Abstract
Strongyloides stercoralis chronic infections are usually asymptomatic and underestimated. We used direct fresh stool examination, Ritchie's method, and agar plate culture for diagnosis in patients with eosinophilia and previous residence in endemic areas. The frequency of strongyloidosis detected among these patients was high: 21 of 42 were positive. Among them, 10 were positive only by agar plate culture. After ivermectin treatment, patients resulted negative for parasitological tests and reduced their eosinophil counts. Half of the submitted patients that were followed 4–12 months after treatment remained negative without eosinophilia, except one who showed an eosinophil ascending curve before reappearance of larvae in stools. The high frequency of strongyloidosis found in this group emphasizes the relevance of including this parasitosis among differential diagnosis in patients with eosinophilia and past risk of S. stercoralis infection to prevent disseminated infections secondary to corticoid therapy.
Introduction
Strongyloides stercoralis is an intestinal nematode, endemic in tropical and subtropical regions. The humidity and clay soils favor the development of larvae stages of the parasite in the environment. The filariform larvae (L3) are the infective stage. Upon skin penetration, they travel to the bloodstream and reach the lung. After ascending the tracheobronchial tree, they arrive in the small intestine, where they evolve to adult stages and females begin the oviposition in the intestinal wall. Rhabditoid larvae emerge from these eggs; they may differentiate into L3 in the environment or to autoinfected filariform stage (aL3) in the host intestine, the latter being able to penetrate through the bowel mucose or perianal skin overinfecting the host.1,2
Strongyloidosis is usually not suspected because patient exposure may be remote and physicians often do not include this entity among differential diagnosis out of endemic areas. Moreover, the parasite is difficult to detect in chronic infections because of the low parasite burden.
Most chronic infections are asymptomatic and the only laboratory finding may be eosinophilia.3,4 In patients without any other underlying causes of eosinophilia but at high epidemiological risk, strongyloidosis should be ruled out. The various chronic manifestations include nausea, vomiting, epigastric pain with tenderness, diarrhea, constipation, weight loss, asthma-like symptoms, urticaria, and distinctive larva currens rash from the subcutaneous migration of larvae.4,5
Inmunosupressed people may experience accelerated autoinfection, with the consequent hyperinfection syndrome. This clinical presentation can be observed after starting immunosuppressive therapy (e.g., corticosteroids alone or administered contemporarily with vinca alkaloids, methotrexate, antithymocyte globulin, chlorambucil), in carriers of HTLV-1, alcoholics, patients with diabetes, and in patients with hypoclorhydria, hematological malignancies (especially lymphoma), impaired gut motility, and protein caloric malnutrition as well as transplantation recipients.2–7
The diagnosis of this parasitosis is usually performed by direct microscopic examination of stool specimens looking for the rhabditoid larvae. However, in chronic infection, larvae excretion may be low and fluctuating. For this reason, microscopic observation is not sensitive enough and multiple stool specimens should be analyzed to increase the sensitivity of the test. It has been reported that a single stool examination only detects larvae in as much as 30% of the cases.8,9 Different methods such as Baerman concentration, Harada Mori filter paper culture, formalin ethyl acetate concentration technique, and nutrient agar plate culture are used to improve the direct diagnosis. The latest, proved to be the best to detect S. stercoralis infection.10–15 Serology is also used for screening and diagnosis out of endemic areas.16–18
In hyperinfection and disseminated strongyloidosis, patients are usually symptomatic and parasitological diagnosis is easy, because larvae are frequently found in stool, sputum, and even in other samples (ascitic fluid, bronchoalveolar lavage).7,19
The aim of this study was to estimate the frequency of strongyloidosis among patients with eosinophilia and past residence in endemic areas, and to find out whether its frequency justifies the systematic use of a highly sensitive practical diagnosis method in areas where specific serology is difficult to be performed.
Materials and Methods
Patients.
We conducted a prospective study from May 2005 to December 2008. Patients more than 18 years of age, that showed ≥ 450 eosinophils/mL and were at risk of S. stercoralis infection because of past residence in endemic areas, were submitted to the Department of Microbiology, Parasitology and Immunology from the Divisions of Hematology and Infectious Diseases of the Medical School Hospital (Hospital de Clínicas), University of Buenos Aires. One pregnant woman was included for parasitological diagnosis and followed up, but antihelmintic treatment was not supplied to avoid the embryo-fetal iatrogenic risks. Patients who received any antiparasitic treatment up to 3 months before the study were excluded and any patient who returned to the endemic area during the last 12 months.
Information was collected by a standardized questionnaire, which included the data about demographic characteristics, current and past occupation, history of past exposure in the endemic area, underlying medical conditions, and risk of recent infection or reinfection. The immunological status was determined reviewing history of acquired immunodeficiency syndrome (AIDS), the presence of a chronic illness, immunosuppressive drug treatment, malignancy disease, and transplantation or connective tissue diseases. Informed consent was obtained from all the participants.
This study was approved by the Ethics Committee of the Medical School Hospital and the Independent Ethics Committee in Research, Medical School, University of Buenos Aires.
Samples.
Fresh stools in phosphate-buffered saline (PBS) and feces collected in formalin 5% for 7 days were obtained from each patient at the first visit. Fresh samples were proceeded after emission and studied by triplicates. Eosinophil values were registered. In those patients in which the first stool sample was negative, a second sample was studied at Day 15 to discard false negatives. Thirty days after the first visit, parasitological studies and eosinophil count were conducted again in all patients.
When possible, positive patients were followed up between 4 and 12 months after the end of the treatment. Similarly, patients under corticoid treatment were followed up to avoid risks of false negatives, even when they were informed as negative for strongyloidosis.
Microscopic diagnosis.
Fresh stools were centrifuged and the pellets were analyzed by triplicate under a light microscope after adding lugol stain. Stools preserved in formalin were studied by the Ritchie's method.20 There was a search for rhabditoid larvae of S. stercoralis and other parasites in both fresh and fixed samples. When rhabditoid S. stercoralis larvae were detected samples were considered positive for strongyloidosis. Larvae or eggs of other helminths and cysts of protozoa detected in formalin preserved samples and/or in fresh stools were informed as well. The Kinyoun stain was used to discard intestinal coccidian parasites.
Culture procedure.
Three grams of fresh stools/plate were seeded in the center of three agar plates. They were incubated at 37°C for up to 7 days and examined daily under a stereomicroscope to search for the tracks generated by the larvae migration. The surface of each microscopically positive dish was washed with 10% formalin solution, one at the moment that samples are detected as positives and the rest at the seventh day. Morphology of the worms collected was observed under light microscopy to differentiate between larvae of hookworms and S. stercoralis. The principal keys for morphological differences of rhabditoid stage between the latter and the hookworm larvae are the extremely short bucal capsule and the prominent genital primordium, which is fairly obvious and is located in about two-thirds of the larvae length in S. stercoralis. For the filariform larvae the difference between both species is the length of the esophagus and tip of the tail.20,21
Pharmacological treatment.
Patients with diagnosis of strongyloidosis received ivermectin 200 μg/kg/day, for 2 days.3,4 The therapy was directly observed. The first dose was administered by the physician and the second by a companion of the patient. Patients with other intestinal parasitic infections were administered with adequate therapy.
Statistical method.
Data consistency analysis was performed. Mean, median, and confidence interval (CI) were estimated. Sensitivity and specificity for Ritchie's method plus PBS fresh stools compared with the summatory results of the three methods used were calculated. A similar procedure was performed for the agar plate culture method. Mean changes in eosinophilia before and after treatment were assessed by the Wilcoxon signed-rank test and were considered significant when P < 0.05. Data analysis was performed by SPSS (version 13.0, SPSS Inc., Chicago, IL, 1989–1999).
Results
Patients included in this report (42) ranged from 18 to 83 years of age (mean 46.1, 95% CI 38.7–53.4) of which 41.2% were females. General features and eosinophil values at the admission moment are shown in Table 1. None of the patients were at risk of recent S. stercoralis infections because they had not returned to the endemic area at least for 1 year before they were included in this study.
Table 1.
Demographic and clinical characteristics of patients
| Patient no. | Sex*- age | Country | Underlying medical conditions | Immunosuppressive drugs | Clinical manifestation** | Eosinophilia (mm3)** |
|---|---|---|---|---|---|---|
| 1 | F-83 | Argentina | Myelodysplastic syndromes | None | Anemia | 464 |
| 2 | F- 28 | Paraguay | Ocular toxoplasmosis | Prednisone | None | 1,177 |
| 3 | M-30 | Paraguay | Absent | None | None | 500 |
| 4 | M-19 | Paraguay | Absent | None | None | 650 |
| 5 | F-71 | Argentina | Trombocytopenia | None | None | 1,024 |
| 6 | F-33 | Argentina | Hypereosinophilic syndrome | Prednisone | Dyspnea | 2,555 |
| 7 | F-82 | Argentina | Rheumatoid arthritis | Methotrexate -prednisone | Dyspnea | 1,500 |
| 8 | F-35 | Argentina | Absent | None | Fever unknown origin | 950 |
| 9 | F-71 | Argentina | Hiatus hernia | None | None | 1,080 |
| 10 | M-19 | Perú | Absent | None | Rash | 513 |
| 11 | M-20 | Uruguay | Asthma | Fluticasone inhaled | None | 1,080 |
| 12 | M-59 | Uruguay | Hypereosinophilic syndrome | Prednisone | None | 10,250 |
| 13 | M-19 | Argentina | Myeloid leukemia | Daunomycin - cytarabine | None | 1,030 |
| 14 | M-67 | Argentina | Septic arthritis | None | Loss weight | 950 |
| 15 | M-71 | Argentina | Multiple myeloma | Prednisone | None | 650 |
| 16 | F-54 | Argentina | Multiple myeloma | Prednisone - melphalan | None | 450 |
| 17 | M-77 | Argentina | Hepatitis C | None | None | 1,320 |
| 18 | M-43 | Argentina | Absent | None | Unknown fever | 8640 |
| 19 | M-80 | Argentina | Severe aortic stenosis | None | Dyspnea | 920 |
| 20 | M-38 | Argentina | AIDS | None | Absent | 867 |
| 21 | F-45 | Argentina | Absent | None | Absent | 1,200 |
| 22† | F-24 | Paraguay | Absent | None | Absent | 635 |
| 23 | M-30 | Argentina | Absent | None | Absent | 910 |
| 24 | M-23 | Argentina | Non-Hodgkin lymphoma | CHOP*** | Absent | 570 |
| 25 | F-53 | Argentina | Hypereosinophilic syndrome | Prednisone | Absent | 3960 |
| 26 | F-59 | Argentina | Absent | None | Abdominal pain | 3,061 |
| 27 | M-23 | Argentina | Absent | None | Diarrhea-vomiting | 5,090 |
| 28 | M-31 | Paraguay | AIDS | None | None | 467 |
| 29 | F-68 | Argentina | Non-Hodgkin lymphoma | CHOP*** | None | 1,144 |
| 30 | M-31 | Argentina | Absent | None | Chronic diarrhea | 855 |
| 31 | M-50 | Argentina | AIDS | None | Abdominal pain-itching | 10,152 |
| 32 | M-59 | Argentina | Larynx cancer | None | None | 8,466 |
| 33 | M-46 | Paraguay | Absent | None | None | 507 |
| 34 | F-26 | Paraguay | AIDS | None | None | 900 |
| 35 | M30 | Argentina | Renopancreatic transplant | Rapamicyn-prednisone | Diarrhea | 2000 |
| 36 | M67 | Argentina | Herpes zoster | Prednisone | Acute abdomen | 1,280 |
| 37 | M59 | Argentina | Absent | None | Itching | 1,122 |
| 38 | M56 | Argentina | Ulcerative colitis | Prednisone | Diarrhea | 500 |
| 39 | M43 | Paraguay | Absent | None | Absent | 2,090 |
| 40 | M40 | Argentina | Renopancreatic transplant | Rapamicyn-prednisone | Absent | 650 |
| 41 | M49 | Argentina | Multiple myeloma | Prednisone | Diarrhea | 650 |
| 42 | M52 | Argentina | Psoriasis | Methotrexate | Absent | 3,172 |
;F = female; M = male.
Clinical signs related to strongyloidosis and eosinophil values, both at the admission moment.
Cyclophosphamide hydroxydaunomycin, vincristine, and prednisone.
Pregnant.
Twenty-one patients out of 42 presented strongyloidosis. Ten patients showed S. stercoralis larvae by direct observation of the fresh feces. When agar plate cultures were performed, 19 out of 42 patients, comprising 9 of the 10 already mentioned, proved to be positive for this parasitosis. When formalin-fixed feces were observed by light microscopy after periodical collection (Ritchie's method), six samples were positive in coincidence with the other two methods, and another sample was positive only by Ritchie's method (patient 19). These results were obtained with the first sample and were not modified by those performed at Day 15 with the purpose to discard possible false negatives. The percentage of positive samples for each technique is represented in Figure 1. If we consider larvae detection by any of the tests used here as the gold standard, the sensitivity and negative predictive values for Ritchie's plus fresh stools were 0.52 and 0.67, respectively, whereas for agar culture the sensitivity and negative predictive values were 0.90 and 0.91.
Figure 1.
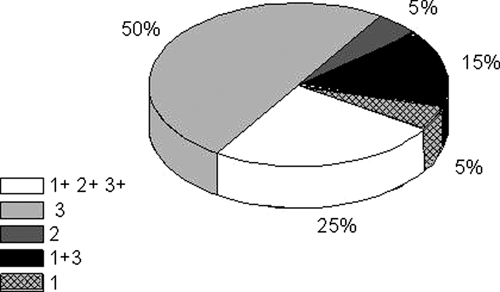
Detection of Strongyloides stercoralis larvae in stool samples: 1: Fresh stools. 2: Ritchie's method. 3: Agar plate cultures.
All larvae recovered from the surface agar cultures were assigned as S. stercoralis rhabditoid/filariform larvae. The lack of hookworm infection was confirmed because no hookworm eggs were ever detected. Cysts of Blastocystis hominis and Entamoeba coli were detected in most patients (except in patients 1, 3, 5, 8, and 22), whereas Giardia lamblia was only observed in patient 3. The absence of Isospora belli oocyst and Cryptosporidium spp. was confirmed by the Kinyoun staining.
As shown in Figure 2, cultures became positive at different days of the observation period starting from Day 1 (patients 1, 3, 8, 14, 15, 16, 37, and 41) up to Day 6 (patient 27). Furthermore, the elapsed time during which they remained positive differed among patients. For example, patients 3 and 41 were positive only 1 day, whereas patients 1 and 37 remained positive throughout the observation period. When cumulative values were considered, the highest number of positive samples was registered on Day 2.
Figure 2.
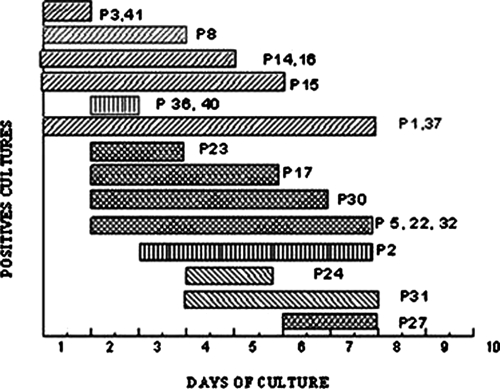
Follow-up of cultures by microscopical observation of rhabditoid larvae. Period of larvae detection in individual patients. Cultures were considered positive as described in Materials and Methods section. P = patients.
All patients with diagnosis of strongyloidosis (except the pregnant woman who was periodically checked during pregnancy but failed to return for controls after delivery) received ivermectin treatment. Parasitological studies performed 30 days after treatment were negative and eosinophil counts diminished significantly (median Day 0: 950, Day 30: post treatment 344, P < 0.0001) (Figure 3). The patient with giardiasis was treated with metronidazole and became negative thereafter. Those with diagnosis of E. coli and B. hominis were not treated.
Figure 3.
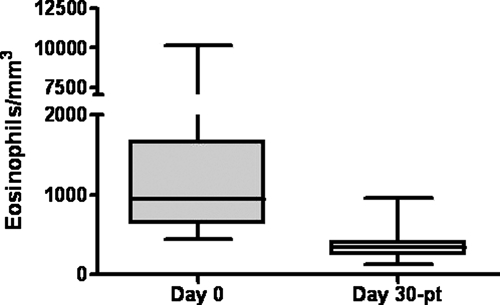
Eosinophil count before and after ivermectin treatment. Median eosinophils/mm3 pre- and post-treatment is represented as box and whiskers plot. (P < 0.0001, pre-treatment vs. post-treatment by Wilcoxon signed-rank test).
Ten out of the 20 patients treated with ivermectin were followed up for 4 to 12 months after the end of ivermectin treatment: three for 1 year, four for 6 months, and three for 4 months. With the only exception of patient 30, the rest remained negative for parasitological studies and their eosinophil counts showed normal values, which were similar to those recorded immediately after treatment. Patient 30 showed a persistent increase of eosinophil numbers since the fourth month post-treatment, reaching eosinophilia after 10 months of follow-up (Figure 4). For this reason, 3–4 consecutive stool samples were periodically requested, which were exhaustively examined for the presence of larvae. It was only after 10 months of control and in the third cultured feces sample that S. stercoralis larvae were observed. The patient was not immunosupressed and he had no evidence of any previous illness or immunosuppressant treatment and was negative for human immunodeficiency virus (HIV) and HTLV-1.
Figure 4.
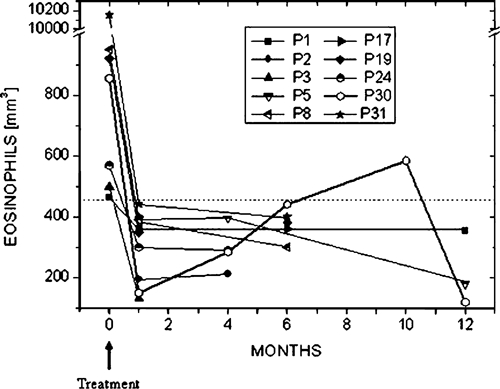
Eosinophil counts in individual patients before and at different times after treatment. Values over the dashed line represent eosinophilia.
Discussion
Strongyloides stercoralis often causes chronic clinically asymptomatic infections. The diagnosis is difficult because of the low larvae excretion in stools. It is usually performed by the microscopic examination of fresh and fixed enriched stool samples. The agar plate culture has been reported as more sensitive than the fresh stool observation and the Ritchie's methods. Sato, who assayed 1,350 samples using direct fecal smear, formalin-ether concentration, Harada-Mori filter paper culture, and agar plate culture, reported the highest sensitivity for the agar plate culture method (96%).15 In another study performed to evaluate the cost-effectiveness in recovering S. stercoralis larvae, the direct smear, a modified Baermann technique and the agar plate culture method were compared, being the last 4.4 times more sensitive than the others.12,13 The agar plate culture is not routinely performed in all laboratories to diagnose this enteroparasitosis. Results presented here reinforce the convenience to introduce this assay, or a similarly sensitive method, to search for S. stercoralis in patients with eosinophilia and past risks of infection, who presently live outside of endemic areas. In our hands the agar plate culture follow-up during 7 days enhanced the sensitivity of the test, proving to be more suitable than the observation at a single scheduled day.
On the other hand, serology was reported to be useful for screening and follow-up after treatment, because enzyme immunoassay (EIA) was used in many laboratories out of endemic areas. Loutfy and others had reported for the Centers for Disease Control and Prevention (CDC) the Strongyloides EIA 94.6% sensitivity.17,22 Van Doorn and others16 reported for EIA a specificity ranging from 95% to 97.7% and a sensitivity of 83–93% depending on the EIA test used. These authors pointed out that serology are less time-consuming and does not require fresh stool samples, which they consider difficult to obtain in routine practices. However, this situation, that is real in developed countries, may be different for other regions. In this sense, serology may be difficult to carry out in areas where commercial kits are not available. Moreover, if the samples to test are few, the importation of these kits cannot be justified. In these cases the agar plate cultures are an option because it may be performed in the bacteriological laboratories, which usually exist in every hospital. Additionally, cross-reactivity may be observed in endemic areas, but also in those regions out of endemic areas where infections with parasites including other nematodes, can coexist in patients suspected of strongyloidosis. Serological tests may have false negative results in immunosupressed patients, too.5,16,17,23 In our country, infections with other intestinal parasites is frequently out of the endemic areas and the enzyme-linked immunosorbent assay (ELISA) kit is not available commercially at present.
Strongloides stercoralis possesses a peculiar life cycle caused by the inherent ability of the aL3 to penetrate the intestinal epithelium resulting in an increase of larvae migration.1,24,25 If such migration persists, it is associated with persistent eosinophilia.2,26 In a percentage of patients eosinophilia may be absent. Because of this, the present study may possess a bias caused by the fact that only patients with eosinophilia were included. However, this sign is considered a warning by different authors.18,22,23 In this study the eosinophil counts diminished significantly immediately after ivermectin therapy. As the screening was performed among patients living out of endemic areas, eosinophil values proved to be useful, to include strongyloidosis among the possible differential diagnosis for those patients at risk for this parasitic infection. The British Infection Society recommends that all returning travelers and migrants from the tropics with eosinophilia should be investigated with concentrated stool microscopy and Strongyloides serology.18
Patients with negative results for strongyloidosis may suffer comorbidities that justify the eosinophilia. However, this is not the case for all the negative parasitological samples presented here. Hence, it is possible that, at least a part of these patients may possibly be false negatives. This possibility is strongly suggested by one patient with negative cultures that was positive by the Ritchie's method. Therefore, even when the culture practically duplicates the sensitivity of the Ritchie's method plus fresh stools examination and the negative predictive value is nearly 30% higher; it is worth continuing this work to develop an even more sensitive test.
The follow-up of patients after treatment that shows, for the majority of them, both persistently negative cultures and decreased eosinophil counts indicates that ivermectin therapy was effective. Regardless, one of the patients showed reactivation of infection after treatment, although the patient received the proper therapy, did not return to endemic areas, and was not immunocompromised. This outcome was unexpected as the drug of choice against S. stercoralis is the ivermectin whose cure rates reach 100% as referred by different studies.4,27,28 Nevertheless, some authors reported values as low as 67%.29 Uncomplicated infection may be treated with ivermectin 200 µg/kg of body weight single dose, although some reports suggested that two doses of ivermectin on consecutive days provided the best results with regard to efficacy.4,19,27,28 However, to certainly evaluate the results of treatment of S. stercoralis, stool examinations should be performed for at least 1 or 2 years when possible.27
Even when cure cannot be assured, parasite burden drastically decreased below the threshold of detection after the parasiticide treatment. One patient had a rebound of eosinophil count, which prompted us to exhaustively search for the presence of larvae in stools. This patient had such low parasite burden that the collection of three consecutive samples to detect a positive culture was required. This result reinforces the proposal that eosinophilia may be a good predictive marker of parasite reactivation.22,23
To summarize, our results indicate the need to include strongyloidosis as a presumptive diagnosis in patients with past risk of infection and especially if they develop eosinophilia out of endemic areas. A suitable diagnosis should include a test with high sensitivity such agar culture, Baerman's, or serology test depending on the laboratory facilities. These procedure facts may avoid subjecting these patients to unnecessary invasive techniques required to diagnose other pathologies.
Acknowledgments
We thank Maricel Repetto for helping with the figures and José Oubiña and Gerardo Mirkin for carefully reading the manuscript.
Footnotes
Financial support: This work was supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) of Argentina and the Universidad de Buenos Aires.
Disclosure: SMGC is a member of the Research Career from CONICET, and SAR is a fellow of UBA, Argentina.
Authors' addresses: Silvia A. Repetto and Stella M. González-Cappa, Departamento de Microbiología, Parasitología e Inmunología, Facultad de Medicina, Universidad de Buenos Aires, Ciudad Autónoma de Buenos Aires, Argentina, E-mails: silvia_repetto@yahoo.com.ar, smgcappa@fmed.uba.ar, and smgcappa@gmail.com. Pablo A. Durán, Departamento de Salud Pública, Facultad de Medicina, Universidad de Buenos Aires, Ciudad Autónoma de Buenos Aires, Argentina, E-mail: apduran@intramed.net. María B. Lasala, Hospital de Clínicas José de San Martín, Universidad de Buenos Aires, Ciudad Autónoma de Buenos Aires, Argentina, E-mail: mblasala@fibertel.com.ar.
References
- 1.Brigandi RA, Rotman HL, Nolan TJ, Schad GA, Abraham D. Chronicity in Strongyloides stercoralis infections: dichotomy of the protective inmune response to infective and autoinfective larvae in a mouse model. Am J Trop Med Hyg. 1997;56:640–646. doi: 10.4269/ajtmh.1997.56.640. [DOI] [PubMed] [Google Scholar]
- 2.Concha R, Harrington W, Jr, Rogers AI. Intestinal strongyloidiasis: recognition, management and determinants of outcome. J Clin Gastroenterol. 2005;39:203–211. doi: 10.1097/01.mcg.0000152779.68900.33. [DOI] [PubMed] [Google Scholar]
- 3.Vadlamudi RS, Chi DS, Krishnaswamy G. Intestinal strongyloidiasis and hyperinfection syndrome. Clin Mol Allergy. 2006;30:4–8. doi: 10.1186/1476-7961-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segarra-Newnham M. Manifestations, diagnosis, and treatment of Strongyloides stercoralis infection. Ann Pharmacother. 2007;41:1992–2001. doi: 10.1345/aph.1K302. [DOI] [PubMed] [Google Scholar]
- 5.Marcos LA, Terashima A, Dupont HL, Gotuzzo E. Strongyloides hyperinfection syndrome: an emerging global infectious disease. Trans R Soc Trop Med Hyg. 2008;102:314–318. doi: 10.1016/j.trstmh.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Keiser PB, Nutman TB. Strongyloides stercoralis in the inmunocompromised population. Rev Clin Microbiol Rev. 2004;17:208–217. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Hasan MN, McCormick M, Ribes JA. Invasive enteric infections in hospitalized patients with underlying strongyloidiasis. Am J Clin Pathol. 2007;128:622–627. doi: 10.1309/PK0RDQWB764C3WQ2. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040–1047. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 9.Lim S, Katz K, Krajden S, Fuksa M, Keystone JS, Kain KC. Complicated and fatal Strongyloides infection in Canadians: risk factors, diagnosis and management. CMAJ. 2004;171:479–484. doi: 10.1503/cmaj.1031698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Intapan PM, Maleewong W, Wongsaroj T, Singthong S, Morakote N. Comparison of the quantitative formalin ethyl acetate concentration technique and agar plate culture for diagnosis of human strongyloidiasis. J Clin Microbiol. 2005;43:1932–1933. doi: 10.1128/JCM.43.4.1932-1933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salazar SA, Gutierrez C, Berk SL. Value of the agar plate method for the diagnosis of intestinal strongyloidiasis. Diagn Microbiol Infect Dis. 1995;23:141–145. doi: 10.1016/0732-8893(95)00247-2. [DOI] [PubMed] [Google Scholar]
- 12.De Kamisky RG. Evaluation of three methods for laboratory diagnosis of Strongyloides stercoralis infection. J Parasitol. 1993;79:277–280. [PubMed] [Google Scholar]
- 13.Jongwutiwes S, Charoenkorn M, Sitthichareonchai P, Akaraborvorn P, Putaporntip C. Increased sensitivity of routine laboratory detection of Strongyloides stercoralis and hookworm by agar-plate culture. Trans R Soc Trop Med Hyg. 1999;93:398–400. doi: 10.1016/s0035-9203(99)90132-3. [DOI] [PubMed] [Google Scholar]
- 14.Hirata T, Nakamura H, Kinjo N, Hokama A, Kinjo F, Yamane N, Fujita J. Increased detection rate of Strongyloides stercoralis by repeated stool examinations using the agar plate culture method. Am J Trop Med Hyg. 2007;77:683–684. [PubMed] [Google Scholar]
- 15.Sato Y, Kobayashi J, Toma H, Shiroma Y. Efficacy of stool examination for detection of Strongyloides infection. Am J Trop Med Hyg. 1995;53:248–250. doi: 10.4269/ajtmh.1995.53.248. [DOI] [PubMed] [Google Scholar]
- 16.van Doorn HR, Koelewijn R, Hofwegen H, Gilis H, Wetsteyn JC, Wismans PJ, Sarfati C, Vervoort T, Van Gool T. Use of enzyme-linked immunosorbent assay and dipstick assay for detection of Strongyloides stercoralis infection in humans. J Clin Microbiol. 2007;45:438–442. doi: 10.1128/JCM.01735-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention Diagnostic findings: strongyloidiasis antibody detection. 2009. http:/www.dpd.cdc.gov/dpdx/html/frames/S-Z/Strongyloidiasis/ Available at. Accessed August 28, 2009.
- 18.Checkley AM, Chiodini PL, Dockrell DH, Bates I, Thwaites GE, Booth HL, Brown M, Wrigght SG, Grant AD, Mabey DC, Whitty C, Sanderson F. Eosinophilia in returning travelers and migrants from the tropics: UK recommendations for investigation and initial management. J Infect. 2010;60:1–20. doi: 10.1016/j.jinf.2009.11.003. British Infection Society and Hospital for Tropical Diseases. [DOI] [PubMed] [Google Scholar]
- 19.Ramanathan R, Nutman T. Strongyloides stercoralis infection in the immunocompromised host. Curr Infect Dis Rep. 2008;10:105–110. doi: 10.1007/s11908-008-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González Cappa SM, Paulin P. Diagnóstico Parasitológico. Argentina: Asociación Argentina de Microbiología, Colegio de Bioquímicos de la Provincia de Entre Ríos, Facultad de Bioquímica y Ciencias Biológicas; 1997. (Microbiología clínica). [Google Scholar]
- 21.Garcia LS. Diagnosis Medical Parasitology. Fifth edition. Washington, DC: ASM Press; 2007. [Google Scholar]
- 22.Loutfy M, Wilson M, Keystone J, Kain K. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am J Trop Med Hyg. 2002;66:749–762. doi: 10.4269/ajtmh.2002.66.749. [DOI] [PubMed] [Google Scholar]
- 23.Olsen A, van Lieshout L, Marti H, Polderman T, Polman K, Steinmann P, Stothard R, Thybo S, Verweij JJ, Magnussen P. Strongyloidiasis–the most neglected of the neglected tropical diseases? Trans R Soc Trop Med Hyg. 2009;103:967–972. doi: 10.1016/j.trstmh.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol Rev. 1992;5:345–355. doi: 10.1128/cmr.5.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neva FA. Biology and immunology of human strongyloidiasis. J Infect Dis. 1986;153:397–406. doi: 10.1093/infdis/153.3.397. [DOI] [PubMed] [Google Scholar]
- 26.Mansfield LS, Niamatali S, Bhopale V, Volk S, Smith G, Lok JB, Genta RM, Schad GA. Strongyloides stercoralis: maintenance of exceedingly chronic infections. Am J Trop Med Hyg. 1996;55:617–624. doi: 10.4269/ajtmh.1996.55.617. [DOI] [PubMed] [Google Scholar]
- 27.Boulware DR, Stauffer WM, Hendel-Paterson BR, Rocha JL, Seet RC, Summer AP, Nield LS, Supparatpinyo K, Chaiwarith R, Walker PF. Maltreatment of Strongyloides infection: case series and worldwide physicians-in-training survey. Am J Med. 2007;120:545.e1–545.e8. doi: 10.1016/j.amjmed.2006.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Igual-Adell R, Oltra-Alcaraz C, Soler-Company E, Sánchez-Sánchez P, Matogo-Oyana J, Rodríguez-Calabuig D. Efficacy and safety of ivermectin and thiabendazole in the treatment of strongyloidiasis. Expert Opin Pharmacother. 2004;5:2615–2619. doi: 10.1517/14656566.5.12.2615. [DOI] [PubMed] [Google Scholar]
- 29.Intapan P, Prasongdee T, Laummaunwai P, Sawanyawisuth K, Singthong S, Maleewong W. A modified filter paper culture technique for screening of Strongyloides stercoralis ivermectin sensitivity in clinical specimens. Am J Trop Med Hyg. 2006;75:563–564. [PubMed] [Google Scholar]


