Abstract
On the military training facility of Tekong Island, Singapore, a comprehensive vector-borne disease control program was started in end-2006 to reduce mosquito populations and negate the need for anti-malaria chemoprophylaxis. The program was based on 1) preventing importation of malaria through screening of visitors, 2) preventing human-to-mosquito transmission through early case detection and mosquito control, 3) preventing mosquito-to-human transmission through personal protection, and 4) contingency plans. Systematic environmental works were performed to reduce breeding sites, and insecticide use targeted both adult mosquitoes and larvae. Mosquito populations declined from 103 mosquitoes per sampling site in January 2007 to 6 per site by March 2007 (P < 0.001). The proportion of positive ovitraps declined from 93% in January 2007–2% in March 2007 (P < 0.001). There were no malaria cases on the island despite chemoprophylaxis termination, showing that comprehensive combination vector-control strategies were effective in reducing the risk of malaria.
Introduction
Vector-borne disease control programs have been a major global challenge, especially with the emergence of insecticide resistance among insect vectors, which resulted in the limited field success of single-method vector control programs.1,2 Integrated vector control programs, which involve combination strategies have been successful in some locations in reducing the impact of vector-borne diseases,3,4 but there are insufficient studies in different tropical settings where many vector-borne diseases reside.
In Singapore, a tropical city-state in South-East Asia, malaria was a main vector-borne disease resulting in substantial morbidity and mortality in the early 20th Century. Singapore was declared malaria-free in 1982 after meeting the World Health Organization (WHO) assessment of having 1) a comprehensive and efficacious case detection mechanism; 2) reliable microscopic diagnosis of blood smears; 3) thorough epidemiological investigations and a satisfactory epidemiological situation; 4) adequate preventive and remedial actions upon detection of cases; 5) adequate general health services, effective system of case notification, and epidemiological follow-up for prevention of re-establishment of malaria.5
Although Singapore was declared malaria-free, Tekong Island (a forested island of approximately 5,900 acres located to the north-east of Singapore that houses a military training facility, Figure 1) remained malaria-receptive because of the presence of Anopheles sp. mosquitoes. From 1996 to 2003, there were 19 cases of malaria on the island attributable to secondary transmission on the island (5 in May/June 1996, 8 in July to September 1997, and 6 in August to October 2003), despite the systematic use of anti-malaria prophylaxis for visitors to the island since the 1980s. Because of the side effects of anti-malaria chemoprophylaxis regimes6–8 and the operational costs of chemoprophylaxis, an attempt was made through a comprehensive integrated vector-borne disease management program to reduce the mosquito population and negate the need for chemoprophylaxis. This work describes the program's success and provides additional evidence for the use of combination strategies against mosquito-borne diseases in forested environments.
Figure 1.
Map of Singapore and Pulau Tekong.
Methods
To reduce the mosquito population and need for chemoprophylaxis, which had been in place since the 1980s, the Singapore Armed Forces (SAF) performed an integrated vector-borne disease management program on Tekong Island from December 2006. Surveillance programs were simultaneously set up to determine the effectiveness of the interventions.
Tekong Island is located about 2 miles from the main island of Singapore and is only accessible by sea or air (Figure 1). The island is forested, with mangrove swamps in the coastal areas—ideal for the breeding of Anopheles mosquitoes. The resident human population comprises several thousand conscript military personnel who reside in the south of the island and use the island for training. In addition, tens of thousands of SAF military personnel visit the island annually for field and jungle training, and thousands of foreign workers work on construction projects on the island.
Four Rings of Prevention
To reduce the threat from malaria to individuals on the island, we developed an integrated program to address the different aspects of disease transmission. This is encapsulated in the four prevention rings shown in Figure 2, with the aim of reducing the risk of local transmission of malaria to a negligible level where chemoprophylaxis can be removed and training can continue without substantial risk for malaria infections. These comprise the following measures.
Figure 2.
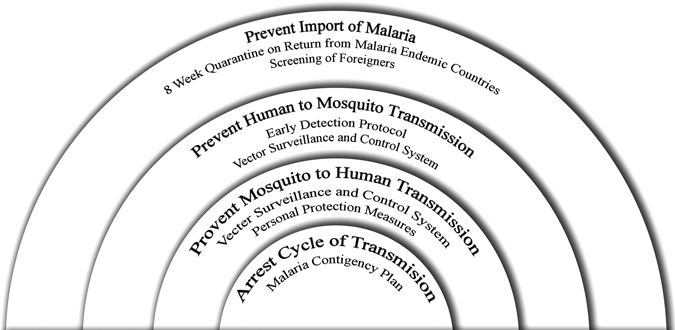
The four rings of prevention of the malaria control program on Tekong Island.
Prevent importation.
A restriction period was imposed on all visitors to the island who had visited malaria endemic areas over the past 8 weeks (the long end of the average incubation period for malaria). All foreign workers who work on construction projects on the island would also be screened for malaria using reverse transcription-polymerase chain reaction (RT-PCR) after the quarantine period. In addition, any military serviceman with fever of unknown origin would be screened using real-time fluorescence-based RT-PCR for malaria parasites performed by a certified laboratory, DSO National Laboratories (Singapore). Positive cases were referred for clinical management and prevented from entry to the island.
Early detection of human cases.
All servicemen and medical staff on the island and military camps in Singapore were educated on malaria and the need for early reporting of symptoms. Cases of fever (≥ 37.5°C) with no localizing symptoms were tested for malaria and other common vector-borne diseases in Singapore, and all confirmed malaria cases were temporarily removed from the island.
Mosquito control program.
The vector-control program consists of environmental works to reduce mosquito breeding habitats and an intense insecticide regimen. Malaria vectors in Singapore were previously identified as Anopheles sundaicus and Anopheles maculates.9 Anopheles sinensis has also recently been suspected to be a vector. Their respective habitats, marshland with brackish water, streams in hilly areas, and small pools, were thus specially targeted.
Systematic environmental and infrastructural improvements were made to reduce breeding sites. They include shoreline works to reduce the mixing of salt and fresh water, improvements to drainage around built-up areas to reduce water pooling, maintenance of drains, including clearance of vegetation and undergrowth to allow accessibility across the island, and filling up pools of water. Clearance of vegetation and undergrowth was maintained on a 3-monthly cycle to prevent rapid re-growth in a jungle habitat. Residents were also encouraged to reduce mosquito breeding habitats within the built-up areas through education programs.
Insecticide use targeted mosquito larvae through the use of Bacillus thuringiensis israelensis (Bti) larvicide by ultra-low volume mist fogging. Bacillus thuringiensis israelensis has been shown to be effective in sustained reduction of mosquito populations in the control of malaria.10–12 This strategy used a combination of vehicle-mounted ultra-low volume mist-foggers for treatment of large areas along roads/tracks, and man-packed mist-foggers for reaching deep forested areas inaccessible to vehicles. Before the application of Bti, conventional fogging using a combination of s-bioallethrin, permethrin, and piperonyl butoxide (Resigen, Bayer Cropscience, Kuala Lumpur, Malaysia) and cypermethrin (New Cyper, Agrolex Agrochemicals Manufacturing, Jiangsu, China) was done daily for 3 consecutive days to reduce the adult mosquito population. Bacillus thuringiensis israelensis was then administered across the island covering 200 g per acre of the entire island at least four times per month. Handheld Global positional systems (GPS) were used to ensure systematic coverage of the entire island. In addition, indoor residual spraying with alphacypermethrin 1.5 sc (Fendona, BASF, Malaysia) or Resigen was applied by mist blowers in the existing buildings to reduce exposure of the resident population to mosquitoes.
Personal protection.
Existing personal protection measures were continued to prevent mosquito-to-human transmission. These included the use of bed nets, pre-treatment of uniforms with permethrin, and application of N,N-Diethyl-meta-toluamide (DEET) insect repellent regularly during jungle training.
Malaria contingency plan.
A response plan involving multiple agencies was developed in the event of an outbreak of malaria (defined as a single case of malaria on the island) despite the previous measures. In addition to routine clinical management, this plan comprised active surveillance for malaria cases and intensified vector surveillance. This was done in conjunction with the DSO National Laboratories, tertiary hospitals in Singapore, the Ministry of Health (MOH), and the National Environment Agency (NEA).
Mosquito Surveillance
To monitor the outcomes of reduction potential mosquito vectors, which are most likely anthropophillic and anthrozoophillic, human bare leg catch was conducted by a commercial pest control contractor without any soldiers being involved. A systematic surveillance at 60 fixed sites across the island was conducted on a weekly basis using 20 men from 1800 to 0700 hrs the following day, from November 2006 to April 2007. This was reduced to fortnightly intervals from April 2007, and the number of sites was then reduced to 40 from June 2007 when the initial vector-control target of 90% reduction was achieved. Ovitraps were also placed in 50 fixed sites and any immatures (ova or larvae) found in the trap would classify the trap as positive for breeding.
This operational program, including use of the human bait method for collection of mosquitoes, was approved by the Singapore Armed Forces Medical Review Committee. The program cost about SGD1 million per year (1 SGD = US$0.664 in 2007).
Statistical Methods
The χ2 and Fisher's exact tests were used to compare the categorical outcomes for bivariate analysis. The non-parametric Mann-Whitney test was used for two-group comparisons continuous outcomes. Statistical analyses were performed using Stata (version 9.0, Stata Corp., College Station, TX), and all tests were conducted at the 5% level of significance.
Results
By the end of 2008, 8,303 foreign workers had been screened by DSO National Laboratories by RT-PCR before entry to the island (100% compliance rate based on documented entry into the island), and seven individuals tested positive for malaria. They were barred from entering the island pending successful treatment and evaluation.
The coastal construction work was completed by January 2007 with 12.5 km of silted and earthen drains cleared and concretized, respectively, and 187 large permanent water pools identified and filled across the island. For the insecticide program, after one round of adulticide fogging, an initial 9 weekly cycles of Bti were applied across the entire island from December 2006 to February 2007, and maintained at two cycles per month thereafter.
Two months after program initiation, the mosquito catch rate declined from a mean of 103 mosquitoes per human baiting sampling site in January to 6 per site in March 2007 (P < 0.001), and maintained at between 2 and 6 per site through December 2008 (Figure 3 and Figure 4). This represents a decline of more than 94% of initial mosquito catch rate before the program was instituted in December 2006. The proportion of ovitraps that were positive declined from 93% in January 2007 to a nadir of 2% in March 2007 (P < 0.001), and maintained at a median of 12% through December 2008 (Figure 5). The proportion of Anopheles mosquitoes (the main vector of concern) remained low and did not change significantly throughout the treatment period—from 1.63% in end 2006 and early 2007 to 1.32% in 2008 (P = 0.41). This suggests a similar reduction in the Anopheles population.
Figure 3.
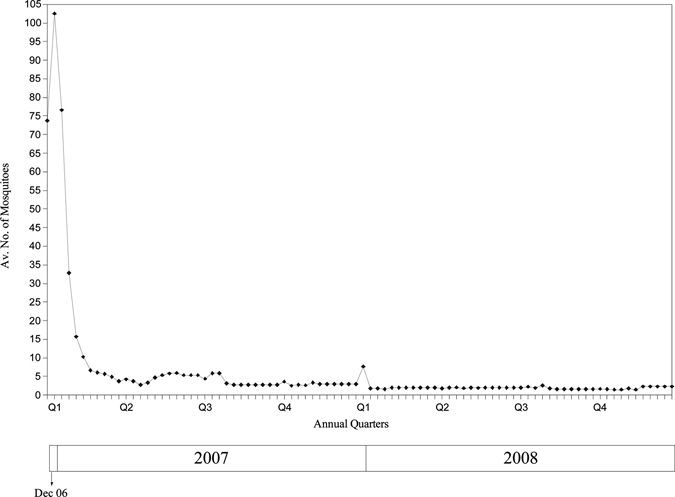
Mean number of mosquitoes trapped by live human baiting per site from December 2006 to December 2008 (sixty sites from Dec 2006 to Jun 2007, 40 sites after Jun 2007).
Figure 4.
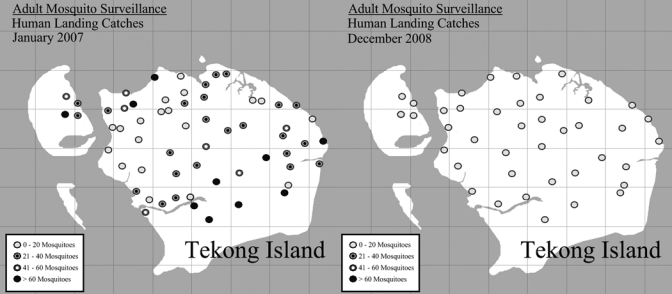
Adult mosquito surveillance through human baiting catches—at the start of the vector control program in January 2007 and in December 2008.
Figure 5.
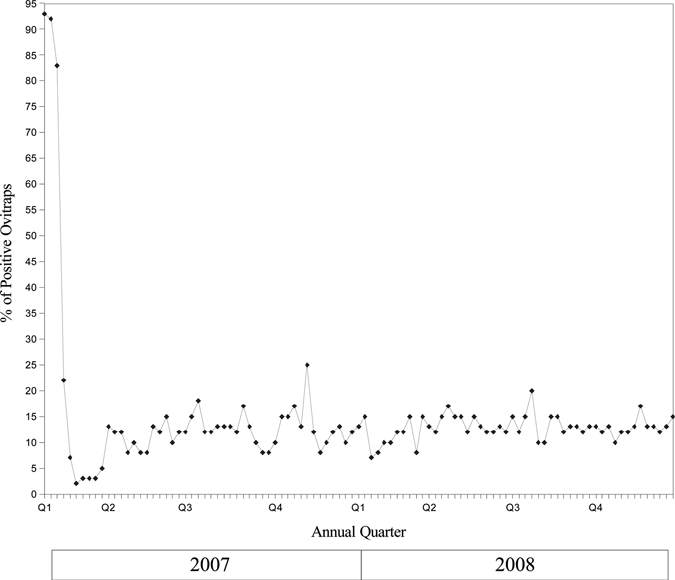
Percentage of ovitraps that was positive for mosquito ova from January 2007 to December 2008.
Subjective surveys on visitors to the island suggested a substantial reduction in the number of mosquitoes and mosquito bites on the island compared with previous visits before December 2006 when the program was started.
Because of the successful vector control efforts and stringent malaria screening policies for visitors, the requirement for malaria chemoprophylaxis on the island was lifted from April 2007. Since April 2007 to the end of 2009, there had not been any reported case of malaria on Tekong Island despite the absence of chemoprophylaxis.
Discussion
We have shown that a comprehensive combination approach can reduce the overall mosquito population in a densely forested area, and this success can reasonably be maintained across time. Although our circumstances and geography are unique, different portions of the program can be extrapolated in other forested areas where vector-borne disease control is desirable. Elimination of breeding sites in forested areas is challenging, but we have done so through a systematic approach using traditional methods together with more advanced technologies such as vehicle-mounted Bti application and GPS mapping. The application of Bti together with environmental works gave the program success in achieving control. Results of our program show a likely systematic reduction of all mosquitoes before and after intervention.
Previous studies have also showed the efficacy of biological larvicides alone in the control of malaria vector populations, established the treatment doses, and frequencies of Bti.10 This program, which led to a significant reduction in mosquito populations, is a translation of these results to show how Bti can be incorporated into a comprehensive vector-control program and effectively mitigate vector-borne disease risk. Many other countries have also used Bti as part of vector-control programs to control various mosquito species such as Anopheles gambiae, Anopheles sundaicus, and Aedes agypti, with success rates of 50–99%.11–24
Bacillus thuringiensis israelensis treatment, although effective in reducing mosquito populations, did not totally eradicate them and additional measures, such as screening for malaria cases, was effective in identifying previously undetected cases. Other combination programs were effective in different environments. One was in the Region of Peel, Ontario, North America, a region struck by multiple West Nile virus epidemics.25 In 2004, several initiatives were implemented including the treatment of water with larvicide (Bti and methoprene), using Larvasonic devices with acoustic energy to kill mosquito larvae, and using fathead minnow fish for vector larvae control.25 The post-treatment pupal monitoring indicated the methoprene pellet's and Larvasonic device's efficacy rate was 90% each.25 For treatment of stagnant water using Bti products, pre- and post-treatment counts of mosquito larva revealed an efficacy of 100% at one site, and an average efficacy of 76.5% in nine other monitored sites. Between 1998 and 2004, the incidence of vector-borne malaria cases in Eriteria was reduced substantially with the implementation of insecticide-impregnated mosquito nets, indoor residual spraying, and larval control measures.16,26 As part of the WHO initiative, Intergrated vector management (IVM) programs are currently being implemented in multiple countries in Africa, and early results of mosquito eradication and disease incidence reduction appears promising.27
From a public health policy standpoint, this comprehensive program not only reduced the potential threat of mosquito-borne diseases but also the need for anti-malaria chemoprophylaxis. Systematic chemoprophylaxis of a large number of individuals with drugs that have substantial side-effects will result in constant morbidity and occasional severe effects. Side effects for mefloquine include neurological and cardiac distubances; for doxycycline include gastrointestinal disturbances and hypersensitivty reactions; for malarone include gastrointestinal disturbances and blood abnormalities. Reducing the need for chemoprophylaxis was therefore a desirable outcome of the program. The four rings allowed for the entire program to work even if individual rings are not 100% effective. For example, although the mosquito population has been significantly reduced, mosquitoes are still present. The additional screening program, which identified human malaria cases, helped to reduce the risk of parasite introduction to the island. The importance of program maintenance must be emphasized. Bacillus thuringiensis israelensis needs to be continually applied to all breeding and potential breeding sites at regular intervals and environmental works must be regularly maintained. Our results have also shown that low vector populations are sustainable with a consistent, comprehensive, and multi-faceted program that is maintained across time.
Compared with the occasional clusters of locally transmitted malaria despite anti-malaria chemoprophylaxis previously, we have not had a case with this program in more than 2 years with the increased surveillance. The seven individuals identified to have possible malaria infection may have resulted in outbreaks if they were allowed to enter the island and if mosquito populations were at previous levels. However, the number of human infections of malaria attributable to Tekong Island during the period of chemoprophylaxis were low and longer term follow-up is necessary to determine if the preventive measures and mosquito population is below the threshold required for such rare outbreaks to occur.
There were some limitations encountered in this program. The substantial vector control and environmental works may have affected the eco-system of the surrounding areas. However, the combination strategy with Bti (which has less ecological impact) and other larvicides may have helped to reduce the impact of excessive use of any single method. The use of the human baiting method for surveillance also meant that the mosquitoes caught were mainly of the human-biting variety. However, these are the mosquito species that are most relevant to the spread of vector-borne diseases. Finally, the program used multiple layers of protection to ensure the reduction of risk from vector-borne diseases, and may not be easily reproduced in its entirety in rural regions. However, components of the program such as visitor screening, Bti spraying, and personal protection can be combined for use in various settings.
Conclusion
We have shown that comprehensive combination vector-control strategies are effective in reducing and maintaining low mosquito populations and reduce the risk of disease transmission. These lessons can be extrapolated in other forested environments to reduce the threat of vector-borne infectious diseases.
Acknowledgments
We thank the staff of the Headquarters Army Medical Services; Biodefence Centre, Singapore Armed Forces; DSO National Laboratories; and Environmental Health Institute, National Environmental Agency for their support for this study.
Disclaimer: The authors do not have any conflicts of interest, financial or otherwise, in this study.
Footnotes
Authors' addresses: Vernon J. Lee, Biodefence Center, Singapore Armed Forces, Singapore, Centre for Health Services Research, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, and Department of Epidemiology and Public Health, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, E-mail: vernonljm@hotmail.com. Samuel Ow, Patrick Lam, and Abdul Qadir Imran, Biodefence Center, Singapore Armed Forces, Singapore, E-mails: samuel_ow@yahoo.co.uk, lam_hong_yeong_patrick_damian@starnet.gov.sg, and jedi_didi@hotmail.com. Harold Heah, Meng Yaw Tan, and Benjamin Seet, Headquarters Army Medical Services, Singapore Armed Forces, Singapore, E-mails: haroldheah@gmail.com, my_inspiration82@yahoo.com, and seet_hun_yew_benjamin@starnet.gov.sg. Lee-Ching Ng and Sai Gek Lam-Phua, Environmental Health Institute, National Environmental Agency, Singapore, E-mails: ng_lee_ching@nea.gov.sg and phua_sai_gek@nea.gov.sg.
References
- 1.Hargreaves K, Hunt RH, Brooke BD. Anopheles arabiensis and An. quadriannulatus resistance to DDT in South Africa. Med Vet Entomol. 2003;17:417–422. doi: 10.1111/j.1365-2915.2003.00460.x. [DOI] [PubMed] [Google Scholar]
- 2.Hargreaves K, Koekemoer LL, Brooke BD. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol. 2000;7:181–189. doi: 10.1046/j.1365-2915.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- 3.Filliner U, Kannady K, William G. A tool box for operational mosquito larval control: preliminary results and early lessons from the Urban Malaria Control Programme in Dar es Salaam, Tanzania. Malar J. 2008;7:20. doi: 10.1186/1475-2875-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawaguchi I, Sasaki A, Mogi M. Combining zooprophylaxis and insecticide spraying: a malaria-control strategy limiting the development of insecticide resistance in vector mosquitoes. Proc Biol Sci. 2004;271:301–309. doi: 10.1098/rspb.2003.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goh KT. Eradication of malaria from Singapore. Singapore Med J. 1983;25:255–268. [PubMed] [Google Scholar]
- 6.Tee AK, Oh HM, Wee IY. Dapsone hypersensitivity syndrome masquerading as a viral exanthem: three cases and a mini-review. Ann Acad Med Singapore. 2004;33:375–378. [PubMed] [Google Scholar]
- 7.Teo RY, Tay YK, Tan CH. Presumed dapsone-induced drug hypersensitivity syndrome causing reversible hypersensitivity myocarditis and thyrotoxicosis. Ann Acad Med Singapore. 2006;53:385–391. [PubMed] [Google Scholar]
- 8.Hussien OA. Antimalarial drug toxicity: a review. Chemotherapy. 2007;53:385–391. doi: 10.1159/000109767. [DOI] [PubMed] [Google Scholar]
- 9.Lee YC, Tang CS, Ang LW, Han HK, James L, Goh KT. Epidemiological characteristics of imported and locally-acquired malaria in Singapore. Ann Acad Med Singapore. 2009;38:840–849. [PubMed] [Google Scholar]
- 10.Lancey LA. Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. J Am Mosq Control Assoc. 2007;23:133–163. doi: 10.2987/8756-971X(2007)23[133:BTSIAB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Fillinger U, Knols BGJ, Becker N. Efficacy and efficiency of new Bacillus thuringiensis var. israelensis and Bacillus sphaericus formulations against Afrotropical anophelines in Western Kenya. Trop Med Int Health. 2003;8:37–47. doi: 10.1046/j.1365-3156.2003.00979.x. [DOI] [PubMed] [Google Scholar]
- 12.Majambere, Lindsay S, Green C, Kandeh B, Fillinger U. Microbial larvicides for malaria control in The Gambia. Malar J. 2007;6:76. doi: 10.1186/1475-2875-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pepperell C, Rau N, Krajden S. West Nile virus infection in 2002: morbidity and mortality among patients admitted to hospital in south central Ontario. CMAJ. 2003;168:1399–1405. [PMC free article] [PubMed] [Google Scholar]
- 14.Kroeger A, Horstick O, Riedl C. The potential for malaria control with the biological larvicide Bacillus thuringiensis israelensis (Bti) in Peru and Ecuador. Acta Trop. 1995;60:47–57. doi: 10.1016/0001-706x(95)00101-j. [DOI] [PubMed] [Google Scholar]
- 15.Brown M, Carter J, Watson TM. Evaluation of liquid Bacillus thuringiensis var. israelensis products for control of Australian Aedes arbovirus vectors. J Am Mosq Control Assoc. 2001;17:8–12. [PubMed] [Google Scholar]
- 16.Russell TL, Brown MD, Purdie DM. Efficacy of VectoBac (Bacillus thuringiensis variety israelensis) formulations for mosquito control in Australia. J Econ Entomol. 2003;96:1786–1791. doi: 10.1093/jee/96.6.1786. [DOI] [PubMed] [Google Scholar]
- 17.Nyarango PM, Gebremaeskel T, Mebrahtu G. A steep decline of malaria morbidity and mortality trends in Eritrea between 2000 and 2004: the effect of combination of control methods. Malar J. 2006;5:33. doi: 10.1186/1475-2875-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunasekaran K, Doss PS, Vaidyanathan K. Laboratory and field evaluation of Teknar HP-D, a biolarvicidal formulation of Bacillus thuringiensis spp. israelensis, against mosquito vectors. Acta Trop. 2004;92:109–118. doi: 10.1016/j.actatropica.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Zhakhongirov ShM, Yarbaboyev MKh, Khamraeyeva ASh, Bekker N, Lebedeva NI, Ponomarev IM, Muminov MS, Tsoĭ EG. Testing the efficiency of Bacillus thuringien israelesis against mosquito larvae in Uzbekistan. Med Parazitol (Mosk) 2004:28–31. [PubMed] [Google Scholar]
- 20.Sharma SN, Shukla RP, Mittal PK. Efficacy of a new formulation of Bacillus thuringiensis var. israelensis (Bti) in laboratory and field conditions of Kumaun foothills of Uttaranchal, India. J Commun Dis. 2003;35:290–299. [PubMed] [Google Scholar]
- 21.Teng HJ, Lu LC, Wu YL. Evaluation of various control agents against mosquito larvae in rice paddies in Taiwan. J Vector Ecol. 2005;30:126–132. [PubMed] [Google Scholar]
- 22.Lee YW, Zairi J. Field evaluation of Bacillus thuringiensis H-14 against Aedes mosquitoes. Trop Biomed. 2006;23:37–44. [PubMed] [Google Scholar]
- 23.Setha T, Chantha N, Socheat D. Efficacy of Bacillus thuringiensis israelensis, VectoBac WG and DT, formulations against dengue mosquito vectors in cement potable water jars in Cambodia. Southeast Asian J Trop Med Public Health. 2007;38:261–268. [PubMed] [Google Scholar]
- 24.Becker N. Microbial control of mosquitoes: management of the upper rhine mosquito population as a model programme. Parasitol Today. 1997;13:485–487. doi: 10.1016/s0169-4758(97)01154-x. [DOI] [PubMed] [Google Scholar]
- 25.Region of Peel West Nile Virus in the Region of Peel 2004 Report. 2004. http://www.peelregion.ca/health/westnile/resources/reports.htm#report2004 Available at. Accessed April 9, 2009.
- 26.Graves PM, Osgood DE, Thomson MC. Effectiveness of malaria control during changing climate conditions in Eritrea, 1998. Trop Med Int Health. 2003;13:218–228. doi: 10.1111/j.1365-3156.2007.01993.x. [DOI] [PubMed] [Google Scholar]
- 27.Manga L, Toure A, Shililu J. Implementation of Integrated Vector Management in the WHO-African Region: Progress Report 2000–2003. Washington, DC: Environmental Health Project, U.S. Agency for International Development; 2004. pp. 1–26. [Google Scholar]


