Abstract
We examined the use of megadoses of VectoBac WG for residual control of Aedes aegypti in 2-L plastic buckets. Doses of 10×, 20×, and 50× the recommended rate of 8 mg/L provided ≥ 90% control for 8, 8, and 23 weeks, respectively. There was no significant difference in mortality between dry (neat) or aqueous mixture of VectoBac WG. Pretreatment of dry containers up to 8 weeks before flooding did not significantly decrease efficacy through 11 success weeks. Thus, megadoses of dry formulations of Bti can be used for residual control of Ae. aegypti in small containers. Furthermore, these doses use small amounts of product (0.08–0.4 g/L) that is more practical to measure than the minute amounts (0.008 g/L) required by the recommended rate, and cost US$2.18 to treat 50 Cairns yards containing an average total of 80 containers. This method could also be used to control Aedes albopictus.
Introduction
Aedes aegypti1,2 and Aedes albopictu3,4are important vectors of dengue and chikungunya viruses. Both species breed in artificial containers, often in small items such as buckets and plastic food containers that hold less than 5 L of water. Dengue control programs often do not have the resources or personnel to revisit properties and treat containers at frequent intervals. Thus, residual larvicides are needed to control mosquitoes in small ground containers when removal of the containers is not possible. Several products offer residual control of Ae. aegypti including the insect growth regulators (IGRs) s-methoprene5 and pyriproxyfen,6 the chitin synthesis inhibitors triflumuron7 and novaluron,8 and the organophosphate temephos9 and synthetic pyrethroid insecticides.10 However, IGR efficacy is difficult to assess and requires collection of pupae to assess emergence inhibition. Resistance has developed to both temephos11 and synthetic pyrethroids12 in Ae. aegypti, and insecticides can also have significant nontarget effects.
Bacillus thuringiensis var. israelensis (Bti) is a bacterial agent that kills mosquito larvae when ingested. Generally, it is used for broad acreage and small plot treatment, with little persistence in the environment.13 Indeed, the label rates are based on bioassays and field trials with 24-hr mortality as a key measure. Thus, the rates are not designed for residual treatment. However, some studies indicate that residual efficacy lasting up to 16 weeks can occur in containerized water,14 especially when higher doses are used. Indeed, some higher rates (in excess of 100,000 international toxic units; ITUs/L) have been used.15,16 Tablet formulations have been developed that provide several weeks control of Ae. aegypti in water storage containers. Armengol and others.17 found that a 4.8% technical powder Bti tablet provided 12 weeks of > 80% reduction in pupae production within 70-L containers exposed to sunlight. Use of VectoBac DT (dispersible tablet) significantly reduced pupal numbers in large cement jars for up to 3 months.18 Benjamin and others19 obtained > 90% control of Ae. aegypti in earthenware jars for up to 5.5 months using VectoBac DT. Granular formulations of Bti have been used to control Ae. aegypti in tires for up to 33 days,9 and wettable powder and tablet Bti formulations provided 80% control for up to 6 months.20
VectoBac WG (water dispersible granule; WG) has a potency of 3,000 international toxic units (ITU)/mg for Ae. aegypti and is especially suited for control of Ae. aegypti in containers. This is the only Bti formulation evaluated and specifications published by the World Health Organization (WHO, 2007).21 Vilarinhos and Monnerat22 found that a dose of 4 mg/L of VectoBac WDG (equivalent to Vectobac WG) provided > 90% control of Ae. aegypti in 250-L fiberglass jars for up to 12 weeks when the trial was terminated. However, Lima and others23 only obtained 9–36 days control, but this was in 50-L jars treated at a rate of only 2 mg/L. Lee and Zairi24 found that application of VectoBac ABG6561 (i.e., VectoBac WG) at 5 mg/L (15,000 ITU/L) provided > 90% control of Ae. aegypti for 49 and 56 days, respectively, in ceramic jars and glass jars. Subsequent field trials showed that using 2 mg/L VectoBac WG (6,000 ITU/L) decreased Ae. aegypti production by > 90% for 35 to 40 days in both container types.25
The WD formulation readily suspends in the water column26 and gradually settles to the base of the container where it is ingested by larvae.27 Only a very small dose is required for small to medium sized (< 20 L capacity) containers. Indeed, using a recommended rate of 8 g/1,000 L (Benjamin S, Valent Biosciences, personal communication), a typical 1-L container would only require 8 mg of product (24,000 ITU/L). These minute amounts are impractical to measure in the field for the treatment of individual containers. Thus, it is possible that by using high doses of product (“megadoses”), practicality could be improved while extending efficacy to several months. The excellent dispersive qualities of the WG formulation may also allow us to treat flooded containers by simply adding dry formulation.26 This would greatly improve the speed and logistics of treating containers during dengue control operations as water will not have to be carried and dosages mixed by field staff.
Initially, we conducted a small trial to see if simple application of dry VectoBac WG to the water gave comparable results as treatment using an aqueous solution. From this, a large field trial using “megadoses” of 10×, 20×, and 50×, the recommended manufacturer's rate of 8 mg/L, were conducted against Ae. aegypti. In many field situations containers are dry, and workers need a product that can be used to reliably “pre-treat” dry containers for up to 1–2 months. We also measured the efficacy of VectoBac WG in buckets that were not flooded for 1 and 2 months.
Materials and Methods
Will a dry dose of VectoBac WG provide comparable control as an aqueous solution?
A standard dose of 16 mg of VectoBac WG was mixed with 100 mL of water and sprayed to runoff inside a 2-L white plastic bucket creating a dose of 8 mg/L (24,000 ITU/L). We also applied the same amount of dry formulation to a 2-L bucket containing 2 L of water. An untreated bucket served as a control. Five longan tree (Dimocarpus longan Lour.) leaflets were added to each bucket to provide natural organic matter. Each week, 10 second instar Ae. aegypti (F2–5 Cairns strain) were added to each bucket and 24-hr mortality determined. Five replicates were conducted, and each bucket was placed into a crate that was covered with fine cloth mesh to prevent oviposition by mosquitoes (Figure 1). Alfalfa pellets (rabbit chow) were provided ad libitum as larval food. The crates were placed under a veranda to provide shade but were exposed to rain. The trial was continued until treatment mortality was < 80%.
Figure 1.

Experimental buckets containing 2 L of water and longan leaflets were held in crates in an outdoor shaded environment exposed to rainfall. Mesh screening was used to prevent oviposition by wild mosquitoes.
Can a megadose of VectoBac WG provide residual control of Ae. aegypti?
A similar exposure method used in the formulation trial was used. The crates were placed outside in a well-shaded area under a mango (Magnifera indica L.) tree but were exposed to rainfall. The dry treatment method was used in the following treatments:
-
1.
untreated control;
-
2.
dose of 80 mg/L (240,000 ITU/L) unmixed VectoBac WG/container (10× recommended rate);
-
3.
dose of 160 mg/L (480,000 ITU/L) unmixed VectoBac WG/container (20× recommended rate); and
-
4.
dose of 400 mg/L (1,200,000 ITUs/L) unmixed VectoBac WG/container (50× recommended rate).
Doses of VectoBac WG were weighed on a balance (precision of 0.001) and then immediately sprinkled into the test bucket. There were five replicates and the trial was run for 33 weeks from March 25 to November 11, 2007. Rainfall at the site was measured using a rain gauge, and temperature from a Bureau of Meteorology station located 5 km away.
Each week, 20 third instar Ae. aegypti (Cairns strain, F2–F8) were added to each bucket. The 0.5 g of alfalfa was added for food when needed. Larval mortality was determined at 24 hr, and all pupae were collected within 3 to 5 days of exposure. Pupae were placed in 70-mL jars and percent emergence inhibition determined and used to calculate overall (total) mortality of each treatment cohort. For each Bti treatment, efficacy was assessed until control was less than 50% for two consecutive weeks. Efficacy was assessed every 2 weeks after Week 22 for the 50× dose. An analysis of variance (ANOVA) (SPSS, Inc., version 14, Chicago, IL) was used to compare treatment means for each week, with Tukey's honestly significant difference (HSD) used to separate means at P < 0.05 level. An unpaired t test was used to compare the number of larvae surviving 24-hr post treatment and the overall mortality for each dose.
Pretreatment of dry containers using VectoBac WG for residual control of Aedes aegypti.
The efficacy of VectoBac WG as a pretreatment was tested for small containers against Ae. aegypti for 0, 4, and 8 weeks before the containers were flooded with 2 L of water. Pretreatment exposure was undertaken in a covered carport to prevent flooding by rainfall, but allowed exposure to prevailing ambient temperatures. Two doses were used, a 1× and 10× manufacturers' recommended rate (8 and 80 mg/L, respectively = 16 and 160 mg in each 2-L bucket). Furthermore, untreated buckets were included in the experimental design as a Bti control. For each treatment five replicates were undertaken. After the completion of pretreatment exposure each test bucket was flooded with 2 L of water and the crates of buckets were placed in an outdoor shaded area that was exposed to rainfall. Longan leaflets were added as with the previous trial. Test buckets were “challenged” each week, starting on the day buckets were flooded with 10 third instar Ae. aegypti larvae. Mortality was assessed 24 hr after larvae had been introduced into the test buckets and again at adult emergence (overall mortality). Both 24 hr and overall mortality were monitored until overall mortality in each bucket for a given treatment was < 50% for two consecutive weeks. Rainfall and temperature were recorded as in previous trials. The trial was conducted for 11 weeks from December 5, 2007 to February 20, 2008. A one-way ANOVA and Tukey's HSD were used to separate treatment effects for overall mortality each week after buckets had been flooded. We used an unpaired t test to compare the 24 hr survival and overall mortality between 1× and 10× treatments.
Results
Will a dry dose of VectoBac WG provide comparable control as an aqueous solution?
The wet and dry application treatments provided excellent control for up to 3 weeks post treatment (Figure 2), despite nearly 1 m (952 mm) of rain during the trial (28 Jan–4 Mar 2007). There was no significant difference (t test, P > 0.05) between mortality of the wet and dry treatments for each of the five exposures. Beyond 3 weeks post treatment, dry application provided about 20% higher mortality than wet application, although this was not significantly higher (t = 1.25, P = 0.22). Mean control mortality was only 6%. These data indicate that a simple direct application of dry VectoBac WG to containers will provide comparable control as an aqueous mixture. The direct dry application was used to test the megadoses.
Figure 2.
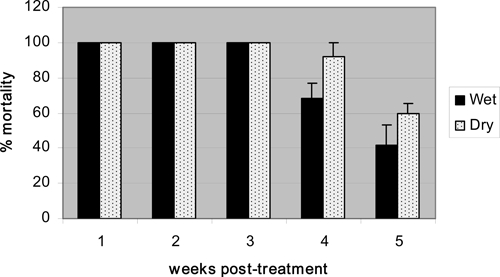
Mean (±SE) percent 24-hr mortality of second instar Ae. aegypti larvae exposed to dry and wet formulations of VectoBac WG applied at 8 mg/L.
Can a megadose of VectoBac WG provide residual control of Ae. aegypti?
Megadoses of VectoBac WG provided persistent control of Ae. aegypti in the buckets. A total of 377 mm of rainfall fell during this trial. The 10×, 20×, and 50× treatments provided at least 100% control for 7, 7, and 22 weeks, respectively (Figure 3; Table 1). Interestingly, after week 13 some larvae exposed to the 50× dose survived 24-hr post-exposure but died before pupating. Overall, mortality was significantly greater than 24-hr mortality for the 20× (t = 2.28, P = 0.02) and 50× (t = 2.15, P = 0.03) doses but not for the 10× dose (t = 0.96, P = 0.34). Thus, 24-hr larval mortality underestimates actual mortality. We also found there was little impact on pupal emergence (Table 2), with a very high percentage of adults successfully eclosing in all treatments.
Figure 3.
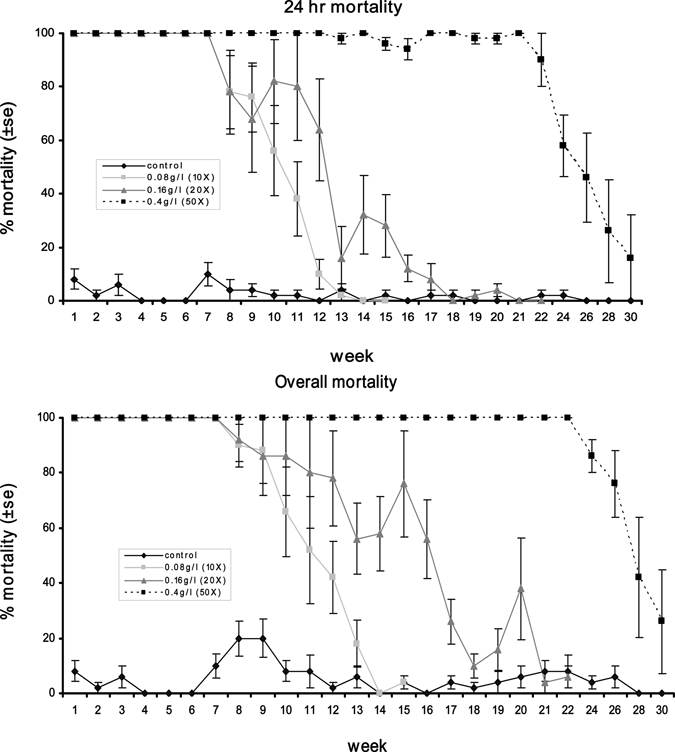
Mean (±SE) percentage 24 hr and overall mortality (through adult emergence) of Aedes aegypti third instar larvae exposed to 8, 16, and 40 mg/L of VectoBac WG in an outdoor container trial.
Table 1.
The effect of 10×, 20×, and 50× recommended dose (8 mg/L) treatments of VectoBac WG on (a) 24-hr mortality, and (b) overall mortality (until adult emergence) of third instar Aedes aegypti in 2-L buckets*
| (a) | |||||||||||||||||||
| Week | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 24 | 26 | 28 | 30 |
| P value | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0.02 | 0.22 | 0.35 |
| Control | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a |
| 10× dose | b | b | b | ab | a | a | a | a | – | – | – | – | – | – | – | – | – | – | – |
| 20× dose | b | b | b | bc | b | a | b | b | a | a | a | a | a | a | a | – | – | – | – |
| 50× dose | b | b | b | c | b | b | c | c | b | b | b | b | b | b | b | b | b | a | a |
| (b) | |||||||||||||||||||
| Week | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 24 | 26 | 28 | 30 |
| P value | 0 | 0 | 0 | 0.02 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0.09 | 0.21 |
| Control | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a |
| 10× dose | b | b | b | ab | ab | a | a | a | – | – | – | – | – | – | – | – | – | – | – |
| 20× dose | b | b | b | b | bc | b | b | b | b | b | a | a | a | a | a | – | – | – | – |
| 50× dose | b | b | b | b | c | c | c | b | c | c | b | b | b | b | b | b | b | a | a |
Means analyzed using a one-way analysis of variance and significantly different treatments were identified using Tukey's HSD post-hoc test; P < 0.05. Treatment means in the same row with the same letter were not significantly different; – infers treatment ceased.
Table 2.
Percent emergence of Aedes aegypti pupae developed from larvae exposed to 10×, 20×, and 50× label rate (8 mg/L) treatment of VectoBac WG over different lengths of exposure
| Control (30 weeks) | 10× Dose (15 weeks) | 20× Dose (22 weeks) | 50× Dose(30 weeks) | |
|---|---|---|---|---|
| Pupae picked | 896 | 220 | 366 | 136 |
| Adults emerged | 890 | 215 | 360 | 135 |
| % Emergence | 99.3% | 97.7% | 98.4% | 99.3% |
Pretreatment of dry containers.
The manufacturer's recommended rate of 8 mg/L (1× dose) VectoBac WG was a very effective pretreatment of the control Ae. aegypti regardless of whether the test bucket was treated 0, 4, or 8 weeks before the bucket was flooded (Figure 4; Table 3). Mortality (both 24 and overall) was significantly higher than the control for at least 7 weeks for all pretreatment periods (Figure 4; Table 3). For all pretreatment periods the 1× dose provided 100% overall mortality for 6 weeks. The only significant difference in mortality to Ae. aegypti larvae among the three pretreatments lengths occurred after 8 weeks when pretreatment of 4 weeks produced significantly lower overall mortality than a pretreatment of 0 or 8 weeks (Figure 4B).
Figure 4.
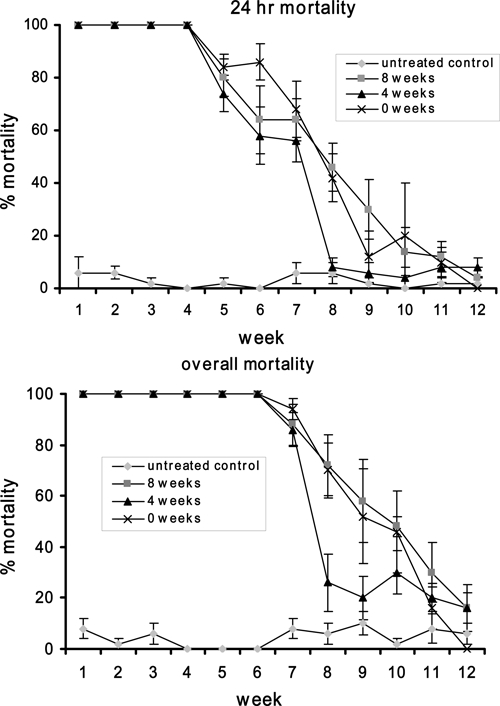
Percentage 24-hr mortality and overall mortality of Aedes aegypti third instar larvae provided by the pretreatment of test buckets with standard rate of VectoBac WG (8 mg/L) 0, 4, and 8 weeks before buckets were flooded.
Table 3.
Treatment effects for (a) 24-hr mortality, and (b) overall mortality (until adult emergence) of third instar Aedes aegypti in 2-L buckets pretreated 0, 4, and 8 weeks before flooding with 8 mg/L VectoBac WG*
| (a) | ||||||||||||
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| P value | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.10 | 0.59 | 0.55 | 0.29 |
| Control | a | a | a | a | a | a | a | a | a | a | a | a |
| 0 weeks | b | b | b | b | b | b | b | b | a | a | a | a |
| 4 weeks | b | b | b | b | b | b | b | a | a | a | a | a |
| 8 weeks | b | b | b | b | b | b | b | b | a | a | a | a |
| (b) | ||||||||||||
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| P value | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.05 | 0.01 | 0.33 | 0.19 |
| Control | a | a | a | a | a | a | a | a | a | a | a | a |
| 0 weeks | b | b | b | b | b | b | b | b | a | b | a | a |
| 4 weeks | b | b | b | b | b | b | b | a | a | ab | a | a |
| 8 weeks | b | b | b | b | b | b | b | b | a | b | a | a |
Data (Figure 4) analyzed with a one-way analysis of variance. Significantly different pretreatment lengths were identified using Tukey's HSD post-hoc test and have been identified with a different letter (P < 0.05).
The high dose (80 mg/L) also proved effective over the extended pretreatment period (Figure 5, Table 4). There was no significant difference in 24 hr and overall larval mortality between the 0, 4, and 8 week pretreatment until Weeks 10 and 12, respectively. The 80 mg/L dose provided greater efficacy than the 8 mg/L dose against third instar Ae. aegypti over the three pretreatment periods (Figures 4 and 5). Mortality (24 hr) for pretreatment periods of 0 (t = 4.68, P < 0.001), 4 (t = 4.37, P < 0.001), and 8 (t = 2.40, P < 0.018) weeks was significantly higher for the 80 mg/L dose. Overall, mortality was significantly higher for pretreatment periods 0 (t = 4.66, P < 0.001) and 4 (t = 3.72, P < 0.001) weeks but not for 8 weeks (t = 1.40, P = 0.16). The 10× dose provided 100% mortality for 7 weeks for all pretreatment lengths and the 0 pretreatment period was still providing significantly greater overall mortality compared with the untreated control 14 weeks after containers had been flooded. Similar to the 1× dose the residual effect of the 10× dose was not greatly influenced by the length of pretreatment, although there was some evidence that a pretreatment of 0 weeks caused higher 24 hr and overall mortality 10 to 12 weeks after buckets were flooded. Indeed, 13 weeks after buckets were flooded the pretreatment of 0 weeks provided significantly higher 24 hr and overall mortality (Figure 5; Table 4B) compared with the pretreatments of either 4 or 8 weeks in length.
Figure 5.
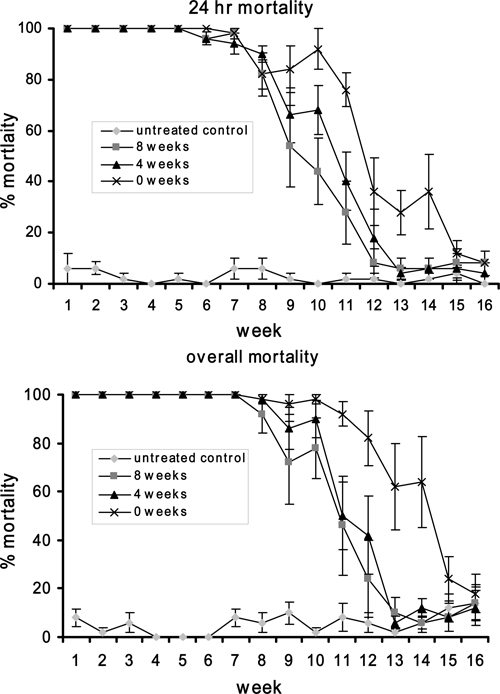
Percentage 24 hr and overall mortality of Aedes aegypti third instar larvae provided by the pretreatment of test buckets with VectoBac WG (80 mg/L) 0, 4, and 8 weeks before buckets were flooded.
Table 4.
Treatment effects for (a) 24-hr mortality, and (b) overall mortality (until adult emergence) of third instar Ae. aegypti in 2-L buckets pretreated 0, 4, and 8 weeks before flooding with 80 mg/L VectoBac WG*
| (a) | ||||||||||||||||
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| P value | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.09 | 0 | 0.03 | 0.71 | 0.46 |
| Control | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a |
| 0 weeks | b | b | b | b | b | b | b | b | b | c | c | a | b | b | a | a |
| 4 weeks | b | b | b | b | b | b | b | b | b | bc | bc | a | a | ab | a | a |
| 8 weeks | b | b | b | b | b | b | b | b | b | b | ab | a | a | ab | a | a |
| (b) | ||||||||||||||||
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| P value | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0.29 | 0.95 |
| Control | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a | a |
| 0 weeks | b | b | b | b | b | b | b | b | b | b | b | b | b | b | a | a |
| 4 weeks | b | b | b | b | b | b | b | b | b | b | ab | ab | a | a | a | a |
| 8 weeks | b | b | b | b | b | b | b | b | b | b | ab | a | a | a | a | a |
Data (Figure 4) analyzed with a one-way analysis of variance. Significantly different pretreatment lengths were identified using Tukey's HSD post-hoc test and have been identified with a different letter (P < 0.05).
Discussion
We have showed that a dry dispersible granule Bti formulation (VectoBac WG) can be used to provide residual control of Ae. aegypti in small ground containers. Megadose Bti application is intended for situations where discarded containers such as buckets and tires, rather than water storage containers (e.g., drums, jars, and tanks), are a key container for Aedes. Many tropical urban areas subject to dengue outbreaks (e.g., North Queensland, Singapore) have piped water supply, and do not store water in large containers. In these areas, small ground containers such as tires, buckets, ornamental plant bowls, and vases are important producers of Ae. aegypti.28
Our results do reflect semi-field conditions, and have greater validity than laboratory assays. The containers were exposed to rainfall, ambient temperature fluctuations, and longan leaflets were added to the buckets to simulate accumulation of detritus. However, the screening and location of the crates in deep shade would have minimized exposure to UV light, which can reduce efficacy of Bti.23 It is likely that efficacy in containers exposed to partial or full sunlight would not be as persistent. When used in an operational situation VectoBac WG may be subjected to higher temperatures, increased sunlight, and more cyclical flooding and drying compared with those recorded in the current study. How these environmental variables affect its larvicidal persistence is unknown. If they cause a reduction in efficacy vector control, officers will require training with regards to the most appropriate application sites (i.e., shaded) for VectoBac WG in the field.
The formulation trial showed that VectoBac WG can be applied directly to flooded containers with no loss of efficacy. The WG formulation rapidly spreads across the water surface before gradually sinking. We suspect that most Bti endospores and crystals become attached to the sides and bottom of the bucket where they are ingested by grazing Ae. aegypti larvae, as confirmed by Su and Mulla.27 Indeed, larvae were still killed in one bucket after the water was tipped out and the bucket rinsed. Benjamin and others19 and Lee and Zairi25 describe a similar situation where replenishing water in large earthen (ceramic) jars and 2-L glass jars did not significantly reduce efficacy against Ae. aegypti. Megadoses of VectoBac WG provided residual control of Ae. aegypti for several months even in heavy rain. No pupal emergence inhibition was noted. The persistence of control may also be linked to recycling of Bti toxins; perhaps as cadavers are ingested.20 Furthermore, variability in mortality as efficacy declines was noted. This may be caused by the increasing stochastic processes involved with the interception and ingestion of a declining number of lethal Bti crystals and spores by grazing larvae.
We also showed that VectoBac WG can be used as a reliable pretreatment of dry containers for at least 8 weeks before the container is flooded with little reduction in larvicidal efficacy. Operationally, this means that vector control officers can treat dry containers in the field knowing that if the container is flooded sometime in the next 1–2 months mosquito mortality will be as effective within the container as would have been achieved by treating the container at the time of flooding. A higher dose provides longer residual control.
Environmental factors could also impact the efficacy of dry Bti used as a pretreatment of dry containers. For instance, efficacy may be affected by multiple flooding and drying events within a container. Furthermore, although containers exposed to direct sunlight in the field are not preferred oviposition sites for dengue vectors, a trial to determine the impact of varying amounts of sunlight on efficacy may be of value. We have showed that the persistence of VectoBac WG increases when it is used as a pretreatment “mega-dose” of 80 mg/L versus 8 mg/L (Figures 4 and 5). Thus, the manufacturer's recommended rate may need to be changed for use against container breeding mosquitoes in non-potable water. Operationally, dry Vectobac WG can be applied from a 275-mL plastic sauce bottle. By tipping the bottle upside down approximately 100 mg of product is released through the nozzle; squeezing the bottle increases this to 300–400 mg (Davis J, unpublished data). Finally, tablet formulations may be even easier to apply in the field and should be considered for use in large ground containers.
Is the use of megadoses of dry formulations of Bti cost-effective for dengue control? Certainly use of megadoses of Bti would be prohibitively expensive where very large containers such as a 1,000-L tank, treated at 0.4 g/L, requires 400 g of product! However, for treatment of small to medium sized ground containers that hold < 20 L of water, it is potentially cost-effective. During container surveys in Cairns, Australia during an epidemic of DENV-3 in 2009, premise yards (N = 29,841) had an average of 1.64 small to medium sized containers, with an average capacity of 1.3 L per container (Queensland Health, unpublished data). Treating these with a 50× dose (0.4 g/L) would cost $AUS0.03 per container or $AUS0.05 per yard, based on a retail price of $AUS900 for a 25-kg container of VectoBac WG. A two person team can typically inspect 50 premises/day, treating an average of 80 containers at a material cost of $AUS2.42 (US$2.18) per day. This could potentially provide > 90% control of Ae. aegypti for up to 22 weeks (Figure 3). Pupal surveys in Merida, Mexico revealed that small to medium sized containers accounted for 87% of Ae. aegypti pupae, with a mean of 4.2 wet containers/premise.29 Thus, the estimated cost to treat 50 yards is US$5.72. Further trials should be conducted to confirm efficacy and cost effectiveness of Bti megadose interventions under operational field conditions.
Finally, there is legal precedent for registering of products for megadose use in nonpotable water within containers for residual control of mosquitoes. The Australian label for Prolink Pellets (4% s-methoprene) states “ProLink Pellets may be used in artificial containers, which breed mosquitoes, such as water tanks, plant pot holders, tyres, gutters, and catch basins, and in ornamental plants such as bromeliads. For residual treatment of water in small containers or wet areas, the following is recommended: 1 pellet/L or m2 for control up to 3 months; 3 pellets/L or m2 for control up to 6 months.” Thus, it is hoped that a similar strategy could be developed for Bti use against container-breeding mosquitoes such as Ae. aegypti and Ae. albopictus.
Acknowledgments
We thank Michelle Rapley, Petrina Johnson, Rebecca Silcock, and Michelle Janes for helping with the field study and Sharron Long for accessing weather data.
Footnotes
Financial support: This research was funded by a grant from Valent Biosciences to the Edward Koch Foundation.
Disclosure: S. Benjamin is the technical development specialist for Valent Biosciences Corporation, which funded this research. S. Ritchie receives travel funds from the Edward Koch Foundation. These statements are made in the interest of full disclosure and not because the authors consider this to be a conflict of interest.
Authors' addresses: Scott Ritchie, School of Public Health, Tropical Medicine and Rehabilitation Sciences, James Cook University, Cairns, Queensland, 4870 Australia. Luke Rapley, Ross, Tasmania 7209 Australia. Seleena Benjamin, Public Health, Valent BioSciences Corporation, Singapore.
References
- 1.Gubler DJ. In: Dengue and Dengue Hemorrhagic Fever. Gubler DJ, Kuno E, editors. New York: CAB International; 1997. (Dengue and dengue hemorrhagic fever: its history and resurgence as a global health problem). [Google Scholar]
- 2.Ritchie SA, Long S, Smith G, Pyke A, Knox TB. Entomological investigations in a focus of dengue transmission in Cairns, Queensland, Australia, by using the sticky ovitraps. J Med Entomol. 2004;41:1–4. doi: 10.1603/0022-2585-41.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Gratz N. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 4.Hawley WA. The biology of Aedes albopictus. J Am Mosq Control Assoc. 1998;4:1–40. [PubMed] [Google Scholar]
- 5.Ritchie SA, Broadsmith G. Efficacy of Altosid pettets and granules against Aedes aegypti in ornamental bromeliads. J Am Mosq Control Assoc. 1997;13:201–202. [PubMed] [Google Scholar]
- 6.Sihuincha M, Zamora-Perea E, Orellana-Rios W, Stancil JD, Lopez-Sifuentes V, Vidal-Ore C, Devine GJ. Potential use of pyriproxzfen for control of Aedes aegypti (Diptera: Culicidae in Iquitos, Peru. J Med Entomol. 2005;42:620–630. doi: 10.1093/jmedent/42.4.620. [DOI] [PubMed] [Google Scholar]
- 7.Batra CP, Mittal PK, Adak T, Ansari MA. Efficacy of IGR compound Starycide 480 SC (Triflumuron) against mosquito larvae in clear and polluted water. J Vector Borne Dis. 2005;42:109–116. [PubMed] [Google Scholar]
- 8.Arredondo-Jimenez JI, Valdez-Delgado KM. Effect of Novaluron (Rimon 10 EC) on the mosquitoes Anopheles albimanus, Anopheles pseudopunctipennis, Aedes aegypti, Aedes albopictus and Culex quinquefasciatus from Chipas Mexico. Med Vet Entomol. 2006;20:377–387. doi: 10.1111/j.1365-2915.2006.00656.x. [DOI] [PubMed] [Google Scholar]
- 9.Novak RJ, Gubler DJ, Underwood D. Evaluation of slow-release formulations of temephos (Abate) and Bacillus thuringiensis var. israelensis for the control of Aedes aegypti in Puerto Rico. J Am Mosq Control Assoc. 1985;1:449–453. [PubMed] [Google Scholar]
- 10.Ritchie SA, Montgomery B, Walsh I, Long S, Hart A. Efficacy of an aerosol surface spray against container-breeding Aedes. J Am Mosq Control Assoc. 2001;17:147–149. [PubMed] [Google Scholar]
- 11.Seccacini E, Lucia A, Zerba E, Licastro S, Masuh HC. Aedes aegypti resistance to temephos in Argentina. J Am Mosq Control Assoc. 2008;24:608–609. doi: 10.2987/5738.1. [DOI] [PubMed] [Google Scholar]
- 12.Ponlawat A, Scott JG, Harrington LC. Insecticide susceptibility of Aedes aegypti and Aedes albopictus across Thailand. J Med Entomol. 2009;42:821–825. doi: 10.1603/0022-2585(2005)042[0821:ISOAAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Lacey LA. Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. J Am Mosq Control Assoc. 2009;23:133–163. doi: 10.2987/8756-971X(2007)23[133:BTSIAB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Mulla MS, Thavara U, Tawatsin A, Chompoosri J. Procedures for the evaluation of fiels efficacy of slow-release formulations of larvicides against Aedes aegypti in water storage containers. J Am Mosq Control Assoc. 2004;20:64–73. [PubMed] [Google Scholar]
- 15.Lee MH, Pe TH, Cheong WH. Laboratory evaluation of the persistence of Bacillus thuringiensis var. israelensis against Aedes aegypti larvae. Mosquito borne Dis Bull. 1986;2:61–66. [Google Scholar]
- 16.Batra CP, Mittal PK, Adak T. Control of Aedes aegypti breeding in desert coolers and tires by use of Bacillus thuringiensis var. israelensis formulation. J Am Mosq Control Assoc. 2000;16:321–323. [PubMed] [Google Scholar]
- 17.Armengol G, Hernandez J, Velez JG, Orduz S. Long-lasting effects of a Bacillus thuringiensis serovar israelensis experimental tablet formulation for Aedes aegypti (Diptera: Culicidae) control. J Econ Entomol. 2006;99:1590–1595. doi: 10.1603/0022-0493-99.5.1590. [DOI] [PubMed] [Google Scholar]
- 18.Setha T, Chantha N, Socheat D. Efficacy of Bacillus thuringiensis israelensis, VectoBac WG and DT formulations against dengue mosquito vectors in cement potable water jars in Cambodia. Southeast Asian J Trop Med Public Health. 2007;38:261–268. [PubMed] [Google Scholar]
- 19.Benjamin S, Rath A, Fook CY, Lim LH. Efficacy of a Bacillus thuringiensis israelensis tablet formulation, Vectorbac DT, for control of dengue mosquito vectors in potable water containers. Southeast Asian J Trop Med Public Health. 2005;36:879–892. [PubMed] [Google Scholar]
- 20.Melo-Santos MA, de Araujo AP, Rios EMM, Regis L. Long lasting persistence of Bacillus thuringiensis servar. israelensis larvicidal activity in Aedes aegypti (Diptera: Culicidae) breeding places is associated to bacteria recycling. Biol Control. 2009;49:186–191. [Google Scholar]
- 21.World Health Organization . WHO Specifications and Evaluations for Public Health Pesticides: Bacillus thuringiensis Subspecies israelensis Strain AM65-52. Geneva: World Health Organization; 2007. [Google Scholar]
- 22.Vilarinhos PT, Monnerat R. Larvicidal persistence of formulations of Bacillus thuringiensis var. israelensisto control larval Aedes aegypti. J Am Mosq Control Assoc. 2004;20:311–314. [PubMed] [Google Scholar]
- 23.Lima JB, Melo NV, Valle D. Residual effect of two Bacillus thuringiensis var. israelensis products assayed against Aedes aegypti (Diptera: Culicidae) in laboratory and outdoors at Rio de Janeiro, Brazil. Rev Inst Med Trop Sao Paulo. 2005;47:125–130. doi: 10.1590/s0036-46652005000300002. [DOI] [PubMed] [Google Scholar]
- 24.Lee YW, Zairi J. Laboratory evaluation of Bacillus thuringiensis H-14 against Aedes aegypti. Trop Biomed. 2005;22:5–10. [PubMed] [Google Scholar]
- 25.Lee YW, Zairi J. Field evaluation of Bacillus thuringiensis H-14 against Aedes aegypti. Trop Biomed. 2006;23:37–44. [PubMed] [Google Scholar]
- 26.Clark JD, Devisetty BN, Krause SC, Novak RJ, Warrior P. A novel method for evaluating the particle size distribution behavior of a spray-dried technical concentrate and a water-dispersable granule formulation of Bacillus thuringiensis subsp. israelensis in an aqueous column. J Am Mosq Control Assoc. 2007;23:60–65. doi: 10.2987/8756-971X(2007)23[60:ANMFET]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 27.Su T, Mulla MS. Field evaluation of new water-dispersible granular formulations of Bacillus thuringiensis spp. israelensis and Bacillus sphaericus against Culex mosquitoes in microcosms. J Am Mosq Control Assoc. 1999;27:356–365. [PubMed] [Google Scholar]
- 28.Baker-Hudson P, Jones R, Kay BH. Categorizarion of domestic breeding habits of Aedes aegypti (Diptera: Culicidae) in Northern Queensland, Australia. J Med Entomol. 1988;25:178–182. doi: 10.1093/jmedent/25.3.178. [DOI] [PubMed] [Google Scholar]
- 29.Manrique-Saide P, Davies CR, Coleman PG, Rebollar-Tellez E, Che-Medoza A, Dzul-Manzanilla F, Zapata-Peniche A. Pupal surveys for Aedes aegypti surveillance and potential targeted control in residential aresa of Merida, Mexico. J Am Mosq Control Assoc. 2008;24:289–298. doi: 10.2987/5578.1. [DOI] [PubMed] [Google Scholar]


