mM (squared symbols). (B) Time autocorrelation functions of light scattered by the same vesicle suspensions at L/P = 14.3, 23.7 and 33 (continuous, short-dashed, and long-dashed line, respectively). (C) Corresponding size distributions after inversion of the data shown in panel B.
Fig. 1 B displays DLS measurements performed on LUVs in the presence of LAH4 at the same L/P ratios as above. For the sake of clarity, only three intensity autocorrelation functions are displayed, corresponding to L/P ratios equal to (a) 14.3, (b) 23.7, and (c) 33 (indicated by arrows in Fig. 1 A). Fig. 1 C represents the particle size distributions generated with the nonnegative least-squares fit algorithm (37) developed by the instrument manufacturer. Curve a in Fig. 1 B shows that at L/P = 14.3, the self-correlation of the scattered light intensity is a quasi-perfect monoexponential decay on the linear-log scale. This confirms that the LUVs used in this study are monodisperse. Their low PDI (0.152) is comparable to that of the control without peptide (PDI = 0.145; data not shown). Hence, the presence of LAH4 does not alter the shape, size, or dispersion characteristics of the liposomes in a significant manner. The low PDI value means that mechanical extrusion produces quasi-spherical LUVs. Data inversion (see Fig. 1 C) reveals that the intensity distribution is centered near the mean value DH = 106 nm with a full width at half maximum of 85 nm. Both values are very close to pure LUVs (DH = 107.7 nm, full width at half maximum = 85 nm) and are consistent with the absence of detectable aggregates. Other solutions with L/P ratios below 20 gave the same result.
Curve b in Fig. 1 B shows the DLS signal for an L/P ratio of 23.7. In addition to the fast exponential decay (τd ≈ 185 μs, which corresponds to the autocorrelation intensity of photons scattered by monomeric liposomes), there is a slower relaxation decay structure appearing at longer timescales (τ > 500 μs) that can be attributed to aggregated liposomes. No well-defined cluster size explains the shape of this signal. It corresponds to particles with sizes ranging from DH ≈ 1 to several microns. As shown by the inverted data represented as a volume particle size distribution in Fig. 1 C, the aggregated liposomes coexist with a minor population of monomeric LUVs.
Curve c in Fig. 1, B and C, shows an example of DLS measurements performed after the dissociation of liposome aggregates at L/P = 33. For this L/P stoichiometry, the autocorrelation function almost superimposes on that of nonaggregated LUVs. Most aggregates have dissociated and the liposomes are back in their initial monodisperse state. The few aggregates left in solution disappear completely within 30 min.
Binding of LAH4 to liposomes
The association isotherm of LAH4 to the POPC/POPS 3:1 liposomes was measured by CD spectroscopy, as shown in Fig. 2. A membrane insertion model (7) was used to analyze the experimental data, resulting in the following expression for the fraction F of peptides bound to the vesicles:
where CL is the concentration of phospholipids and Kass is the surface association constant of the binding process. A single parameter least-square fit of the data (solid line) gives an estimate of the surface association constant Kass ≈ 4130 ± 464 mM−1. The arrow indicates the position where liposome association is the highest (L/P = 23.6), which corresponds to a lipid concentration of CL = 0.6 mM and F = 70%. Because of the artifacts generated by scattered light in the direction of the photodetector, no CD measurements could be performed in this vicinity (38). To test whether the scatter of data points caused significant deviations, we also performed a simulation when the two downshifted points at 1.2 and 1.4 mM were deleted from the data set. In this case, Kass ≈ 4200 ± 482 mM−1 was obtained, which is only marginally different from calculations including the full data set and does not affect our conclusions from the experiment shown in Fig. 2.
Figure 2.

Titration curve of a 23 μM LAH4 solution with an increasing amount of SUVs (POPC/POPS: 3:1) in 10 mM acetate buffer pH = 5.0. The percentage of membrane-associated peptide was estimated by circular dichroism spectroscopy.
Effect of peptide concentration on liposome aggregation
The left- and right-hand columns in Fig. 3 respectively display SLS and DLS measurements performed in the presence of four different LAH4 solutions to which liposomes were titrated. As above, the depletion in the SLS intensity signal is caused by the formation of aggregates, and the lowest intensity locates the position where association is the strongest. In panels A–D, this occurs at L/P = 23.7, 22.6, 19.7, and 20.1, respectively. In panels E–H, the time autocorrelation functions of scattered light measured at these ratios are displayed as dashed lines. For comparison, the autocorrelation functions of nonaggregated LUVs are shown as solid lines. As mentioned above, the fast-decay structure with τd ≈ 185 μs corresponds to the autocorrelation intensity of photons scattered by monomeric liposomes. The slow-relaxation structure with τ > 500 μs is attributed to a distribution of large aggregates. Furthermore, when the relative intensities of the slow and fast decays are compared with each other, it becomes obvious that the association process is enhanced by the increase in peptide concentration.
Figure 3.

Variation of the intensity of light scattered in static mode by LUV suspensions (POPC/POPS: 3:1) as a function of the phospholipid-to-peptide ratio. In panels A–D the initial LAH4 concentration was (A) CLAH4 = 230, (B) 170, (C) 120, and (D) 75 μM. Panels E–H display the corresponding time autocorrelation functions of light scattered by free LUVs (solid lines) and associated LUV at the maximum aggregation (dashed lines).
Effect of membrane composition on liposome aggregation
The solid lines in the panels of Fig. 4 correspond to the time autocorrelation functions of light scattered by LUVs composed of various amounts of POPC and POPS, and thus differing in membrane surface charge density. LUVs with POPC/POPS = 1:1, 3:1, and 9:1 were prepared by mechanical extrusion through 100 nm pore filters and had average hydrodynamic diameters of DH = 91.3, 105.7, and 141.8 nm, respectively. All three samples had a PDI value < 0.2, which confirms the homogeneity of particles in suspension having likely a near-to-spherical shape.
Figure 4.

Time autocorrelation functions of light scattered by suspensions of free LUVs (solid lines) and LUVs associated in the presence of LAH4 (dashed lines). In panels A–C, the membranes were composed of mixtures of POPC and POPS at ratios equal to (A) 1:1, (B) 3:1, and (C) 9:1.
SLS measurements performed on LAH4 solutions titrated with liposomes made of POPC/POPS at ratios of 1:1, 3:1, and 9:1 indicate that association is maximal at L/P ratios of 10.4 (see Fig. 5 A), 23.7 (see Fig. 1 A), and 58.9 (data not shown), respectively. In Fig. 4, the time autocorrelation functions of light scattered by aggregates of LUVs are plotted as dashed lines. The data indicate that the transition between monodisperse vesicles and aggregated clusters is strongly dependent on membrane lipid composition. The formation of clusters is more favorable at the lowest POPC/POPS ratio, i.e., when the initial negative surface charge is the highest. In Fig. 4 A, the absence of a fast relaxation decay at the maximum charge surface density (POPC/POPS = 1:1) means that all LUVs are aggregated.
Figure 5.

(A) Variation of the intensity of light scattered in static mode by a mixture of LUVs (POPC/POPS: 1:1) and LAH4 in 200 mM acetate buffer pH = 5. The P/L ratio ranges from 0 to 26 (round symbols) and the lipid (LUV) concentration ranges from CL = 16 μM to 4 mM (squared symbols). (B) Percentage of fluorescence due to resonance energy transfer between NBD-PC and Rhodamine-DHPE. The titration was made on a 40 μL sample of LAH4 (CLAH4 = 230 μM) in 200 mM acetate buffer pH = 5, by an increased amount of LUVs (5% of POPC/POPS/NBD-PC/Rhodamine-DHPE: 49.5/49.5/0.5/0.5 with 95% of POPC/POPS: 1:1) in the same buffer. Two illustrative fluorescence spectra (λfluo. = 465 - 650 nm) are displayed on the figure recorded at L/P = 10.4 and 25.1.
Fusion of the vesicles (POPC/POPS = 1:1) induced by LAH4
Fig. 5 A displays the intensity of backscattered light (round symbols) recorded during the titration of a 40 μL LAH4 solution (CLAH4 = 230 μM) with highly anionic LUVs composed of POPC/POPS at a 1:1 ratio (diameter ∼ 100 nm). Except for the very low lipid concentrations (L/P = 1.6 and 2.5), the SLS data strongly deviate from the square symbols of the control experiment, highlighting the presence of macroscopic structures in the sample. Even at the lowest L/P ratios, the autocorrelation functions reflect the presence of enlarged structures (data not shown). Their diameter of ∼315 nm indicates that vesicles have already aggregated. When lipid is added, the SLS intensity decreases to a minimum centered at L/P = 10.4 and the turbidity of the sample increases. At L/P > 11.9, the scattering signal increases slowly and in a linear fashion with the addition of new vesicles, but it never reaches the level of the control experiment, suggesting that the larger structures do not disassemble. Therefore, using fluorescence spectroscopy (emission induced by RET), we sought to determine whether the vesicles fuse during the aggregation process. To monitor the fusion process, vesicles were prepared so that a high density of lipids labeled with both rhodamine and NBD would ensure an efficient RET. When mixed with unlabeled lipid vesicles, fusion led to the dilution of the fluorophores and a concomitant increase of the emission at 533 nm in combination with the loss of intensity at ∼587 nm (see the two spectra inset in Fig. 5 B). Fig. 5 B shows that at low L/P ratios, energy transfer between the two fluorophores was absent, indicating fusion of the labeled and unlabeled liposomes. At L/P > 12, a linear increase in the RET emission shows that the supplementation of additional liposomes does not cause further membrane fusion. Notably, the addition of peptide to vesicles that were uniformly labeled with rhodamine and NBD had only a small effect on the RET efficiency. Furthermore, the results of another control experiment indicate that fusion of labeled and unlabeled vesicles is observed only in the presence of peptide.
Conformation of LAH4 bound to liposomes
Spectra of LAH4 and computed second derivatives of the absorption in the vicinity of the amide I band are displayed in Fig. 6, A and B, respectively. The solid line is the spectrum of pure LAH4. As revealed by CD spectroscopy, the peptide is not structured at pD = 5 in solution. The maximum of the absorption structure at 1645 cm−1 correlates with random coil conformations (39,40).
Figure 6.

(A) FTIR absorption spectra of LAH4 in solution (solid line) and of LAH4 mixed with SUVs at phospholipid-to-peptide ratios equal to 12 (short dashed lines), 24 (medium dashed lines) and 36 (long dashed lines). (B) Second derivative of the absorption spectra in panel A.
The dashed lines in Fig. 6 are measurements of the LAH4 amide I band in the presence of SUVs (membrane composition: POPC/POPS = 3:1) for L/P = 12, 24, and 36 corresponding to the conditions of nonaggregation, aggregation, and after dissociation of the vesicles. The absence of any significant difference among the three absorption spectra indicates that the conformation of LAH4 does not change during either association or dissociation of the aggregates. The analysis of the second derivative of the absorption spectra led us to assign the spectral intensities centered at 1645 and 1647 cm−1 to the random coil and the α-helical structures of LAH4, respectively (39,40).
Discussion
Electrostatic consideration
A major result of this study is that the presence of LAH4 leads to the association of anionic vesicles made of mixtures of POPC and POPS. The aggregates are stable over time and can dissociate when the fraction of anionic liposomes is increased. Related observations have been made with divalent ions (41), polyions (42,43), and penetratin, a cell-penetrating peptide with a very high density of cationic residues (44,45).
Aggregation of the LAH4-LUV complexes occurs at or close to electric neutrality of the complexes, and electrostatic repulsion explains their stable monodisperse nature as well as their disassembly. This is well documented for the POPC/POPS 3:1 membranes, where vesicle aggregation is observed within a narrow range of L/P ratios and is maximal at L/P = 23.7 (Fig. 1 A). The association constant between the peptide and the LUVs was determined by a CD titration experiment (Fig. 2). Therefore, the fraction F of peptides bound to the liposomes was evaluated in a quantitative manner. Following this approach, the ratio between positive and negative charges, N+/N−, over the liposome surface was calculated according to:
where qLAH4 is the number of positive charges carried by LAH4, qPOPS is the number of negative charges carried by one molecule of POPS, and CPOPS and CLAH4 are, respectively, the concentrations of POPS and LAH4 in solution. Taking F = 70% (see Fig. 2), assuming that there are eight positive charges on the peptide (qLAH4 = 8) and all POPS phospholipid headgroups are fully ionized (qPOPS = 1), gives N+/N− = 1.06 at L/P = 23.7, where aggregation is the strongest. At L/P < 23.7 the excess of LAH4 adsorbed by the bilayers saturates the liposomes with positive charges, whereas at L/P > 23.7 the lipid charges dominate and the membrane surface carries an overall negative surface potential. In both cases, the net density of charges of the membranes (positive or negative) keeps the LAH4-LUV complexes nonaggregated in suspension. For the membranes composed of POPC/POPS = 9:1 and 1:1, the F-factor could not be measured due to the high turbidity of the sample. Nevertheless, assuming an F-value close to 70%, where maximum aggregation is the strongest, gives N+/N− = 1.06 and 0.94 for POPC/POPS = 9:1 and 1:1, respectively.
Therefore, in all cases tested, the maximum aggregation occurs in the vicinity of N+/N− = 1, and the overall charge neutralization of the anionic membranes by the cationic peptide seems to be an important prerequisite for the efficient formation of clusters. The formation of the macroscopic aggregates involves at least two steps, namely, peptide membrane association concomitant with charge neutralization, followed by vesicle association.
For membranes composed of POPC/POPS = 9:1 and 3:1, the liposome complexes dissociate when more LUVs are added to the sample. This observation suggests that the further addition of pure lipid vesicles captures a fraction of the free peptides. A decrease in peptide concentration also affects the established equilibrium between membrane-associated and free LAH4 (Fig. 3) (46), thereby reducing the density of peptides in the existing liposome aggregates. Proton-decoupled 15N solid-state NMR spectra of linear cationic antimicrobial peptides indeed show that resonances for membrane-associated and free peptides coexist (47). Furthermore, membrane-associated LAH4 can be removed from ATR-FTIR samples when they are incubated with acidic buffer solutions (21). Recently, the effect of crowding on the complex equilibrium between transmembrane, in-plane oriented LAH4 and LAH4 in solution was studied in the presence of zwitterionic membranes (48). Furthermore, it has been demonstrated that LAH4 redistributes between solution and peptide-nucleic acid complexes in a pH-dependent manner, and such equilibria have been suggested to be essential for transfection of cells with genetic information (24,57).
A different behavior was observed with vesicles made of POPC/POPS = 1:1. As with PC/PS membranes in which the zwitterionic component dominates (see above), LAH4 also induces very efficient association of these more-anionic vesicles (see Fig. 4). However, upon further addition of lipid vesicles, the aggregates do not dissociate, as membrane fusion has occurred (Fig. 5 B). Indeed, previous investigators have studied the peptide-induced fusion of model membranes to better understand this important biological process (25–31). Segregation of anionic lipids by cationic peptides has been observed for LAH4 (49) and other antimicrobial peptides (50), and may be crucial for fusion since such lipid segregation phenomena have been shown to result in line defects and/or destabilize the membranes (51,52). In this context, the reversible vesicle aggregation can be considered a preliminary state before irreversible membrane fusion occurs.
Structure and topology of LAH4
Although we have identified charge neutralization as a prerequisite of vesicle aggregation, this by itself is not sufficient to explain such a transition. Of interest, POPC vesicles alone remain in suspension, even though they may accumulate at the bottom of a vial after centrifugation or a long period of storage. For vesicle aggregation to occur, attractive interactions must be established via the amphipathic peptides. As a consequence, lipid aggregation is more efficient when the membranes carry a high density of POPS and consequently a much higher P/L ratio at charge neutrality (Fig. 4).
To gain more insight into the role of the peptide in this process, we investigated the structure of LAH4 in the presence of vesicles. Analyses by ATR-FTIR (see Fig. 6), CD, and multidimensional solution NMR spectroscopy reveal that the peptide adopts an α-helical structure when associated with free liposomes or with detergent micelles (Vogt and Bechinger (7), as well as A. Marquette, and B. Bechinger, unpublished data)). Moreover, proton-decoupled 15N solid-state NMR and ATR-FTIR measurements on uniaxially aligned bilayers have demonstrated that at pH = 5 the peptides adopt a stable in-plane orientation (19–21,53). Previous solid-state NMR spectroscopic investigations of POPC/POPS/cholesterol 70:15:15 bilayers in the presence of LAH4 revealed a strong decrease in the order parameter of the POPS, but not the POPC, fatty acyl chains. This observation is indicative of a selective interaction with the anionic lipid and suggestive of a burial of the hydrophobic face of the amphipathic LAH4 (49,54) and other amphipathic peptides (55) within the membrane interface. The resulting exposure to the aqueous environment of the histidine and lysine side chains, as well as some hydrophobic residues, enables a wide variety of interactions with the adjacent membrane. Our data do not completely exclude the possibility that the topology of the peptide is different upon vesicle aggregation or when the POPS content is increased, or that a transmembrane orientation of a fraction of peptides might exist in a crowded environment (48) or upon a change in local pH (19–21).
Our FTIR investigations indicate that LAH4 keeps its amphipathic α-helical structure during liposome aggregation (see Fig. 6). In this conformation, two lysines are located at each terminus and the four histidines all line up on one face of the helix, and a hydrophobic surface is formed by leucine and alanine residues on the opposite face. Previous investigations using DLS indicated that LAH4 is prone to self-aggregation at neutral pH when the charges of its four histidines are neutralized (56). Therefore, it seems possible that peptide-peptide interactions are enhanced when the charges of membrane-associated LAH4 are screened due to the presence of POPS. A lowering of the charge density reduces the peptide-peptide long-distance repulsion and facilitates the formation of, for example, hydrophobic interactions between peptides that are associated with adjacent bilayers. Furthermore, a peptide carrying eight positive charges is prone to generate electrostatic forces, attract lipids from adjacent membranes, and establish van der Waals contacts and hydrophobic interactions through its nonpolar residues.
Conclusions
We have examined the association of anionic vesicles made of three mixtures of phospholipids (POPC/POPS = 9:1, 3:1, and 1:1) in the presence of the cationic amphipathic peptide LAH4. The peptide adopts α-helical conformations in membranes regardless of the aggregation state of the vesicles. Our results indicate that before vesicle aggregation can occur, the negative charges of the POPS lipids have to be neutralized by the membrane-association of the cationic peptides. Aggregate formation requires the presence of peptides and is therefore due to interactions between peptides and/or between peptides and phospholipids belonging to adjacent bilayers. For the two higher PC/PS ratios, associated and free vesicles are in equilibrium and aggregation is reversible upon redistribution of the peptides onto more vesicles, whereas at the highest concentration of anionic lipids, fusion occurs. To the best of our knowledge, vesicle aggregation due to the presence of cationic antimicrobial peptides has not been reported previously. Our observations reveal a new layer of complexity in antimicrobial actions, as the outer surface of many bacteria and fungi is also characterized by a high negative surface charge density.
Acknowledgments
We thank the reviewers for valuable comments that helped us improve our analysis and discussion of the data. The Institut de Sciences et d'Ingénierie Supramoléculaire, University of Strasbourg, is acknowledged for hosting the Laboratory of Membrane Biophysics and NMR.
This study was supported by grants from Vaincre la Mucoviscidose (TG 0501), the Agence National de Recherche (ANR-PCV, TRANSPEP), the University of Strasbourg, and the French Ministry for Research (ACI-BCMS 042358 and PIR-MME).
References
- 1.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 2.Boman H.G. Antibacterial peptides: basic facts and emerging concepts. J. Intern. Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 3.Henriques S.T., Melo M.N., Castanho M.A.R.B. Cell-penetrating peptides and antimicrobial peptides: how different are they? Biochem. J. 2006;399:1–7. doi: 10.1042/BJ20061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.http://www.bbcm.units.it/∼tossi/pag1.htm. Accessed April 21, 2010.
- 5.Tossi A., Sandri L. Molecular diversity in gene-encoded, cationic antimicrobial polypeptides. Curr. Pharm. Des. 2002;8:743–761. doi: 10.2174/1381612023395475. [DOI] [PubMed] [Google Scholar]
- 6.Blondelle S.E., Houghten R.A. Novel antimicrobial compounds identified using synthetic combinatorial library technology. Trends Biotechnol. 1996;14:60–65. doi: 10.1016/0167-7799(96)80922-X. [DOI] [PubMed] [Google Scholar]
- 7.Vogt T.C.B., Bechinger B. The interactions of histidine-containing amphipathic helical peptide antibiotics with lipid bilayers. The effects of charges and pH. J. Biol. Chem. 1999;274:29115–29121. doi: 10.1074/jbc.274.41.29115. [DOI] [PubMed] [Google Scholar]
- 8.Hilpert K., Elliott M.R., Hancock R.E. Sequence requirements and an optimization strategy for short antimicrobial peptides. Chem. Biol. 2006;13:1101–1107. doi: 10.1016/j.chembiol.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Mason A.J., Gasnier C., Bechinger B. Enhanced membrane disruption and antibiotic action against pathogenic bacteria by designed histidine-rich peptides at acidic pH. Antimicrob. Agents Chemother. 2006;50:3305–3311. doi: 10.1128/AAC.00490-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler A. Thermodynamic studies and binding mechanisms of cell-penetrating peptides with lipids and glycosaminoglycans. Adv. Drug Deliv. Rev. 2008;60:580–597. doi: 10.1016/j.addr.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Morris M.C., Deshayes S., Divita G. Cell-penetrating peptides: from molecular mechanisms to therapeutics. Biol. Cell. 2008;100:201–217. doi: 10.1042/BC20070116. [DOI] [PubMed] [Google Scholar]
- 12.Novak R., Henriques B., Tuomanen E. Emergence of vancomycin tolerance in Streptococcus pneumoniae. Nature. 1999;399:590–593. doi: 10.1038/21202. [DOI] [PubMed] [Google Scholar]
- 13.Bechinger B. The structure, dynamics and orientation of antimicrobial peptides in membranes by multidimensional solid-state NMR spectroscopy. Biochim. Biophys. Acta. 1999;1462:157–183. doi: 10.1016/s0005-2736(99)00205-9. [DOI] [PubMed] [Google Scholar]
- 14.Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by α-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta. 1999;1462:55–70. doi: 10.1016/s0005-2736(99)00200-x. [DOI] [PubMed] [Google Scholar]
- 15.Lohner K., Blondelle S.E. Molecular mechanisms of membrane perturbation by antimicrobial peptides and the use of biophysical studies in the design of novel peptide antibiotics. Comb. Chem. High Throughput Screen. 2005;8:241–256. doi: 10.2174/1386207053764576. [DOI] [PubMed] [Google Scholar]
- 16.Brogden K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 17.Mason A.J., Bechinger B., Kichler A. Rational design of vector and antibiotic peptides using solid-state NMR. Mini Rev. Med. Chem. 2007;7:491–497. doi: 10.2174/138955707780619563. [DOI] [PubMed] [Google Scholar]
- 18.Powers J.P., Hancock R.E. The relationship between peptide structure and antibacterial activity. Peptides. 2003;24:1681–1691. doi: 10.1016/j.peptides.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 19.Bechinger B. Towards membrane protein design: pH-sensitive topology of histidine-containing polypeptides. J. Mol. Biol. 1996;263:768–775. doi: 10.1006/jmbi.1996.0614. [DOI] [PubMed] [Google Scholar]
- 20.Aisenbrey C., Kinder R., Bechinger B. Interactions involved in the realignment of membrane-associated helices. An investigation using oriented solid-state NMR and attenuated total reflection Fourier transform infrared spectroscopies. J. Biol. Chem. 2006;281:7708–7716. doi: 10.1074/jbc.M513151200. [DOI] [PubMed] [Google Scholar]
- 21.Aisenbrey C., Goormaghtigh E., Bechinger B. Translocation of amino acyl residues from the membrane interface to the hydrophobic core: thermodynamic model and experimental analysis using ATR-FTIR spectroscopy. Mol. Membr. Biol. 2006;23:363–374. doi: 10.1080/09687860600738742. [DOI] [PubMed] [Google Scholar]
- 22.Kichler A., Leborgne C., Bechinger B. Histidine-rich amphipathic peptide antibiotics promote efficient delivery of DNA into mammalian cells. Proc. Natl. Acad. Sci. USA. 2003;100:1564–1568. doi: 10.1073/pnas.0337677100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kichler A., Leborgne C., Bechinger B. Characterization of the gene transfer process mediated by histidine-rich peptides. J. Mol. Med. 2007;85:191–201. doi: 10.1007/s00109-006-0119-4. [DOI] [PubMed] [Google Scholar]
- 24.Prongidi-Fix L., Sugawara M., Bechinger B. Self-promoted cellular uptake of peptide/DNA transfection complexes. Biochemistry. 2007;46:11253–11262. doi: 10.1021/bi700766j. [DOI] [PubMed] [Google Scholar]
- 25.Nomura F., Inaba T., Takiguchi K. Microscopic observations reveal that fusogenic peptides induce liposome shrinkage prior to membrane fusion. Proc. Natl. Acad. Sci. USA. 2004;101:3420–3425. doi: 10.1073/pnas.0304660101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peisajovich S.G., Shai Y. Viral fusion proteins: multiple regions contribute to membrane fusion. Biochim. Biophys. Acta. 2003;1614:122–129. doi: 10.1016/s0005-2736(03)00170-6. [DOI] [PubMed] [Google Scholar]
- 27.Murata M., Nagayama K., Ohnishi S. Membrane fusion activity of succinylated melittin is triggered by protonation of its carboxyl groups. Biochemistry. 1987;26:4056–4062. doi: 10.1021/bi00387a047. [DOI] [PubMed] [Google Scholar]
- 28.Kitamura A., Kiyota T., Sugihara G. Morphological behavior of acidic and neutral liposomes induced by basic amphiphilic α-helical peptides with systematically varied hydrophobic-hydrophilic balance. Biophys. J. 1999;76:1457–1468. doi: 10.1016/S0006-3495(99)77306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cummings J.E., Vanderlick T.K. Aggregation and hemi-fusion of anionic vesicles induced by the antimicrobial peptide cryptdin-4. Biochim. Biophys. Acta. 2007;1768:1796–1804. doi: 10.1016/j.bbamem.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Matsuzaki K., Fukui M., Miyajima K. Permeabilization and morphological-changes in phosphatidylglycerol bilayers induced by an antimicrobial peptide, tachyplesin-I. Colloid. Polym. Sci. 1993;271:901–908. [Google Scholar]
- 31.Fujii G., Selsted M.E., Eisenberg D. Defensins promote fusion and lysis of negatively charged membranes. Protein Sci. 1993;2:1301–1312. doi: 10.1002/pro.5560020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hope M.J., Nayar R., Cullis P.R. Reduction of liposome size and preparation of unilamellar vesicles by extrusion techniques. In: Gregoriadis G., editor. Liposome Technology. 2nd ed. CRC Press; Boca Raton, FL: 1993. pp. 124–139. [Google Scholar]
- 33.Frisken B.J. Revisiting the method of cumulants for the analysis of dynamic light-scattering data. Appl. Opt. 2001;40:4087–4091. doi: 10.1364/ao.40.004087. [DOI] [PubMed] [Google Scholar]
- 34.Koppel D.E. Analysis of macromolecular polydispersity in intensity correlation spectroscopy: the method of cumulants. J. Chem. Phys. 1972;57:4814–4820. [Google Scholar]
- 35.Marchand P., Marmet L. Binomial smoothing filter—a way to avoid some pitfalls of least-squares polynomial smoothing. Rev. Sci. Instrum. 1983;54:1034–1041. [Google Scholar]
- 36.Struck D.K., Hoekstra D., Pagano R.E. Use of resonance energy transfer to monitor membrane fusion. Biochemistry. 1981;20:4093–4099. doi: 10.1021/bi00517a023. [DOI] [PubMed] [Google Scholar]
- 37.Twomey S. Dover Publications; Mineola, NY: 2002. Introduction to the Mathematics of Inversion of Remote Sensing and Indirect Measurements. [Google Scholar]
- 38.Shindo Y. On the problem of CD spectropolarimeter (IV) artifacts due to the light scattering by small particles. Appl. Spectrosc. 1985;39:713–715. [Google Scholar]
- 39.Barth A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta. 2007;1767:1073–1101. doi: 10.1016/j.bbabio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 40.Kong J., Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. (Shanghai). 2007;39:549–559. doi: 10.1111/j.1745-7270.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- 41.Ohki S., Düzgünes N. Divalent cation-induced interaction of phospholipid vesicle and monolayer membranes. Biochim. Biophys. Acta. 1979;552:438–449. doi: 10.1016/0005-2736(79)90188-3. [DOI] [PubMed] [Google Scholar]
- 42.Sennato S., Bordi F., Cametti C., Di Biasio A., Diociaiuti M. Polyelectrolyte-liposome complexes: an equilibrium cluster phase close to the isoelectric condition. Colloids Surf. A Physicochem. Eng. Asp. 2005;270:138–147. [Google Scholar]
- 43.Bordi F., Cametti C., Sennato S. Polyions act as an electrostatic glue for mesoscopic particle aggregates. Chem. Phys. Lett. 2005;409:134–138. [Google Scholar]
- 44.Persson D., Thorén P.E.G., Nordén B. Vesicle membrane interactions of penetratin analogues. Biochemistry. 2004;43:11045–11055. doi: 10.1021/bi036054d. [DOI] [PubMed] [Google Scholar]
- 45.Persson D., Thorén P.E.G., Nordén B. Penetratin-induced aggregation and subsequent dissociation of negatively charged phospholipid vesicles. FEBS Lett. 2001;505:307–312. doi: 10.1016/s0014-5793(01)02843-5. [DOI] [PubMed] [Google Scholar]
- 46.Bechinger B. A dynamic view of peptides and proteins in membranes. Cell. Mol. Life Sci. 2008;65:3028–3039. doi: 10.1007/s00018-008-8125-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prongidi-Fix L., Bertani P., Bechinger B. The membrane alignment of helical peptides from non-oriented 15N chemical shift solid-state NMR spectroscopy. J. Am. Chem. Soc. 2007;129:8430–8431. doi: 10.1021/ja072668k. [DOI] [PubMed] [Google Scholar]
- 48.Aisenbrey C., Bechinger B., Gröbner G. Macromolecular crowding at membrane interfaces: adsorption and alignment of membrane peptides. J. Mol. Biol. 2008;375:376–385. doi: 10.1016/j.jmb.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 49.Mason A.J., Martinez A., Bechinger B. The antibiotic and DNA-transfecting peptide LAH4 selectively associates with, and disorders, anionic lipids in mixed membranes. FASEB J. 2006;20:320–322. doi: 10.1096/fj.05-4293fje. [DOI] [PubMed] [Google Scholar]
- 50.Pukala T.L., Boland M.P., Bowie J.H. Solution structure and interaction of cupiennin 1a, a spider venom peptide, with phospholipid bilayers. Biochemistry. 2007;46:3576–3585. doi: 10.1021/bi062306+. [DOI] [PubMed] [Google Scholar]
- 51.Bechinger B., Lohner K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim.Biophys. Acta. 2006;1758:1529–1539. doi: 10.1016/j.bbamem.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 52.Jean-François F., Castano S., Dufourc E.J. Aggregation of cateslytin β-sheets on negatively charged lipids promotes rigid membrane domains. A new mode of action for antimicrobial peptides? Biochemistry. 2008;47:6394–6402. doi: 10.1021/bi800448h. [DOI] [PubMed] [Google Scholar]
- 53.Bechinger J.M.R., Goormaghtigh E. Membrane helix orientation from linear dichroism of infrared attenuated total reflection spectra. Biophys. J. 1999;76 doi: 10.1016/S0006-3495(99)77223-1. A353–A353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salnikov E.S., Mason A.J., Bechinger B. Membrane order perturbation in the presence of antimicrobial peptides by (2)H solid-state NMR spectroscopy. Biochimie. 2009;91:734–743. doi: 10.1016/j.biochi.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Marcotte I., Wegener K.L., Separovic F. Interaction of antimicrobial peptides from Australian amphibians with lipid membranes. Chem. Phys. Lipids. 2003;122:107–120. doi: 10.1016/s0009-3084(02)00182-2. [DOI] [PubMed] [Google Scholar]
- 56.Marquette A., Mason A.J., Bechinger B. Aggregation and membrane permeabilizing properties of designed histidine-containing cationic linear peptide antibiotics. J. Pept. Sci. 2008;14:488–495. doi: 10.1002/psc.966. [DOI] [PubMed] [Google Scholar]
- 57.Langlet-Bertin B., Leborgne C., Kichler A. Design and evaluation of histidine-rich amphipathic peptides for siRNA delivery. Pharm. Res. 2010 doi: 10.1007/s11095-010-0138-2. Apr. 15. Epub ahead of print. [DOI] [PubMed] [Google Scholar]


