Abstract
Corneal scarring from trauma and inflammation disrupts vision for millions worldwide, but corneal transplantation, the primary therapy for corneal blindness, is unavailable to many affected individuals. In this study, stem cells isolated from adult human corneal stroma were examined for the ability to correct stromal opacity in a murine model by direct injection of cells into the corneal stroma. In wild-type mice, injected human stem cells remained viable for months without fusing with host cells or eliciting an immune T-cell response. Human corneal-specific extracellular matrix, including the proteoglycans lumican and keratocan, accumulated in the treated corneas. Lumican-null mice have corneal opacity similar to that of scar tissue as a result of disruption of stromal collagen organization. After injection with human stromal stem cells, stromal thickness and collagen fibril defects in these mice were restored to that of normal mice. Corneal transparency in the treated mice was indistinguishable from that of wild-type mice. These results support the immune privilege of adult stem cells and the ability of stem cell therapy to regenerate tissue in a manner analogous to organogenesis and clearly different from that of normal wound healing. The results suggest that cell-based therapy can be an effective approach to treatment of human corneal blindness.
Keywords: Side Population, Cornea, Scarring, Cellular therapy, Tissue regeneration, Tissue-specific stem cells, Xenogeneic stem cell transplantation, Mouse
Introduction
The cornea serves as the optical portal to the visual system, maintaining both transparency and the remarkably tough connective tissue barrier protecting the eye. The corneal stroma is a dense collagenous tissue populated by keratocytes, quiescent cells responsible for secretion of the specialized stromal extracellular matrix. This matrix is characterized by layers of tightly packed, highly uniform collagen fibrils associated with a unique class of keratan sulfate-containing proteoglycans [1]. Trauma, inflammation, or infection induce keratocytes to differentiate into fibroblasts, mitotic cells that secrete altered extracellular matrix components, resulting in stromal scar formation and reduced transparency [2]. The altered collagenous matrix is stable and visual disruption from corneal scars can persist for decades in human corneas. Such corneal opacity is a significant cause of blindness worldwide [3].
Corneal scars show profound alterations in collagen fibril diameter and organization, and also loss of specialized corneal keratan sulfate proteoglycans [4, 5]. Proteoglycans, particularly lumican, a protein linked to about half of the keratan sulfate in stroma, interact with collagen fibrils and regulate fibril growth and diameter [6]. Small, uniform diameter collagen fibrils are essential for corneal transparency. The important role for lumican in the maintenance of collagen organization is shown in corneas of Lum−/− mice, which have large collagen fibril aggregates, altered lamellar architecture in the posterior stroma, and increased corneal light scatter [7].
We recently described stem cells from adult human corneas that maintain the ability to produce stromal matrix after extensive expansion in vitro [8]. In the current study, we examined the ability of these stem cells to restore organization and transparency to the corneas of mice with scar-like disruption of corneal transparency as a result of lumican deficiency.
Methods and Materials
Animals
Female C57BL/6 mice (Charles River Laboratories International, Inc., Wilmington, MA, http://www.criver.com), 8–10 weeks of age, and lumican-null mice [9] were used in these experiments. Enhanced green fluorescent protein (EGFP) chimeric mice were generated as previously described [10]. In brief, C57BL/6 recipient mice received a total of 1,200 rad (12 Gy) whole body irradiation delivered in two doses of 600 rad (6 Gy) 3 hours apart and were then injected i.v. via the tail vein with 5 × 106 bone marrow cells from C57BL/6-TgN (ACTbEGFP) mice (Jackson Laboratory, Bar Harbor, ME, http://www.jax.org). EGFP-chimeric mice were used 2 weeks after the bone marrow transplant to allow for bone marrow reconstitution. All experimental procedures were reviewed and approved by Institutional Animal Care and Use Committees and handled according to guidelines provided in the Association for Research in Vision and Ophthalmology Resolution on the Use of Animals in Ophthalmic and Vision Research.
Cell Injection Into Mouse Corneal Stroma
Human corneas were obtained from the Center for Organ Recovery & Education (Pittsburgh, PA, http://www.core.org). Human corneal stromal stem cells were isolated as side population cells by fluorescence-activated cell sorting (FACS) and were cloned and passaged as described previously [8]. Passage 18 cells were used for intrastromal injection. To produce fibroblasts as controls, cultures of human corneal stromal keratocytes were expanded in Dulbecco’s modified Eagle’s medium (DMEM)/F-12 with 10% fetal bovine serum. Passage 8 fibroblasts were used for injection. The cells were prelabeled with the membrane dyes Vybrant DiO or Vybrant DiI (Invitrogen, Carlsbad, CA, http://www.invitrogen.com). The cells were suspended in DMEM/F-12 at 1 × 106 cells/ml; DiO or DiI was added at a dilution 50 μg/ml for 20 minutes at 37°C. Then the cells were washed twice with DMEM/F-12 and resuspended in serum-free DMEM/F-12 at 2.5 × 104 cells/μl for injection.
All mice were anesthetized by i.p. injection of 2 mg of ketamine hydrochloride and 0.04 mg of xylazine (IVX Animal Health, Inc., St. Joseph, MO, http://www.tevaanimalhealth.com) in 0.2 ml of Dulbecco’s phosphate buffered salt solution (PBS). The eyes were washed with PBS with antibiotics. The eye was anesthetized with topical proparacaine. The right cornea of each mouse was used for stem cell or fibroblast injection and the left cornea was used as a sham or noninjected control. A small intrastromal tunnel in the center of the cornea was created using a 33-gauge needle (Hamilton Company, Reno, NV, http://www.hamiltoncompany.com). Cells in 2 μl of 2.5 × 104 cells/μl, or medium only (sham), were injected into the cornea through a blunt 33-gauge needle attached to a 25-μl Hamilton syringe. After injection, the eyes were treated once with gentamicin sulfate ophthalmic drops.
In Vivo Imaging and Corneal Opacity Analysis
At different time points after cell injection, in vivo imaging of mouse corneas was performed using a Leica MZFLIII high-resolution stereo fluorescence biomicroscope with vertical fluorescence illuminator (Leica Microsystems Inc., Bannockburn, IL, http://www.leica-microsystems.com). Mice were anesthetized and immobilized with a three-point stereotactic mouse restrainer. Each whole cornea was focused, and images were obtained at a magnification of 25×.
Opacity and neovascularization developed concurrently and were monitored by slit-lamp examination. Six weeks after injection, corneal opacity was scored based on microscope examination as: 1+, complete corneal transparency; 2+, mild corneal haze; 3+, severe opacity obscuring the iris; and 4+, severe corneal opacity with neovascularization. The corneal opacity differences among sham, stem cells, and fibroblasts were analyzed statistically by one-way analysis of variance (ANOVA) followed by the Tukey post-test to assess the significance of differences.
Examination of Stromal Thickness and Haze
In vivo analysis of mouse corneal stromal thickness and haze was accomplished by in vivo confocal microscopy (Confo-scan3; Nidek Technologies America, New Orleans, LA, http://www.nidek-intl.com). Mice were sacrificed by phenobarbitol overdose and immobilized on a secure platform. A 40× objective optically coupled to the cornea with transparent gel (Viscotirs; Novartis Ophthalmics, Duluth, GA, http://www.novartis.com) was focused onto the corneal endothelial surface. The software (NAVIS; Lucent Technologies, Murray Hill, NJ, http://www.alcatel-lucent.com) captured images from the corneal endothelium through the corneal epithelium every 1.7 μm and stored them as an image stack for analysis of corneal thickness and haze. Image stacks were inspected individually to determine the stromal-endothelium and stromal-epithelium transitions. Thickness of the stroma was determined by the number of stromal images × 1.7. Light reflectance (brightness) of a central circular region of each 12-bit image was calculated using Metamorph software (Molecular Devices, Downingtown, PA, http://www.moleculardevices.com). Stromal light scatter was defined as the sum of reflectance of all images in the stroma as a proportion of total reflectance. Statistical analyses were carried out by ANOVA with Prism Graph Pad software (Prism; Graph Pad Software, San Diego, http://www.graphpad.com).
Wholemount Corneal Staining for Lumican and Keratocan
Mouse eyes were enucleated and corneas were dissected from the eyes. Radial incisions were made in the corneas to produce four wedges allowing flat-mounting of the tissue. The corneas were fixed for 30 minutes in 1% paraformaldehyde in PBS at room temperature, rinsed in PBS, and stored at 4°C in 50% glycerol and 50% PBS (v/v) until further processing. Tissues were washed in PBS and incubated in anti-mouse CD16/CD32 Fc γ III/II (BD Pharmingen, San Jose, CA, http://www.bdbiosciences.com/index_us.shtml) at 1:100 for 30 minutes to block nonspecific antigens. Anti-human keratocan [11] (a kind gift from Dr. Chia-Yang Liu) or anti-lumican [12] (a kind gift from Dr. Bruce Caterson) was added and incubated for 48–72 hours at 4°C. After five washes with PBS, secondary antibody Alexa Fluor 488-conjugated goat anti-rabbit or goat anti-mouse (1:1,500) (Invitrogen-Molecular Probes, Eugene, OR, http://probes.invitrogen.com) together with 4′,6-diamidino-2-phenylindole (DAPI) (0.5 μg/ml) (Roche Molecular Biochemicals, Indianapolis, IN, http://www.roche.com) was added and tissues were incubated for 2 hours at room temperature. Omission of the primary antibody served as a negative control. The stained wholemounts were placed in aqueous mounting medium (Thermo Fisher Scientific, Pittsburgh, PA, http://www.thermofisher.com) and examined using an Olympus FluoView FV1000 confocal microscope (Olympus, Tokyo, http://www.olympus-global.com).
Western Blotting for Human Keratocan and Lumican Detection
The proteoglycans in mouse corneas were extracted in 6M urea, pH 7.4, with protease inhibitors, 150 μl/cornea [13]. Extract supernatants were digested with keratanase, 0.1 U/ml (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com) overnight at 37°C. SDS-PAGE and immunoblotting for detection of keratocan and lumican were carried out as previously described [10].
Viability and Gene Expression of Injected Cells
Seven weeks after injection of DiO-labeled stem cells, enucleated mouse eyes were incubated with 20 mM EDTA in PBS, pH 7.4, for 20 minutes at 37°C, and then corneal epithelium was removed with forceps. Corneas were dissected from the eyes and the endothelium was removed. The stroma was cut into four pieces and digested in collagenase I (Sigma-Aldrich) at 82 units/cornea in DMEM-10% FBS for 1–1.5 hours until no visible tissue remained. The digest was filtered through 70-μm nylon mesh and cells were collected by centrifugation and washed in PBS. The cells were stained with Cell Trace calcein red-orange AM (Invitrogen-Molecular Probes) according to the manufacturer’s instructions. FACS was performed on the BD FACS-Aria Cell Sorter (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) and live green cells were collected. DNA from the sorted cells was extracted using the DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA, http://www1.qiagen.com). Polymerase chain reaction (PCR) to detect human TATA binding protein (sense, 5′-CCC ACA GCC TAT TCA GAA CAC C; antisense, 5′-CAA TCC CAG AAC TCT CCG AAG C) and mouse TATA binding protein (sense, 5′-AGA ACA ACA GCC TTC CAC CTT ATG; antisense, 5′-CAA GTT TAC AGC CAA GAT TCA CGG) was carried out on genomic DNA as previously described [14].
Detection of Inflammation by Flow Cytometry
At 1, 2, 3, and 7 days after injection, sham-, stem cell-, and fibroblast-injected corneas were dissected and collagenase digested as above. The stromal cells were filtered and incubated in anti-mouse CD16/CD32 Fc γ III/II (BD Pharmingen) at 1:50 on ice for 10 minutes to block nonspecific antigens. Fluorescein isothiocyanate (FITC)-conjugated CD45 (BD Pharmingen) was added at 1:50 and cells were incubated on ice for 30 minutes. After a PBS wash, 7-amino-actinomycin D (BD Pharmingen) was added to exclude non-viable cells. Flow cytometry was performed to gate CD45+ cells. Noninjected normal mouse stroma and FITC IgG iso-type served as controls. The CD45+ cell numbers from each cornea (at least three corneas from each group) were counted and the data were analyzed statistically by one-way ANOVA followed by the Tukey post-test to assess the significance of differences.
Transmission Electron Microscopy
Four corneas per group were analyzed by transmission electron microscopy. The corneas were processed as previously described [15]. Briefly, fixation was with 4% paraformaldehyde, 2.5% glutaraldehyde, 0.1 M sodium cacodylate, pH 7.4, and 8.0 mM CaCl2 followed by postfixation with 1% osmium tetraoxide and en bloc staining with uranyl acetate/50% ethanol. After dehydration in an ethanol series followed by propylene oxide, the corneas were infiltrated and embedded in a mixture of EMbed 812, nadic methyl anhydride, dodecenyl succinic anhydride, and DMP-30 (Electron Microscopy Sciences, Hatfield, PA, http://www.emsdiasum.com). Thin sections were cut using a Reichert UCT ultramicrotome (Leica, Wein, Austria, http://www.leica.com) equipped with a diamond knife and were stained with 2% aqueous uranyl acetate and 1% phosphotungstic acid, pH 3.2. Sections taken from the central cornea and the anterior and posterior stroma were analyzed independently using electron microscopy. Corneas were examined and photographed at 80 kV using a Tecnai 12 transmission electron microscope (Fei Company, Hillsboro, OR, http://www.fei.com) with a Gatan 2K Ultrascan bottom mount charge-coupled device camera (Gatan Inc., Pleasanton, CA, http://www.gatan.com). Collagen fibril diameters were determined in a masked fashion for >1,000 fibrils in random regions of five separate micrographs.
Results
Differentiation of Human Stem Cells in the Murine Cornea
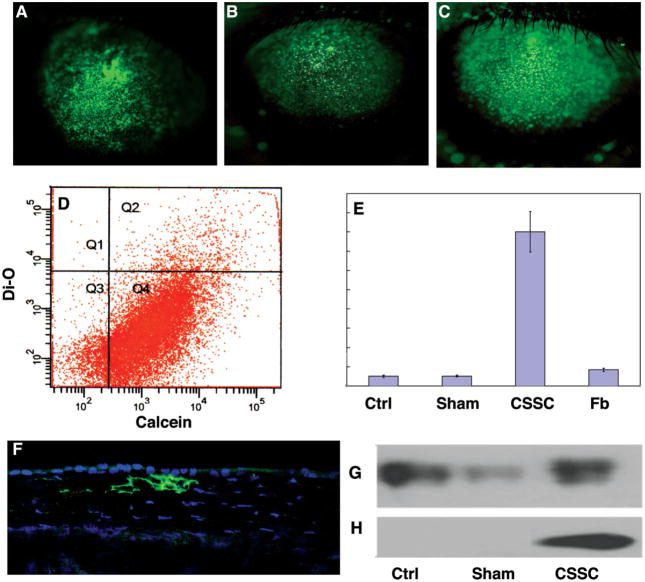
To determine if human cells survive and secrete extracellular matrix in mouse corneas, human corneal stromal stem cells were injected into the corneal stroma of 8- to 10-week-old C57BL/6 mice. Human corneal fibroblasts (stromal keratocytes expanded in serum-containing medium) and medium alone served as controls. Cells were labeled with the green fluorescent dye DiO before transplantation by direct injection into the mouse corneal stroma. We observed that the injection produced a transient corneal swelling and rapid diffusion of the labeled cells throughout the cornea. Photographs of the injected eyes after 48 hours (Fig. 1A) show the initial distribution of the injected fluorescent cells. The number and distribution of cells remained stable for at least 10 weeks (Fig. 1B, 1C). To determine survival of the human cells, excised corneas were collagenase digested 7 weeks after injection, and the cells were labeled with the vital dye calcein red-orange. Analysis by flow cytometry showed that 88% of the DiO-labeled cells were also calcein red-orange positive, indicating that they remained viable (Fig. 1D, quadrant Q2), a proportion similar to that of unlabeled resident mouse corneal cells (Fig. 1D, Q4). Cells captured from this Q2 population by FACS expressed mRNA for human keratocan (Fig. 1E), a cornea-specific keratan sulfate proteoglycan. Secretion of this protein, a highly tissue-specific marker of corneal stromal matrix, was confirmed by immunostaining corneas with antibody to human keratocan (Fig. 1F) and by Western blotting using mouse-specific (Fig. 1G) and human-specific (Fig. 1H) antibodies. The FACS-isolated green cell population (Fig. 1D, Q2) contained genomic DNA for human but not mouse TATA binding protein (Fig. 2), demonstrating that there was no cell fusion or dye transfer between human donor cells and resident murine corneal cells. These results demonstrate that, when injected directly into the corneal stroma, human stem cells remain viable for long periods and secrete extracellular matrix components characteristic of human corneal stroma.
Figure 1.
Fate of human corneal stromal stem cells in mouse cornea. (A): Green fluorescent human CSSCs injected into mouse corneal stroma spread throughout the stroma within 48 hours and were readily detected at 1 week (B) and 10 weeks (C) after injection. (D): At 7 weeks after injection, corneas were collected and viable cells were labeled with calcein red-orange vital dye. After collagenase digestion, flow cytometry showed live corneal cells (Q2, Q4) to be 88% of the total cells, and injected stem cells (Q1, Q2) to have a similar proportion of viable cells. (E): Stromal cells expressed increased mRNA for human keratocan after injection with human CSSCs but not human corneal stromal Fbs. (F): Human-specific antibody for keratocan protein showed stromal accumulation of keratocan in CSSC-injected stroma (green). Blue stain shows cell nuclei. (G): Mouse keratocan was detected by immunoblotting in untreated mouse corneas (Ctrl), corneas injected with serum-free culture medium (Sham), and those injected with CSSCs. (H): Human keratocan was only detected in those corneas injected with the human CSSCs. Abbreviations: CSSC, corneal stromal stem cell; Ctrl, control; Fb, fibroblast; Q, quadrant.
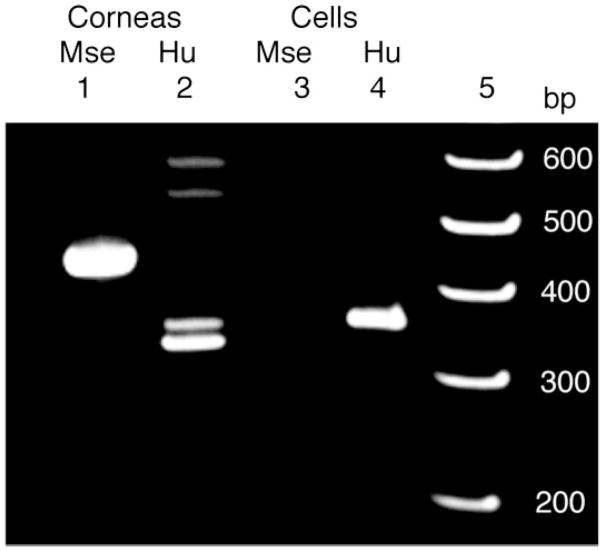
Figure 2.
Injected CSSCs do not fuse with endogenous mouse cells. PCR of cellular DNA detected genes for TATA binding protein using species-specific primers for mouse (Mse, lane 1, 465 bp) and human (Hu, lane 2, 368 bp), with corneal DNA as templates. In lane 3 and lane 4, amplification was carried out on DNA from the viable green-labeled CSSCs recovered by fluorescence-activated cell sorting from digests of Lum+/− mouse corneas (Fig. 1D) 7 weeks after injection. Human DNA was evident (lane 4) but mouse DNA was not detected (lane 3) in the recovered cells. PCR products were separated by gel electrophoresis and stained next to a ladder of DNA size standards (lane 5). The higher three Mr bands in lane 2 are nonspecific ampli-cons. Abbreviations: CSSC, corneal stromal stem cell; PCR, polymerase chain reaction.
Absence of Host Immunity to Transplanted Human Stem Cells
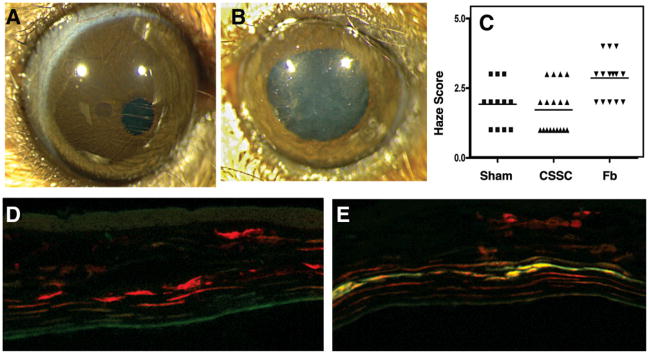
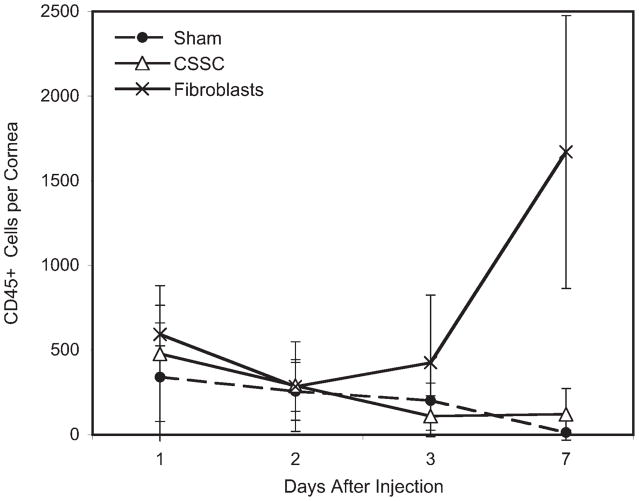
One month after injection, mouse corneas injected with human corneal stromal stem cells were transparent, whereas those injected with corneal fibroblasts had a slight haze detectable by direct microscopic observation (Fig. 3A, 3B). Masked scoring of the intensity of haze in 60 eyes injected with corneal stromal stem cells, fibroblasts, or culture medium found a statistically significant (p < .01) increase in the haze only in the fibroblast-injected eyes (Fig. 3C). Cytometric analysis of immune cells in the cornea showed a rapid influx of neutrophils into the stroma after all of the injections (Fig. 4). Within 1 week these cells were cleared from the tissue, except in the case of injected fibroblasts, in which the population of inflammatory cells increased significantly. Using chimeric mice in which bone marrow cells express EGFP, we found that the transient inflammation documented in Figure 4 was associated with the injection site (Fig. 5), and that the response resolved within a few days in eyes injected with stem cells, though not in those injected with fibroblasts. After 2 weeks, CD3+ T cells were detected in fibroblast-injected corneas, but not in stem cell-injected corneas (Fig. 3D, 3E). These results show that xenotransplantation of adult human stem cells does not evoke a CD3+ T-cell response and indicate the likelihood that these stem cells will also survive in allogeneic human transplantation therapy.
Figure 3.
Transparency of CSSC-injected corneas. (A): Mouse corneas injected with CSSCs retained transparency. (B): Injection of corneal Fbs produced visible corneal haze. (C): Visual scoring of corneal transparency in sham-injected, CSSC-injected, and Fb-injected stromas showed a significant increase in haze (p < .01) only in Fb-injected corneas. (D): Two weeks after injection with DiI-labeled (red) CSSCs, staining for CD3 T-cell antigen (green) was not detected. (E): In Fb-injected corneas, CD3 T-cell staining was observed. Abbreviations: CSSC, corneal stromal stem cell; Fb, fibroblast.
Figure 4.
Inflammatory cells in mouse corneas after injection with human cells. Mouse corneas were digested with collagenase and CD45+ cells were stained and quantified by flow cytometry after injection with serum-free culture medium (Sham), CSSCs, or passaged human corneal keratocytes (fibroblasts). CD45+ cells are expressed as number per cornea. Control (not injected) corneas had <50 positive cells (not shown). Differences between the number of inflammatory cells detected were not statistically significant except at day 7, when cells from fibroblast-injected corneas were greater (p < .05) than either of the other samples. Abbreviation: CSSC, corneal stromal stem cell.
Figure 5.
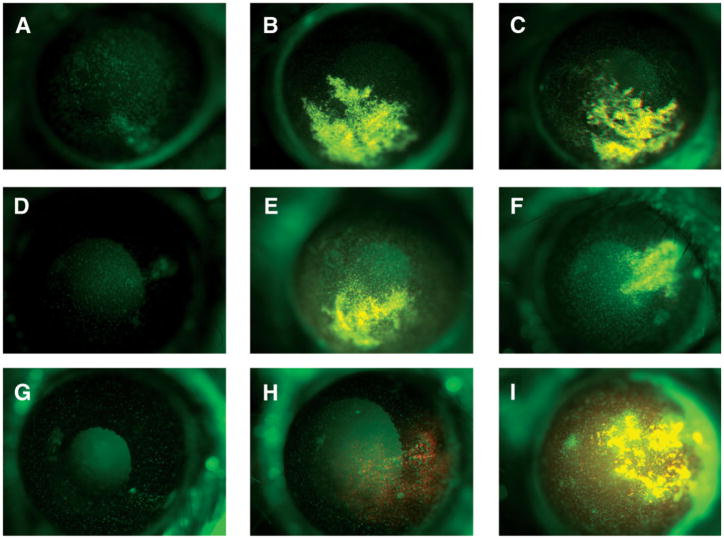
Inflammatory response to stromal cell injections. Corneal stromas of chimeric mice rescued with EGFP-expressing bone marrow were injected with 2 μl of serum-free culture medium (A, D, G), 2 μl medium containing 50,000 red fluorescent DiI-labeled CSSCs (B, D, H), or 2 μl medium with 50,000 DiI-labeled human stromal fibroblasts (C, F, I). In vivo biomicroscopy documented fluorescent cells at 24 hours (A–C), 72 hours (D–F), and 2 weeks (G–I). Bone marrow-derived (green) infiltrate in response to CSSCs is localized mostly near needle tracks (B) and, unlike the response to fibroblasts, is transient. Abbreviations: CSSC, corneal stromal stem cell; EGFP, enhanced green fluorescent protein.
Rescue of the Lum−/− Phenotype by Human Stem Cells
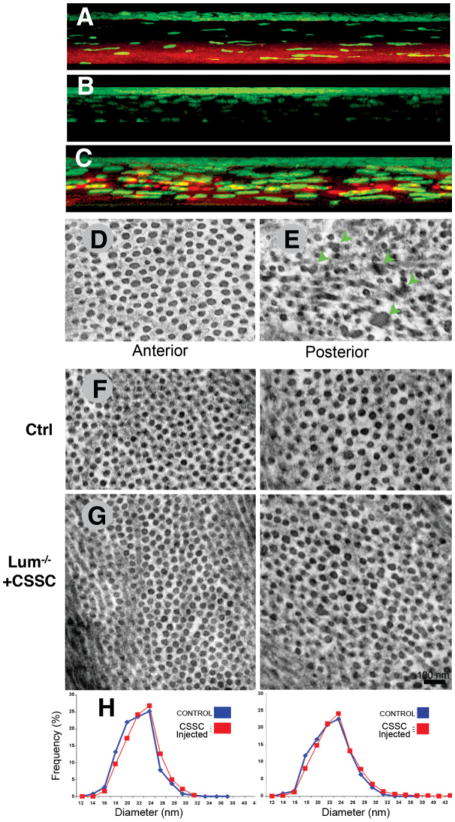
Human stromal stem cells were injected into 14-week-old Lum−/− mice, and after 12 weeks, expression of lumican in the corneal stroma was examined by immunostaining. In wild-type mice, but not Lum−/− mice, lumican was present in the posterior corneal stroma (Fig. 6A, 6B). In mice injected with human stem cells (Fig. 6C), lumican was restored throughout the posterior stromal extracellular matrix. Electron microscopic examination of the stromal matrix in wild-type mice showed the tightly packed uniform array of collagen fibrils characteristic of normal cornea (Fig. 6D), whereas Lum−/− mice had a distinct population of large, abnormal, fused fibrils in the posterior stroma associated with disruption of the stromal architecture (Fig. 6E, arrows). In marked contrast, Lum−/− mice 12 weeks after injection of human stem cells exhibited normal fibrils in both the anterior and posterior stroma (Fig. 6G), which were similar to those of their normal, heterozygous littermates (Fig. 6F). A fibril diameter analysis in anterior and posterior Lum−/− corneas (Fig. 6H) showed identical size distribution profiles between controls and injected Lum−/− stromas.
Figure 6.
CSSC injection rescues collagen phenotype of lumican null mice. (A): Lumican (red) is largely localized in the posterior stroma of WT mice. (B): Lumican is absent in lumican-null mutant (Lum−/−) mice. (C): After injection of CSSCs, lumican was distributed throughout the posterior cornea of Lum−/− mice. Nuclear stain (4′,6-diamidino-2-phenylindole [DAPI]) of all corneal cells is shown in green (A–C). (D): Electron microscopy of the posterior stroma of WT mice revealed aligned collagen fibrils with highly regular diameters. (E): Lum−/− posterior stroma collagen had numerous aggregated fibrils (green arrows). (G): Twelve weeks after CSSC injection, both the anterior and posterior stroma of Lum−/− mice exhibited regular collagen architecture, lacking fibril aggregates and indistinguishable from stromas of noninjected littermate heterozygote controls (shown in (F)). (H): Fibril diameter analysis showed injected Lum−/− (red lines) eyes to have regained distribution profiles identical to those of noninjected eyes of normal mice (blue lines) in both the anterior (left) and posterior (right) corneas. Abbreviations: CSSC, corneal stromal stem cell; WT, wild-type.
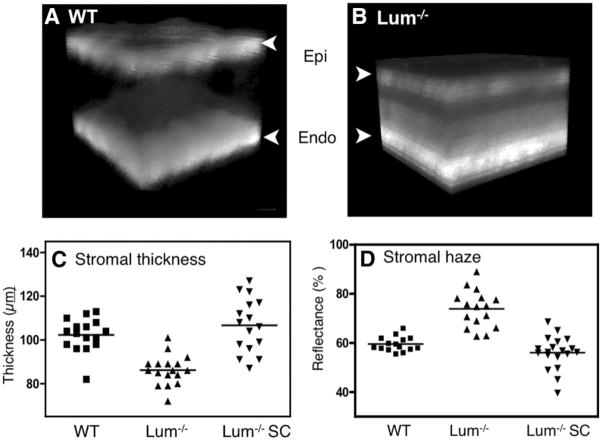
In addition to electron microscopy, we examined the ability of the corneal stem cells to reverse the corneal haze that results from disruption of the stromal ultrastructure in Lum−/− corneas. Direct photography of these eyes with a biomicroscope (as in Fig. 3) was not effective in imaging the stromal haze; therefore, light scatter and stromal thickness were determined using in vivo confocal scanning microscopy. Three-dimensional reconstruction of image stacks (Fig. 7A) showed that, as in previous studies [1, 7], Lum−/− corneas exhibited increased light scatter (haze) in the posterior stroma and were significantly thinner (Fig. 7B). Quantitative analysis of the stromal light scatter and stromal thickness show a significant decrease (p < .001) in Lum−/− stromal thickness (Fig. 7C) and increase in stromal haze (p < .001) (Fig. 7D). However, Lum−/− mice injected with human stroma cells had a stromal thickness (Fig. 7C) that was indistinguishable from that of wild-type control corneas (102 μm ± 8 μm versus 107 μm ± 12 μm) and was significantly (p < .001) different from that of untreated Lum−/− eyes. Similarly, stromal haze in recipient Lum−/− corneas was restored to the range of wild-type corneas (59% ± 7% versus 62% ± 14%) (Fig. 4D) and was significantly different from that of Lum−/− mice (93% ± 19%; p < .001). In separate experiments, we also examined the effects of fibroblast injections of Lum−/− mice. The results (not shown) were consistent with the injections of wild-type mice shown in Figure 3, in which fibroblasts increased stromal haze rather than decreased it. Together, these findings clearly demonstrate, by ultrastructural and functional analyses, that adult human stem cells restore both matrix organization and transparency of the Lum−/− cornea.
Figure 7.
Rescue of corneal transparency in Lum−/− mice. (A): Three-dimensional projection of confocal microscope images from normal mouse cornea shows reflectance primarily at the Epi and Endo cell interfaces. (B): Images of Lum−/− mice show a thinner cornea with significant light scatter in the posterior. (C): Stromal thickness was significantly less in Lum−/− mice than in WT (p < .01) mice but was significantly increased (p < .01) in Lum−/− eyes 12 weeks after injection of CSSCs. (D): Stromal light scatter was significantly greater in Lum−/− mice (p < .001) but was reduced to a normal range after CSSC injection (p < .001). Abbreviations: CSSC, corneal stromal stem cell; Endo, endothelial; Epi, epithelial; WT, wild-type.
Discussion
Corneal transparency is dependent on the small fibril diameter, parallel alignment, and regular interfibrillar spacing of collagen in the corneal stroma. Disruption of stromal matrix structure is a common outcome of corneal disease and trauma, and the resulting corneal dysfunction is a major cause of blindness worldwide. The disorganization of the extracellular matrix in the stroma of Lum−/− mice closely resembles that of human corneal scars. Because mouse cornea is fully developed by 12 weeks, the Lum−/− mice used in the current study provided a functional model of permanent human corneal scars. The ability of stem cells to remodel this matrix into a tissue essentially indistinguishable from that of wild-type matrix demonstrates a novel aspect of stem cell function. During morphogenesis, corneal collagen and proteoglycans self-assemble into a highly organized ultrastructure, resulting in a transparent tissue [16]. Such reorganization does not take place during wound healing, resulting in opaque scar tissue. Remodeling of a stable, nonregenerating tissue by stem cells supports the idea that corneal scarring is not irreversible but is dependent on the resident cells. Our results suggest, in fact, that the persistence of corneal scarring is a result of the inability of cells populating the tissue to produce the correct spectrum of molecular components. Many organ systems depend on the three-dimensional architecture of connective tissue elements to achieve correct function. The ability of adult stem cells to remodel tissue in a manner consistent with organogenesis and clearly different from wound healing is a new and important observation.
Corneal xenotransplantation is not successful in animal models [17], and our data show that xenotransplantation of human corneal fibroblasts elicits a T-cell-mediated adaptive immune response together with development of corneal haze (Figs. 3–5). However, xenotransplantation of human stromal stem cells elicits only a transitory inflammation similar in scope and course to the innate host response produced by the trauma of the injection. This conclusion was confirmed using three separate experimental approaches—subjective haze assessment (Fig. 3), flow cytometric measurement of CD45+ cells in collagenase-digested corneas (Fig. 4), and direct observation of invading immune cells in chimeric mice with EGFP-labeled bone marrow cells (Fig. 5). These results are consistent with a recent study in which human adult adipose-derived stem cells maintained viability after injection into rabbit corneal stroma [18], and the absence of a host response is consistent with reports that adult stem cells induce immune suppression [19]. Together, the observations that human stem cells survive and function in a xenogenic environment provide an argument that these cells, either alone or as part of a bioengineered tissue, would be tolerated in human allogeneic transplantation. Even though corneal allografts exhibit a low incidence of immune rejection [20], this is not a universal phenomenon, and rejection is a problem in inflamed eyes and ones in which a corneal graft has been previously rejected. Although a procedure for biopsy and expansion of autologous stromal stem cells might be feasible, our current study suggests that the use of autologous stem cells may not be necessary for cell-based therapy of stromal opacity. A bioengineered tissue containing a pure population of immune privileged cells could be an advantage in such situations. Such engineered tissue would be an important supplement of the supplies of donated corneal tissue, which are limited in many parts of the world [21, 22].
Whether the direct cell-based therapeutic approach used in this study will be applicable to treatment of human corneal opacities remains to be established. The mouse cornea differs from the human in at least two important properties. Mouse cornea is much thinner than human cornea and as such is more readily accessible to cell-based therapy. Additionally, the collagen in human corneas is extensively crosslinked, resulting in a tougher, more stable tissue, possibly less amenable to remodeling than that of the mouse. However, even if these experiments do not define the best methodological approach for human corneal stem cell therapy, they do provide a new paradigm demonstrating unequivocally that a stem cell-based therapy in some form can provide a powerful approach to treatment for corneal opacity, a major cause of blindness.
Acknowledgments
The authors appreciate the help of Kira Lathrop and Danny Roh with imaging. The work was supported by National Institutes of Health grants EY016415 and EY09368 to J.L.F., EY10320 and EY11373 to E.P., EY005129 to D.E.B., EY11845 to W.W.K., and core grant P30-EY08098, and by Research to Prevent Blindness and the Eye and Ear Foundation of Pittsburgh. J.L.F. is a Jules and Doris Stein Research to Prevent Blindness Professor.
Footnotes
Disclosure of Potential Conflicts Of Interest
The authors indicate no potential conflicts of interest.
Author contributions: Y.D.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; E.C.C.: conception and design, provision of study material, collection and assembly of data, data analysis and interpretation, final approval of manuscript; M.L.F.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; D.E.B.: conception and design, financial support, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; E.P.: provision of study material, financial support, administrative support, manuscript writing, final approval of manuscript; Winston W-Y Kao: provision of study material, manuscript writing, final approval of manuscript; J.L.F.: conception and design, financial support, administrative support, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; N.G.: collection and assembly of data, final approval of manuscript.
References
- 1.Kao WW, Liu CY. Roles of lumican and keratocan on corneal transparency. Glycoconj J. 2002;19:275–285. doi: 10.1023/A:1025396316169. [DOI] [PubMed] [Google Scholar]
- 2.Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18:529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- 3.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: A global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson EC, Wang IJ, Liu CY, et al. Altered KSPG expression by keratocytes following corneal injury. Mol Vis. 2003;9:615–623. [PubMed] [Google Scholar]
- 5.Funderburgh JL. Keratan sulfate: Structure, biosynthesis, and function. Glycobiology. 2000;10:951–958. doi: 10.1093/glycob/10.10.951. [DOI] [PubMed] [Google Scholar]
- 6.Chakravarti S, Petroll WM, Hassell JR, et al. Corneal opacity in lumican-null mice: Defects in collagen fibril structure and packing in the posterior stroma. Invest Ophthalmol Vis Sci. 2000;41:3365–3373. [PMC free article] [PubMed] [Google Scholar]
- 7.Chakravarti S, Magnuson T, Lass JH, et al. Lumican regulates collagen fibril assembly: Skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du Y, Funderburgh ML, Mann MM, et al. Multipotent stem cells in human corneal stroma. Stem Cells. 2005;23:1266–1275. doi: 10.1634/stemcells.2004-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saika S, Shiraishi A, Liu CY, et al. Role of lumican in the corneal epithelium during wound healing. J Biol Chem. 2000;275:2607–2612. doi: 10.1074/jbc.275.4.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson EC, Lin M, Liu CY, et al. Keratocan and lumican regulate neutrophil infiltration and corneal clarity in lipopolysaccharide-induced keratitis by direct interaction with CXCL1. J Biol Chem. 2007;282:35502–35509. doi: 10.1074/jbc.M705823200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pellegata NS, Dieguez-Lucena JL, Joensuu T, et al. Mutations in KERA, encoding keratocan, cause cornea plana. Nat Genetics. 2000;25:91–95. doi: 10.1038/75664. [DOI] [PubMed] [Google Scholar]
- 12.Carlson EC, Liu CY, Chikama T, et al. Keratocan, a cornea-specific keratan sulfate proteoglycan, is regulated by lumican. J Biol Chem. 2005;280:25541–25547. doi: 10.1074/jbc.M500249200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funderburgh JL, Funderburgh ML, Mann MM, et al. Arterial lumican. Properties of a corneal-type keratan sulfate proteoglycan from bovine aorta. J Biol Chem. 1991;266:24773–24777. [PubMed] [Google Scholar]
- 14.Muotri AR, Nakashima K, Toni N, et al. Development of functional human embryonic stem cell-derived neurons in mouse brain. Proc Natl Acad Sci U S A. 2005;102:18644–18648. doi: 10.1073/pnas.0509315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du Y, Sundarraj N, Funderburgh ML, et al. Secretion and organization of a cornea-like tissue in vitro by stem cells from human corneal stroma. Invest Ophthalmol Vis Sci. 2007;48:5038–5045. doi: 10.1167/iovs.07-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birk DE, Fitch JM, Babiarz JP, et al. Collagen fibrillogenesis in vitro: Interaction of types I and V collagen regulates fibril diameter. J Cell Sci. 1990;95:649–657. doi: 10.1242/jcs.95.4.649. [DOI] [PubMed] [Google Scholar]
- 17.Pindjáková J, Vitová A, Krulová M, et al. Corneal rat-to-mouse xeno-transplantation and the effects of anti-CD4 or anti-CD8 treatment on cytokine and nitric oxide production. Transpl Int. 2005;18:854–862. doi: 10.1111/j.1432-2277.2005.00112.x. [DOI] [PubMed] [Google Scholar]
- 18.Arnalich-Montiel F, Pastor S, Blazquez-Martinez A, et al. Adipose-derived stem cells are a source for cell therapy of the corneal stroma. Stem Cells. 2008;26:570–579. doi: 10.1634/stemcells.2007-0653. [DOI] [PubMed] [Google Scholar]
- 19.Patel SA, Sherman L, Munoz J, et al. Immunological properties of mesenchymal stem cells and clinical implications. Arch Immunol Ther Exp (Warsz) 2008;56:1–8. doi: 10.1007/s00005-008-0001-x. [DOI] [PubMed] [Google Scholar]
- 20.Niederkorn JY. The immunology of corneal transplantation. Dev Ophthalmol. 1999;30:129–140. doi: 10.1159/000060740. [DOI] [PubMed] [Google Scholar]
- 21.Garg P, Krishna PV, Stratis AK, et al. The value of corneal transplantation in reducing blindness. Eye. 2005;19:1106–1114. doi: 10.1038/sj.eye.6701968. [DOI] [PubMed] [Google Scholar]
- 22.Williams KA, Coster DJ. The immunobiology of corneal transplantation. Transplantation. 2007;84:806–813. doi: 10.1097/01.tp.0000285489.91595.13. [DOI] [PubMed] [Google Scholar]