Abstract
Data on the effect of trajectories in long-term glycemia and all-cause mortality are lacking. The authors studied the effect of trajectories in long-term glycemic control on all-cause mortality in patients with type 2 diabetes. A cohort of 8,812 veterans with type 2 diabetes was assembled retrospectively using Veterans Affairs registry data. For each veteran in the cohort, a 3-month person-period data set was created from April 1997 to May 2006. The average duration of follow-up was 4.5 years. The overall mortality rate was 15.3%. Using a novel approach for joint modeling of time to death and longitudinal measurements of hemoglobin A1c (HbA1c) level, after adjustment for all significant baseline covariates, baseline HbA1c was found to be significantly associated with mortality (hazard ratio = 2.1, 95% confidence interval: 1.3, 3.6) (i.e., a 1% increase in baseline HbA1c level was associated with an average 2-fold increase in mortality risk). Similarly, the slope of the HbA1c trajectory was marginally significantly associated with mortality (hazard ratio = 7.3, 95% confidence interval: 0.9, 57.1) after adjustment for baseline covariates (i.e., a 1% increase in HbA1c level over 3 months was associated with a 22% increase in mortality risk). The authors conclude that a positive trajectory of long-term hyperglycemia is associated with increased mortality.
Keywords: cohort studies; diabetes mellitus, type 2; hemoglobin A, glycosylated; mortality; retrospective studies; veterans
Diabetes affects approximately 7.8% of the US population or 23.6 million people (1). Diabetes is the leading cause of cardiovascular disease, stroke, blindness, and nontraumatic lower limb amputations (1). It was the seventh leading cause of death listed on US death certificates in 2006, and persons with diabetes have a 2-fold increased risk of death compared with persons without diabetes (1). Diabetes is also associated with significant health care costs. In 2007, the total cost of diabetes-related health care was $174 billion, including $116 billion in direct costs and $58 billion in indirect costs (1).
Multiple studies have established that poor glycemic control as measured by hemoglobin A1c (HbA1c) level is associated with increased mortality in persons with type 2 diabetes (2–10). Recently, in the Norfolk, United Kingdom, component of the European Prospective Investigation into Cancer and Nutrition (EPIC-Norfolk), Khaw et al. (10) demonstrated that HbA1c was continuously related to subsequent all-cause, cardiovascular, and ischemic heart disease mortality through the whole population distribution, with the lowest rates being seen among persons with HbA1c concentrations below 5%. In the EPIC-Norfolk study, an HbA1c level of 5% was used as the reference category, and there was a significant linear relation between HbA1c and risk of death, such that there was an almost 3-fold increased risk of death in men with HbA1c concentrations greater than or equal to 7% (10).
However, few studies have examined the effect of trajectories of long-term glycemic control (i.e., increasing, stable, or decreasing) on mortality in adults with type 2 diabetes. Therefore, using a novel 2-stage, semiparametric regression approach, we sought to study the relation between the trajectory of glycemic control over time and the risk of death among persons with type 2 diabetes. Our main hypothesis was that after adjustment for baseline HbA1c and other measured confounders, an increasing glycemic trajectory would be significantly associated with all-cause mortality.
MATERIALS AND METHODS
Creation of study data set
We retrospectively assembled a cohort of 8,812 veterans with type 2 diabetes in the Charleston, South Carolina, Veterans Affairs catchment area. The Charleston Veterans Affairs catchment area includes 1 tertiary-care center and 5 community-based outpatient clinics. Multiple patient and administrative files from the Veterans Health Administration Decision Support System, linked by Social Security number, were merged to create the database. Social Security numbers were removed after the database was created. The Veterans Health Administration Decision Support System is a national automated management information system based on commercial software with which to integrate data from clinical and financial systems for both inpatient and outpatient care (11). The following Decision Support System data sets were merged in developing the diabetes data set: 1) discharge files; 2) outpatient files; 3) laboratory files—laboratory results data sets for specific tests, separated into inpatient and outpatient files; 4) pharmacy files—prescription, unit dose, and intravenous pharmacy detail for inpatient and outpatient files; 5) treating specialty—treatment specialty upon admission and treatment specialty upon discharge; and 6) cost—costs by Diagnosis-Related Group, readmissions within certain numbers of days, average patient costs, and cost details for selected clinic stops.
Creation of the longitudinal data set
We used a previously validated algorithm to identify persons with type 2 diabetes (12). Subjects were identified as having diabetes if they had at least 2 International Classification of Diseases, Ninth Revision (ICD-9) codes for diabetes in either outpatient or inpatient files and also had made 2 or more medical visits each year since diagnosis. We created a person-period data set for each subject to cover 3-month intervals from April 1997 to May 2006. We chose 3-month intervals because HbA1c is generally measured every 3 or 6 months in clinical practice as the standard of medical care, depending on the stability of glycemic control (13). HbA1c concentration is a measure of the extent of glycemia (blood glucose levels) over a period of 3 months and therefore provides a more accurate and stable measurement of long-term glycemic control (14). Therefore, our choice of the 3-month period (quarterly assessment) for HbA1c values was supported by clinical practice guidelines. HbA1c values for each subject in the 3-month time interval were used for analysis. When HbA1c levels were not observed during a 3-month period, values were considered missing. We did not anticipate that missing HbA1c values would depend on the unobserved HbA1c values; hence, we assumed missingness at random in order to analyze the data, using generalized linear mixed models which are valid under the assumption of missingness at random (15–17). For subjects with 2 or more HbA1c values in a given 3-month time interval, the most recent HbA1c value for that interval was used. Subjects were followed from the time of entry into the study until death, loss to follow-up, or May 2006. From a total of 11,803 subjects who were identified by the above algorithm, the analysis data set included 8,812 non-Hispanic white and non-Hispanic black veterans with type 2 diabetes. Veterans with unknown race/ethnicity were excluded. The study was approved by our institutional review board (Medical University of South Carolina) and the local Veterans Affairs research and development committee.
Outcome measures
For the first stage of analysis, the outcome variable was HbA1c concentration, measured in 3-month intervals from the date of entry into the cohort until the date last seen, the date of death, or the end of the study. For the second stage of analysis, the main outcome variable was time to death from all causes. Time to death was defined in 2 different ways. First, calendar time was used, and time to death was defined as time in quarters (3-month periods) between date of entry into the cohort and time of exit from the cohort (date of death, date last seen, or study end). Second, age was used as the time scale and time to death was defined as number of years from age at time of entry into the cohort to age at time of exit (time of death, date last seen, or study end) as previously described (18).
Predictor variables
Three of the predictor variables in the Cox model were outputs from the first-stage mixed model for longitudinal HbA1c. First, the baseline value of the trajectory of HbA1c was defined as the intercept of the first-stage mixed model. Second, the slope or rate of change of the HbA1c trajectory was defined as the estimated value of the coefficient for time in the mixed model. Third, the last value of HbA1c for each subject was estimated by means of the mixed model to reflect the value of the HbA1c trajectory at the time of death or censoring for that subject. For subjects who were alive at the end of the study, their last HbA1c value at the time the study ended was computed. We also considered mean HbA1c level, baseline HbA1c level, and the last observed HbA1c value for each patient as main covariables to make a comparison of the 2-stage approach, which accounts for the overall HbA1c trajectory, with approaches that use a cross-sectional summary of the observed HbA1c profile of each patient.
Other risk factors (or covariates) included age, gender, race/ethnicity, marital status, employment status, and comorbidity (stroke, hypertension, coronary heart disease, or depression). On the basis of clinical relevance, age was categorized into 4 groups (<50, 50–64, 65–74, or ≥75 years). These age groups were chosen to reflect the demographic characteristics of veterans who use the Veterans Affairs health care system. Gender was treated as a categorical variable. Race/ethnicity was dichotomized as non-Hispanic white or non-Hispanic black, after exclusion of subjects with unknown race/ethnicity. Differences in patient characteristics between subjects with and without race/ethnicity information were not significant (see Web Table 1, which is posted on the Journal’s Web site (http://aje.oxfordjournals.org/)). Marital status was classified as never married, married, or separated/widowed/divorced. Employment was classified as employed, not employed, or retired. Comorbidity variables (stroke, coronary heart disease, hypertension, and depression) were selected as those conditions causing the highest rates of complications and the majority of deaths among persons with type 2 diabetes (1) and were defined on the basis of enhanced ICD-9 codes using validated algorithms (19). Stroke was defined as ICD-9 codes 430–438, coronary heart disease as ICD-9 codes 410–414, hypertension as ICD-9 codes 401–405, and depression as ICD-9 codes 296.2, 296.3, 296.5, 300.4, 309.4, and 311.
Statistical analysis
In preliminary analyses, we examined crude associations between mortality (alive/dead) and all measured covariates in our study population of patients with type 2 diabetes, using chi-squared tests for categorical variables and t tests for continuous variables. We also studied the association between mortality and 3 cross-sectional measures of HbA1c—mean HbA1c level, baseline HbA1c level, and the last observed HbA1c value—using Cox regression.
We used a 2-stage approach (20–24) to jointly model a longitudinal time-varying covariate for HbA1c and time to death. In the first stage, a longitudinal model for HbA1c is assumed to follow a parametric linear mixed model, where both covariate effects and subject-specific random effects are modeled parametrically. In the second stage, a Cox model for time to death is assumed. The mixed model for the longitudinal process and the Cox model for the survival process are associated through common covariates in both models and the stochastic dependence between the random-effects terms in both models. This novel 2-stage regression calibration approach has been shown to lead to less biased and more efficient inferences when an association between the 2 processes exists (20–24). Inference on the parameter estimates of the Cox model is based on the robust sandwich variance estimator (21, 25–27).
Stage 1.
Suppose that i = 1, …, n subjects are followed over an interval of time [0, τ]. For each subject, we observe HbA1c level every 3 months (j = 1, …, sij) from entry into the study until death or the last day seen and a censored survival time ti. Covariates are denoted by X. The mixed-effects model for HbA1c values is
where the random intercept (b0i) and random slope (b1i) are independent and assumed to be normally distributed with mean 0 and a 2 × 2 covariance matrix Σ. The β’s are parameters for the fixed effects of the covariates. This specification allows different subjects to have different baseline HbA1c values and different time trends for HbA1c over the follow-up period.
Stage 2.
The Cox proportional hazards model includes the estimated baseline value (U0i), the current value (U2i), and the rate of change (U1i) of the underlying individual trajectories of HbA1c from the estimated mixed model (stage 1) as covariates, with further adjustment for baseline variables. The Cox model for time to death is specified as
where
Inference on (γ0–γ5) will be of primary interest, since the main focus is to study the association between mortality and HbA1c over time and whether this relation differs by race/ethnicity. In this joint model, the parameters (γ0–γ2) measure the association between the 2 models induced by the random intercepts, slopes, and fitted value of HbA1c at the time of death of each subject. In other words, the parameters are used to indicate the level and direction of association between initial level, slope, and fitted current values of HbA1c with the hazard of death. For example, the coefficient of the slope (γ1) is the log-hazard ratio for a unit increase in the rate of change of HbA1c over 3 months’ time. For all covariates, including the random terms U0i–U2i, we used likelihood ratio tests to evaluate whether to include interaction or quadratic terms. Model adequacy was assessed via the Akaike and Bayesian information criteria (25, 28).
For the Cox model, the appropriateness of the assumption of proportionality was determined by examining log{−log(time)} plots and by testing the coefficients of the interactions of time with the respective covariate in multivariate analyses. Initially, we created 3 models to calculate hazard ratios for mortality risk, all adjusted for age and then sequentially for a specific set of covariates, termed sequentially built Cox models. In the first model, results were adjusted for age only; in the second model, results were adjusted for age and the set of demographic variables; and in the third model, results were adjusted for age and the set of comorbid conditions. In the final Cox model, results were adjusted for all covariates (age, demographic factors, and comorbid conditions). The Kaplan-Meier method was used to plot the survival function. Residual analysis was used to assess the goodness of fit of the models from each stage (26). All data analyses were conducted using SAS, version 9.1.3 (SAS Institute Inc., Cary, North Carolina).
There are alternative approaches with which to jointly model time-to-event and survival data (20–24, 28). The 2-stage semiparametric regression calibration method (20) provides 2 variations of the regression calibration method: the risk set regression calibration and a computationally simpler ordinary regression calibration. Simulation results showed that the 2-stage regression calibration approach performs well in practice and effectively corrects the bias from the naive method (20). We used the 2-stage regression calibration approach and implemented it on the basis of prior research (20, 21, 23) and standard knowledge of time-to-event analysis (26). The issue of how to estimate the variance of the “plug-in” random effects needs careful consideration. While the sandwich estimator does not guarantee the best unbiased estimate of the variance, it provides a more reliable estimate of the variance than standard likelihood variance estimates. In fact, Ye et al. (20) mentioned that to make inferences for the risk coefficient estimators, the standard errors calculated on the basis of the induced partial likelihood, as if all of the true covariate values were known, do not take into account the uncertainty of these estimated time-varying covariates. Therefore, the estimated standard errors for the risk coefficients are likely to be biased and tend to be smaller than the true variance of these risk coefficient estimates. Other alternatives are to use a bootstrap estimate of variance, suggested by Li et al. (21), and the Cox-frailty model (26), with the random slope and intercept estimates treated as frailties in the Cox model. We are not aware of any head-to-head comparisons that have been made, so we decided to use the methods described above.
RESULTS
Our cohort comprised 8,812 veterans with type 2 diabetes who were followed over a 9-year period (from April 1997 to May 2006). Table 1 shows the demographic and clinical characteristics of the study cohort by censoring status (alive or dead) in May 2006. A total of 1,348 veterans in the cohort died during this period. The overall mortality rate was 15.3%, with a statistically significant difference by race/ethnicity: Approximately 16% of non-Hispanic whites and 14% of non-Hispanic blacks died. The mean length of follow-up for the cohort was 4.5 years. Non-Hispanic whites comprised 64% of the sample; 97% were male. At baseline, the distribution of comorbid conditions among veterans showed that 31% had hypertension, 17% had coronary heart disease, 7% had preexisting depression, and 4% had suffered a stroke. Web Figures 1–3 provide additional relevant information, including the distribution of HbA1c measurements by quarter (Web Figure 1), the frequency of HbA1c measurements over time (Web Figure 2), and the distribution of censoring times (Web Figure 3).
Table 1.
Characteristics of Veterans With Type 2 Diabetes (n = 8,812) by Vital Status as of May 31, 2006, Charleston, South Carolina, April 1997–May 2006
| Living |
Deceased |
P Value | |||||
| No. | Row % | Mean (SD) | No. | Row % | Mean (SD) | ||
| Sample size | 7,464 | 84.7 | 1,348 | 15.3 | <0.01 | ||
| Age at study entry, years | 60.5 (11.2) | 66.8 (10.3) | <0.01 | ||||
| Age group, years | <0.01 | ||||||
| <50 | 1,270 | 92.6 | 101 | 7.4 | |||
| 50–64 | 3,265 | 89.5 | 384 | 10.5 | |||
| 65–74 | 2,090 | 80.3 | 512 | 19.7 | |||
| ≥75 | 839 | 70.5 | 351 | 29.5 | |||
| Gender | <0.01 | ||||||
| Female | 209 | 93.7 | 14 | 6.3 | |||
| Male | 7,255 | 84.5 | 1,334 | 15.5 | |||
| Race/ethnicity | 0.03 | ||||||
| Non-Hispanic black | 2,700 | 85.8 | 446 | 14.2 | |||
| Non-Hispanic white | 4,764 | 84.1 | 902 | 15.9 | |||
| Baseline hemoglobin A1c concentration, % | 7.24 (1.89) | 7.40 (1.82) | <0.01 | ||||
| Average glycemic control | <0.01 | ||||||
| Good | 4,903 | 85.7 | 821 | 14.3 | |||
| Poor | 2,561 | 82.9 | 527 | 17.1 | |||
| Marital status | 0.06 | ||||||
| Never married | 476 | 84.7 | 86 | 15.3 | |||
| Married | 4,865 | 85.3 | 836 | 14.7 | |||
| Separated/divorced/widowed | 2,123 | 83.3 | 426 | 16.7 | |||
| Employment status | <0.01 | ||||||
| Active military or employed | 1,720 | 95.5 | 81 | 4.5 | |||
| Not employed | 3,515 | 80.6 | 846 | 19.4 | |||
| Retired | 2,229 | 84.1 | 421 | 15.9 | |||
| Hypertension | <0.01 | ||||||
| Yes | 2,127 | 78.5 | 584 | 21.5 | |||
| No | 5,337 | 87.5 | 764 | 12.5 | |||
| Coronary heart disease | <0.01 | ||||||
| Yes | 1,150 | 75.9 | 365 | 24.1 | |||
| No | 6,314 | 86.5 | 983 | 13.5 | |||
| Depression | 0.04 | ||||||
| Yes | 515 | 80.7 | 123 | 19.3 | |||
| No | 6,949 | 85.0 | 1,225 | 15.0 | |||
| Stroke | <0.01 | ||||||
| Yes | 262 | 76.4 | 81 | 23.6 | |||
| No | 7,202 | 85.0 | 1,267 | 15.0 | |||
Abbreviation: SD, standard deviation.
Several factors assessed at baseline, such as age (P < 0.001), race/ethnicity (P = 0.03), gender (P = 0.002), marital status (P = 0.06), and employment status (P < 0.001), were independently associated with mortality. Hypertension, coronary heart disease, stroke, and depression also had statistically significant associations with mortality (all P’s < 0.05); therefore, each of these covariates was included in modeling analyses.
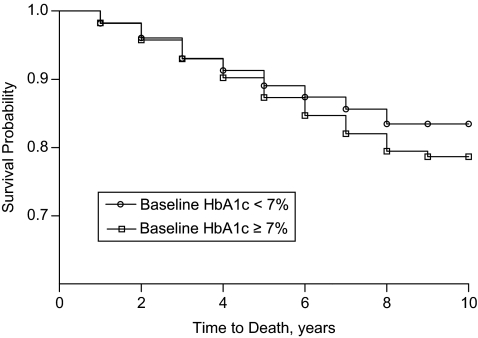
As expected, subjects with poor baseline glycemic control (HbA1c ≥ 7.0%) had significantly lower survival rates than those with good baseline control (HbA1c < 7.0%) after approximately 5 years (Figure 1). Table 2 provides hazard ratio estimates for the association between all-cause mortality and 3 measures of glycemia (the mean, baseline, and last observed value of the HbA1c profile of each patient), adjusted for other potential risk factors. The hazard ratios for mean HbA1c, baseline HbA1c, and the last observed value of the HbA1c profile were 1.06 (95% confidence interval (CI): 1.02, 1.11), 1.07 (95% CI: 1.04, 1.15) and 0.99 (95% CI: 0.95, 1.04), respectively.
Figure 1.
Kaplan-Meier survival curves for veterans with type 2 diabetes (n = 8,812) by baseline hemoglobin A1c (HbA1c) concentration, Charleston, South Carolina, April 1997–May 2006.
Table 2.
Hazard Ratios From 3 Different Cox Models for the Association Between Mortality and Mean, Baseline, and Last Observed Hemoglobin A1c Value Among Veterans With Type 2 Diabetes (n = 8,812), Charleston, South Carolina, April 1997–May 2006
| Parameter | Hazard Ratioa | 95% Confidence Interval | P Value |
| Mean HbA1c level over time | 1.06 | 1.02, 1.11 | 0.007 |
| Baseline HbA1c level | 1.07 | 1.04, 1.15 | 0.001 |
| Last observed HgbA1c value | 0.99 | 0.95, 1.02 | 0.455 |
| Age group, years (referent: <50) | |||
| 50–64 | 1.43 | 1.15, 1.79 | 0.0014 |
| 65–74 | 2.49 | 1.99, 3.11 | <0.0001 |
| ≥75 | 4.37 | 3.46, 5.52 | <0.0001 |
| Non-Hispanic black race/ethnicity (referent: non-Hispanic white) | 0.86 | 0.77, 0.97 | 0.0112 |
| Female gender (referent: male) | 0.53 | 0.31, 0.88 | 0.0148 |
| Marital status (referent: never married) | |||
| Married | 0.70 | 0.56, 0.88 | 0.0020 |
| Separated/divorced/widowed | 0.74 | 0.59, 0.94 | 0.0143 |
| Employment status (referent: employed) | |||
| Not employed | 2.68 | 2.12, 3.39 | <0.0001 |
| Retired | 1.96 | 1.52, 2.52 | <0.0001 |
| Coronary heart disease (referent: none) | 1.22 | 1.04, 1.42 | 0.0135 |
| Hypertension (referent: none) | 1.31 | 1.14, 1.51 | 0.0001 |
Abbreviation: HbA1c, hemoglobin A1c.
Results were adjusted for all covariates.
The set of Cox models that were sequentially built to estimate the adjusted association between mortality and baseline HbA1c, the slope of HbA1c, and the last HbA1c value is available on the Journal’s Web site (see Web Table 2 and Web Table 3). The first model (model 1) included adjustment for age at entry and last observed HbA1c value only. The hazard ratio for slope in HbA1c was 6.7 (95% CI: 0.8, 54.9), while the hazard ratio for baseline HbA1c was 2.2 (95% CI: 1.3, 3.7). When gender, race/ethnicity, marital status, and employment status were added (model 2), the hazard ratio for a 0.1-unit change in the slope of HbA1c increased to 8.2 (95% CI: 1.0, 67.8), while the hazard ratio for baseline HbA1c remained the same. Adjusting model 1 results for coronary heart disease and hypertension decreased the hazard ratio for slope to 5.9 (95% CI: 0.8, 45.6) and minimally changed the hazard ratio for baseline HbA1c to 2.1 (95% CI: 1.2, 3.5). The 95% confidence interval for slope indicated that the hazard ratio for a unit increase in the rate of change in HbA1c was expected to lie between 0.8 and 45.6. Neither stroke nor depression exhibited a significant confounding effect. Even though we used a robust sandwich variance estimator for the hazard ratio estimates from the Cox model to adjust for the variability in the random terms obtained from the longitudinal model (baseline, slope, and last HbA1c value), our hazard ratio estimates featured relatively wide confidence intervals, consistent with observations in other studies (23, 24). However, these intervals were narrower than what we observed from the naive partial likelihood estimates.
The final, fully adjusted model included adjustment for both the baseline and last levels of HbA1c, as well as covariates that were statistically significant in earlier models (see Table 3). Results showed that the slope of HbA1c was marginally significantly associated with mortality (hazard ratio = 7.3, 95% CI: 0.9, 57.1). In this model, a 0.1-unit increase in the slope of HbA1c or a 1% increase in HbA1c over 3 months was associated with a 22% increase in risk of mortality, and a 0.5-unit increase in the slope or a 5% increase in HbA1c over 3 months was associated with a hazard ratio of 2.7. Similarly, holding the slope and last value of HbA1c constant, our data suggested that the baseline level of HbA1c was significantly associated with mortality (hazard ratio = 2.1, 95% CI: 1.3, 3.6). Examining the last HbA1c value in a similar manner did not show a significant association with mortality.
Table 3.
Hazard Ratios From a Fully Adjusted Cox Model for Mortality Among Veterans With Type 2 Diabetes (n = 8,812), Charleston, South Carolina, April 1997–May 2006
| Parameter | Hazard Ratioa | 95% Confidence Interval | P Value |
| Slope of HbA1c levelb | 7.28 | 0.93, 57.10 | 0.06 |
| Baseline HbA1c level | 2.14 | 1.27, 3.60 | <0.01 |
| Last observed HgbA1c value | 0.55 | 0.26, 1.20 | 0.13 |
| Last observed HbA1c value squared | 1.00 | 0.97, 1.03 | 0.83 |
| Age group, years (referent: <50) | |||
| 50–64 | 1.39 | 1.11, 1.73 | <0.01 |
| 65–74 | 2.37 | 1.89, 2.96 | <0.01 |
| ≥75 | 4.10 | 3.25, 5.18 | <0.01 |
| Non-Hispanic black race/ethnicity (referent: non-Hispanic white) | 0.87 | 0.78, 0.98 | 0.02 |
| Female gender (referent: male) | 0.53 | 0.31, 0.88 | 0.01 |
| Marital status (referent: never married) | |||
| Married | 0.69 | 0.55, 0.86 | <0.01 |
| Separated/divorced/widowed | 0.74 | 0.58, 0.93 | 0.01 |
| Employment status (referent: employed) | |||
| Not employed | 2.67 | 2.11, 3.38 | <0.01 |
| Retired | 1.95 | 1.51, 2.50 | <0.01 |
| Coronary heart disease (referent: none) | 1.24 | 1.06, 1.45 | 0.01 |
| Hypertension (referent: none) | 1.30 | 1.13, 1.50 | <0.01 |
Abbreviation: HbA1c, hemoglobin A1c.
Results were adjusted for all covariates.
Slope of HbA1c concentration over time from a linear mixed model.
Increased mortality risk in veterans with type 2 diabetes was associated with multiple demographic factors. Persons aged 75 years or older were approximately 4 times more likely to die than those who were younger than age 50 years. Interestingly, our data suggested that the hazard ratio for non-Hispanic blacks was 0.87, indicating that the risk of mortality in non-Hispanic blacks was 13% lower than that in non-Hispanic whites. Subjects who had coronary heart disease and/or hypertension at baseline had an approximately 25%–30% increased risk of mortality. Compared with those who had never married, both married and divorced subjects had at least a 25% lower risk of mortality. Persons who were unemployed or retired had at least twice the risk of mortality as those currently employed (active military duty, fully employed, or partially employed).
DISCUSSION
This study revealed an important relation between a positive trajectory of glycemia and risk of mortality in a large sample of persons with diabetes using a novel approach of joint modeling of longitudinal measurements of HbA1c and mortality data. Our findings demonstrated that every 0.1-unit increase in the slope of HbA1c was associated with a 22% increased risk of mortality and that a 0.5-unit increase in the slope of HbA1c nearly tripled mortality risk. After adjustment for the last observed value and slope of HbA1c, baseline HbA1c level was also significantly associated with mortality. In contrast, we found that using simple approaches that do not account for the trajectory of HbA1c (see Table 2) could lead to underestimation of the association between mortality and HbA1c. As expected, older age and having coronary heart disease or hypertension as a comorbid condition were significant predictors of greater all-cause mortality. However, being of non-Hispanic black race/ethnicity, being married, and being employed were associated with lower risk of dying.
This study demonstrated 2 important associations: first, the relation between glycemia and mortality and, second, the impact of a true trajectory of glycemia (over multiple time points) on mortality. The analytic ability to examine the slope of glycemia revealed that a small increase in HbA1c (0.1 unit) is associated with higher mortality risk (22%) and that a larger HbA1c increase (0.5 unit) is associated with an exponential increase in mortality risk. This suggests a dose-response effect, with increasing hyperglycemia conferring higher risks of mortality, even after adjustment for age, gender, race/ethnicity, socioeconomic status, and multiple cardiovascular disease risk factors (coronary heart disease, depression, hypertension, and stroke). This analysis concurs with many prospective and cross-sectional studies in finding that hyperglycemia is linked to an increased risk of death (2–10). However, in those studies, glycemia was generally based on 1 measurement (either baseline or average) without accounting for multiple measurements.
Earlier randomized clinical trials and prospective cohort studies, such as the United Kingdom Prospective Diabetes Study and EPIC-Norfolk, demonstrated that intensive glycemic control (HbA1c < 7.0%) leads to reductions in mortality rates among adults with type 2 diabetes (8–10). However, data from newer randomized clinical trials have yielded conflicting results. The ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial showed that targeting an HbA1c level less than 6.0% increases the risk of mortality (19); investigators in the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation) trial found no significant association between intensive glycemic control (mean HbA1c = 6.5%) and increased mortality (29, 30); and results from the Veterans Affairs Diabetes Trial demonstrated that intensive glucose control (mean HbA1c = 6.9%) in patients with poorly controlled type 2 diabetes had no significant effect on rates of major cardiovascular disease events or death (31). These recent randomized clinical trials suggest that very stringent glycemic control (HbA1c < 6.0%) may not be ideal for patients with type 2 diabetes. Our study adds clarity to the current debate and shows that an overall increasing trajectory of glycemia in adults with diabetes is associated with an increasing risk of death from all causes. It is relevant to examine all-cause and cardiovascular mortality among persons with type 2 diabetes, because data show that diabetes is a leading cause of mortality in the United States, with a majority of diabetes-related deaths being attributed to cardiovascular disease (1). However, diabetes is probably underreported on death certificates (1), so it becomes highly important to examine all causes of death among persons diagnosed with type 2 diabetes. Further analyses that examined a decreasing glycemic trajectory and a steady trend of good or poor control would provide greater clarification regarding the potential mortality benefit of diabetes control.
The findings of this study were obtained in a very large sample of adults with diabetes. The study was longitudinal in nature, with 9 years of follow-up data and multiple measures of HbA1c from each participant, allowing examination of glycemic trajectory. In addition, a newly established analytic method that improves the validity of results was used to track glycosylated hemoglobin in 3-month intervals, providing more accurate, long-term data on glycemic control and its impact on mortality. Very few researchers have examined longitudinal data on such a large scale using multiple HbA1c measurements, especially in the veteran population.
Despite these strengths, our study had several limitations. First, the study was most representative of veterans who are predominantly male and older and have more comorbid conditions than the general population. Second, target organ damage (e.g., nephropathy with reduced glomerular filtration) can significantly contribute to diabetes-related death (32) but was not accounted for in this study. Third, information about blood pressure and lipid control, other potential confounders (e.g., indices of body composition, smoking, and physical activity), duration of diabetes, and medication adherence (33) was not available in our data sets for the study period and hence was not included. Prior cohort studies have shown that 10% increments in medication adherence lead to improvements in HbA1c in the range of 0.10%–0.16% (34−36). Studies have also shown that glycemic control worsens over time even with treatment intensification (37, 38). Therefore, the effects of these potential confounders need to be further examined in future studies. Finally, our findings could have been biased by the significant proportion of veterans with missing data on race/ethnicity. While we believe that the unreported race/ethnicity information was missing at random, we also conducted a sensitivity analysis to see whether the missingness depended on demographic and clinical characteristics of the persons with missing race/ethnicity data. When we compared demographic and clinical characteristics of persons in our sample with the characteristics of persons with unknown race/ethnicity information, there were no significant differences by age, gender, insulin use at baseline, or mean HbA1c concentration. However, there were significant differences by marital status and comorbidity. Nonetheless, we believe that our estimates are unbiased, because we used generalized mixed modeling, which provides unbiased and efficient effect estimates when the missingness mechanism is missing at random (15–17).
In summary, using a novel approach of joint modeling of longitudinal measurements of HbA1c and mortality data, we showed in this study that a positive trajectory of long-term hyperglycemia is associated with increased mortality. In future research, investigators should adopt the method of evaluating how glycemic trends, created from multiple HbA1c measurements, influence diabetes-related outcomes. This would provide more clinically relevant information about glycemic control that would enable providers to explain the correlated risk or benefit to patients and would provide greater understanding regarding the importance of diligent monitoring and control of diabetes.
Supplementary Material
Acknowledgments
Author affiliations: Center for Disease Prevention and Health Interventions for Diverse Populations, Ralph H. Johnson Veterans Affairs Medical Center, Charleston, South Carolina (Leonard E. Egede, Mulugeta Gebregziabher, Cheryl P. Lynch, Carrae L. Echol, Gregory E. Gilbert); Division of General Internal Medicine and Geriatrics, Medical University of South Carolina, Charleston, South Carolina (Leonard E. Egede, Cheryl P. Lynch, Yumin Zhao); Center for Health Disparities Research, Medical University of South Carolina, Charleston, South Carolina (Leonard E. Egede, Cheryl P. Lynch, Yumin Zhao); and Department of Biostatistics, Bioinformatics, and Epidemiology, Medical University of South Carolina, Charleston, South Carolina (Mulugeta Gebregziabher).
This study was supported by grant REA 08-261 from the Center for Disease Prevention and Health Interventions for Diverse Populations, which is funded by the Department of Veterans Affairs Health Services Research and Development Service (Principal Investigator, Leonard E. Egede).
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- EPIC
European Prospective Investigation into Cancer and Nutrition
- HbA1c
hemoglobin A1c
- ICD-9
International Classification of Diseases, Ninth Revision
References
- 1.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2007. Atlanta, GA: Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 2.Moss SE, Klein R, Klein BE, et al. The association of glycemia and cause-specific mortality in a diabetic population. Arch Intern Med. 1994;154(21):2473–2479. [PubMed] [Google Scholar]
- 3.Andersson DK, Svärdsudd K. Long-term glycemic control relates to mortality in type II diabetes. Diabetes Care. 1995;18(12):1534–1543. doi: 10.2337/diacare.18.12.1534. [DOI] [PubMed] [Google Scholar]
- 4.Wei M, Gaskill SP, Haffner SM, et al. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality. The San Antonio Heart Study. Diabetes Care. 1998;21(7):1167–1172. doi: 10.2337/diacare.21.7.1167. [DOI] [PubMed] [Google Scholar]
- 5.de Vegt F, Dekker JM, Ruhé HG, et al. Hyperglycaemia is associated with all-cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia. 1999;42(8):926–931. doi: 10.1007/s001250051249. [DOI] [PubMed] [Google Scholar]
- 6.Gregg EW, Gu Q, Cheng YJ, et al. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med. 2007;147(3):149–155. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
- 7.Gu K, Cowie CC, Harris MI. Diabetes and decline in heart disease mortality in US adults. JAMA. 1999;281(14):1291–1297. doi: 10.1001/jama.281.14.1291. [DOI] [PubMed] [Google Scholar]
- 8.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khaw KT, Wareham N, Bingham S, et al. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European Prospective Investigation into Cancer in Norfolk. Ann Intern Med. 2004;141(6):413–420. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- 10.Khaw KT, Wareham N, Luben R, et al. Glycated haemoglobin, diabetes, and mortality in men in Norfolk cohort of European Prospective Investigation of Cancer and Nutrition (EPIC-Norfolk) BMJ. 2001;322(7277):15–18. doi: 10.1136/bmj.322.7277.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maynard C, Chapko MK. Data resources in the Department of Veterans Affairs. Diabetes Care. 2004;27(suppl 2):B22–B26. doi: 10.2337/diacare.27.suppl_2.b22. [DOI] [PubMed] [Google Scholar]
- 12.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(suppl 2):B10–B21. doi: 10.2337/diacare.27.suppl_2.b10. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association Executive summary: standards of medical care in diabetes—2009. Diabetes Care. 2009;32(suppl 1):S6–S12. doi: 10.2337/dc09-S006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2003;26(suppl 1):S106–S108. doi: 10.2337/diacare.26.2007.s106. [DOI] [PubMed] [Google Scholar]
- 15.Diggle PJ, Heagerty P, Liang K-Y, et al. Analysis of Longitudinal Data. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2002. [Google Scholar]
- 16.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 17.Ibrahim JG, Molenberghs G. Missing data methods in longitudinal studies: a review. TEST. 2009;18(1):1–43. doi: 10.1007/s11749-009-0138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145(1):73–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 19.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 20.Ye W, Lin X, Taylor JM. Semiparametric modeling of longitudinal measurements and time-to-event data—a two-stage regression calibration approach. Biometrics. 2008;64(4):1238–1246. doi: 10.1111/j.1541-0420.2007.00983.x. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Hu B, Greene T. A semiparametric joint model for longitudinal and survival data with application to hemodialysis study. Biometrics. 2009;65(3):737–745. doi: 10.1111/j.1541-0420.2008.01168.x. [DOI] [PubMed] [Google Scholar]
- 22.Guo X, Carlin BP. Separate and joint modeling longitudinal and event time data using standard computer packages. Am Stat. 2004;58(1):16–24. [Google Scholar]
- 23.Wang Y, Taylor JMG. Jointly modeling longitudinal and event time data with application to acquired immunodeficiency syndrome. J Am Stat Assoc. 2001;96(455):895–905. [Google Scholar]
- 24.Taylor JM, Yu M, Sandler HM. Individualized predictions of disease progression following radiation therapy for prostate cancer. J Clin Oncol. 2005;23(4):816–825. doi: 10.1200/JCO.2005.12.156. [DOI] [PubMed] [Google Scholar]
- 25.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 26.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. Hoboken, NJ: John Wiley & Sons, Inc; 2002. [Google Scholar]
- 27.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distribution. J Am Stat Assoc. 1989;84(408):1065–1073. [Google Scholar]
- 28.Diggle PJ, Sousa I, Chetwynd AG. Joint modelling of repeated measurements and time-to-event outcomes: the fourth Armitage lecture. Stat Med. 2008;27(16):2981–2998. doi: 10.1002/sim.3131. [DOI] [PubMed] [Google Scholar]
- 29.The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.The ADVANCE. Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 31.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 32.Bo S, Ciccone G, Gancia R, et al. Mortality within the first 10 years of the disease in type 2 diabetic patients. Nutr Metab Cardiovasc Dis. 2006;16(1):8–12. doi: 10.1016/j.numecd.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166(17):1836–1841. doi: 10.1001/archinte.166.17.1836. [DOI] [PubMed] [Google Scholar]
- 34.Schectman JM, Nadkarni MM, Voss JD. The association between diabetes metabolic control and drug adherence in an indigent population. Diabetes Care. 2002;25(6):1015–1021. doi: 10.2337/diacare.25.6.1015. [DOI] [PubMed] [Google Scholar]
- 35.Krapek K, King K, Warren SS, et al. Medication adherence and associated hemoglobin A1c in type 2 diabetes. Ann Pharmacother. 2004;38(9):1357–1362. doi: 10.1345/aph.1D612. [DOI] [PubMed] [Google Scholar]
- 36.Rozenfeld Y, Hunt JS, Plauschinat C, et al. Oral antidiabetic medication adherence and glycemic control in managed care. Am J Manag Care. 2008;14(2):71–75. [PubMed] [Google Scholar]
- 37.Riedel AA, Heien H, Wogen J, et al. Loss of glycemic control in patients with type 2 diabetes mellitus who were receiving initial metformin, sulfonylurea, or thiazolidinedione monotherapy. Pharmacotherapy. 2007;27(8):1102–1110. doi: 10.1592/phco.27.8.1102. [DOI] [PubMed] [Google Scholar]
- 38.Jermendy G Hungarian RECAP Group. Erdesz D, et al. Outcomes of adding second hypoglycemic drug after metformin monotherapy failure among type 2 diabetes in Hungary [electronic article] Health Qual Life Outcomes. 2008;6:88. doi: 10.1186/1477-7525-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.