Abstract
Exposure to carbon monoxide (CO) and other ambient air pollutants is associated with adverse pregnancy outcomes. While there are several methods of estimating CO exposure, few have been evaluated against exposure biomarkers. The authors examined the relation between estimated CO exposure and blood carboxyhemoglobin concentration in 708 pregnant western Washington State women (1996–2004). Carboxyhemoglobin was measured in whole blood drawn around 13 weeks’ gestation. CO exposure during the month of blood draw was estimated using a regression model containing predictor terms for year, month, street and population densities, and distance to the nearest major road. Year and month were the strongest predictors. Carboxyhemoglobin level was correlated with estimated CO exposure (ρ = 0.22, 95% confidence interval (CI): 0.15, 0.29). After adjustment for covariates, each 10% increase in estimated exposure was associated with a 1.12% increase in median carboxyhemoglobin level (95% CI: 0.54, 1.69). This association remained after exclusion of 286 women who reported smoking or being exposed to secondhand smoke (ρ = 0.24). In this subgroup, the median carboxyhemoglobin concentration increased 1.29% (95% CI: 0.67, 1.91) for each 10% increase in CO exposure. Monthly estimated CO exposure was moderately correlated with an exposure biomarker. These results support the validity of this regression model for estimating ambient CO exposures in this population and geographic setting.
Keywords: air pollutants, carbon monoxide, carboxyhemoglobin, pregnancy
Ambient carbon monoxide exposure during pregnancy has been associated with preterm delivery, intrauterine growth restriction, and reduced birth weight in diverse settings (1–6). Carbon monoxide exposures have been estimated using data from local air pollutant monitoring networks in several ways: by averaging concentrations across all local monitors (3, 6), using concentrations measured at the monitor nearest the maternal residence (1, 4), or calculating inverse-distance weighted averages of nearby concentrations (2, 5). These approaches are limited by the assumption that all persons residing within a given distance of a monitoring site are equally exposed.
Land-use regression is an exposure estimation method designed to overcome this limitation by exploiting the fact that intraurban variations in many air pollutants are related to nearby environmental characteristics, such as traffic, population density, and land use (7–9). The model quantifies the relations between local characteristics and the concentration of an air pollutant measured at a network of stationary monitors. The model is then used to estimate local residents’ exposures (7–9). Previous regression models have explained 16%–76% of the variability in annual air pollutant concentrations (7, 10–13). Some model-based exposure estimates have been validated against independently obtained air pollutant measurements (11, 14). However, because of the expense and difficulty of this process, most estimates have not been directly compared with other exposure measures.
In contrast to many air pollutants (15), there exists a sensitive and specific biomarker of carbon monoxide exposure: whole blood carboxyhemoglobin concentration, resulting from the displacement of oxygen from hemoglobin by carbon monoxide (16, 17). Carboxyhemoglobin concentrations reflect recent exposures (biphasic elimination, half-lives of 3.6 and 4.5 hours) (18). In previous studies, carboxyhemoglobin concentrations measured in nonsmokers were correlated with declining air pollution levels within 1 city (19) and with differences in pollution across 4 cities (20). In 2 small studies, carboxyhemoglobin levels in infant umbilical cord blood were correlated with ambient carbon monoxide concentrations (21, 22). However, to our knowledge, no investigators have examined the correlation between regression model-based estimates of ambient carbon monoxide exposure and carboxyhemoglobin concentrations.
As part of our agenda to examine pregnancy-related effects of air pollution, we designed a regression model incorporating land use and temporal terms to estimate monthly and trimester-specific ambient carbon monoxide exposures among pregnant women in western Washington State. We aimed to quantify the relation between model-based exposure estimates and contemporaneous carboxyhemoglobin concentrations. This information should provide evidence of the degree to which our model captures biologically meaningful variations in ambient carbon monoxide exposure.
MATERIALS AND METHODS
Study population
In this cross-sectional analysis, we used data collected for the Omega Study (1996–2008), a previously described prospective cohort study (23). Participants were women attending prenatal care clinics affiliated with Swedish Medical Center in Seattle, Washington, and Tacoma General Hospital in Tacoma, Washington. Women who initiated prenatal care before 20 weeks’ gestation were eligible to participate. Women were ineligible if they were less than 18 years old, did not speak and read English, or did not plan to deliver at either hospital. Participants completed a questionnaire administered by a trained interviewer (mean gestational age = 15.9 weeks (standard deviation (SD), 4.8)). This questionnaire gathered information on sociodemographic, anthropometric, behavioral, medical, and reproductive characteristics. Participants provided a 20-mL nonfasting blood sample (mean gestational age = 14.8 weeks (SD, 3.1)). Blood was fractionated using standard procedures and stored at −80°C. After delivery, medical records were abstracted for information on the course and outcome of pregnancy. Study procedures were approved by the institutional review boards of both hospitals. All participants provided written informed consent.
In this analysis, we used data from women recruited between 1996 and 2004. During this period, 3,000 (77%) of the 3,899 invited women participated. Of these, 60 experienced early pregnancy losses and 152 were lost to follow-up because of an unknown delivery outcome or a missing medical record.
Analytical sample
Carboxyhemoglobin level was measured in 789 of the remaining 2,788 participants’ blood samples. As part of our research agenda, we aimed to examine carboxyhemoglobin in relation to preeclampsia and preterm delivery. Therefore, we oversampled women who experienced these outcomes for carboxyhemoglobin measurement. We selected all women who developed preeclampsia or preterm delivery (n = 390) and a random sample of 399 who remained normotensive and delivered at term. From the 789 women with carboxyhemoglobin measurements, we excluded 35 women who did not complete an interview, from whom we obtained covariate data. We excluded 36 women with a nongeocodable address, which prevented estimation of carbon monoxide exposures. We also excluded 10 women who lived outside the Puget Sound region (King, Kitsap, Pierce, or Snohomish county) because the carbon monoxide exposure model may not be generalizable to other locations (8). The analytical population included 708 women.
Carboxyhemoglobin measurement
We measured carboxyhemoglobin in whole blood collected in tubes containing ethylenediaminetetraacetic acid. We used head-space capillary gas chromatography-mass spectrometry with an HP-Molesieve PLOT column (Agilent Technologies, Santa Clara, California). The assay provides a lower detection limit of 0.2% (16). Carboxyhemoglobin is expressed as percent hemoglobin. Hemoglobin was measured with a Quantichrom colorimetric assay (BioAssay Systems, Hayward, California) (24).
Estimation of ambient carbon monoxide exposure
We estimated monthly ambient carbon monoxide exposure using a multivariable linear regression model that included terms for land-use characteristics, month, and year. Our model was constructed using carbon monoxide measurements collected from 1996 to 2006 at 15 regional monitoring sites administered by the Puget Sound Clean Air Agency (see Web Figure 1, which is posted on the Journal’s Web site (http://aje.oxfordjournals.org/)) (25). The Puget Sound Clean Air Agency describes 12 sites as urban and 2 as suburban (1 is not described). Fourteen sites are in commercial areas; 3 of these areas are also described by the Puget Sound Clean Air Agency as residential. The sites are located using Environmental Protection Agency criteria to ensure a consistent and representative measure of air quality (25). We collapsed daily measurements into 890 monthly average concentrations. The average number of measurements taken per month was 29.9 (range, 18–31). The average number of monthly measurements taken per site was 59.3 (range, 4–123). Four sites operated for 10 years or more, 5 sites operated for 4–9 years, and 6 sites operated for less than 4 years. Within each month, the average difference in carbon monoxide concentrations across monitoring sites was 0.78 ppm (range, 0.18–1.67). Within each monitoring site, the average difference across months was 1.19 ppm (range, 0.31–2.01).
We evaluated local characteristics as potential predictors of monthly carbon monoxide concentrations at the monitoring sites. Characteristics were mapped and measured at each site using ArcMap 9.2 software (ESRI, Redlands, California). Using 2001 traffic-count data from the Washington State Department of Transportation, we estimated annual average traffic volume on the nearest major road (federal or state highway) within circular buffers with radii of 250 m and 500 m (26). We also measured distance to the nearest major road. Using US Census 2000 TIGER line files, we estimated street density (km/km2) within 100-, 250-, 500-, and 1,000-m buffers (27). We used census measures of population density (persons/km2) and housing density (housing units/km2) within each site's census block group (27). We used monthly averages of daily high and low temperatures and precipitation collected by the Western Regional Climate Center at 31 weather stations (28). We used measurements taken at the nearest weather station; the average distance between each monitoring site and the nearest station was 6.8 km (range, 0.7–32 km). We used year and month terms to capture secular and seasonal variations in carbon monoxide concentrations.
We fitted multivariable linear regression models for the relation between each environmental characteristic (independent variables) and monthly average carbon monoxide (dependent variable). We used a stepwise procedure to determine the final model. First, we fitted models including a single environmental characteristic, year, and month as predictors. (All of these “single-predictor” models included year and month to allow estimation of monthly carbon monoxide concentrations). We coded continuous predictors linearly, log-transformed, and as a set of indicators or splines based on quartiles or other cutpoints suggested by exploratory analyses. First, for each predictor, we selected the model with the largest adjusted R2 value. If multiple coding schemes produced similar R2 statistics, the most parsimonious model was selected. Second, we chose the most predictive spatial scale (buffer) for street and bus densities and traffic volume by comparing single-predictor models and choosing the model with the largest adjusted R2. Third, we included in the final model all characteristics with a Wald P value less than 0.10 in single-predictor models.
We measured the environmental characteristics included in the final model at each participant's self-reported, geocoded residential address (Web Figure 1). We used model coefficients and these measurements to estimate participants’ monthly carbon monoxide exposures. We estimated exposures within each calendar month of pregnancy after approximating the date of conception to the nearest calendar month. We measured date of conception using the maternal report of the date of the last menstrual period and ultrasonography performed at ≤20 weeks’ gestation. Information on the last menstrual period and ultrasound results was gathered by interview and medical record abstraction, respectively. If both the last-menstrual-period and ultrasound-based dates agreed within 14 days, we used the former date. Among 4% of participants with dates differing by more than 14 days, we used the ultrasound-based date.
For this analysis, we used carbon monoxide exposures estimated in the calendar month and trimester of blood draw (e.g., the first trimester was defined as pregnancy calendar months 1–3). We chose these exposure windows because many studies suggest that adverse outcomes are most strongly associated with monthly- or trimester-specific exposures (1–6).
Statistical analysis
Because the carboxyhemoglobin distribution was skewed, we examined median concentrations and interquartile ranges according to participant characteristics (shown in Table 1), assessed during early-pregnancy interviews. We also examined distributions across groups classified by year, season, and trimester of blood draw.
Table 1.
Characteristics of Pregnant Women Receiving Prenatal Care and Distribution of Median Carboxyhemoglobin Concentrations According to Those Characteristics, Western Washington, 1996–2004
| Characteristic | No. of Subjects | % | Median Carboxyhemoglobin Concentration, % Hemoglobin |
| Entire study population | 708 | 100.0 | 0.85 (0.72–1.04)a |
| Age, years | |||
| ≤20 | 10 | 1.4 | 0.89 (0.82–1.23) |
| 21–34 | 466 | 65.8 | 0.86 (0.72–1.05) |
| 35–39 | 180 | 25.4 | 0.84 (0.74–1.05) |
| ≥40 | 52 | 7.3 | 0.81 (0.68–0.99) |
| Parity | |||
| Nulliparous | 461 | 65.1 | 0.88 (0.75–1.09) |
| Parous | 247 | 34.9 | 0.79 (0.68–0.94) |
| Prepregnancy body mass indexb,c | |||
| <18.5 | 32 | 4.6 | 0.78 (0.68–0.95) |
| 18.5–24.9 | 460 | 65.4 | 0.85 (0.72–1.05) |
| 25.0–29.9 | 117 | 16.6 | 0.86 (0.73–1.00) |
| ≥30.0 | 94 | 13.4 | 0.86 (0.73–1.14) |
| Race/ethnicityc | |||
| Non-Hispanic white | 601 | 85.1 | 0.84 (0.71–1.03) |
| Non-Hispanic black | 19 | 2.7 | 0.93 (0.79–1.13) |
| Hispanic | 19 | 2.7 | 0.93 (0.72–1.15) |
| Asian/Pacific Islander | 50 | 7.1 | 0.85 (0.75–1.05) |
| Other | 17 | 2.4 | 0.86 (0.78–1.08) |
| Education | |||
| Completion of high school | 29 | 4.1 | 0.90 (0.72–1.16) |
| Vocational school or some college | 138 | 19.5 | 0.87 (0.75–1.09) |
| Completion of college | 299 | 42.2 | 0.83 (0.71–1.01) |
| Postgraduate education | 242 | 34.2 | 0.85 (0.72–1.03) |
| Employed during early pregnancyc | |||
| Yes | 584 | 82.8 | 0.87 (0.73–1.06) |
| No | 121 | 17.2 | 0.79 (0.69–0.90) |
| Marital status | |||
| Married | 644 | 91.0 | 0.84 (0.72–1.02) |
| Unmarried | 64 | 9.0 | 0.93 (0.80–1.26) |
| Annual household incomec | |||
| <$30,000 | 23 | 3.3 | 0.87 (0.71–1.38) |
| $31,000–$69,999 | 170 | 24.3 | 0.88 (0.75–1.11) |
| ≥$70,000 | 492 | 70.2 | 0.84 (0.71–1.01) |
| Refused to respond | 16 | 2.3 | |
| Smoking statusc | |||
| Never smoked | 508 | 71.8 | 0.84 (0.71–1.01) |
| Smoked before pregnancy | 154 | 21.8 | 0.85 (0.75–1.08) |
| Smoked before and during pregnancy | 46 | 6.5 | 0.91 (0.76–1.41) |
| SHS exposure in home during year before pregnancy | |||
| No | 680 | 96.0 | 0.85 (0.72–1.04) |
| Yes | 28 | 4.0 | 0.93 (0.78–1.31) |
| SHS exposure outside home during year before pregnancy | |||
| No | 430 | 60.7 | 0.82 (0.71–1.00) |
| Yes | 278 | 39.3 | 0.89 (0.74–1.11) |
| Regular recreational physical activity during early pregnancyc | |||
| Yes | 642 | 90.8 | 0.85 (0.72–1.05) |
| No | 65 | 9.2 | 0.84 (0.73–1.00) |
| Year of blood draw | |||
| 1996–1998 | 144 | 20.3 | 1.09 (0.84–1.29) |
| 1999–2001 | 249 | 35.2 | 0.88 (0.76–1.01) |
| 2002–2004 | 315 | 44.5 | 0.78 (0.66–0.91) |
| Season of blood draw | |||
| Winter (December–February) | 166 | 23.5 | 0.88 (0.76–1.06) |
| Spring (March–May) | 167 | 23.6 | 0.81 (0.69–0.95) |
| Summer (June–August) | 183 | 25.9 | 0.84 (0.71–1.08) |
| Autumn (September–November) | 192 | 27.1 | 0.86 (0.73–1.08) |
| Gestational week of blood draw | |||
| 5.7–12.9 (first trimester) | 402 | 56.8 | 0.87 (0.74–1.10) |
| 13.0–27.7 (second trimester) | 306 | 43.2 | 0.82 (0.71–0.98) |
Abbreviation: SHS, secondhand smoke.
Numbers in parentheses, interquartile range (25th–75th percentiles).
Weight (kg)/height (m)2.
Numbers in subgroups do not sum to overall number because of missing data.
We quantified the fit of the model in several ways. We calculated R2 to quantify the proportion of the variance in carbon monoxide concentrations explained by the model. We calculated a root mean square prediction error to quantify the average absolute difference between observed and predicted concentrations at monitoring sites. We calculated 2 cross-validated coefficients of correlation between observed and estimated concentrations (11). First, we fitted the model using a randomly selected sample of half of the concentrations measured at the monitoring sites. We used this model to predict the remaining half, used the same procedure to predict concentrations in the first half of the sample, and calculated a “random-half R2.” Second, we fitted the model using data from all but 1 of the carbon monoxide monitoring sites and then applied it to estimate concentrations at the remaining site; we performed this procedure for every site in turn and then calculated the “remaining-site R2.”
We fitted a weighted linear regression model to quantify the relation between log-transformed carboxyhemoglobin concentration and the characteristics used as predictors of ambient carbon monoxide exposure in the land-use regression model. We report model coefficients (β) as the X% (i.e., (1 + X/100)-fold) difference in median carboxyhemoglobin levels observed when comparing 2 groups of participants classified according to each predictor (29). We used frequency weights to account for the overrepresentation of preterm delivery and preeclampsia cases in the study sample. Subgroup analyses provided no evidence that the relations of interest differed according to preterm delivery or preeclampsia (data not shown). We calculated R2 to quantify the proportion of variance in log-transformed carboxyhemoglobin concentration explained by the model predictors.
We quantified the agreement between estimated carbon monoxide exposure and carboxyhemoglobin concentration using a Pearson's ρ (Spearman's ρ values were similar). We used a multivariable linear regression model weighted to account for the overrepresentation of preterm delivery and preeclampsia, with log-transformed carboxyhemoglobin level as the dependent variable and log-transformed estimated carbon monoxide exposure as the independent variable. We estimated the percent difference in median carboxyhemoglobin concentration associated with each 10% increase in estimated carbon monoxide exposure. We log-transformed carbon monoxide values to maintain consistency in scale with carboxyhemoglobin values. We evaluated the characteristics listed in Table 1 as potential confounders. We included those that changed the association of interest by 10% or more (cigarette smoking, secondhand smoke exposure, and gestational age at blood draw) in the final model. We repeated analyses after stratifying women according to smoking and/or secondhand smoke exposure.
RESULTS
Participants were typically 21–34 years old, nulliparous, white, and married. Only 7% reported current smoking (Table 1). Median carboxyhemoglobin levels were higher among younger women, women without previous livebirths, black or Hispanic women, employed women, unmarried women, current smokers, women reporting secondhand smoke exposure, earlier participants, and those with first-trimester blood draws. As expected given the low prevalence of smoking and the geographic setting, distributions of local ambient carbon monoxide concentrations, estimated carbon monoxide exposures, and carboxyhemoglobin concentrations were generally low (Table 2).
Table 2.
Ambient Carbon Monoxide Concentrationsa and Study Participants’ Estimated Carbon Monoxide Exposures and Carboxyhemoglobin Concentrations (n = 708), Western Washington, 1996–2004
| Mean | Minimum | Percentile |
Maximum | ||||
| 25th | 50th | 75th | 95th | ||||
| Local monthly CO concentration, ppm | 1.08 (0.4)b | 0.38 | 0.80 | 1.08 | 1.38 | 1.87 | 2.64 |
| Estimated CO exposure in month of blood draw, ppm | 1.00 (0.40) | 0.12 | 0.71 | 0.97 | 1.28 | 1.70 | 2.14 |
| Estimated CO exposure in trimester of blood draw, ppm | 1.01 (0.39) | 0.16 | 0.73 | 0.99 | 1.27 | 1.68 | 2.07 |
| Carboxyhemoglobin level, % hemoglobin | 0.93 (0.43) | 0.17 | 0.72 | 0.85 | 1.04 | 1.55 | 5.92 |
Abbreviation: CO, carbon monoxide.
Local concentrations measured at 15 monitoring sites.
Numbers in parentheses, standard deviation.
The exposure estimation model included terms for year and month (indicators), street density within 500 m (quartile-based indicators), distance to the nearest major road (<100 m, 101–1,000 m, >1,000 m), and census tract population density (continuous). R2 was 0.73. The root mean square error was 0.22 ppm (10% of the range). A scatterplot of observed versus predicted concentrations is shown in Figure 1. The random-half and remaining-site R2 values were 0.71 and 0.31, respectively. Model coefficients showed that concentrations at monitoring sites were highest in 1996 and declined thereafter (Table 3). Concentrations were highest in February and lowest in July, on average. Concentrations were highest at sites with third-quartile street density but lower at sites with fourth-quartile density. Concentrations were inversely related to distance to the nearest major road and were not strongly related to population density.
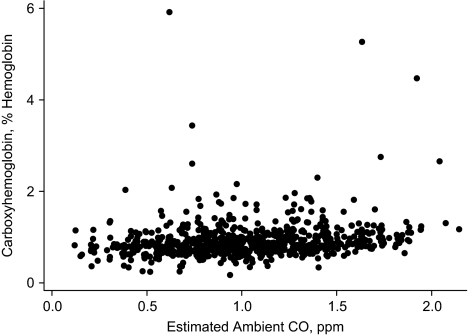
Figure 1.
Carboxyhemoglobin concentration versus estimated ambient carbon monoxide (CO) concentration in the month of blood draw, Western Washington, 1996–2004.
Table 3.
Comparison of the Exposure Model and Predictor/Biomarker Relations, Western Washington, 1996–2006a
| CO Model Predictor Term | Coefficient for the CO Exposure Estimation Model (n = 890 Monthly Concentrations) |
Adjustedb % Difference in Median Carboxyhemoglobin Concentration Across Participants (n = 708 Participants) |
|||||
| No. of Site-Months | No. of Sites | β, ppm | 95% CI | No. of Study Participants | % Hemoglobin | 95% CI | |
| Year | |||||||
| 1996 | 103 | 8 | 0.77 | 0.68, 0.85 | 3 | 78.2 | 50.0, 106.6 |
| 1997 | 96 | 8 | 0.73 | 0.64, 0.81 | 75 | 56.6 | 46.8, 66.4 |
| 1998 | 102 | 10 | 0.60 | 0.51, 0.68 | 66 | 21.1 | 13.0, 29.3 |
| 1999 | 81 | 8 | 0.57 | 0.48, 0.65 | 76 | 37.7 | 27.9, 47.4 |
| 2000 | 102 | 9 | 0.57 | 0.48, 0.66 | 60 | 13.9 | 5.2, 22.6 |
| 2001 | 83 | 8 | 0.39 | 0.30, 0.49 | 113 | 6.8 | −0.9, 14.5 |
| 2002 | 92 | 9 | 0.25 | 0.17, 0.34 | 104 | 4.4 | −4.6, 13.3 |
| 2003 | 63 | 6 | 0.17 | 0.08, 0.26 | 128 | 10.9 | 1.4, 20.4 |
| 2004 | 64 | 6 | 0.17 | 0.08, 0.26 | 83 | 0 | |
| 2005 | 67 | 6 | 0.14 | 0.05, 0.23 | 0 | ||
| 2006 | 37 | 6 | 0 | 0 | |||
| Month | |||||||
| January | 78 | 14 | 0 | 59 | 0 | ||
| February | 77 | 14 | 0.03 | −0.04, 0.10 | 41 | 1.0 | −10.9, 12.9 |
| March | 77 | 14 | −0.18 | −0.25, −0.11 | 55 | −13.1 | −24.6, −1.6 |
| April | 73 | 15 | −0.28 | −0.35, −0.21 | 64 | −11.7 | −25.3, 1.9 |
| May | 73 | 15 | −0.37 | −0.44, −0.30 | 48 | −11.9 | −26.0, 1.9 |
| June | 73 | 15 | −0.43 | −0.50, −0.36 | 58 | −7.6 | −21.6, 6.4 |
| July | 73 | 15 | −0.45 | −0.52, −0.38 | 64 | −13.2 | −25.8, −0.7 |
| August | 70 | 13 | −0.36 | −0.43, −0.29 | 61 | −4.6 | −17.2, 8.0 |
| September | 68 | 13 | −0.21 | −0.28, −0.13 | 59 | −10.1 | −24.1, 3.8 |
| October | 73 | 13 | −0.03 | −0.10, 0.04 | 61 | −6.9 | −19.7, 5.9 |
| November | 73 | 13 | 0.10 | 0.03, 0.17 | 72 | −9.4 | −21.7, 2.9 |
| December | 73 | 13 | 0.03 | −0.04, 0.10 | 66 | −6.9 | −20.3, 6.4 |
| Street density within a 500-m bufferc, km/km2 | |||||||
| ≤9.07 | 226 | 4 | 0 | 278 | 0 | ||
| 9.08–11.87 | 246 | 4 | 0.31 | 0.25, 0.36 | 178 | −0.2 | −7.4, 7.2 |
| 11.88–14.37 | 114 | 4 | 0.66 | 0.56, 0.76 | 189 | −3.3 | −10.4, 3.7 |
| ≥14.38 | 304 | 3 | 0.19 | 0.13, 0.24 | 63 | −5.6 | −18.0, 6.8 |
| Distance to the nearest major roadd, m | |||||||
| ≤100 | 89 | 2 | 0 | 28 | 0 | ||
| 101–1,000 | 759 | 12 | −0.30 | −0.36, −0.23 | 326 | 6.1 | −3.8, 16.1 |
| ≥1,001 | 42 | 1 | −0.49 | −0.57, −0.40 | 354 | 0.9 | −8.7, 10.6 |
| Population densitye per 1,000 persons/km2 | 0.40 | −1.52, 2.35 | 0.6 | −1.8, 2.9 | |||
Abbreviations: CI, confidence interval; CO, carbon monoxide.
CO exposure model coefficients and percent differences in median carboxyhemoglobin concentration across study participants according to exposure model predictor terms.
Adjusted for all other covariates in the table. Estimates and confidence intervals are weighted to account for oversampling of preeclampsia and preterm delivery cases from the underlying cohort.
Street density ranged from 1.90 km/km2 to 17.93 km/km2 at monitoring sites and from 1.90 km/km2 to 18.65 km/km2 at participants’ residences.
Distance to the nearest major road ranged from 0.3 m to 29,786 m at monitoring sites and from 3 m to 1,449 m at participants’ residences.
Population density ranged from 237 persons/km2 to 4,872 persons/km2 at monitoring sites and from 1.3 persons/km2 to 17,035 persons/km2 at participants’ residences.
Participants’ carboxyhemoglobin concentrations were generally similarly related to model predictors (Table 3). This set of characteristics explained 28% of the variance in carboxyhemoglobin level (adjusted R2 = 0.28). Year and month accounted for almost all of the explained variance in carboxyhemoglobin: The adjusted R2 statistic for a model without the land-use terms was 0.27. Concentrations were generally higher among earlier participants, those who had winter blood draws, and those with higher street or population density. Unlike ambient carbon monoxide, carboxyhemoglobin was not strongly associated with distance to the nearest major road.
Carboxyhemoglobin was correlated with estimated carbon monoxide exposures in the calendar month (ρ = 0.22, 95% confidence interval (CI): 0.15, 0.29) and trimester (ρ = 0.21, 95% CI: 0.14, 0.28) of blood draw (Table 4). Each 10% increase in estimated carbon monoxide exposure during the month of blood draw was associated with a 1.42% (95% CI: 0.83, 2.02) difference in median carboxyhemoglobin concentration. This relation weakened slightly after adjustment (β = 1.12%, 95% CI: 0.54, 1.69). The adjusted relation with estimated carbon monoxide in the trimester of blood draw was similar (per 10% increase in exposure, β = 1.10%, 95% CI: 0.52, 1.69). Comparing 2 groups with 75th percentile carbon monoxide exposure versus 25th percentile carbon monoxide exposure in the month of blood draw, the adjusted difference in median carboxyhemoglobin level was 6.92% (95% CI: 3.35, 10.52). Relations of interest did not meaningfully differ after exclusion of the highest 1% of carboxyhemoglobin concentrations (data not shown).
Table 4.
Relations Between Estimated Ambient Carbon Monoxide Exposure and Carboxyhemoglobin Concentration, According to Cigarette Smoke Exposure, Western Washington, 1996–2004
| Analytic Sample and Window of CO Exposure | Pearson's ρ | 95% CI | Difference in Median CBH Level per 10% Increase in CO Exposure | |||
| Unadjusted |
Adjusted |
|||||
| % | 95% CI | % | 95% CI | |||
| Entire study population (n = 708)a,b | ||||||
| Month of blood draw | 0.22 | 0.15, 0.29 | 1.42 | 0.83, 2.02 | 1.12 | 0.54, 1.69 |
| Trimester of blood draw | 0.21 | 0.14, 0.28 | 1.41 | 0.81, 2.01 | 1.10 | 0.52, 1.69 |
| Nonsmokers with no SHS exposure (n = 422)a,c | ||||||
| Month of blood draw | 0.24 | 0.15, 0.33 | 1.44 | 0.80, 2.08 | 1.29 | 0.67, 1.91 |
| Trimester of blood draw | 0.21 | 0.12, 0.30 | 1.27 | 0.62, 1.91 | 1.12 | 0.49, 1.76 |
| Women reporting smoking and/or SHS exposure (n = 286)a,c | ||||||
| Month of blood draw | 0.16 | 0.04, 0.27 | 1.11 | 0.01, 2.23 | 0.90 | −0.01, 2.02 |
| Trimester of blood draw | 0.17 | 0.05, 0.28 | 1.30 | 0.04, 2.57 | 1.16 | −0.01, 2.41 |
Abbreviations: CBH, carboxyhemoglobin; CI, confidence interval; CO, carbon monoxide; SHS, secondhand smoke.
Number included in adjusted model. Estimates and confidence intervals are weighted to account for oversampling of preeclampsia and preterm delivery cases from the underlying cohort.
Adjusted model included covariates for smoking (never, before, or during early pregnancy), SHS exposure (yes or no), and gestational age at blood draw (weeks; continuous).
Adjusted model included a covariate for gestational age at blood draw (weeks; continuous).
Current smoking or exposure to secondhand smoke during the year before pregnancy did not strongly affect the association (Table 4). Among unexposed women, each 10% increase in estimated carbon monoxide exposure during the month of blood draw was associated with a 1.29% (95% CI: 0.67, 1.91) increase in median carboxyhemoglobin concentration. The relation within the subgroup of smoke-exposed women was somewhat weaker (β = 0.90%, 95% CI: −0.01, 2.02).
DISCUSSION
Within this population of pregnant women in western Washington State, estimated residence-based carbon monoxide exposures were moderately correlated with carboxyhemoglobin concentrations. The association was slightly stronger within the subgroup of women who reported no smoking or secondhand smoke exposure.
Ours is the first study, to our knowledge, to demonstrate a relation between an air pollutant exposure estimated using a regression model and a contemporaneous exposure biomarker. However, 5 previous studies have suggested that ambient carbon monoxide concentrations influence carboxyhemoglobin concentrations. In a study by Stewart et al. (20), carboxyhemoglobin concentrations among nonsmoking blood donors in Chicago (Illinois), Los Angeles (California), Milwaukee (Wisconsin), and New York City were higher (0.7% ≤ median ≤ 2.7% across locations) than those measured among study volunteers breathing carbon monoxide-free air (median, 0.5%). The median carboxyhemoglobin concentration declined from 1.7% to 1.4% in nonsmoking Chicago blood donors from 1970 to 1974, correlating with reduced ambient carbon monoxide concentrations (19). Among 176 nonsmoking traffic police in Milan, Italy, Bono et al. (30) showed that each 10% increase in ambient carbon monoxide exposure was associated with a 2.4% increase in median carboxyhemoglobin level (95% CI: 0.0, 4.9). Ambient exposure (geometric mean = 3.0 ppm) was estimated using the average of the past-24-hour values measured at 6 monitoring sites. Ziaei et al. (22) measured umbilical cord blood carboxyhemoglobin in newborns delivered to 41 women living in a high-pollution area of Tehran, Iran, and 32 women living in a lower-pollution area. Average carbon monoxide exposures, measured at the nearest of 2 sites in the month of delivery, were 16 ppm (SD, 6.7) and 2.7 ppm (SD, 0.8) in the 2 groups. Cord-blood carboxyhemoglobin was correlated with carbon monoxide exposures (ρ = 0.86 and ρ = 0.36 in high- and low-pollution groups; both P’s ≤ 0.01). In the highly polluted and less polluted areas, each 0.1-ppm increase in carbon monoxide was associated with 0.07% and 0.06% rises in carboxyhemoglobin level, respectively (confidence intervals were not reported). As Pereira et al. (21) reported, among 47 newborns of nonsmoking women in São Paulo, Brazil, each 1-ppm increase in carbon monoxide was associated with a 0.29% (95% CI: 0.17, 0.40) increase in cord-blood carboxyhemoglobin. Carbon monoxide exposure was measured as the past 24-hour average of concentrations at 5 monitoring sites. Average exposure was 5.7 ppm (SD, 1.9).
Because of differences in modeling of carboxyhemoglobin concentrations and carbon monoxide exposures, it is difficult to quantitatively compare relations in the studies by Ziaei et al. (22) and Pereira et al. (21) with those estimated here. The association in Bono et al.’s study (30) was stronger than ours, perhaps because carbon monoxide concentrations were much higher in that setting. Nonetheless, like our findings, these studies show a positive relation between ambient carbon monoxide exposure and blood carboxyhemoglobin levels.
Two cross-validation procedures show that our model explained 31%–71% of the variance in local carbon monoxide concentrations. The R2 value for the non-cross-validated model, 0.73, was similar to or higher than others in the literature (7, 10–12). The average error in our estimates was small compared with the range of concentrations. Both traffic measures were associated with carbon monoxide, though distance to the nearest major road explained little of their variance (adjusted R2 values were 0.19 and 0.01, respectively). In 1996–2005, on-road vehicles caused approximately 65% of regional ambient carbon monoxide emissions (31, 32). Street density was not linearly associated with carbon monoxide. This may be due to the fact that all monitoring sites with highest-quartile density were located within 1 km of the waterfront; traffic may influence local concentrations differently near large bodies of water. However, in post hoc analyses, adding an interaction term for nearness to water and street density neither improved model fit nor meaningfully changed the carbon monoxide–street density relation.
Month and year were the strongest predictors of carbon monoxide concentration: Adjusted R2 statistics were 0.20 and 0.30, respectively. The final model including temporal and land-use terms explained more variance in ambient carbon monoxide than a model with only temporal terms (adjusted R2 values were 0.73 and 0.50, respectively). However, the temporal terms accounted for nearly the same amount of variance in carboxyhemoglobin concentrations as the full model (adjusted R2 values were 0.28 and 0.27, respectively). Regional carbon monoxide concentrations are typically highest in winter, and concentrations declined steadily from 1996 to 2006 (25). These secular and seasonal fluctuations probably improved the model's ability to predict carbon monoxide concentrations. Models designed for short or fixed time periods in regions without declining carbon monoxide levels, or models designed to estimate annual concentrations, may be less strongly predictive.
Endogenous and exogenous carbon monoxide sources influence carboxyhemoglobin concentrations. Endogenous carboxyhemoglobin (typically 0.4%–0.7%) results primarily from hemolysis (33, 34). Exogenous sources include automobile exhaust, industrial combustion, tobacco smoke, and home appliances (35). In the absence of indoor sources, indoor and ambient concentrations are highly correlated (36, 37). Carboxyhemoglobin concentrations in urban nonsmokers are approximately 1%–2%; concentrations in smokers are typically 4%–7% (17, 34).
Given carboxyhemoglobin's short half-lives, incidental carbon monoxide exposure shortly before blood draw may have influenced concentrations, resulting in misclassification of longer-term exposures that may have weakened our estimates of association. Inaccuracies in residential geocoordinates may have introduced misclassification. Errors in self-reported smoking and secondhand smoke exposure may have also influenced our results. To the extent that such errors are unrelated to ambient carbon monoxide exposure, they would attenuate estimated associations toward the null.
The random-half R2 between observed and estimated carbon monoxide levels at monitoring sites was stronger than the remaining-site R2, suggesting that individual sites influenced model fit. Within monitoring sites, R2 ranged from 0.03 to 0.85. Excluding the 2 sites with the smallest R2 values did not meaningfully improve the model fit. Sources of error in our model included rounding of exposure windows to the nearest month, imprecision in geocoding, and inaccurate land-use measures. Changes in traffic characteristics during the study period may not have been captured because of our reliance on 2001 traffic density data. Furthermore, the accuracy and precision of our residence-based model was probably hampered by our inability to estimate exposures outside the home. If these errors were unrelated to ambient exposures, they would have biased our estimates of association toward the null.
Another limitation of the model is its reliance on data collected from monitors sited for local monitoring purposes. While this approach is convenient and cost-effective because it takes advantage of existing data, the monitoring sites may not be optimally located for purposes of prediction. For instance, few monitors were located in residential areas, only 2 were located near a major road, and some participants lived in areas more densely populated than the monitoring sites. Differences between sites and participants’ residences such as these may have led to exposure misclassification. The small numbers of sites in close proximity to a major road may have adversely limited our ability to capture traffic-related variations in carbon monoxide concentrations.
Our relatively simple model probably does not capture all of the spatial and temporal dependence in ambient carbon monoxide concentrations. The model's predictive ability may be improved by incorporating residual dependence using universal kriging or other methods. Nevertheless, measures of model fit and the relations between exposure estimates and carboxyhemoglobin are strong, though they appear to be driven primarily by temporal rather than spatial characteristics. These results support the validity of this easily implementable model for estimating monthly and trimester-specific carbon monoxide exposures and examining their influences on pregnancy-related health outcomes in this setting. We look forward to future studies designed to relate model-based air pollutant exposure estimates to other exposure biomarkers such as DNA adducts and carbon in airway macrophages (38, 39). Such studies may provide additional evidence to support the increasingly common use of air pollution prediction models in estimating exposures for epidemiologic research.
Supplementary Material
Acknowledgments
Author affiliations: Department of Social and Preventive Medicine, School of Public Health and Health Professions, University at Buffalo, State University of New York, Buffalo, New York (Carole B. Rudra); Department of Obstetrics-Gynecology, School of Medicine, University at Buffalo, State University of New York, Buffalo, New York (Carole B. Rudra); Center for Perinatal Studies, Swedish Medical Center, Seattle, Washington (Carole B. Rudra, Michelle A. Williams, Ihunnaya O. Frederick); Department of Epidemiology, School of Public Health, University of Washington, Seattle, Washington (Michelle A. Williams, Melissa A. Schiff); Department of Biostatistics, School of Public Health, University of Washington, Seattle, Washington (Lianne Sheppard); Department of Environmental and Occupational Health Sciences, School of Public Health, University of Washington, Seattle, Washington (Lianne Sheppard, Russell Dills); and Harborview Injury Prevention and Research Center, Harborview Medical Center, Seattle, Washington (Melissa A. Schiff).
This work was supported by the National Institutes of Health (grants R01-ES-14716 and R01-HD-32562).
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- SD
standard deviation
References
- 1.Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115(7):1118–1124. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brauer M, Lencar C, Tamburic L, et al. A cohort study of traffic-related air pollution impacts on birth outcomes. Environ Health Perspect. 2008;116(5):680–686. doi: 10.1289/ehp.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu S, Krewski D, Shi Y, et al. Association between maternal exposure to ambient air pollutants during pregnancy and fetal growth restriction. J Expo Sci Environ Epidemiol. 2007;17(5):426–432. doi: 10.1038/sj.jes.7500503. [DOI] [PubMed] [Google Scholar]
- 4.Ritz B, Wilhelm M, Hoggatt KJ, et al. Ambient air pollution and preterm birth in the Environment and Pregnancy Outcomes Study at the University of California, Los Angeles. Am J Epidemiol. 2007;166(9):1045–1052. doi: 10.1093/aje/kwm181. [DOI] [PubMed] [Google Scholar]
- 5.Salam MT, Millstein J, Li YF, et al. Birth outcomes and prenatal exposure to ozone, carbon monoxide, and particulate matter: results from the Children's Health Study. Environ Health Perspect. 2005;113(11):1638–1644. doi: 10.1289/ehp.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilhelm M, Ritz B. Local variations in CO and particulate air pollution and adverse birth outcomes in Los Angeles County, California, USA. Environ Health Perspect. 2005;113(9):1212–1221. doi: 10.1289/ehp.7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brauer M, Hoek G, van Vliet P, et al. Estimating long-term average particulate air pollution concentrations: application of traffic indicators and geographic information systems. Epidemiology. 2003;14(2):228–239. doi: 10.1097/01.EDE.0000041910.49046.9B. [DOI] [PubMed] [Google Scholar]
- 8.Jerrett M, Arain A, Kanaroglou P, et al. A review and evaluation of intraurban air pollution exposure models. J Expo Anal Environ Epidemiol. 2005;15(2):185–204. doi: 10.1038/sj.jea.7500388. [DOI] [PubMed] [Google Scholar]
- 9.Hoek G, Beelen R, de Hoogh K, et al. A review of land-use regression models to assess spatial variation of outdoor air pollution. Atmos Environ. 2008;42(33):7561–7578. [Google Scholar]
- 10.Clougherty JE, Wright RJ, Baxter LK, et al. Land use regression modeling of intra-urban residential variability in multiple traffic-related air pollutants [electronic article] Environ Health. 2008;7:17. doi: 10.1186/1476-069X-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson SB, Beckerman B, Jerrett M, et al. Application of land use regression to estimate long-term concentrations of traffic-related nitrogen oxides and fine particulate matter. Environ Sci Technol. 2007;41(7):2422–2428. doi: 10.1021/es0606780. [DOI] [PubMed] [Google Scholar]
- 12.Moore DK, Jerrett M, Mack WJ, et al. A land use regression model for predicting ambient fine particulate matter across Los Angeles, CA. J Environ Monit. 2007;9(3):246–252. doi: 10.1039/b615795e. [DOI] [PubMed] [Google Scholar]
- 13.Beelen R, Hoek G, Pebesma E, et al. Mapping of background air pollution at a fine spatial scale across the European Union. Sci Total Environ. 2009;407(6):1852–1867. doi: 10.1016/j.scitotenv.2008.11.048. [DOI] [PubMed] [Google Scholar]
- 14.Briggs DJ, de Hoogh C, Gulliver J, et al. A regression-based method for mapping traffic-related air pollution: application and testing in four contrasting urban environments. Sci Total Environ. 2000;253(1–3):151–167. doi: 10.1016/s0048-9697(00)00429-0. [DOI] [PubMed] [Google Scholar]
- 15.Ritz B, Wilhelm M. Ambient air pollution and adverse birth outcomes: methodologic issues in an emerging field. Basic Clin Pharmacol Toxicol. 2008;102(2):182–190. doi: 10.1111/j.1742-7843.2007.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Dam J, Daenens P. Microanalysis of carbon monoxide in blood by head-space capillary gas chromatography. J Forensic Sci. 1994;39(2):473–478. [PubMed] [Google Scholar]
- 17.Widdop B. Analysis of carbon monoxide. Ann Clin Biochem. 2002;39(4):378–391. doi: 10.1258/000456302760042146. [DOI] [PubMed] [Google Scholar]
- 18.Bruce MC, Bruce EN. Analysis of factors that influence rates of carbon monoxide uptake, distribution, and washout from blood and extravascular tissues using a multicompartment model. J Appl Physiol. 2006;100(4):1171–1180. doi: 10.1152/japplphysiol.00512.2005. [DOI] [PubMed] [Google Scholar]
- 19.Stewart RD, Hake CL, Wu A, et al. Carboxyhemoglobin trend in Chicago blood donors, 1970–1974. Arch Environ Health. 1976;31(6):280–285. doi: 10.1080/00039896.1976.10667236. [DOI] [PubMed] [Google Scholar]
- 20.Stewart RD, Baretta ED, Platte LR, et al. Carboxyhemoglobin concentrations in blood from donors in Chicago, Milwaukee, New York, and Los Angeles. Science. 1973;182(119):1362–1364. doi: 10.1126/science.182.4119.1362. [DOI] [PubMed] [Google Scholar]
- 21.Pereira LA, Loomis D, Conceição GM, et al. Association between air pollution and intrauterine mortality in São Paulo, Brazil. Environ Health Perspect. 1998;106(6):325–329. doi: 10.1289/ehp.98106325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziaei S, Nouri K, Kazemnejad A. Effects of carbon monoxide air pollution in pregnancy on neonatal nucleated red blood cells. Paediatr Perinat Epidemiol. 2005;19(1):27–30. doi: 10.1111/j.1365-3016.2004.00619.x. [DOI] [PubMed] [Google Scholar]
- 23.Butler CL, Williams MA, Sorensen TK, et al. Relation between maternal recreational physical activity and plasma lipids in early pregnancy. Am J Epidemiol. 2004;160(4):350–359. doi: 10.1093/aje/kwh223. [DOI] [PubMed] [Google Scholar]
- 24.Green P, Teal CF. Modification of the cyanmethemoglobin reagent for analysis of hemoglobin in order to avoid precipitation of globulins. Am J Clin Pathol. 1959;32:216–217. doi: 10.1093/ajcp/32.3.216. [DOI] [PubMed] [Google Scholar]
- 25.Puget Sound Clean Air Agency. 2006 Air Quality Data Summary. Seattle, WA: Puget Sound Clean Air Agency; 2007. [Google Scholar]
- 26.Washington State Department of Transportation. 2001 Annual Traffic Report: Revision #1. Olympia, WA: Washington State Department of Transportation; 2002. [Google Scholar]
- 27.Geography Division, Bureau of the Census, US Department of Commerce. TIGER/Line Files, 108th CD Census 2000. Washington, DC: US Census Bureau; 2003. ( http://www.census.gov/geo/www/tiger/tgrcd108/tgr108cd.html). (Accessed April 14, 2009) [Google Scholar]
- 28.Western Regional Climate Center. Western U.S. Climate Historical Summaries: Washington Climate Summaries. Reno, NV: Western Regional Climate Center; 2008. ( http://www.wrcc.dri.edu/summary/climsmwa.html). (Accessed July 24, 2008) [Google Scholar]
- 29.Flanders WD, DerSimonian R, Freedman DS. Interpretation of linear regression models that include transformations or interaction terms. Ann Epidemiol. 1992;2(5):735–744. doi: 10.1016/1047-2797(92)90018-l. [DOI] [PubMed] [Google Scholar]
- 30.Bono R, Piccioni P, Traversi D, et al. Urban air quality and carboxyhemoglobin levels in a group of traffic policemen. Sci Total Environ. 2007;376(1–3):109–115. doi: 10.1016/j.scitotenv.2007.01.086. [DOI] [PubMed] [Google Scholar]
- 31.Puget Sound Clean Air Agency. Final Report of the Puget Sound Clean Air Agency CO/Ozone Stakeholders Group. Olympia, WA: Puget Sound Clean Air Agency; 2001. [Google Scholar]
- 32.Puget Sound Clean Air Agency. 2005 Air Emission Inventory for King, Kitsap, Pierce, and Snohomish Counties. Olympia, WA: Puget Sound Clean Air Agency; 2008. [Google Scholar]
- 33.Rodgers PA, Vreman HJ, Dennery PA, et al. Sources of carbon monoxide (CO) in biological systems and applications of CO detection technologies. Semin Perinatol. 1994;18(1):2–10. [PubMed] [Google Scholar]
- 34.Scherer G. Carboxyhemoglobin and thiocyanate as biomarkers of exposure to carbon monoxide and hydrogen cyanide in tobacco smoke. Exp Toxicol Pathol. 2006;58(2-3):101–124. doi: 10.1016/j.etp.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Thomassen Ø, Brattebø G, Rostrup M. Carbon monoxide poisoning while using a small cooking stove in a tent. Am J Emerg Med. 2004;22(3):204–206. doi: 10.1016/j.ajem.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 36.De Bruin YB, Carrer P, Jantunen M, et al. Personal carbon monoxide exposure levels: contribution of local sources to exposures and microenvironment concentrations in Milan. J Expo Anal Environ Epidemiol. 2004;14(4):312–322. doi: 10.1038/sj.jea.7500327. [DOI] [PubMed] [Google Scholar]
- 37.Polidori A, Arhami M, Sioutas C, et al. Indoor/outdoor relationships, trends, and carbonaceous content of fine particulate matter in retirement homes of the Los Angeles Basin. J Air Waste Manag Assoc. 2007;57(3):366–379. doi: 10.1080/10473289.2007.10465339. [DOI] [PubMed] [Google Scholar]
- 38.Kulkarni N, Pierse N, Rushton L, et al. Carbon in airway macrophages and lung function in children. N Engl J Med. 2006;355(1):21–30. doi: 10.1056/NEJMoa052972. [DOI] [PubMed] [Google Scholar]
- 39.Perera FP, Tang D, Rauh V, et al. Relationships among polycyclic aromatic hydrocarbon-DNA adducts, proximity to the World Trade Center, and effects on fetal growth. Environ Health Perspect. 2005;113(8):1062–1067. doi: 10.1289/ehp.7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.