Abstract
In mammals, sweet taste is mediated largely by a single receptor. New work shows that polymorphisms in the promoter region of one subunit contribute to variation in sweet perception.
Sweet taste perception is the source of both great pleasure and terrible public health problems: triple chocolate blackout cake and tooth decay; Meyer lemon ice cream profiteroles and obesity; passion fruit pavlova with lemon verbena cream and diabetes. Humans were experimenting with methods for altering our sweet taste perception long before we knew the identity of the sweet receptor, whether we were chewing on miracle fruit to make sour foods taste sweet or drinking gymnema sylvestre tea to make sugar taste like sand [1]. Earlier this decade, several groups [2–8] showed that in mammals, sweet taste is mediated largely by a single receptor composed of the two subunits TAS1R2 and TAS1R3. Understanding how this receptor responds to sugars may lead to the development of new sweeteners and inhibitors that would be useful to both the food industry and medicine. In this issue, Fushan et al. [9] examine how polymorphisms in this receptor, the point of convergence for both desert and disease, alter our perception of sucrose.
We have known for some time that the taste world of one individual is different from that of another--humans' sensitivity to sweet and bitter compounds can show dramatic variation--but the basis for this variability has been elusive. As information from the human genome became available, the field of chemoreception found a number of cases where receptor genes show polymorphisms among individuals. In some cases, researchers showed that these variations in the primary receptor alter perception of the sensory world. For example, previous work on bitter receptors in vitro showed that three one-letter changes in the hTAS2R38 receptor lead to much higher sensitivity to certain bitter compounds known as glucosinolates [10]. Consequently, humans with this variant receptor are more sensitive to glucosinolates [11, 12]. While humans have over 20 receptors for bitter tastes, they have only one for sweet taste, making it an attractive place to look for genetic changes that lead to variation in sweet perception. Indeed, variations in the sweet receptor across species have already been shown to have effects on sweet perception—domestic house cats have a defect in the TAS1R2 gene, and are therefore indifferent to the taste of sucrose [13].
In this issue, Fushan et al. [9] measured the ability of 144 individuals to detect various concentrations of sugar solutions and searched for polymorphisms in the TAS1R2 and TAS1R3 genes in these individuals that correlate with perception. They found several variations that change amino acid sequences in both the TAS1R2 and TAS1R3 subunits of the receptor. Surprisingly, however, variations in the two subunits did not correlate with a shift in the subjects' sweet perception. The authors confirmed in vitro that despite all of these variations, the function of these receptors, namely activation by sweet compounds, was largely unaffected. What, then, was causing some people to be more sensitive to sweet compounds? The answer was not in the receptor sequence, but in an upstream flanking region of DNA. People with two one-letter changes in the promoter sequence of the TAS1R3 gene have a decreased sensitivity to sucrose.
The promoter sequence interacts with transcription factors to regulate the amount of receptor transcripts. The authors verified in vitro that the promoter variant resulting in lower amounts of the TAS1R3 transcript correlated with reduced sensitivity to sucrose. This strengthened the case that polymorphisms in the promoter region cause changes in sweet taste perception. However, some caution is warranted here, as in vitro studies of taste receptors are not carried out in taste cells, due to the fact that there are no available taste-cell derived cell lines. Instead, the in vitro work is performed in cells derived from the bile duct, which endogenously expresses TAS1R3. It is possible that the proteins interacting with this promoter region could be quite different in taste cells, thus causing different effects in mediating TAS1R3 transcript levels.
In addition, an evolutionary analysis indicated that the variations were not just neutral genetic drift, suggesting they may have a role in the receptor's function [14]. What is the selective advantage of a change in sweet perception? Here, the ethnic variation may hold some clue. The T alleles, associated with a decreased sensitivity to sucrose, are most common in sub-Saharan Africa, while the C allele is the major variant in all geographic regions except Africa. The authors hypothesize that in tropical climates, where sugar sources are plentiful, the ability to taste a small amount of sugar was less important than in cold climates, where sugar sources are scarce.
Interestingly, the subunit affected by this promoter variation (TAS1R3) plays a dual role, partnering with a third subunit to form the umami receptor (umami translates from Japanese as “delicious” but in this context is perhaps closer to “savory”). TAS1R2, in contrast, is believed to only form a sweet receptor complex. This raises the possibility that the same changes that affect sweet taste may also affect the perception of umami-tasting amino acids such as monosodium glutamate.
This evidence that sweet taste perception may be under partial genetic control is exciting because it may allow for more personalized health interventions. Those interventions in turn will hopefully ensure that you spend less time in the dentist's chair and more time enjoying your brandied cherry truffle torte.
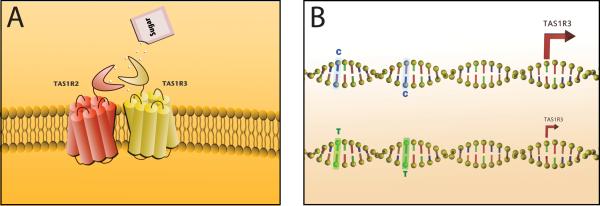
Figure 1.
A) The mammalian sweet receptor is made up of two subunits, TAS1R2 and TAS1R3. B) Two one-letter changes in the promoter region, from C-C to T-T, reduce transcription of the TAS1R3 subunit. Humans with the T-T allele have a reduced taste sensitivity to sucrose relative to humans with the C-C allele.
References
- 1.Bartoshuk LM. Taste illusions: some demonstrations. Ann N Y Acad Sci. 1974;237:279–285. doi: 10.1111/j.1749-6632.1974.tb49862.x. [DOI] [PubMed] [Google Scholar]
- 2.Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, et al. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses. 2001;26:925–933. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 4.Montmayeur JP, Liberles SD, Matsunami H, Buck LB. A candidate taste receptor gene near a sweet taste locus. Nat Neurosci. 2001;4:492–498. doi: 10.1038/87440. [DOI] [PubMed] [Google Scholar]
- 5.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 6.Kitagawa M, Kusakabe Y, Miura H, Ninomiya Y, Hino A. Molecular genetic identification of a candidate receptor gene for sweet taste. Biochem Biophys Res Commun. 2001;283:236–242. doi: 10.1006/bbrc.2001.4760. [DOI] [PubMed] [Google Scholar]
- 7.Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- 8.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 9.Fushan AA, Simons CT, Slack JP, Manichaikul A, Drayna D. Allelic Polymorphism within the TAS1R3 Promoter is Associated with Human Taste Sensitivity to Sucrose. Curr Biol. 2009 doi: 10.1016/j.cub.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bufe B, Breslin PA, Kuhn C, Reed DR, Tharp CD, Slack JP, Kim UK, Drayna D, Meyerhof W. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 2005;15:322–327. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandell MA, Breslin PA. Variability in a taste-receptor gene determines whether we taste toxins in food. Curr Biol. 2006;16:R792–794. doi: 10.1016/j.cub.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 12.Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Li W, Wang H, Cao J, Maehashi K, Huang L, Bachmanov AA, Reed DR, Legrand-Defretin V, Beauchamp GK, et al. Pseudogenization of a sweet-receptor gene accounts for cats' indifference toward sugar. PLoS genetics. 2005;1:27–35. doi: 10.1371/journal.pgen.0010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim UK, Wooding S, Riaz N, Jorde LB, Drayna D. Variation in the human TAS1R taste receptor genes. Chem Senses. 2006;31:599–611. doi: 10.1093/chemse/bjj065. [DOI] [PubMed] [Google Scholar]