Abstract
There is a high incidence of cardiac arrest and poorer post-resuscitation outcome in the elderly population. Cardiac arrest and resuscitation results in ischemia/reperfusion injury associated with oxidative stress, leading to post-resuscitation mortality and delayed selective neuronal cell loss. In this study we investigated recovery following cardiac arrest and resuscitation in the aged rat brain. Male Fischer 344 rats (6, 12 and 24 months old) underwent 7 minute cardiac arrest before resuscitation. Overall survival and hippocampal neuronal counts were determined at 4 days of recovery. Brainstem function was assessed by hypoxic ventilatory response (HVR). Mitochondria of brainstem, cortex and hippocampus were isolated and assessed for respiratory function. Effect of an antioxidant, alpha-phenyl-tert-butyl-nitrone (PBN) was used as a treatment strategy against oxidative stress in the 6 and 24-month old rats. The time course of mitochondrial function was established using 3-month old Wistar rats with 12-minute cardiac arrest. In the 24-month old rats, overall survival rate, hippocampal CA1 neuronal counts, HVR, and brain mitochondrial respiratory control ratio were significantly reduced following cardiac arrest and resuscitation compared to the younger rats, and PBN treatment improved outcome. The data suggest that (i) there was increased susceptibility to ischemia/reperfusion in aged rat brain; (ii) HVR was decreased in the aged rats; (iii) brain mitochondrial respiratory function related to coupled oxidation was decreased following cardiac arrest and resuscitation in rats, more so in the aged; and (iv) treatment with an antioxidant, such as PBN, reduced the oxidative damage following cardiac arrest and resuscitation.
1. Introduction
Cardiac arrest is a leading cause of death in the United States. About 180-250,000 Americans die as a result of sudden cardiac arrest every year (Chugh et al., 2004). About 50% of short-term survivors die in permanent coma and 10% to 30% of long-term survivors suffer permanent brain damage as a result of global brain ischemia (Safar, 1993). Most of the mortality and morbidity after initially successful resuscitation can be assigned to the immediate and delayed effects of reperfusion injury in the central nervous system. With aging, there is an increased incidence of cardiac arrest and subsequent death or residual neurologic deficit secondary to reperfusion injury. Elderly patients (≥ 65 years old) account for 84% of patients with cardiac arrest and, not surprisingly, have the worst outcomes (Zheng et al., 2001). The age-related changes in the brain could alter the outcome of diseases, as well as the approach to therapeutic strategies (Toescu, 2005).
Transient global brain ischemia induced by cardiac arrest and resuscitation results in reperfusion injury associated with oxidative stress (Vereczki et al., 2006). Despite the increased understanding of ischemic brain damage, the study of brain ischemia/reperfusion injuries in aged animals is still lacking. Most of current models of brain ischemia use younger animals. This might explain why many clinical trials that are designed based on animal models have failed to identify clinically effective drug-based treatments for stroke. Therefore, it is important to study cardiac arrest and resuscitation in the elderly animals to further understand the mechanisms of brain ischemia/reperfusion injuries and to provide information for developing potential treatment strategies.
We have previously reported that in the young adult rats post-resuscitation mortality is associated with decreased hypoxic ventilatory response (HVR) (Xu and LaManna, 2009). HVR reflects brainstem regulation of respiration and thus can be used to assess brainstem function (LaManna et al., 1996). It remains debated whether there is an age-related change in HVR in rats (Schlenker and Goldman 1985; Wenninger et al., 2009). Thus it is unknown if cardiac arrest and resuscitation results in a further impairment in brainstem function that is associated with decreased HVR in the aged.
The poorer outcome following cardiac arrest and resuscitation in the aged may be due to an increase in free radical production and a decrease in defense mechanisms that exacerbates reperfusion injury (Floyd and Carney, 1991). Since mitochondria are both a major source and target of free radicals, oxidative damage is, therefore, likely to play a role in the decline of mitochondrial function, especially following an ischemic-reperfusion insult by cardiac arrest and resuscitation. To test this hypothesis, we measured mitochondrial respiratory function in brains before and following cardiac arrest and resuscitation. A free radical scavenger, alpha-phenyl-tert-butyl-nitrone (Phillis and Clough-Helfman, 1990), PBN, was used as a treatment strategy. PBN has been shown to protect the brain from ischemia / reperfusion insult in both focal global ischemia models (Cao and Phillis 1994; Folbergrova et al., 1995; Pazos et al., 1999) by scavenging certain specific highly deleterious free radicals and suppressing overall oxidative stress (Floyd, 1999).
Aging is associated with increased susceptibility to ischemia/reperfusion injury induced by cardiac arrest and resuscitation; thus, an antioxidant treatment with an agent such as PBN in aged rats would result in improved brainstem function and mitochondrial function, as well as, overall recovery following cardiac arrest and resuscitation. In this study, we investigated the recovery from cardiac arrest and resuscitation in rats of 3 different age groups (6, 12 and 24 months old). Effect of PBN was also tested in 6 and 24-month old rats. Physiological variables, overall survival rate and hippocampal CA1 neuronal survival were determined in 4-day recovery rats. HVR was measured daily to assess brainstem function; mitochondrial oxidative capacity was assessed by measuring respiratory rates (ADP-stimulated state 3 rates and state 4 rates) from freshly isolated brain mitochondria.
2. Results
2.1. Physiological variables
Physiological variables before and after cardiac arrest (7 minutes) and resuscitation were determined in Fischer 6, 12 and 24- month old untreated rats and 24-month old PBN-treated rats (Table 1). These rats were allowed to survive for 4 days after resuscitation for the determination of survival rate and hippocampal counts. There were no significant differences in body weights among any of the experimental groups before cardiac arrest. At the end of 4 days of recovery, body weights in all groups were significantly lower compared to the pre-arrest baseline values. The 24-month untreated rats had the lowest body weights of all groups, while the 24-month PBN-treated rats showed similar weights compared to the 6-month untreated rats. Pre-arrest mean arterial blood pressure (MABP) values were similar among all groups. The post-resuscitation MABP values in all untreated groups were slightly lower than the pre-arrest values. However, in the PBN-treated 24-month old rats the MABP completely returned to the pre-arrest baseline values. The rats regained spontaneous respiration 2 to 6 hours after resuscitation. The aged rats trended toward a longer time before weaning them off the ventilator. Blood gases (pH, PaO2 and PaCO2) taken pre-arrest and at 1 hour post-resuscitation were in the normal range in all groups. At 1 hour post-resuscitation, plasma glucose and lactate concentrations in all groups were significantly higher than their pre-arrest values; the plasma lactate value was significantly higher in the 24-month old untreated rats compared to the 6 and 12-month old untreated groups and the 24-month old PBN-treated group.
Table 1.
Physiological variables of the long-term recovery rats
| Untreated groups |
PBN-treated |
|||
|---|---|---|---|---|
| Variable | 6 month (n = 13) | 12 month (n = 9) | 24 month (n = 13) | 24 month (n = 9) |
| Body weight (g) | ||||
| Pre | 391 ± 26 | 399 ± 46 | 397 ± 22 | 398 ± 40 |
| 4 d | 362 ± 14 (n = 9)* | 348 ± 54 (n = 5)* | 327 ± 10 (n = 5)*‡ | 352 ± 33 (n = 6)* |
| MABP (mmHg) | ||||
| Pre | 111 ± 12 | 114 ± 8 | 113 ± 6 | 118 ± 4 |
| 1 hr | 102 ± 17 | 105 ± 11 | 104 ± 19 | 119 ± 7 |
| Arterial pH (unit) | ||||
| Pre | 7.42 ± 0.03 | 7.41 ± 0.03 | 7.43 ± 0.01 | 7.44 ± 0.04 |
| 1 hr | 7.43 ± 0.08 | 7.38 ± 0.07 | 7.44 ± 0.03 | 7.41 ± 0.06 |
| PaO2 (mmHg) | ||||
| Pre | 91 ± 11 | 91 ± 5 | 91 ± 15 | 96 ± 20 |
| 1 hr | 100 ± 14 | 97 ± 16 | 96 ± 12 | 96 ± 11 |
| PaCO2 (mmHg) | ||||
| Pre | 43 ± 4 | 42 ± 2 | 43 ± 4 | 40 ± 3 |
| 1 hr | 36 ± 4* | 37 ± 3* | 36 ± 3* | 38 ± 6 |
| Hematocrit (%) | ||||
| Pre | 47 ± 3 | 49 ± 1 | 48 ± 4 | 48 ± 2 |
| 1 hr | 47 ± 3 | 49 ± 1 | 49 ± 3 | 48 ± 3 |
| Glucoseplasma (mM) | ||||
| Pre | 7.7 ± 0.8 | 7.5 ± 1.1 | 7.9 ± 1.9 | 7.8 ± 1.6 |
| 1 hr | 9.1 ± 1.5* | 9.9 ± 2.6* | 9.6 ± 2.9* | 9.2 ± 1.5* |
| Lactateplasma (mM) | ||||
| Pre | 1.6 ± 0.5 | 1.7 ± 0.3 | 1.5 ± 0.4 | 1.6 ± 0.6 |
| 1 hr | 2.8 ± 0.9* | 2.8 ± 0.7* | 4.4 ± 1.4*‡ | 2.5 ± 0.7*§ |
Values are mean ± SD
indicates significant difference (t-test, p < 0.05) from the pre-arrest value in the same experimental group.
indicates significant difference (ANOVA, p < 0.05) from the 6 -month old untreated group
indicates significance (t-test, p < 0.05) between the untreated and PBN-treated 24 -month old groups.
2.2. Overall survival rate
Overall survival rates over the 4-day period following cardiac arrest (7 minutes) and resuscitation were determined in Fischer 6, 12 and 24- month old untreated rats and 24-month old PBN-treated rats (Table 2). The overall survival rates decreased with age in the untreated rats. There was greater mortality during the first 2 days of recovery in all age groups. The survival rate in the 24-month old untreated rats was significantly less than that of the 6-month old group (39% vs. 69%, Wilcoxon survival analysis, p < 0.05). However, with PBN treatment, overall survival rate was significantly improved (67%) in 24-month old, which was similar to that of the youngest 6-month old group.
Table 2.
Survival rates (4 days) after cardiac arrest and resuscitation
| Age (months) | Treatment | Number of deaths |
Survival rate |
|||
|---|---|---|---|---|---|---|
| < 1 d | 1-2 d | 2-3 d | 3-4 d | 4d/total | ||
| 6 | Untreated | 0 | 1 | 1 | 2 | 69% (9/13) |
| 12 | Untreated | 2 | 1 | 1 | 0 | 56% (5/9) |
| 24 | Untreated | 1 | 5 | 2 | 0 | 39% (5/13)* |
| 24 | PBN-treated | 0 | 0 | 0 | 3 | 67% (6/9)§ |
indicates significant difference from the 6-month old untreated group, Wilcoxon survival analysis, p < 0.05.
indicates significant difference between the untreated and PBN-treated 24-month old groups, Wilcoxon survival analysis, p < 0.05.
2.3. Hippocampal neuronal counts
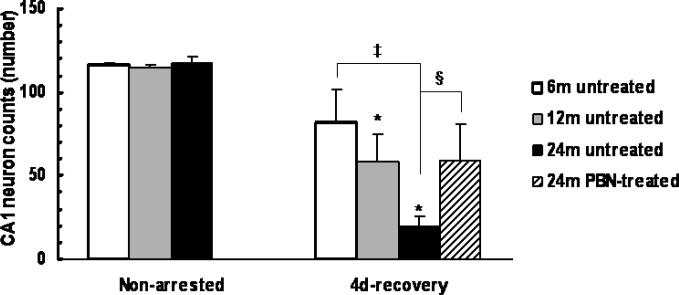
The hippocampal neuronal cell counts were determined at 4 days of recovery from 7-min cardiac arrest in Fischer 6, 12 and 24- month old untreated rats and 24-month old PBN-treated rats (Fig. 1). The hippocampal neuronal counts in non-arrested rats were similar among all age groups (116 ± 1, 114 ± 2 and 117 ± 5 for 6, 12 and 24-month old groups, respectively, n = 4 each). Hippocampal neuronal loss occurred at 4 days after resuscitation in all age groups with greater loss in the 24-month old rats. The hippocampal cell counts were 82 ± 19 (n = 7), 58 ± 17 (n = 4) and 20 ± 6 (n = 5) in the 6, 12 and 24 month old groups, respectively; the 24-month old group had significantly lower hippocampal CA1 counts than that of the 6-month old rats. In the 24-month old with PBN treatment, the hippocampal CA1 cell counts (59 ± 21, n = 6) were significantly higher compared to the untreated rats.
Fig.1.
Hippocampal CA1 neuronal counts 4 days after cardiac arrest and resuscitation, in 6, 12 and 24-month old untreated groups and 24-month old PBN treated group. Values are means ± SD; * indicates significant difference (ANOVA, p < 0.05) from the non-arrested group in the same age group, ‡ indicates significant difference (ANOVA, p < 0.05) from the 6-month old untreated group; indicates significant difference (ANOVA, p < 0.05) between the untreated and the PBN-treated 24-month old groups.
2.4. Hypoxic ventilatory response (HVR)
2.4.1. HVR as a function of age
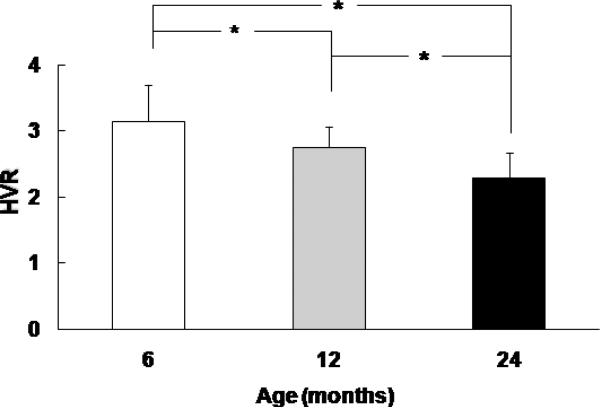
Brainstem function before cardiac arrest in 6, 12 and 24-month old Fischer rats was assessed by measuring the hypoxic ventilatory response (Fig.2). The pre-arrest HVR values decrease with age, the pre-arrest HVR in 6, 12 and 24-month were 3.1 ± 0.5, n = 22; 2.7 ± 0.3, n =13; and 2.3 ± 0.3, n = 31, respectively, and there was significance between any two age groups (ANOVA, p<0.05).
Fig.2.
Pre-arrest values of hypoxic ventilatory response (HVR) in 6, 12, and 24-month old rats. HVR is represented by ratio of minute volume, hypoxic (10% oxygen) vs. normoxic baseline. Values are means ± SD, n = 22, 13, and 31 for 6, 12 and 24 month-old rats, respectively. * indicates significant difference (ANOVA, p < 0.05) between the two groups.
2.4.2. HVR after cardiac arrest and resuscitation
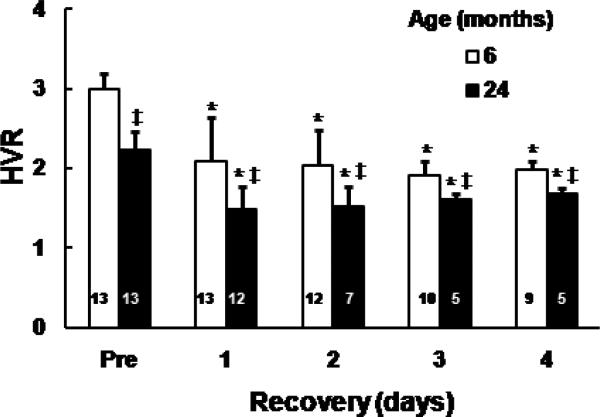
HVR was measured in the Fischer 6 and 24-month old untreated rats before and during the first 4 days of recovery from 7-min cardiac arrest. The pre-arrest baseline HVR values in the two age groups were consistent with values shown in previous figure. After day 1, in both 6 and 24-month old groups HVR values decreased significantly compared to their pre-arrest values. In addition, the 24-month old rats had significantly lower HVR values compared to the 6-month old rats at each time point (Fig. 3).
Fig.3.
Hypoxic ventilatory response (HVR) in 6 and 24-month old untreated rats before and following cardiac arrest and resuscitation. HVR is represented by ratio of minute volume, hypoxic (10% oxygen) vs. normoxic baseline. Values are means ± SD, number on the bar indicates number of rats survived at each time point. * indicates significant difference (t-test, p < 0.05) from the pre-arrest value (Pre) in the same age, ‡ indicates significant difference (t-test, p < 0.05) from the 6-month old untreated group.
2.4.3. PBN and HVR after cardiac arrest and resuscitation
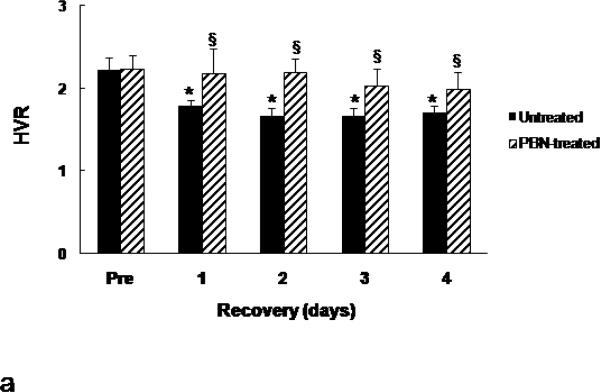
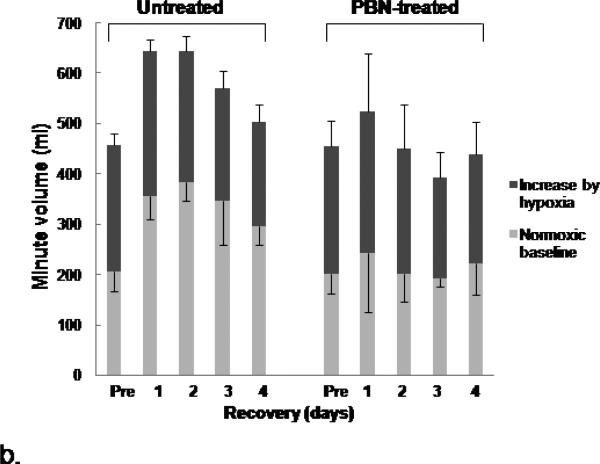
The values of HVR (pre-arrest and post resuscitation from 7-min arrest) were compared in the 4-day survivors in the untreated and the PBN-treated 24-month old Fischer rats (Fig. 4a). The untreated group and PBN-treated group had similar HVR values before cardiac arrest. Post-resuscitation HVR values were significantly higher in the PBN-treated group throughout the 4 recovery days (Fig.4a). We further compared the normoxic and hypoxic ventilation between these two groups and as expected, there were no differences in either the normoxic baseline or the hypoxic minute ventilation before cardiac arrest (Fig. 4b). However, following resuscitation, the normoxic baseline in the untreated group increased significantly by 44-87% during the first 4 days of recovery compared to the pre-arrest values, however, the normoxic baseline in the PBN-treated rats remained unchanged (Fig. 4b). The hypoxia-induced minute volume increase (hypoxic minute volume minus normoxic minute volume) remained similar in both groups following resuscitation, as there were no group differences at each time points. Since HVR is calculated by the ratio of hypoxic minute volume (numerator) to normoxic minute volume (denominator), therefore, compared to the untreated group, the higher post-resuscitation HVR in the PBN-treated group was due to the attenuated normoxic baseline minute ventilation rather than the increased hypoxic response.
Fig.4.
Hypoxic ventilatory response (HVR) and minute volume in 24-month old rats before and following cardiac arrest and resuscitation. (a): HVR, represented by ratio of minute volume, hypoxic (10% oxygen) vs. normoxic baseline. Values are means ± SD; n = 5 each. * indicates significant difference (t-test, p < 0.05) from the pre-arrest values (Pre). § indicates significant difference (t-test, p < 0.05) between the untreated and PBN-treated groups. (b): Minute volume, values are means ± SD; n = 5 each. The normoxic baseline is indicated by light gray bar with negative error bar. The increase of minute volume (hypoxic minute volume - normoxic minute volume) is indicated by dark gray bar with positive error bar.
2.5. Mitochondrial Respiratory Function
2.5.1. Time course of mitochondrial respiratory function
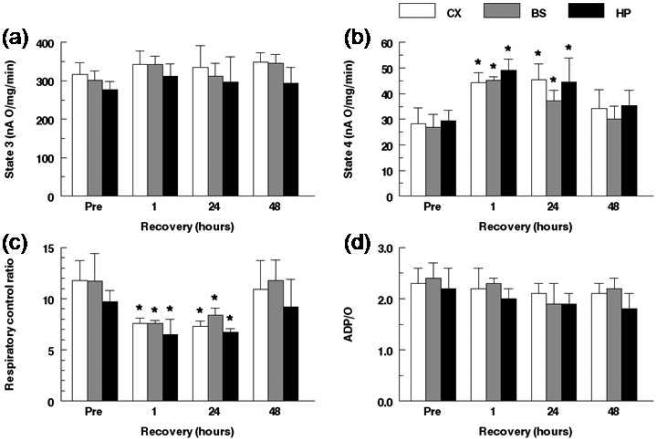
To establish a time course of mitochondrial function following cardiac arrest and resuscitation, mitochondrial oxidative capacity was measured using polarographic analysis in the 3 month old adult Wistar rat before and at 1, 24 and 48 hours of recovery from 12-min cardiac arrest. Oxygen consumption in the presence of glutamate/malate, pre-arrest and over 48 hours of recovery following resuscitation in cortex, brain stem and hippocampus was measured (Fig. 5). Mitochondria isolated from cortex exhibited slightly higher state 3 rates than mitochondria from brainstem and hippocampus (316 ± 30, 301 ± 25, and 278 ± 22 nA O/mg/min for cortex, brainstem and hippocampus, respectively; n = 7 each) with no significant differences among the regions. Over the 48 hour recovery period the state 3 rates trended higher in all regions following resuscitation, with no significant changes in any of the three regions (Fig 5a). There were no differences in the state 4 rates in cortex, brainstem and hippocampus pre-arrest (28 ± 6, 27 ± 5, 28 ± 4 for cortex, brainstem and hippocampus, respectively, n = 7 each, Fig. 5b). Compared to the pre-arrest values the state 4 rates significantly increased at 1 and 24 hours (59-68% and 39-61%, respectively) following resuscitation in all regions. At 48 hours following resuscitation, the state 4 rates in all regions returned to pre-arrest baseline values (Fig. 5b). RCR values were significantly decreased at 1 hour (about 35%) and at 24 hour recovery (28-37%) in all regions compared to their non-arrested control values (Fig. 5c). The respiratory control ratios (RCR, state 3/ state 4) before cardiac arrest were similar among regions (11.8 ± 1.9, 11.7 ± 2.6 and 9.7 ± 1.1; cortex, brainstem and hippocampus, respectively). At 48 hours of recovery, RCR values in all regions were completely recovered to the pre-arrest baseline values (Fig. 5c). RCR is calculated by the ratio of state 3 to state 4 rates and with the state 3 oxidative rates being similar, the lower respiratory control ratios at 1 and 24 hour recovery were as a result of an increase in state 4 rates. Lower RCR's with unchanged state 3 and higher state 4 rates suggest that oxidative phosphorylation was not well coupled. The efficiency of mitochondria were evaluated by the ADP to oxygen ratios, which showed no regional differences at any time point or between post-resuscitation values compared to pre-arrest values (Fig. 5d). In the pre-arrest condition the mitochondrial protein yield (MPY, mg protein /g wet weight) was 6.2 ± 0.5, 5.2 ± 0.5, 7.0 ± 1.4 in the cortex, brainstem and hippocampus, respectively, and remained unchanged following cardiac arrest and resuscitation.
Fig. 5.
Mitochondrial respiratory function in cortex, brainstem, and hippocampus before and following cardiac arrest and resuscitation, measured polarographically, in the presence of glutamate + malate. Values are mean ± SD. Pre: non-arrested control values, n = 7 for each regions; 1, 24 and 48 h recovery time points: n = 5 for each region. (a) ADP-stimulated state 3 respiratory rates and (b) State 4 respiratory rates, expressed as nA O/mg/min; (c) Respiratory control ratio (RCR); (d): ADP to oxygen ration (ADP: O). * indicates significant difference (ANOVA, p < 0.05) compared to the pre-arrest baseline values of respective region.
2.5.2. PBN and mitochondrial respiratory function in the 6 and 24-month old rats
To determine the effects of PBN treatment, the mitochondrial respiratory function was measured in three regions (cortex, brainstem and hippocampus) in 6 and 24 month old Fischer rats before and 1 hour after resuscitation from 7-min cardiac arrest (Table 3). The 1 hour post-resuscitation time point was chosen because in our model the greatest changes in mitochondrial function occurs in the early stage post resuscitation, as shown by the higher state 4 oxidative rates and thus lower RCR values (Fig. 5b and c). There were age differences between 6 and 24 month old with respect to the mitochondrial protein yields. The MPY was significantly lower in the 24-month old rats compared to the 6-month old group in all regions (Table 3). Among the three regions, the highest yield was observed in hippocampus and lowest yield was in brainstem. Following cardiac arrest and resuscitation the MPY were similar to the pre-arrest values.
Table 3.
Mitochondrial respiratory function in isolated brain mitochondria of 6 and 24-month old rats
| Region | Age (mos) | Condition | n | MPY | State 3 | State 4 | ADP/O |
|---|---|---|---|---|---|---|---|
| Cortex | 6 | Non-arrested | 8 | 5.9 ± 0.4 | 316 ± 38 | 30 ± 3 | 2.6 ± 0.6 |
| Untreated 1h | 7 | 5.7 ± 0.3 | 315 ± 31 | 40 ± 5* | 2.5 ± 0.8 | ||
| PBN 1h | 7 | 6.0 ± 0.5 | 307 ± 29 | 33 ± 3§ | 2.8 ± 0.8 | ||
| | |||||||
| 24 | Non-arrested | 10 | 4.5 ± 0.8‡ | 307 ± 12 | 30 ± 3 | 2.1 ± 0.3 | |
| Untreated 1h | 10 | 4.4 ± 0.8‡ | 296 ± 43 | 43 ± 5* | 2.4 ± 0.7 | ||
| PBN 1h | 8 | 4.6 ± 0.4‡ | 301 ± 53 | 33 ± 9§ | 2.5 ± 0.6* | ||
| | |||||||
| Brainstem | 6 | Non-arrested | 8 | 5.0 ± 0.8 | 294± 22 | 32 ± 6 | 2.6 ± 0.6 |
| Untreated 1h | 8 | 5.0 ± 0.8 | 299 ± 44 | 44 ± 7* | 2.6 ± 0.6 | ||
| PBN 1h | 6 | 5.1 ± 0.4 | 307 ± 55 | 33 ± 3§ | 2.6 ± 0.3 | ||
| | |||||||
| 24 | Non-arrested | 11 | 3.9 ± 0.8‡ | 285 ± 28 | 32 ± 7 | 2.2 ± 0.2‡ | |
| Untreated 1h | 10 | 4.0 ± 0.9‡ | 287 ± 45 | 48 ± 11* | 2.3 ± 0.3 | ||
| PBN 1h | 7 | 3.9 ± 0.6‡ | 288 ± 51 | 33 ± 6§ | 2.4 ± 0.4 | ||
| | |||||||
| Hippocampus | 6 | Non-arrested | 5 | 7.2 ± 0.8 | 263 ± 48 | 36 ± 6 | 2.4 ± 0.3 |
| Untreated 1h | 4 | 6.9 ± 0.6 | 263 ± 38 | 49 ± 7* | 2.4 ± 0.1 | ||
| PBN 1h | 6 | 7.2 ± 0.6 | 262 ± 36 | 37 ± 9§ | 2.3 ± 0.4 | ||
| | |||||||
| 24 | Non-arrested | 6 | 5.4 ± 0.6‡ | 264 ± 38 | 35 ± 7 | 1.8 ± 0.2‡ | |
| Untreated 1h | 5 | 5.2 ± 0.4‡ | 257 ± 53 | 51 ± 10* | 2.3 ± 0.4 | ||
| PBN 1h | 6 | 5.5 ± 0.5‡ | 277 ± 35 | 39 ± 2§ | 2.2 ± 0.2* | ||
Values are mean ± SD. MPY: mitochondrial protein yield, mg/g wet weight. Respiratory rates (state 3 and state 4) are expressed as nA O/mg/min; ADP/O, ADP-to-oxygen ratio.
indicates significant difference from the pre-arrest value in the same age group
indicates significant difference (ANOVA, p < 0.05) from the 6-month old rats with same condition
indicates significant difference (ANOVA, p < 0.05) between the untreated and PBN-treated groups with same age.
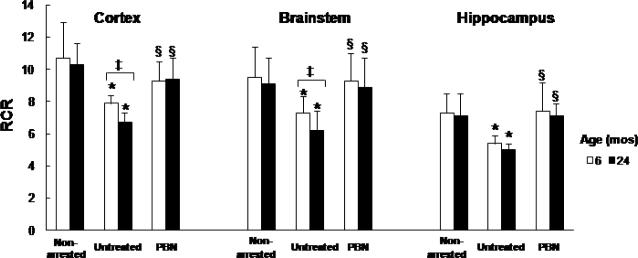
There were no significant age-related changes in the 24 month old rats in state 3 and state 4 before arrest (Table 3). As with the younger rats, the state 3 rates did not change following cardiac arrest and resuscitation in both age groups (6 and 24 month). Compared to the pre-arrest control values, the state 4 rates significantly increased in all regions in the untreated groups. Post-resuscitation the state 3 rates trended lower and the state 4 rates trended higher in the aged, 24 month compared to 6 month. There was no age-related on RCR before cardiac arrest, however, RCR values were significantly lower at 1 h recovery compared to the pre-arrested values in both age groups, with a greater decrease in the 24-month (Fig 6).
Fig. 6.
Respiratory control ratio (RCR) in cortex, brainstem and hippocampus of 6 and 24-month old rats, before (non-arrested) and 1 h post-resuscitation (Untreated or PBN-treated). Values are mean ± SD, number of each group is indicated in Table 3. * indicates significant difference (t-test, p<0.05) from the non-arrested values, ‡ indicates significant difference (t-test, p < 0.05) between 6 and 24 month old groups, § indicates significant difference (t-test, p < 0.05) between the untreated group and the PBN-treated group.
In the PBN-treated rats the RCR rates improved (to the pre-arrest values) in both age groups (Fig 6). This is most likely as a result of the lower state 4 rates observed with PBN treatment (Table 3). There appeared to be an age-related effect (pre-arrest) with mitochondrial efficiency, as the ADP/O values were lower in the 24-month old compared to the 6-month in all regions.
3. Discussion
In this study, post-resuscitation outcome was established in 6, 12 and 24 months old rats with 7 minute cardiac arrest. The major aim of this study was to determine brain recovery in the aged rat following cardiac arrest and resuscitation. In our 3-month old adult rat model of cardiac arrest resuscitation we have demonstrated a mortality of 50% at day four of recovery with the 12-minute cardiac arrest ischemia time (Xu et al., 2006; Xu and LaManna 2009). To match a mortality of 50% in the 24-month old rat, as in the 3-month rat, we shortened the ischemia time to 7 minutes, as the 12-minute cardiac arrest time resulted in no survivors. Our data have shown that overall survival rate and hippocampal CA1 neuronal survival, hypoxic ventilatory response and mitochondrial respiratory function (RCR) were significantly decreased in the 24-month old rats and with PBN treatment, the post-resuscitation recovery was improved. Our findings are consistent with the survival data in gerbils (3-4 months versus 18 months) following transient cerebral ischemia (Floyd and Carney, 1991). The increase in mortality and hippocampal CA1 neuronal death in the 24 month old aged group is likely due to increased vulnerability to ischemia/reperfusion injury with aging.
The hypoxic ventilatory response (HVR) is mediated by the peripheral chemoreflex, a reflex arc from the carotid body sensor to brainstem, then to the respiratory muscle effectors such as diaphragm. Ventilatory response to hypoxia is primarily modulated in brainstem (e.g. nucleus tractus solitatius in medulla oblongata) (Finley and Katz 1992; Koshiya and Guyenet 1996; Bianchi et al., 1995). Impairment of brainstem function may result in altered HVR. In a previous study we used the HVR to assess brainstem function and reported that decreased HVR was associated with greater long-term mortality (Xu and LaManna, 2009). Nevertheless, a possible cortical contribution to the HVR differences cannot be ruled out. In this study we found that HVR significantly decreased with aged. A further decrease was observed after cardiac arrest and resuscitation in the aged. These results suggest that the impairment of brainstem function with respect to respiratory regulation was exacerbated in the aged, which may contribute to the increased mortality and morbidity following cardiac arrest and resuscitation.
Evidence has shown an age-related alteration in energy metabolism and mitochondrial function (Roberts, Jr. and Chih 1995; Sastre et al., 2003). The brain requires a continuous delivery of oxygen to maintain normal function, making it particularly vulnerable to oxidative stress resulting from ischemia/reperfusion (Nita et al., 2001). Increased production of reactive oxygen species may also contribute to the greater vulnerability of aged brain to ischemia/reperfusion damage, as the defense systems are known to be limited. Mitochondria are major sites of free radical production, as well as targets for free radicals. Mitochondrial respiration in rat brain has been reported to be reduced during both ischemia and reperfusion in a focal ischemia model (Anderson and Sims, 1999), there has been show regional difference in recovery of mitochondrial function following transient ischemia (Sims, 1991). In the present study mitochondrial respiratory function was measured in rats pre- and post-cardiac arrest and resuscitation to determine if the aged brain is more susceptible to oxidative stress. The mitochondria were isolated from whole homogenate of cortex, hipppcampus and brainstem (portion of medulla obligata), respectively. There were no age-related differences in mitochondrial function before cardiac arrest. There were no significant changes in state 3 respiration following cardiac arrest and resuscitation irrespective of age. However, state 4 rates increased post resuscitation in all ages. In the 24 month old compared to the 6 month, during the early recovery phase (1 h post-resuscitation), a greater decrease in RCR was observed. This decrease in RCR was as a result of increased state 4 rather than decreased state 3 rates. This response also appeared to be age related, as the decrease in RCR was significantly lower in the 24 month old compared to the 6 month. Thus, these data suggest that ischemic reperfusion injury acutely affects mitochondrial oxidative function through uncoupling and that the aged are more susceptible to oxidative stress following cardiac arrest and resuscitation. With PBN treatment brain mitochondrial function was improved in the aged rats following cardiac arrest and resuscitation; this was also observed in the 6 month old. In addition, the overall survival rates, hippocampal CA1 neuronal survival, as well as, hypoxic ventilatory response were improved with PBN treatment. 4-hydroxy-2-nonenal (HNE), a major reactive product of lipid peroxidation, is considered to be cytotoxic and can be used as a marker of oxidative stress (Hall et al., 1993). HNE reacts with proteins leading to enzyme inactivation and subsequence cellular dysfunction (Humphries and Szweda, 1998; Lucas and Szweda, 1998). Levels of 4-hydroxy-2-nonenal (HNE) modified protein adducts, was found to be elevated in freshly isolated brain mitochondria of after cardiac arrest and resuscitation in both 6 and 24-month-old rats. However, the increase of HNE production was more evident in the aged group and was reduced in the PBN- treated group (Xu et al., 2008).
In conclusion, mortality and morbidity following cardiac arrest and resuscitation was increased in the aged rat. Treatment with the antioxidant PBN improved the outcome following cardiac arrest and resuscitation in both age groups, suggesting increased susceptibility to oxidative stress in the aged. The decrease in brainstem response and greater mitochondrial uncoupling support these findings and point to the protective properties of antioxidants as a therapeutic approach.
4. Experimental procedures
4.1. Animal preparation
The experimental protocol employed by this study was approved by the Institutional Animal Care and Use Committee (IACUC) at Case Western Reserve University. Male Fischer 344 rats (aged 6, 12 and 24 months) were purchased from the National Institute of Aging These animals were used for studying aging effects in rat model of cardiac arrest and resuscitation. In another set of experiment, male Wistar rats (aged 3 months, Charles River) were used for studying the time courses of mitochondrial respiratory function following cardiac arrest and resuscitation. Rats were allowed to acclimate in the animal facility at Case Western Reserve University for one week before being utilized. Surgical procedures were as follows (Xu and LaManna, 2009): anesthesia was induced by isoflurane (2.5% isoflurane in medical air) and maintained with 1-2% isoflurane through a nasal cone. Cannulae were placed in: (i) Ventral tail artery using polyethylene tubing (PE-50, 0.023” i.d., 0.038” o.d.) for the purpose of monitoring of arterial blood pressure and collecting samples. (ii) External jugular vein into the right atrium using a Silastic catheter (0.025” i.d., 0.047” o.d.) for administration of drug. The rats were allowed to recover for at least 1 hour after surgery while restrained in plastic cages. The body temperature was maintained at 37°C by an infrared heat lamp (250W, 45 cm above the body) regulated by feedback from a rectal probe during the surgery and the following experiment.
4.2. Induction of transient global brain ischemia in rat
Transient global brain ischemia was achieved using a rat model of cardiac arrest and resuscitation as described previously (Xu et al., 2006; Xu and LaManna 2009). In brief, for the adult Wistar rats (3-month old), cardiac arrest was induced in the conscious rat by rapid sequential intra-atrial injection of d-tubocurare (0.3mg) and ice-cold KCl solution (0.5M; 0.12 ml/100g of body weight). Resuscitation was initiated 7 min after arrest. The animal was intubated orotreacheally with a 14-gauge catheter attached to a rodent ventilator (100% O2; tidal volume: 10 ml/kg; respiratory rate: 80 breaths / min). Simultaneous chest compressions and the infusion of normal saline (0.5 ml/min) were given until a spontaneous heartbeat returned. Epinephrine (4-10 μg) was given to establish a mean blood pressure greater than 80% of the pre-arrest value, at which point the animal was considered to be resuscitated. Ventilation was then adjusted to 20-30% O2 in air, depending on the normal range of blood gas, until spontaneous respiration was regained. Non-arrested rats went through the same surgical procedures except cardiac arrest. The arterial and venous catheters were removed and the corresponding vessels were ligated about 3 hours after resuscitation prior to weaning of artificial ventilation. Rats were sacrificed at 1, 24 and 48 hours after resuscitation with non-arrested controls for brain mitochondria isolation. For the aging study (Fischer 344 rats, 6, 12 and 24 months old), procedures were slightly modified for the purpose of achieving approximately 50 % 4-day survival rate in the 24-month old rats. Resuscitation was initiated 5 min after arrest and the total ischemia time were shortened to 7 min. For the PBN-treated rats, PBN (100 mg/kg) was infused intravenously immediately after resuscitation for 60 min. The untreated rats were given normal saline for same period of time. For overall survival and hippocampal neuronal survival determinations, rats were allowed to survive for 4 days after resuscitation. For mitochondrial study, the untreated rats and PBN-treated rats were sacrificed at 1 hour post-resuscitation with non-arrested controls.
4.3. Measurement of Hypoxic Ventilatory Response (HVR)
Hypoxic ventilatory response was measured in rats before and daily after resuscitation for 4 days using a plethysmograph system (Buxco Electronics, Troy, NY), as described previously (Xu and LaManna 2009). In brief, conscious, unrestrained rats were placed individually in a pre-calibrated 3 L barometric chamber and continuously ventilated with humidified room air humidified room air or gas mixtures passing through the chamber at a rate of 2 L/min. The room air and 10% O2 in nitrogen were ventilated for 10 min each to determine the normoxic baseline and hypoxic ventilation, respectively. Tidal volume, breath frequency, and minute volume were computed and stored for subsequent analysis. HVR was represented as ratio of hypoxic minute volume vs. normoxic minute volume baseline; minute volume = tidal volume × frequency.
4.4. Hippocampal neuron counts
Rats surviving 4 days and non-arrested controls were deeply anesthetized with isoflurane, perfused through the heart with about 200 ml 0.1 M PBS, and perfusion fixed with 4% paraformaldehyde in 0.1 M PBS (pH 7.4). The brains were removed and embedded with paraffin and sectioned on a microtome. Neuronal cell counts were made from H & E stained 5 μm coronal sections through anterior hippocampus. At the level of atlas plate 30 (Palkovits M and Brownstein MJ, 1988) the entire length of the pyramidal cell layer in the hippocampus was viewed under a high-power light microscope (magnification, 400x). Neurons with rounded cell bodies and clearly visible nucleoli were considered to have survived. The number of neurons surviving was evaluated in CA1 region of hippocampus (Xu et al., 2006).
4.5. Isolation of brain mitochondria
Brain mitochondria were isolated using a method previously described with slight modifications (Lesnefsky and Hoppel, 2006). Rats were deeply anesthetized with isoflurane and decapitated. The brains were quickly removed; the brainstem (portion of medulla obligate), cortex, and hippocampus were dissected. Brain tissue of each region was rinsed in ice-cold isolation buffer (200 mM Mannitol, 70 mM Sucrose, and 5.0 mM MOPS, pH 7.4) and blotted dry, freed of visible blood vessels, then weighed and minced thoroughly. The approximate weight of brainstem, cortex, and hippocampus were 0.3, 0.8 and 0.1g, respectively. The tissue was suspended in isolation buffer (10 ml / g tissue) containing defatted bovine albumin (BSA, 0.2%) and EDTA (0.2 mM) and then treated with the protease Subtilisin A (5 mg/g tissue), for 30 second with light shaking. The suspension was then homogenized with a Teflon pestle (4-6 strokes) and the homogenate was centrifuged at 4 °C. The resulting mitochondrial pellet was washed twice with isolation buffer, centrifuged and resuspended. The final protein concentration of brainstem, cortex, and hippocampus were approximately 10, 25 and 7 mg/ml, respectively.
4.6. Measurement of Mitochondrial Respiratory Rates
Oxidative rates were assessed by measuring oxygen consumption using a polarographic system consisting of a Clark-type electrode in the presence of the substrates glutamate plus malate (Kerner et al., 2001). The NADH-linked oxidative rates (state 3: ADP-stimulated; state 4: resting state, ADP-limited) were then calculated (natom oxygen/min/mg protein). The respiratory control ratio (RCR) was determined by equation: RCR = state 3 / state 4. ADP-to-oxygen (ADP/O) ratios (nmol ADP per nanoatom O) were calculated as previously described (Kerner et al., 2001).
4.7. Statistical Methods
All values were presented as mean ± SD. Statistical analyses were performed using SPSS v13.0 for Windows. Group comparisons were made by one-way analysis of variance (ANOVA) using Tukey's statistic. The comparison between any two groups was analyzed with a t-test for paired sample, two-tailed. The survival analysis was performed using a Wilcoxon (Gehan) survival analysis. Significance was considered at the level of p < 0.05.
5. ACKNOWLEGEMENTS
This work was supported by NIH grants NS 46074 and GM 066309.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. REFERENCES
- Anderson MF, Sims NR. Mitochondrial respiratory function and cell death in focal cerebral ischemia. J Neurochem. 1999;73:1189–1199. doi: 10.1046/j.1471-4159.1999.0731189.x. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Cao X, Phillis JW. alpha-Phenyl-tert-butyl-nitrone reduces cortical infarct and edema in rats subjected to focal ischemia. Brain Res. 1994;644:267–272. doi: 10.1016/0006-8993(94)91689-6. [DOI] [PubMed] [Google Scholar]
- Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–1275. doi: 10.1016/j.jacc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Floyd RA. Antioxidants, oxidative stress, and degenerative neurological disorders. Proc Soc Exp Biol Med. 1999;222:236–245. doi: 10.1046/j.1525-1373.1999.d01-140.x. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Carney JM. Age influence on oxidative events during brain ischemia/reperfusion. Arch Gerontol Geriatr. 1991;12:155–177. doi: 10.1016/0167-4943(91)90025-l. [DOI] [PubMed] [Google Scholar]
- Folbergrova J, Zhao Q, Katsura K, Siesjo BK. N-tert-butyl-alpha-phenylnitrone improves recovery of brain energy state in rats following transient focal ischemia. Proc Natl Acad Sci U S A. 1995;92:5057–5061. doi: 10.1073/pnas.92.11.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Andrus PK, Althaus JS, Von Voigtlander PF. Hydroxyl radical production and lipid peroxidation parallels selective post-ischemic vulnerability in gerbil brain. J Neurosci Res. 1993;34:107–112. doi: 10.1002/jnr.490340111. [DOI] [PubMed] [Google Scholar]
- Humphries KM, Szweda LI. Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochem. 1998;37:15835–15841. doi: 10.1021/bi981512h. [DOI] [PubMed] [Google Scholar]
- Kerner J, Turkaly PJ, Minkler PE, Hoppel CL. Aging skeletal muscle mitochondria in the rat: decreased uncoupling protein-3 content. Am J Physiol Endocrinol Metab. 2001;281:E1054–E1062. doi: 10.1152/ajpendo.2001.281.5.E1054. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Guyenet PG. NTS neurons with carotid chemoreceptor inputs arborize in the rostral ventrolateral medulla. Am J Physiol. 1996;270:R1273–R1278. doi: 10.1152/ajpregu.1996.270.6.R1273. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Haxhiu MA, Kutina-Nelson KL, Pundik S, Erokwu B, Yeh ER, Lust WD, Cherniack NS. Decreased energy metabolism in brain stem during central respiratory depression in response to hypoxia. J Appl Physiol. 1996;81:1772–1777. doi: 10.1152/jappl.1996.81.4.1772. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Hoppel CL. Oxidative phosphorylation and aging. Ageing Res Rev. 2006;5:402–433. doi: 10.1016/j.arr.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Lucas DT, Szweda LI. Cardiac reperfusion injury: aging, lipid peroxidation, and mitochondrial dysfunction. Proc Natl Acad Sci U S A. 1998;95:510–514. doi: 10.1073/pnas.95.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nita DA, Nita V, Spulber S, Moldovan M, Popa DP, Zagrean AM, Zagrean L. Oxidative damage following cerebral ischemia depends on reperfusion - a biochemical study in rat. J Cell Mol Med. 2001;5:163–170. doi: 10.1111/j.1582-4934.2001.tb00149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palkovits M, Brownstein MJ. Maps and guide to microdissection of rat brain. Elsevier; New York: 1988. [Google Scholar]
- Pazos AJ, Green EJ, Busto R, McCabe PM, Baena RC, Ginsberg MD, Globus MY, Schneiderman N, Dietrich WD. Effects of combined postischemic hypothermia and delayed N-tert-butyl-alpha-pheylnitrone (PBN) administration on histopathologicaland behavioral deficits associated with transient global ischemia in rats. Brain Res. 1999;846:186–195. doi: 10.1016/s0006-8993(99)02010-7. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Clough-Helfman C. Protection from cerebral ischemic injury in gerbils with the spin trap agent N-tert-butyl-alpha-phenylnitrone (PBN). Neurosci Lett. 1990;116:315–319. doi: 10.1016/0304-3940(90)90093-o. [DOI] [PubMed] [Google Scholar]
- Roberts EL, Jr., Chih CP. Age-related alterations in energy metabolism contribute to the increased vulnerability of the aging brain to anoxic damage. Brain Res. 1995;678:83–90. doi: 10.1016/0006-8993(95)00168-p. [DOI] [PubMed] [Google Scholar]
- Safar P. Cerebral resuscitation after cardiac arrest: research initiatives and future directions. Ann Emerg Med. 1993;22:324–349. doi: 10.1016/s0196-0644(05)80463-9. [DOI] [PubMed] [Google Scholar]
- Sastre J, Pallardo FV, Vina J. The role of mitochondrial oxidative stress in aging. Free Radic Biol Med. 2003;35:1–8. doi: 10.1016/s0891-5849(03)00184-9. [DOI] [PubMed] [Google Scholar]
- Schlenker EH, Goldman M. Ventilatory responses of aged male and female rats to hypercapnia and to hypoxia. Gerontology. 1985;31:301–308. doi: 10.1159/000212713. [DOI] [PubMed] [Google Scholar]
- Sims NR. Selective impairment of respiration in mitochondria isolated from brain subregions following transient forebrain ischemia in the rat. J Neurochem. 1991;56:1836–1844. doi: 10.1111/j.1471-4159.1991.tb03438.x. [DOI] [PubMed] [Google Scholar]
- Toescu EC. Normal brain ageing: models and mechanisms. Philos Trans R Soc Lond B Biol Sci. 2005;360:2347–2354. doi: 10.1098/rstb.2005.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereczki V, Martin E, Rosenthal RE, Hof PR, Hoffman GE, Fiskum G. Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal death. J Cereb Blood Flow Metab. 2006;26:821–835. doi: 10.1038/sj.jcbfm.9600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenninger JM, Olson EB, Jr., Cotter CJ, Thomas CF, Behan M. Hypoxic and hypercapnic ventilatory responses in aging male vs. aging female rats. J Appl Physiol. 2009;106:1522–1528. doi: 10.1152/japplphysiol.90802.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, LaManna JC. The loss of hypoxic ventilatory responses following resuscitation after cardiac arrest in rats is associated with failure of long-term survival. Brain Res. 2009;1258:59–64. doi: 10.1016/j.brainres.2008.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Puchowicz MA, Lust WD, LaManna JC. Adenosine treatment delays postischemic hippocampal CA1 loss after cardiac arrest and resuscitation in rats. Brain Res. 2006;1071:208–217. doi: 10.1016/j.brainres.2005.11.060. [DOI] [PubMed] [Google Scholar]
- Xu K, Puchowicz MA, Sun X, LaManna JC. Mitochondrial dysfunction in aging rat brain following transient global ischemia. Adv Exp Med Biol. 2008;614:379–386. doi: 10.1007/978-0-387-74911-2_42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]