Abstract
Purpose of review
Recent literature in inflammatory myopathies suggests that both immune (cell-mediated and humoral) and non-immune (endoplasmic reticulum (ER) stress and autophagy) mechanisms play a role in muscle fiber damage and dysfunction. This review describes these findings and discusses their relevance to disease pathogenesis and therapy.
Recent findings
Recent data highlights the role of ER stress response especially the roles of Hexose-6-phosphate dehydrogenase and ER-anchored RING finger E3 ligase in the activation of unfolded protein response and the formation of vacuoles and inclusions in myopathies. Several studies investigated the link between inflammation and the beta amyloid associated muscle fiber degeneration and loss of muscle function. Likewise, the roles of ER stress and autophagy in skeletal muscle damage have been explored in multiple muscle diseases.
Summary
Current data indicate that the ER stress, NF-kB pathway and autophagy are active in the skeletal muscle of myositis patients, and the pro-inflammatory NF-kB pathway connects the immune and non-immune pathways of muscle damage. The relative contributions of each of these pathways to muscle fiber damage are presently unclear. Therefore further defining the role of these pathways in disease pathogenesis should help to design effective therapeutic agents for these diseases.
Keywords: endoplasmic reticulum, idiopathic myopathy, skeletal muscle, cell death, autophagy and NF-kB activation
INTRODUCTION
Muscle weakness and inflammation are characteristic features of idiopathic inflammatory myopathies (IIMs), but the molecular pathways that initiate and perpetuate the muscle damage are currently unclear. It is generally thought that IIMs are autoimmune in origin because of the presence of autoantibodies, frequent association with other autoimmune diseases and favorable response in some patients to immunosuppressive therapies. Current literature supports two major immune mediators of muscle damage in myositis: one mediated through T lymphocytes (cytotoxic T cells) directed against muscle fibers, predominating in polymyositis (PM) and inclusion body myositis (IBM), and the other mediated through humoral factors (antibodies and complement) directed against vessels, predominating in patients with dermatomyositis (DM). The relative contribution of immune pathways to disease pathogenesis is undefined. On the other hand, several studies have shown evidence that non-immune processes may also have a role in the pathogenesis of myositis. For example: a) The degree of inflammation in skeletal muscle does not consistently correlate with the severity of the structural changes observed in the muscle fibers or with the severity of the clinical disease [1,2], b) Striking structural changes in the muscle fibers occur even in the absence of any inflammatory cells in muscle [3,4], c) Some myositis patients do not respond even to potent anti-inflammatory therapy [5,6], d) Glucocorticoid treatment may eliminate muscle inflammation without substantial improvement in the clinical disease [7], and e) The clinical disease may still progress when identifiable inflammation has subsided [8]. Collectively, this data suggests a potential role for non-immune mechanisms in the pathogenesis of myositis and the exact nature and roles of these pathways in myositis pathogenesis are becoming evident in the recent literature. This review will discuss recent advances in non-immune mechanism (e.g., the endoplasmic reticulum (ER) stress, autophagy and NF-kB activation) of muscle fiber damage and dysfunction in myositis.
ER stress response pathway
The ER performs important tasks such as Ca2+ release, post-translational maturation, protein folding/quality control, lipids biosynthesis and antigen presentation (Table 1). The ER have inbuilt mechanisms to control malfunction of above processes through a variety of homeostatic responses. However, when this housekeeping response is not sufficient to bring the cell to normal function, intrinsic cell death pathways are automatically triggered.
Table 1.
Endoplasmic and sarcoplasmic reticulum in muscle fibers
| Normal function | Stress response | Dysfunction and Pathology | Relevance to myositis | |
|---|---|---|---|---|
| ER | -Post-translational maturation of proteins | -Increased transcription of housekeeping genes | -Altered Ca2+ levels | -NF-kB activation |
| -Proteins folding control | -Translation attenuation | -Accumulation of unfolded proteins (e.g. APP) | - GRP78 increase | |
| -Ca2+ storage | -Unfolded proteins degradation by proteasomes | - Excess Autophagy | - IL-1 activation | |
| -Lipids synthesis | ||||
| -Loading of peptides onto MHC | -Autophagy | - Autoimmune response | - Autoreactive T cells and autoantibodies | |
| -Docking of cargo proteins | -Antigen presentation | - Abnormal protein accumulation | - Dysfunction or Cell death?? | |
| | ||||
| SR | -Ca2+ storage | - Osmotic shock and sustained Ca2+ release | -Calsequestrin dysfunction | - Skeletal muscle weakness or damage |
| -RyR dysfunction | ||||
As illustrated in figure 1, the ER is intimately connected to other cellular components and likely to affect many cellular functions during homeostasis, cell stress and cell death (Figure 1 and Table 1). It is now known that the ER and sarcoplasmic reticulum (SR) can rapidly be reloaded with Ca2+ from the extracellular environment via the Ca2+ sensors Stromal Interaction Molecules (STIM) and the associated Ca2+ channel Orai in the plasma membrane [9] (Figure 1; Panel 1). Importantly, abnormalities in this pathway have been recently associated with skeletal myopathy and death [10].
Figure 1.
ER and SR interactions within mammalian cells. The ER establishes important physical contact with other organelles and plasma membrane (upper panel). The contact of SR with the membrane permits the rapid reload with Ca2+ from the extracellular environment and is mediated by oligomers of STIM-1 (a Ca2+ sensor) and the channel Orai (panel 1). The ER stress response called UPR relies on the release of Bip from the stress sensors when unfolded proteins accumulate in the organelle. Without Bip, IRE1 and PERK form homodymers and their cytoplasmic portions are free to interact with XBP1 and eIF2α to increase the transcription of housekeeping genes and translation attenuation, respectively. The Bip-free ATF6 in targeted to the Golgi where its cytoplasmic portion is released to migrate to the nucleus for transcriptional activation (panel 2). SR (and ER) and mitochondria also make intimate contact, which is very important for cell function, although only the Ca2+ exchange is represented in panel 3. This exchange is mediated by RyR, VDAC, and Mitochondrial Ca2+ uniport (MCU) for Ca2+ influx from SR to mitochondria. For influx from mitochondria to SR the players are an undefined channel (?, in figure), VDAC and SERCA. SERCA also mediates Ca2+ influx from cytoplasm to SR (panel 3). For ER/autophagy pathway there is the export of unfolded proteins through the Sec61 translocon for degradation by the proteasome, in a process called UPR, and/or the activation of autophagy (upper panel). The Atg1 kinase homologue, unc-51-like kinase (ULK1) in mammals, is one of the initial molecules in autophagy and is silenced by Bcl-2, located on the ER, and m-TOR (panel 4). Then the PI3K complex is formed, composed by Beclin-1, P150, phosphorylated Vps34 (a class III phosphatidylinositol 3-kinase (PI3K)), and UVRAG in mammals. The next step represented in panel 4 is the elongation of the phagophore through the addition of LC3-PE (phosphatidylethanolamine) for lysosomal degradation of sequestered components in the closed autophagosome.
In mammalian cells the ER senses and responds to stress through 3 cellular pathways; 1) Inositol-requiring 1 (IRE1), 2) PKR-like ER kinase (PERK), and 3) Activating Transcription Factor 6 (ATF6) (Figure 1; Panel-2). All 3 pathways under normal conditions are kept inactive by glucose regulated protein-78 (GRP78), also referred to as BiP. However, when unfolded proteins accumulate within the ER, BiP is released from the 3 sensors and helps folding of accumulated proteins. This process is generally known as unfolded protein response (UPR) and the release of BiP from IRE1 and PERK leads to homodimerization and release of their cytoplasmic portions [11]. IRE1 then activates the transcription factor X-box binding protein 1 (XBP1) that induces the production of many ER-stress-inducible genes, including members of the Hsp40 family, ER chaperones and XBP1 itself and degradation of other subsets of mRNA [12] (Figure 1; Panel-2). On the other hand, the immediate consequence of PERK activation is translation attenuation through the phosphorylation of the α-subunit of heterotrimeric eukaryotic initiator factor 2 (eIF2α) [13]. The dissociation of BiP from ATF6 permits its translocation to the Golgi (Figure 1; Panel-2), where its cytoplasmic portion is cleaved by site-1 and site-2 proteases to generate a cytosolic fragment. These fragments then migrate to the nucleus and induce the expression of genes with ER response elements (ERSE) in their promoters (e.g., GRP78, GRP94, calreticulum6, CHOP, XBP1, Orp 150 (Grp170) and protein disulphide isomerase P5) that in turn help to relieve ER stress.
Concomitant to the above biochemical pathways triggered by the ER, unfolded proteins are targeted to retrotransport to cytoplasm, probably through Sec61, ubiquitinylation, and degradation by the 26S proteasome through ER-associated degradation (ERAD) process (Figure 1). This is a highly complex and ill defined process that starts, at least for some glycoproteins, with the action of the ER degradation-enhancing α-mannosidase-like proteins (EDEM) (for more details see [14]).
The role of ER and its associated proteins is becoming clear in several inflammatory muscle diseases including IBM. A recent study showed that the expression of ER-bound RING finger protein 5 RNF5 (aka RMA1), an ER-anchored RING finger E3 ligase, is involved in the recognition and processing of malfolded proteins. This study also demonstrated that expression of RNF5 is increased and localized to cytoplasmic aggregates in sporadic inclusion body myositis (sIBM). Moreover, prolonged expression of RNF results in the formation of muscle fibers containing congophilic material, blue-rimmed vacuoles and inclusion bodies, indicating a role for RNF5 in the ER stress associated myopathies and in muscle physiology [15].
Role of Sarcoplasmic and endoplasmic reticulum in disease
The ER and SR are distinct organelles with different cellular functions. The SR is found in muscle cells and is a specialized form of the ER with a particular distribution of domains and proteins along the contractile unit of muscle fibers that is dedicated to the storage and controlled release of Ca2+. On the other hand, the ER is a netlike labyrinth of tubules that is formed from the nuclear envelope by flattened stacks and budding vesicles that performs more complex cellular processes (Table 1). To understand the role of ER stress in disease conditions, it is important to first understand the function of ER and SR in normal cells. The longitudinal region of SR is specialized in Ca2+ uptake by SR/ER Ca2+-ATPase pumps (SERCA) and the junctional domain of SR is enriched in the Ca2+ releasing channel, ryanodine receptor type 1 (RyR1) and other junctional proteins (Figure 1, panel 3). Ca2+ is stored within the organelle associated with calsequestrin 1, the main calcium buffer of skeletal muscle cells, which also controls the morphological distribution of SR junctional domains, terminal cisternae volume, density and function of RyR through phosphorylation events. Calsequestrin 1 acts as a luminal Ca2+ sensor for RyR via its interactions with triadin and junctin. The close relationship between altered expression and dysfunction of calsequestrin in several skeletal and cardiac disorders highlights its role in maintaining Ca2+ homeostasis and regulation of muscle contraction. Although the SR stress response is not well studied, it appears that volume alteration triggered by osmotic shock in skeletal fibers induces localized and sustained SR Ca2+ release, contributing to skeletal muscle plasticity and/or pathology (Table 1) [16].
Recent studies using mice deficient in Hexose-6-phosphate dehydrogenase (H6PD), an enzyme that generates NADPH via pentose phosphate pathway inside the ER, further illustrate the role of ER stress in skeletal muscle function [17]. H6PD deficient mice showed severe skeletal myopathy and exhibited fasting hypoglycemia, increased insulin sensitivity and increased basal and insulin-stimulated glucose uptake in type II (fast) muscle fibers, suggesting mild insensitivity to glucocorticoids. These studies also showed that affected muscles have normal sarcomeric structures but have large intrafibrillar membranous vacuoles and abnormal triads, suggesting an altered redox state of SR leading to activation of UPR and myopathy [17].
Although the phenotypic consequences of abnormal intracellular accumulation of proteins are not well studied, it was recently showed that skeletal muscle with accumulated beta-amyloid generates less peak force and exhibited transient Ca2+ peaks with lower amplitude. Further, the addition of amyloid-beta peptide (Aβ1-42) to SR vesicles resulted in RyR-mediated Ca2+ release, indicating that altered protein metabolism in IBM changes RyR-mediated Ca2+ release and muscle contractility [18].
Mitochondria is one of the organelles that make intimate contact with ER, in fact about 12% of the outer mitochondria membrane physically contacts the ER in specialized areas called Mitochondria-Associated ER Membrane (MAM) (Figure 1, Panel 3) [19]. This interaction is considered to play major roles in the non-vesicular transport of phospholipids, control of apoptosis and Ca2+ exchange between the two organelles [20]. The MAM is particularly enriched in functionally diverse enzymes including enzymes involved in protein oxidation. The oxidative folding of proteins in the ER is critical for cell function and the abnormal protein oxidation contributes to several diseases [21]. There are evidences that mitochondrial oxidative stress plays a major role in the pathogenesis of neurodegenerative diseases such as Alzheimer or Parkinson disease and probably the interplay between ER and mitochondrial dysfunctions play a role in protein oxidation in IBM and PM [22].
ER stress, autophagy, and cell death
Apoptosis and autophagy cell death have different morphological characteristics and pathways. Although the role of autophagy in different forms of myositis is not clear, in normal mammalian cells it starts with the activation of the phagophore, elongation of this membranous structure and finally fusion with lysosome (Figure 1, Panel 4). Autophagy is a multi step process that sequesters and recycles cytosolic components constitutively, during nutrients deprivation or stress, but can lead to cell death when uncontrolled (see ref [23] for more details). Recently, several studies showed that characteristic apoptotic changes are absent in skeletal muscle of myositis patients, probably due to the expression of anti-apoptotic molecules in the muscle [24, 25]. However, skeletal muscle death and damage occurs in muscle fibers of myositis patients despite the overexpression of various anti-apoptotic molecules, indicating that other forms of cell death, such as autophagy, are involved. Indeed, recent literature provides some evidence that ER stress response in other cells is associated not only with classic apoptotic cell death but also with autophagic cell death. On the other hand, components of apoptotic pathways may modulate autophagy through cross talk interactions, including transcriptional activation of pro-apoptotic Bcl-2 family members or death receptors (DRs) and activation of initiator caspases at the ER. This is illustrated by the down regulation of autophagic cell death through the binding of Bcl-2 to Beclin 1, avoiding its association with PI3 kinase complex (PI3K) (Figure 1, panel 4). Interestingly, the role of Bcl-2 in antagonizing Beclin 1-dependent autophagy is restricted to Bcl-2 located at the ER [26]. It is becoming increasingly clear that ER stress response and autophagy are linked, for example a recent study provided evidence that endogenous ER degradation-enhancing alpha mannosidase−like protein (EDEM1) in non-stressed cells reaches the cytosol and is degraded by basal autophagy [27]. Although caspases activation is not clearly linked to myositis, it has been well demonstrated that caspase-12 is involved in ER stress-induced apoptosis in murine cells, but its role in humans is unclear. It appears that the counterpart of murine caspase-12 in human is the caspase-4.
Autophagy in myopathies
The role of autophagy in diseases is relatively unexplained. A recent study compared clinical and histological features of muscle biopsy of polymyositis with mitochondrial pathology (PM-Mito), steroid-responsive polymyositis, and IBM. It was observed that selective weakness in the quadriceps or finger flexors was common in PM-Mito and IBM and weakness progressed slower in PM-Mito than in IBM. This study showed that autophagy markers LC3 and alpha B-crystallin were found in PM-Mito and IBM, but not in PM biopsies [28]. Another study indicated that Atg8/LC3 colocalizes with amyloid precursor protein (APP) in human muscle cells and APP/beta-amyloid-containing autophagosomes are often observed at increased frequency in muscle fibers of sIBM muscle biopsies, but not in non-myopathic muscle or non-vacuolated myopathic controls. Collectively, these studies suggest that APP/beta-amyloid is targeted for lysosomal degradation via autophagy in sIBM.
Loss of skeletal muscle mass occurs during denervation induced disuse atrophy. Recent studies showed that degradation pathways such as autophagy are highly activated as indicated by the increased expression of Beclin 1 and translocation of LC3-II (LC3 associated with phosphatidylethanolamine) to mitochondrial membranes. These results suggest that autophagy signaling is upregulated in response to denervation, and may preferentially target mitochondria for degradation in skeletal muscle [29]. Recently it was also demonstrated that deficiency of VMA21, a component of V-ATPase proton pump complex leads to mTOR-dependent autophagy and cause vacuolation and atrophy of skeletal muscle in cell X-linked myopathy with excessive autophagy (XMEA) [30]. These findings may be relevant for the perifascicular atrophic phenotype seen in dermatomyositis muscle biopsies.
Muscle inflammation and damage is also seen in drug induced myopathies as well as genetic muscle diseases such as dysferlin deficiency. A recent study demonstrates that the wild-type dysferlin but not the mutant protein is degraded by ERAD. On the other hand, mutant dysferlin spontaneously aggregated in the ER and induced both ER stress and autophagy [31]. Moreover, it appears that accumulation of cell surface proteins such as MHC class I in myositis muscle fibers may also lead to both ER stress and autophagy [32]. Using the model of chloroquine-induced myopathy, it was showed that as early as 3 weeks after drug treatment, autophagic marker LC3-II and amyloid-beta increased in muscle fibers followed by increase in UPR markers such a GRP78 and increase in SERCA by 5 weeks. This study suggests that the rimmed vacuoles observed at 7 weeks after drug treatment are due to autophagy and this process may have a role in diseases related to amyloid-beta accumulation in IBM [33].
Muscle inflammation and NF-kB activation in myositis
Available literature strongly suggests that there is an active interaction between different cell types in the muscle microenvironment in myositis [34]. This interaction between immune and muscle cells results in either stimulation or inhibition of immune cells depending on cell type. MHC class I is highly expressed in myositis muscle fibers and the receptors that interact with MHC class I in myositis are undefined. A recent study shows that leukocyte Ig-like receptor 1 (CD85j) but not CD158 (killer cell Ig-like receptors [KIRs]) is highly expressed in inflammatory cells in muscle, suggesting active interaction between skeletal muscle and immune cells (e.g., dendritic cells) [35]. Also, human skeletal muscle cells not only express common cell surface molecules such as MHC Class I and ICAM-1 but also certain immunoregulatory molecules such as B7 homolog 3 (B7-H3), which may protect from T cell-mediated damage to the skeletal muscle [36].
The precise role of inflammation in muscle fiber damage and dysfunction in myositis is unclear. Although macrophages are the predominant cell type in PM and DM and express iNOS and TGF-β, for example, the relative contribution of these cells and how the differential expression of certain cytokines lead to histological and phenotypic differences in these diseases remain to be studied [37]. A recent study indicates that chronic inflammation (LPS exposure) in a transgenic mouse model increases amyloid beta generation and tau phosphorylation in skeletal muscle through tau kinase and glycogen synthase kinase-3beta (GSK-3beta). They showed that suppression of GSK-3beta activity significantly reduced tau phosphorylation and there was partial recovery of motor impairment in the mouse model [38]. Another independent study also supports the link between expression of amyloid precursor protein and inflammation in the muscle. This study showed colocalization of IL-1 beta with beta-amyloid depositions within myofibers, suggesting that pro-inflammatory mediators specifically induce beta-amyloid-associated skeletal muscle degeneration [39]. Another study also suggested that decreased activity of SIRT1, a member of NAD (+)-dependent histone/protein deacetylases, increases NF-kappaB activation and amyloid beta accumulation in sIBM muscle [36]. All these studies clearly suggest that accumulation of beta amyoid is controlled by inflammatory pathways and targeting specific inflammatory pathways is likely to be beneficial in IBM.
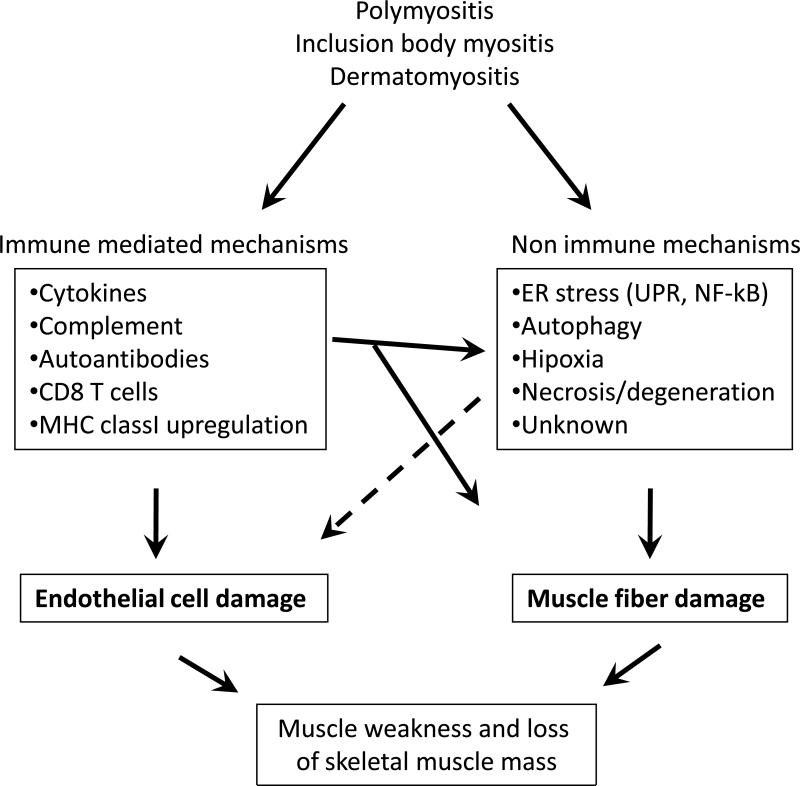
NF-κB is the master regulator of inflammation that is activated not only in immune cells but also in skeletal muscle cells under inflammatory conditions. NF-κB can be activated quickly by a wide variety of stimuli, including inflammatory cytokines (e.g., TNF-α and IL-1), T-cell activation signals, and cell stress inducers. Recent investigations indicated that overexpression of MHC class I on muscle fibers indeed results in activation of the NF-κB and ER stress response pathway in human inflammatory myopathies and in the mouse model of myositis [32,40] (Table 1). Further, there is strong evidence that downstream NF-κB target genes (e.g., MHC class I, ICAM, MCP-1) are highly up-regulated in myositis muscle. It is likely that both classical (proinflammatory cytokine induced) and non-classical (ER stress response induced) pathways activate NF-kB in myositis (Figure 2) [40-43]. Therefore, NF-κB may be a common and potential therapeutic target in all forms of myositis, and use of specific NF-κB pathway inhibitors are likely to be beneficial in these disease conditions.
Figure 2.
Immune and non immune mechanisms of muscle fiber damage in myositis. ER, endoplasmic reticulum; UPR, unfolded protein response; MHC, major histocompatibility complex; PM polymyositis; IBM, inclusion body myositis; DM, dermatomyositis.
Conclusion
Recent studies clearly provide evidence that both immune (cell mediated in PM and IBM and humoral in DM) as well as non-immune (ER stress response and autophagy) mechanisms play a role in muscle fiber damage and dysfunction in myostis. It appears that NF-kB bridges the link between these two pathways of muscle dysfunction in all forms of myositis. These data suggest specific drug(s) that target both immune and non-immune pathways would serve as effective therapeutic agents for these disease conditions.
Acknowledgements
The authors would like to thank Dr.Sree Rayavarapu for critical comments and editorial help with this manuscript.
Funding support: Dr. Nagaraju is supported by the National Institutes of Health (RO1-AR050478 and 5U54HD053177); the Foundation to Eradicate Dystrophy; the US Department of Defense (W81XWH-05-1-0616) and the Myositis Association. Dr.Henriques-Pons is supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended readings
* Of special interest
** Of Outstanding interest
- 1.DeVere R, Bradley WG. Polymyositis: its presentation, morbidity and mortality. Brain. 1975;98:637–666. doi: 10.1093/brain/98.4.637. [DOI] [PubMed] [Google Scholar]
- 2.Plotz PH, Dalakas M, Leff RL, Love LA, Miller FW, Cronin ME. Current concepts in the idiopathic inflammatory myopathies: polymyositis, dermatomyositis, and related disorders. Ann Intern Med. 1989;111:143–157. doi: 10.7326/0003-4819-111-2-143. [DOI] [PubMed] [Google Scholar]
- 3.Emslie-Smith AM, Arahata K, Engel AG. Major histocompatibility complex class I antigen expression, immunolocalization of interferon subtypes, and T cell-mediated cytotoxicity in myopathies. Hum Pathol. 1989;20:224–231. doi: 10.1016/0046-8177(89)90128-7. [DOI] [PubMed] [Google Scholar]
- 4.Englund P, Nennesmo I, Klareskog L, Lundberg IE. Interleukin-1alpha expression in capillaries and major histocompatibility complex class I expression in type II muscle fibers from polymyositis and dermatomyositis patients: important pathogenic features independent of inflammatory cell clusters in muscle tissue. Arthritis Rheum. 2002;46:1044–1055. doi: 10.1002/art.10140. [DOI] [PubMed] [Google Scholar]
- 5.Adams E. A pilot study : use of fludarabine for refractory dermatomyositis and polymyositis, and examination of endpoint measures. J Rheumatol. 1999;26(2):352–360. [PubMed] [Google Scholar]
- 6.Nawata Y, Kurasawa K, Takabayashi K, Miike S, Watanabe N, Hiraguri M, Kita Y, Kawai M, Saito Y, Iwamoto I. Corticosteroid resistant interstitial pneumonitis in dermatomyositis/polymyositis: prediction and treatment with cyclosporine. J Rheumatol. 1999;26:1527–1533. [PubMed] [Google Scholar]
- 7.Lundberg I, Chung Y. Treatment and investigation of idiopathic inflammatory myopathies. Rheumatology (Oxford) 2000;39:7–17. doi: 10.1093/rheumatology/39.1.7. [DOI] [PubMed] [Google Scholar]
- 8.Nyberg P, Wikman AL, Nennesmo I, Lundberg I. Increased expression of interleukin 1alpha and MHC class I in muscle tissue of patients with chronic, inactive polymyositis and dermatomyositis. J Rheumatol. 2000;27:940–948. [PubMed] [Google Scholar]
- *9.Dirksen RT. Checking your SOCCs and feet: the molecular mechanisms of Ca2+ entry in skeletal muscle. J Physiol. 2009;587:3139–3147. doi: 10.1113/jphysiol.2009.172148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *10.Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, Graham V, Ward CC, Seth M, Finch E, Malouf N, et al. STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol. 2008;10:688–697. doi: 10.1038/ncb1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *11.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov. 2008;7:1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 12.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 13.Raven JF, Koromilas AE. PERK and PKR: old kinases learn new tricks. Cell Cycle. 2008;7:1146–1150. doi: 10.4161/cc.7.9.5811. [DOI] [PubMed] [Google Scholar]
- 14.Vembar SS, Brodsky JL. One step at a time: endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delaunay A, Bromberg KD, Hayashi Y, Mirabella M, Burch D, Kirkwood B, Serra C, Malicdan MC, Mizisin AP, Morosetti R, et al. The ER-bound RING finger protein 5 (RNF5/RMA1) causes degenerative myopathy in transgenic mice and is deregulated in inclusion body myositis. PLoS One. 2008;3:e1609. doi: 10.1371/journal.pone.0001609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickering JD, White E, Duke AM, Steele DS. DHPR activation underlies SR Ca2+ release induced by osmotic stress in isolated rat skeletal muscle fibers. J Gen Physiol. 2009 May;133(5):511–24. doi: 10.1085/jgp.200910191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavery GG, Walker EA, Turan N, Rogoff D, Ryder JW, Shelton JM, Richardson JA, Falciani F, White PC, Stewart PM, et al. Deletion of hexose-6-phosphate dehydrogenase activates the unfolded protein response pathway and induces skeletal myopathy. J Biol Chem. 2008;283:8453–8461. doi: 10.1074/jbc.M710067200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shtifman A, Ward CW, Laver DR, Bannister ML, Lopez JR, Kitazawa M, Laferla FM, Ikemoto N, Querfurth HW. Amyloid-beta protein impairs Ca(2+) release and contractility in skeletal muscle. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rizzuto R, Marchi S, Bonora M, Aguiari P, Bononi A, De Stefani D, Giorgi C, Leo S, Rimessi A, Siviero R, et al. Ca(2+) transfer from the ER to mitochondria: When, how and why. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimizu Y, Hendershot LM. Oxidative folding: cellular strategies for dealing with the resultant equimolar production of reactive oxygen species. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2501. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4:600–609. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- 23.Longatti A, Tooze SA. Vesicular trafficking and autophagosome formation. Cell Death Differ. 2009;16:956–965. doi: 10.1038/cdd.2009.39. [DOI] [PubMed] [Google Scholar]
- 24.Nagaraju K, Casciola-Rosen L, Rosen A, Thompson C, Loeffler L, Parker T, Danning C, Rochon PJ, Gillespie J, Plotz P. The inhibition of apoptosis in myositis and in normal muscle cells. J Immunol. 2000;164:5459–5465. doi: 10.4049/jimmunol.164.10.5459. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Dalakas MC. Expression of human IAP-like protein in skeletal muscle: a possible explanation for the rare incidence of muscle fiber apoptosis in T-cell mediated inflammatory myopathies. J Neuroimmunol. 2000;106:1–5. doi: 10.1016/s0165-5728(99)00162-9. [DOI] [PubMed] [Google Scholar]
- 26.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Le Fourn V, Gaplovska-Kysela K, Guhl B, Santimaria R, Zuber C, Roth J. Basal autophagy is involved in the degradation of the ERAD component EDEM1. Cell Mol Life Sci. 2009;66:1434–1445. doi: 10.1007/s00018-009-9038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Temiz P, Weihl CC, Pestronk A. Inflammatory myopathies with mitochondrial pathology and protein aggregates. J Neurol Sci. 2009;278:25–29. doi: 10.1016/j.jns.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 29.O'Leary MF, Hood DA. Denervation-induced oxidative stress and autophagy signaling in muscle. Autophagy. 2009;5:230–231. doi: 10.4161/auto.5.2.7391. [DOI] [PubMed] [Google Scholar]
- **30.Ramachandran N, Munteanu I, Wang P, Aubourg P, Rilstone JJ, Israelian N, Naranian T, Paroutis P, Guo R, Ren ZP, et al. VMA21 deficiency causes an autophagic myopathy by compromising VATPase activity and lysosomal acidification. Cell. 2009;137:235–246. doi: 10.1016/j.cell.2009.01.054. [DOI] [PubMed] [Google Scholar]
- 31.Fujita E, Kouroku Y, Isoai A, Kumagai H, Misutani A, Matsuda C, Hayashi YK, Momoi T. Two endoplasmic reticulum-associated degradation (ERAD) systems for the novel variant of the mutant dysferlin: ubiquitin/proteasome ERAD(I) and autophagy/lysosome ERAD(II). Hum Mol Genet. 2007;16:618–629. doi: 10.1093/hmg/ddm002. [DOI] [PubMed] [Google Scholar]
- 32.Nagaraju K. Role of major histocompatibility complex class I molecules in autoimmune myositis. Curr Opin Rheumatol. 2005;17:725–730. doi: 10.1097/01.bor.0000179947.58271.9a. [DOI] [PubMed] [Google Scholar]
- 33.Ikezoe K, Furuya H, Arahata H, Nakagawa M, Tateishi T, Fujii N, Kira J. Amyloid-beta accumulation caused by chloroquine injections precedes ER stress and autophagosome formation in rat skeletal muscle. Acta Neuropathol. 2009;117:575–582. doi: 10.1007/s00401-009-0488-1. [DOI] [PubMed] [Google Scholar]
- 34.Waschbisch A, Meuth SG, Herrmann AM, Wrobel B, Schwab N, Lochmuller H, Wiendl H. Intercellular exchanges of membrane fragments (trogocytosis) between human muscle cells and immune cells: a potential mechanism for the modulation of muscular immune responses. J Neuroimmunol. 2009;209:131–138. doi: 10.1016/j.jneuroim.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Schleinitz N, Cognet C, Guia S, Laugier-Anfossi F, Baratin M, Pouget J, Pelissier JF, Harle JR, Vivier E, Figarella-Branger D. Expression of the CD85j (leukocyte Ig-like receptor 1, Ig-like transcript 2) receptor for class I major histocompatibility complex molecules in idiopathic inflammatory myopathies. Arthritis Rheum. 2008;58:3216–3223. doi: 10.1002/art.23871. [DOI] [PubMed] [Google Scholar]
- 36.Waschbisch A, Wintterle S, Lochmuller H, Walter MC, Wischhusen J, Kieseier BC, Wiendl H. Human muscle cells express the costimulatory molecule B7-H3, which modulates muscle-immune interactions. Arthritis Rheum. 2008;58:3600–3608. doi: 10.1002/art.23997. [DOI] [PubMed] [Google Scholar]
- 37.Rostasy KM, Schmidt J, Bahn E, Pfander T, Piepkorn M, Wilichowski E, Schulz-Schaeffer J. Distinct inflammatory properties of late-activated macrophages in inflammatory myopathies. Acta Myol. 2008;27:49–53. [PMC free article] [PubMed] [Google Scholar]
- 38.Kitazawa M, Trinh DN, LaFerla FM. Inflammation induces tau pathology in inclusion body myositis model via glycogen synthase kinase-3beta. Ann Neurol. 2008;64:15–24. doi: 10.1002/ana.21325. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt J, Barthel K, Wrede A, Salajegheh M, Bahr M, Dalakas MC. Interrelation of inflammation and APP in sIBM: IL-1 beta induces accumulation of beta-amyloid in skeletal muscle. Brain. 2008;131:1228–1240. doi: 10.1093/brain/awn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vattemi G, Engel WK, McFerrin J, Askanas V. Endoplasmic reticulum stress and unfolded protein response in inclusion body myositis muscle. Am J Pathol. 2004;164:1–7. doi: 10.1016/S0002-9440(10)63089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagaraju K, Casciola-Rosen L, Lundberg I, Rawat R, Cutting S, Thapliyal R, Chang J, Dwivedi S, Mitsak M, Chen YW, et al. Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 2005;52:1824–1835. doi: 10.1002/art.21103. [DOI] [PubMed] [Google Scholar]
- 42.Lundberg I, Brengman JM, Engel AG. Analysis of cytokine expression in muscle in inflammatory myopathies, Duchenne dystrophy, and non-weak controls. J Neuroimmunol. 1995;63:9–16. doi: 10.1016/0165-5728(95)00122-0. [DOI] [PubMed] [Google Scholar]
- 43.Lundberg I, Ulfgren AK, Nyberg P, Andersson U, Klareskog L. Cytokine production in muscle tissue of patients with idiopathic inflammatory myopathies. Arthritis Rheum. 1997;40:865–874. doi: 10.1002/art.1780400514. [DOI] [PubMed] [Google Scholar]