Abstract
Controlling for body size and composition, the authors examined the association between estrogen therapy and bone mineral density in older African-American and Caucasian women. In 1992–1998, 443 African-American and 989 Caucasian women aged 45–87 years were assessed for medication use, laboratory variables, behavioral characteristics, and bone mineral density. The mean age was 61.3 (95% confidence interval: 60.3, 62.3) years in African Americans and 71.0 (95% confidence interval: 70.4, 71.7) years in Caucasians (P < 0.001). All measures of body size and composition were significantly greater in the African-American women compared with Caucasian women (P < 0.001). As expected, African Americans had significantly higher bone mineral density at all 4 sites independent of age, weight, body composition, estrogen use, and lifestyle factors. Although Caucasians were significantly more likely to currently use estrogen (48.9% vs. 33.9%; P < 0.001), African Americans not using estrogen had significantly higher bone mineral density at all sites except the spine than Caucasians who were using estrogen. Regression models including age and lean mass explained the most variation in bone mineral density (R2 range = 0.13–0.37). Results suggest that higher levels of bone mineral density in African-American women were not due to estrogen use.
Keywords: African Americans, body composition, bone density, estrogen replacement therapy
Bone mineral density is higher in African-American women than Caucasian women before and after menopause (1, 2). It is widely accepted that hormone therapy can slow or reverse bone loss in postmenopausal women (3). However, the role of estrogen therapy in the observed differences between African-American and Caucasian women in bone mineral density is not clear.
Few studies address the effect of ethnicity on the relation between hormone therapy and bone mineral density. Some studies show a lower use of hormone therapy in African-American women (4) compared with Caucasian women, while others report no difference (5, 6). In a longitudinal study of women 65 years of age or older, Cauley et al. (5) reported that postmenopausal African-American women using hormone therapy gained bone mineral density but Caucasian women continued to lose bone. The difference in bone mineral density between African-American and Caucasian women has been attributed to differences in body size or composition, including weight (7, 8), as well as fat and lean body mass (2, 6). Although these studies controlled for the use of hormone therapy, the influence of estrogen on body composition and its association with bone mineral density has not been reported.
The purpose of this study was to examine the association between estrogen therapy and bone mineral density in a cohort of community-dwelling older African-American and Caucasian women after controlling for differences in body size and composition.
MATERIALS AND METHODS
Participants
Participants were 1,432 community-dwelling African-American and Caucasian women aged 45–87 years. Caucasian participants were initially enrolled between 1972 and 1974 in the Rancho Bernardo Study, a longitudinal study of cardiovascular risk factors in a middle-class, predominately white, Southern California community (n = 6,339) (9). Between 1992 and 1997, all surviving, locally resident, and noninstitutionalized members of the Rancho Bernardo cohort were invited to attend a follow-up visit focused on osteoporosis (visit 7); a total of 1,732 individuals (69.3%) participated, including 1,082 women aged 30–97 years. Between 1993 and 1998, African-American women aged 45–87 years were enrolled as participants in the Health Assessment Study of African-American Women, a cross-sectional study of chronic disease designed to emulate the Rancho Bernardo Study for ethnic comparison purposes (n = 443). African-American women from the metropolitan San Diego community who were of similar education and social class to the Rancho Bernardo cohort were recruited, in order to have comparable samples rather than a representative sample of African-American women (10).
After exclusion of all women less than age 45 and over 87 years of age (n = 82) and 11 women reporting use of tamoxifen, there remained 1,432 participants who form the basis of this report. All women provided written, informed consent prior to participation; the research protocol was approved by the University of California, San Diego, Human Subjects Protections Program.
Procedure
Identical protocols for data collection were used in both groups. A self-administered questionnaire assessed demographic characteristics (age, education) and behaviors such as smoking status (never/past/current), alcohol use (3 or more drinks per week, yes/no), and physical exercise (3 or more times per week, yes/no). An interviewer-administered questionnaire was used to ascertain menopausal status, as well as past and current hormone replacement therapy usage. Current use of prescription medications (including estrogen) and nonprescription medications was validated by examining prescriptions and pills brought to the clinic for that purpose.
Body size was measured in participants wearing lightweight clothing without shoes. Height was obtained in inches by a stadiometer and converted to meters; weight was assessed in pounds and converted to kilograms. Body mass index was calculated (weight (kg)/height (m)2). Circumference measurements were collected in centimeters at the bending point for the waist and at the largest circumference for the hip. The waist/hip ratio was calculated as waist circumference divided by hip circumference.
In the clinic, a sample of blood was drawn from the antecubital region after a requested overnight fast. A 2-hour oral glucose tolerance test with a 75-g load of glucose was administered, and a 2-hour postchallenge sample of blood was obtained.
Bone mineral density and body composition
For both groups of women, bone mineral density (g/cm2) of the spine, hip, and total body was measured by certified bone density technologists at the University of California, San Diego, Research Clinic using the dual-energy x-ray absorptiometry (DXA) (Hologic QDR-2000; Hologic, Inc., Waltham, Massachusetts) version 5.54 scanning program. The scan of the hip included femoral neck bone mineral density, which was measured at the narrowest point of the femoral neck and perpendicular to the femoral midline, and total hip bone mineral density, which was the mean of the femoral neck, greater trochanter, and intertrochanteric regions. Only 147 scans of the hip using the Hologic QDR-2000 were completed among the Caucasian participants, limiting the sample for those sites. Spine bone mineral density was measured in the anterioposterior view and was reported as the mean of lumbar vertebrae 1–4. Total-body scans consisted of measured bone mineral content and area in 10 regions. Total-body fat mass, total lean mass, and total percentage of body fat was estimated from the total-body DXA. Axial scans were standardized daily against a phantom, with a precision error of 1%. Precision errors for fat mass, lean mass, and percent body fat were approximately 1.2% or less.
Statistical analysis
Comparisons of women from the Health Assessment Study of African-American Women and the Rancho Bernardo Study on demographic and behavioral characteristics were performed with chi-square analysis for categorical variables and analysis of covariance (ANCOVA) for continuous variables after adjustment for age. On the basis of World Health Organization criteria (11), participants were categorized as having type 2 diabetes if their fasting plasma glucose level was 126 mg/dL or more, their 2-hour postchallenge glucose level was 200 mg/dL or more, or they reported a history of type 2 diabetes diagnosed by a physician or treatment with an oral hypoglycemic agent or insulin. Participants were considered to have impaired glucose tolerance if their fasting plasma glucose level was between 110 mg/dL and 125 mg/dL and their 2-hour postchallenge glucose level was between 140 mg/dL and 199 mg/dL; participants were considered to have normal glucose tolerance if their fasting plasma glucose level was less than 110 mg/dL and their 2-hour postchallenge glucose level was less than 140 mg/dL. Estrogen use was grouped by type of estrogen into conjugated estrogens, estradiol, estropipate, esterfied estrogens, ethinyl estradiol, other, and combination therapy. The time since stopping estrogen therapy was calculated as the difference between current age and the age at stopping estrogen therapy, and it was categorized into stopping more and less than 5 years from the visit date. Age-adjusted comparisons of mean body composition measures by current estrogen status were performed separately within each ethnic group by using ANCOVA. Multivariate generalized linear regression models were built in a stepwise method to estimate which set of variables explained the most variation in bone mineral density as indicated by the R2 statistic. Models included age, current estrogen use, body size or composition variables entered separately one at a time (weight, body mass index, lean mass, fat mass, percent body fat, waist circumference, waist/hip ratio), and a set of covariates used together, including current smoking status, alcohol use, exercise frequency, thyroid medication use, calcium use, and diagnosis of type 2 diabetes. On the basis of their associations with bone mineral density, age- and lean body mass-adjusted comparisons of mean bone mineral density by current estrogen status were performed separately in each ethnic group by using ANCOVA. Data were analyzed using SAS, version 8.0, software (SAS Institute, Inc., Cary, North Carolina); all statistical tests were 2-tailed with P ≤ 0.05 considered significant. For all mean values, both significance level and 95% confidence intervals were reported.
RESULTS
The overall age range of these women was 45–87 years, with a mean of 61.3 years in the 443 African-American women and 71.0 years in 989 Caucasian women (P < 0.001) (Table 1). In the age-adjusted analysis, African-American women compared with Caucasians reported a higher level of education (P < 0.001) and were less likely to drink alcohol (P < 0.001) and less likely to exercise (P < 0.001), but were more likely to smoke cigarettes (P = 0.02). African-American women were almost twice as likely to have diabetes compared with Caucasians (P ≤ 0.05). Significantly more Caucasian women reported use of thyroid medication and calcium supplements than did African Americans (P < 0.001); rates of diuretic and steroid use did not differ between the groups. All measures of body size and composition (body mass index, weight, lean and fat mass, body fat percent, waist circumference, and waist/hip ratio) were significantly greater in the African-American women than in Caucasian women (P < 0.001).
Table 1.
Age and Age-adjusted Demographic and Behavioral Characteristics Among African-American and Caucasian Women, San Diego, California, 1992–1998a
| African-American Women (n = 443) |
Caucasian Women (n = 989) |
|||
| Mean | 95% Confidence Interval | Mean | 95% Confidence Interval | |
| Age, years | 61.3 | 60.5, 62.1 | 71.0** | 70.4, 71.7 |
| Body mass index, kg/m2 | 29.8 | 29.3, 30.3 | 25.1** | 24.7, 25.3 |
| Total fat mass, kg | 29.4 | 28.3, 30.3 | 22.4** | 21.7, 22.9 |
| Total lean mass, kg | 43.6 | 43.0, 44.1 | 39.5** | 39.1, 39.8 |
| Total body fat, % | 37.5 | 36.7, 38.2 | 34.1** | 33.6, 34.6 |
| Body weight, kg | 77.4 | 76.0, 78.8 | 65.1** | 64.2, 66.0 |
| Waist circumference, cm | 89.0 | 87.8, 90.1 | 79.1** | 78.4, 79.8 |
| Waist/hip ratio | 0.84 | 0.83, 0.84 | 0.78** | 0.77, 0.78 |
|
No.b |
% |
No.b |
% |
|
| Age category years | ||||
| <50 | 20 | 4.5 | 11 | 11.2 |
| 50–59 | 195 | 44.0 | 170 | 17.2 |
| 60–69 | 160 | 36.1 | 219 | 22.1 |
| 70–79 | 53 | 12.0 | 348 | 35.2 |
| ≥80 | 15 | 3.4 | 241 | 24.3 |
| Education (some college) | 343 | 74.8 | 532** | 68.3 |
| Smoker (current) | 50 | 9.7 | 79* | 8.6 |
| Alcohol (≥3 drinks/week) | 47 | 9.0 | 391** | 37.9 |
| Exercise (≥3 times/week) | 248 | 55.4 | 682** | 67.9 |
| Postmenopausal | 402 | 89.6 | 952 | 89.6 |
| Diabetes (WHO 1999 criteria) | 71 | 17.6 | 114* | 9.8 |
| Medication/supplement (current use) | ||||
| Diuretic | 15 | 3.9 | 27 | 1.6 |
| Thyroid medication | 42 | 8.8 | 208** | 18.8 |
| Steroids | 8 | 2.0 | 23 | 1.7 |
| Calcium | 105 | 21.1 | 501** | 49.0 |
Abbreviation: WHO, World Health Organization.
* P = 0.05; **P = 0.001 (compared with African Americans).
Analysis of covariance for continuous variables with age adjustment and chi-square analysis for categorical variables, with direct age adjustment calculated to estimate these results.
Subject numbers for each variable do not add to total sample because of missing data.
African-American women were less likely to be current hormone users (33.9% vs. 48.9%, respectively; P < 0.05) or ever users of estrogen (53.8% vs. 69.4%, respectively; P < 0.001) (Table 2). The reported duration of hormone use was somewhat shorter in African-American women than in Caucasian women (P < 0.05). African-American women were less likely to use progestin (P < 0.05), but the duration of progestin use did not differ among users in the 2 groups. The type of estrogen preparation used did not differ between groups (data not shown).
Table 2.
Age-adjusted Hormone Replacement Therapy Use in African-American and Caucasian Women, San Diego, California, 1992–1998a
| African-American Women (n = 443) |
Caucasian Women (n = 989) |
|||
| No.b | % | No.b | % | |
| Estrogen replacement therapy | ||||
| Current use | 172 | 33.9 | 463* | 48.9 |
| Ever use | 259 | 53.8 | 687*** | 69.4 |
| Duration ≤5 years | 123 | 52.0 | 211*** | 36.5 |
| Stopped use ≤5 years | 39 | 35.7 | 67** | 44.8 |
| Progestin | ||||
| Ever use | 107 | 20.1 | 304* | 33.8 |
| Mean | 95% Confidence Interval | Mean | 95% Confidence Interval | |
| Estrogen replacement therapy (average duration), years | 11.0 | 9.6, 12.2 | 12.8* | 12.0, 13.5 |
| Progestin (average duration), years | 7.0 | 5.1, 8.8 | 5.7 | 4.6, 6.7 |
* P = 0.05; **P = 0.01; ***P = 0.001 (compared with African Americans).
Analysis of covariance for continuous variables with age adjustment and chi-square analysis for categorical variables, with direct age adjustment calculated to estimate these results.
Subject numbers for each variable do not add to total sample because of missing data.
Age-adjusted comparisons of body size and composition measures by current estrogen status within each group were performed with ANCOVA (Table 3). Among African-American women, those currently using estrogen had a lower body mass index (P = 0.01), fat mass (P = 0.03), percent body fat (P = 0.02), weight (P = 0.05), and waist circumference (P = 0.01) than did those not using estrogen. Among Caucasian women, those currently using estrogen also had a lower body mass index (P = 0.05), weight (P = 0.05), waist circumference (P = 0.007), and waist/hip ratio (P = 0.003) than did those not currently using estrogen. Despite the lower body size and composition measures with estrogen use, African-American women who currently used estrogen had higher levels of all body size measures than did Caucasian women who did not currently use estrogen.
Table 3.
Age-adjusted Comparisons of Mean Body Size and Body Composition Measures by Current Estrogen Use in African-American and Caucasian Women, San Diego, California, 1992–1998a
| African-American Current Estrogen Use |
Caucasian Current Estrogen Use |
|||||||
| Yes (n = 172) |
No (n = 271) |
Yes (n = 463) |
No (n = 526) |
|||||
| Mean | 95% Confidence Interval | Mean | 95% Confidence Interval | Mean | 95% Confidence Interval | Mean | 95% Confidence Interval | |
| Weight, kg | 77.3 | 74.6, 80.0 | 80.8* | 78.6, 82.9 | 63.4 | 62.3, 64.4 | 64.9* | 63.8, 65.8 |
| Body mass index, kg/m2 | 29.1 | 28.2, 30.1 | 30.7** | 30.0, 31.5 | 24.6 | 24.1, 24.9 | 25.2* | 24.8, 25.5 |
| Waist circumference, cm | 86.9 | 85.0, 88.8 | 90.1** | 88.6, 91.6 | 78.2 | 77.1, 79.0 | 80.1** | 79.1, 80.9 |
| Waist/hip ratio | 0.83 | 0.81, 0.83 | 0.83 | 0.82, 0.83 | 0.78 | 0.77, 0.78 | 0.79** | 0.78, 0.79 |
| Total lean mass, kg | 44.5 | 43.6, 45.4 | 44.6 | 43.8, 45.4 | 38.9 | 38.3, 39.3 | 39.2 | 38.7, 39.6 |
| Total fat mass, kg | 28.6 | 26.7, 30.5 | 31.2* | 29.7, 32.8 | 21.4 | 20.7, 22.2 | 22.4 | 21.6, 23.1 |
| Total body fat, % | 36.5 | 35.3, 37.7 | 38.5* | 37.5, 39.5 | 33.5 | 32.8, 34.2 | 34.4 | 33.7, 35.0 |
* P = 0.05; **P = 0.01 (compared with users).
Analysis of covariance with age adjustment was calculated to estimate these results.
In age-adjusted analysis, the mean bone mineral density at all sites (femoral neck (P < 0.001), total hip (P < 0.001), lumbar spine (P = 0.002), and total body (P < 0.001)) was significantly higher in African-American women than in Caucasian women (Table 4). After additional separate adjustment for body size (weight, fat mass, and lean mass), the mean bone mineral density remained significantly higher in African-American women compared with Caucasian women at the femoral neck, total hip, and total body (all P < 0.01) but not at the lumbar spine. Addition of current estrogen use to the models did not materially change these results, except for the association at the total hip, where the association was no longer significant. When other potential covariates (current smoking, drinking alcohol ≥3 times per week, exercise ≥3 times per week, thyroid medication use, calcium use, diagnosis of diabetes) were added to the model with age, lean mass, and current estrogen use, the mean bone mineral density at the femoral neck (P = 0.02), lumbar spine (P = 0.02), and total body (P < 0.001) was significantly higher in African-American women compared with Caucasian women.
Table 4.
Multiply Adjusted Mean Bone Mineral Density Levels for African-American and Caucasian Women, San Diego, California, 1992–1998a,b,c
| African-American Women |
Caucasian Women |
R2 | |||
| Mean | 95% Confidence Interval | Mean | 95% Confidence Interval | ||
| Femoral neck bone mineral density, g/cm2 | |||||
| Model 1 (age) | 0.807 | 0.791, 0.822 | 0.723*** | 0.695, 0.749 | 0.08 |
| Model 2 (age, lean mass) | 0.805 | 0.792, 0.817 | 0.755*** | 0.732, 0.777 | 0.24 |
| Model 3 (age, fat mass) | 0.808 | 0.795, 0.819 | 0.748*** | 0.725, 0.770 | 0.22 |
| Model 4 (age, weight) | 0.799 | 0.783, 0.813 | 0.747** | 0.719, 0.774 | 0.13 |
| Model 5 (age, lean mass, current ERT) | 0.810 | 0.792, 0.827 | 0.760** | 0.732, 0.788 | 0.19 |
| Model 6 (age, fat mass, current ERT) | 0.813 | 0.796, 0.830 | 0.753*** | 0.726, 0.780 | 0.19 |
| Model 7 (age, weight, current ERT) | 0.805 | 0.787, 0.823 | 0.760* | 0.731, 0.789 | 0.19 |
| Model 8 (age, lean mass, covariates) | 0.808 | 0.790, 0.826 | 0.765* | 0.735, 0.793 | 0.20 |
| Total hip bone mineral density, g/cm2 | |||||
| Model 1 (age) | 0.900 | 0.884, 0.915 | 0.818*** | 0.790, 0.845 | 0.07 |
| Model 2 (age, lean mass) | 0.898 | 0.886, 0.910 | 0.855*** | 0.833, 0.876 | 0.27 |
| Model 3 (age, fat mass) | 0.901 | 0.889, 0.913 | 0.846*** | 0.823, 0.868 | 0.24 |
| Model 4 (age, weight) | 0.891 | 0.875, 0.906 | 0.843** | 0.814, 0.870 | 0.13 |
| Model 5 (age, lean mass, current ERT) | 0.901 | 0.884, 0.917 | 0.867* | 0.840, 0.893 | 0.20 |
| Model 6 (age, fat mass, current ERT) | 0.905 | 0.888, 0.921 | 0.858** | 0.831, 0.884 | 0.19 |
| Model 7 (age, weight, current ERT) | 0.896 | 0.877, 0.914 | 0.863 | 0.834, 0.892 | 0.18 |
| Model 8 (age, lean mass, covariates) | 0.899 | 0.881, 0.915 | 0.870 | 0.842, 0.898 | 0.21 |
| Spine bone mineral density, g/cm2 | |||||
| Model 1 (age) | 1.000 | 0.980, 1.019 | 0.963** | 0.949, 0.975 | 0.03 |
| Model 2 (age, lean mass) | 0.985 | 0.967, 1.003 | 0.976 | 0.964, 0.988 | 0.13 |
| Model 3 (age, fat mass) | 0.992 | 0.973, 1.010 | 0.973 | 0.960, 0.984 | 0.10 |
| Model 4 (age, weight) | 0.975 | 0.955, 0.995 | 0.974 | 0.961, 0.986 | 0.07 |
| Model 5 (age, lean mass, current ERT) | 1.016 | 0.992, 1.039 | 0.992 | 0.977, 1.006 | 0.11 |
| Model 6 (age, fat mass, current ERT) | 1.023 | 0.999, 1.046 | 0.989* | 0.975, 1.003 | 0.09 |
| Model 7 (age, weight, current ERT) | 1.000 | 0.976, 1.026 | 0.992 | 0.977, 1.026 | 0.08 |
| Model 8 (age, lean mass, covariates) | 1.024 | 0.999, 1.049 | 0.989* | 0.974, 1.003 | 0.13 |
| Total body bone mineral density, g/cm2 | |||||
| Model 1 (age) | 1.064 | 1.049, 1.079 | 1.029*** | 1.018, 1.039 | 0.11 |
| Model 2 (age, lean mass) | 1.073 | 1.062, 1.083 | 1.038*** | 1.032, 1.045 | 0.37 |
| Model 3 (age, fat mass) | 1.076 | 1.065, 1.086 | 1.037*** | 1.030, 1.043 | 0.35 |
| Model 4 (age, weight) | 1.048 | 1.032, 1.063 | 1.036*** | 1.026, 1.046 | 0.14 |
| Model 5 (age, lean mass, current ERT) | 1.084 | 1.070, 1.096 | 1.050*** | 1.041, 1.057 | 0.32 |
| Model 6 (age, fat mass, current ERT) | 1.086 | 1.073, 1.098 | 1.049*** | 1.041, 1.056 | 0.32 |
| Model 7 (age, weight, current ERT) | 1.063 | 1.047, 1.080 | 1.051 | 1.041, 1.060 | 0.20 |
| Model 8 (age, lean mass, covariates) | 1.083 | 1.069, 1.096 | 1.049*** | 1.041, 1.057 | 0.32 |
Abbreviation: ERT, estrogen replacement therapy; WHO, World Health Organization.
* P = 0.05; **P = 0.01; ***P = 0.001 (significant difference between groups).
Generalized linear regression modeling was calculated to estimate these results.
Model covariates are current smoking, drinking alcohol ≥3 times/week, exercising ≥3 times/week, thyroid medication use, calcium use, and diabetes (WHO 1999 criteria).
Respective numbers of African-American and Caucasian women for both femoral neck and total hip bone mineral density are 440 and 147; for spine bone mineral density, they are 439 and 967; and for total body bone mineral density, 436 and 918.
The overall amount of variation in bone mineral density explained by all the independent variables (R2) varied across the models but was consistently higher in models containing lean mass and age as covariates compared with models containing fat mass or weight (Table 4). When current estrogen use and other covariates were added to the models containing age and either weight, lean mass, or fat mass, the total variation in bone mineral density explained by the models (R2) containing lean mass, fat mass, and weight showed little change, and the goodness-of-fit of the models (R2) was not improved. Additional analyses using body mass index, percent body fat, waist circumference, or waist/hip ratio as the body size or composition variable in the models explained less of the overall variation in bone mineral density compared with models with lean mass as a covariate (data not shown).
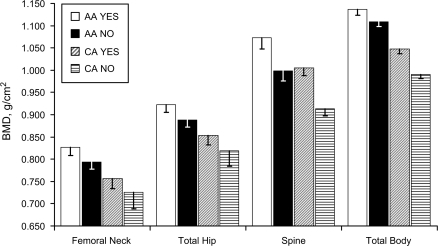
After adjustment for age and lean mass, African-American women who were current estrogen users had higher mean bone mineral density at all bone measurement sites compared with African-American women who were nonusers of estrogen (all P < 0.01) (Figure 1). Caucasian women who currently used estrogen had higher mean bone mineral density at the lumbar spine and total body compared with Caucasian nonusers (all P < 0.001) after adjustment for age and lean body mass. Compared with Caucasian women who were currently using estrogen, African-American women who did not use estrogen had higher mean bone mineral density at all bone measurement sites (all P < 0.01). Compared with Caucasian women who were currently using estrogen, African-American women who did not use estrogen had higher mean bone mineral density at all bone measurement sites (all P < 0.01) except at the lumbar spine, although this difference was not statistically significant at the femur sites.
Figure 1.
Age- and lean body mass-adjusted bone mineral density by estrogen use status in African-American and Caucasian women, San Diego, California, 1992–1998. AA, African American; BMD, bone mineral density; CA, Caucasian. White bars, African-American women who used estrogen; black bars, African-American women who did not use estrogen; bars with diagonal lines, Caucasian women who used estrogen; bars with horizontal lines, Caucasian women who did not use estrogen. T-shaped bars, bottom half of 95% confidence interval.
DISCUSSION
It has been estimated that more than 22 million women in the United States aged 50 years or older have low bone density and that 8 million have osteoporosis (12). African-American women have a higher bone mineral density and a 50% lower risk of hip fractures compared with Caucasian women (2, 8, 13). The present study examined the influence of estrogen replacement therapy on the differences in mean bone mineral density between African-American and Caucasian women. African-American women had higher bone mineral density at the femoral neck, total hip, lumbar spine, and total body than did Caucasian women, after adjustment for age and body size. Although differences in bone mineral density were attenuated with the adjustment for estrogen, body composition, and lifestyle covariates, they continued to be significant at the majority of the body sites. Women of both ethnic groups who used estrogen had higher bone mineral density. Moreover, African-American women who did not use estrogen had a higher bone mineral density at the femoral neck, total hip, and total body than did Caucasian women who used estrogen, although these differences were not statistically significant at the femoral neck or total hip.
Consistent with a previous report (2), body size and composition (weight, waist circumference, waist/hip ratio, body mass index, lean mass, fat mass, percent body fat) were higher in African-American women than in Caucasian women. Most body size measures were also lower among estrogen users in both groups of women. The decreased waist/hip ratio in Caucasian women who used estrogen, not observed in African-American women, likely reflects the higher prevalence of diabetes and its associated increase in waist girth and waist/hip ratio in African-American women. Estrogen users and nonusers in both groups did not differ in lean mass.
Only one previous study has reported the role of estrogen in the bone mineral density difference observed between African-American and Caucasian women. In a longitudinal study of older, postmenopausal women, Cauley et al. (5) found that African-American women gained bone mineral density but that some Caucasian women continued to lose bone mineral density while using hormone replacement therapy. African-American women, who tend to be heavier than white women by 10 years of age (14), are likely to have a higher peak bone mineral density than Caucasian women; these differences may be great enough that bone mineral density in African-American women remains higher in older age even in comparison with Caucasian women who have slower age-related bone loss with hormone therapy.
Several previous studies report lower weight and fat mass and no change in lean mass in estrogen users compared with nonusers. To our knowledge, the present study is the first to report this same observation in a large cohort of African-American women. A randomized trial of over 1,000 women of combined estrogen and progestin in Denmark reported that early postmenopausal women randomized to hormone therapy gained less weight than did controls over the 5-year study (15). Differences in weight gain were primarily due to changes in fat mass, with little change in lean mass over time. However, the final absolute body weight in the 2 groups did not differ significantly. A second arm of this clinical trial, in which women self-selected estrogen use, yielded similar results. In a clinical trial including 875 women, Espeland et al. (16) reported less weight gain in the first year in women randomized to hormone therapy but similar weight gain in the second and third years compared with women assigned to the placebo group.
However, a previous publication from the Rancho Bernardo Study reported no effect of estrogen alone or combined with a progestin on body weight or body mass index (17). Among Rancho Bernardo women aged 65–94 years, intermittent and continuous estrogen users had a lower body mass index at baseline than did never users but 15 years later showed no differences by estrogen use in body mass index, changes in weight and body mass index, waist/hip ratio, or fat mass (17). This previous report included an older sample of women (mean age = 76.3), was longitudinal in nature, and assessed fat mass with bioelectric impedance, a less precise method than DXA. In the Women's Health Initiative, women randomized to the combined estrogen and progestin treatment group showed no significant differences in change in weight, body mass index, or fat mass compared with women in the control group, but only women in the control group lost lean body mass over a 5.6-year period (18).
Body size and composition measures are frequent covariates in research on bone mineral density. Although body weight and body mass index are commonly used to represent the association between body size and bone mineral density, Sowers et al. (19) believe these are inexact measures of body fat and may bias the true association of body composition with bone mineral density, especially in subgroups whose fat and lean mass ratios differ from the typical. Numerous studies have attempted to determine whether fat mass or lean mass is the primary determinant of bone mineral density, with conflicting results. Some studies report that both fat mass and lean mass are independent predictors of bone mineral density (2, 6, 20), while others favor one or the other measure as the primary determinant (21–24). In the present study, lean mass explained the largest amount of variation in whole body, total hip, femoral neck, and lumbar spine bone mineral density compared with other body composition covariates, including weight and body mass index, although the variation in bone mineral density in the models with age and fat mass approached those with age and lean mass. This relation remained after adjustment for estrogen and other covariates in the model. Only one other study assessed whether fat mass and lean mass were independent determinants of bone mineral density in African-American women, with significant correlations observed for both fat and lean mass with bone mineral density of the femoral neck and spine (2).
The association of body composition with bone mineral density is compatible with the theory of mechanical loading. In contrast to weight and fat mass, which provide mechanical strain through gravity alone, lean mass provides additional load to the bone through muscular contractions that provide mechanical force through everyday activities of daily living as well as exercise. The lower volume of muscular tissue surrounding the spine compared with the hip may explain the difference in the influence of lean mass on bone mineral density at the spine and hip. However, differences in lean mass between African-American and Caucasian women in this study cannot be explained by differences in exercise, as African-American women were less likely to exercise than Caucasian women. Because the African-American women weigh considerably more than the Caucasian women, they are doing more weight-bearing exercise with each step.
Several limitations to this study should be noted. The African-American sample was not population-based but was recruited to be comparable to the Rancho Bernardo Cohort. Therefore, results may not generalize to the general population of African-American women. Both groups of women had a high socioeconomic status and greater access to health care compared with the general population. This removes some ethnic differences due to differences in diet and life style, but it may not control for differences in childhood and youth that were not assessed. Because participants self-selected estrogen use in the present study, the difference in the body composition measures could be due to factors other than estrogen use. Overweight women experience more severe hot flashes (25), which may have led to greater hormone use in African-American women, but in our study this was not the case. We can only speculate about ethnic differences in body size and age at puberty on the peak bone mineral density prior to menopause or the subsequent rate of change of bone mineral density or body composition measures after menopause. Sample size was reduced in Caucasian women for hip bone mineral density scans to maintain consistency of measurement methods on the same bone densitometer. Thus, the results observed may be an underestimate of the true association.
This study has a number of strengths, including a relatively large cohort of African-American women compared with other US studies with DXA scans. In addition, the comparability of the data was maximized by using identical data collection procedures for the 2 study populations. Self-reported estrogen use was validated with examination of the participant's prescriptions brought into the clinic for that purpose. Body composition variables were assessed by using state-of-the-art DXA scans rather than less precise older methods, such as bioelectric impedance.
In conclusion, the higher bone mineral density observed in African-American women compared with Caucasian women was expected. However, the difference was significant and suggests that the higher levels of bone mineral density in African-American women were not due to estrogen use. Among the body size and composition measures studied, the parsimonious model of lean mass and age explained the largest amount of variation in bone mineral density, demonstrating that estrogen and many other covariates have minimal effects on bone mineral density. However, given that the best model, which included age and lean mass alone, explained only 13%–37% of the variation in bone mineral density, this emphasizes the need for continued research into other explanatory factors such as genetics, biochemical differences, or unmeasured weight-bearing physical activity in these populations.
Acknowledgments
Author affiliations: San Diego Joint Doctoral Program in Public Health Epidemiology, San Diego State University/University of California, San Diego, California (Susan L. Eskridge); Division of Epidemiology, Department of Family and Preventive Medicine, University of California San Diego, La Jolla, California (Deborah J. Morton, Donna Kritz-Silverstein, Elizabeth Barrett-Connor, Deborah Wingard); and San Diego County Department of Public Health, San Diego, California (Wilma Wooten).
This work was supported by National Institute on Aging grant AG07181 and by National Institute of Diabetes and Digestive and Kidney Diseases grant DK31801.
Conflict of interest: none declared.
Glossary
Abbreviations
- ANCOVA
analysis of covariance
- DXA
dual-energy x-ray absorptiometry
References
- 1.Finkelstein JS, Lee ML, Sowers M, et al. Ethnic variation in bone density in premenopausal and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87(7):3057–3067. doi: 10.1210/jcem.87.7.8654. [DOI] [PubMed] [Google Scholar]
- 2.Perry HM, III, Horowitz M, Morley JE, et al. Aging and bone metabolism in African American and Caucasian women. J Clin Endocrinol Metab. 1996;81(3):1108–1117. doi: 10.1210/jcem.81.3.8772584. [DOI] [PubMed] [Google Scholar]
- 3.Cauley JA, Robbins J, Chen Z, et al. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women's Health Initiative randomized trial. JAMA. 2003;290(13):1729–1738. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 4.Wallace LS, Ballard JE, Holiday DB, et al. Comparison between 60 matched pairs of postmenopausal black and white women: analysis of risk factors related to bone mineral density. Maturitas. 2005;52(3-4):356–363. doi: 10.1016/j.maturitas.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Cauley JA, Lui LY, Stone KL, et al. Longitudinal study of changes in hip bone mineral density in Caucasian and African-American women. J Am Geriatr Soc. 2005;53(2):183–189. doi: 10.1111/j.1532-5415.2005.53101.x. [DOI] [PubMed] [Google Scholar]
- 6.Taaffe DR, Cauley JA, Danielson M, et al. Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res. 2001;16(7):1343–1352. doi: 10.1359/jbmr.2001.16.7.1343. [DOI] [PubMed] [Google Scholar]
- 7.Barrett-Connor E, Siris ES, Wehren LE, et al. Osteoporosis and fracture risk in women of different ethnic groups. J Bone Miner Res. 2005;20(2):185–194. doi: 10.1359/JBMR.041007. [DOI] [PubMed] [Google Scholar]
- 8.Finkelstein JS, Sowers M, Greendale GA, et al. Ethnic variation in bone turnover in pre- and early perimenopausal women: effects of anthropometric and lifestyle factors. J Clin Endocrinol Metab. 2002;87(7):3051–3056. doi: 10.1210/jcem.87.7.8480. [DOI] [PubMed] [Google Scholar]
- 9.Criqui MH, Barrett-Connor E, Austin M. Differences between respondents and non-respondents in a population-based cardiovascular disease study. Am J Epidemiol. 1978;108(5):367–372. doi: 10.1093/oxfordjournals.aje.a112633. [DOI] [PubMed] [Google Scholar]
- 10.Afghani A, Barrett-Connor E, Wooten WJ. Resting energy expenditure: a better marker than BMI for BMD in African-American women. Med Sci Sports Exerc. 2005;37(7):1203–1210. doi: 10.1249/01.mss.0000170080.87526.85. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, Switzerland: World Health Organization; 1999. [Google Scholar]
- 12.National Osteoporosis Foundation. America's Bone Health: The State of Osteoporosis and Low Bone Mass in Our Nation. Washington, DC: National Osteoporosis Foundation; 2002. [Google Scholar]
- 13.Farmer ME, White LR, Brody JA, et al. Race and sex differences in hip fracture incidence. Am J Public Health. 1984;74(12):1374–1380. doi: 10.2105/ajph.74.12.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimm SY, Barton BA, Obarzanek E, et al. Racial divergence in adiposity during adolescence: the NHLBI Growth and Health Study [electronic article] Pediatrics. 2001;107(3):E34. doi: 10.1542/peds.107.3.e34. [DOI] [PubMed] [Google Scholar]
- 15.Jensen LB, Vestergaard P, Hermann AP, et al. Hormone replacement therapy dissociates fat mass and bone mass, and tends to reduce weight gain in early postmenopausal women: a randomized controlled 5-year clinical trial of the Danish Osteoporosis Prevention Study. J Bone Miner Res. 2003;18(2):333–342. doi: 10.1359/jbmr.2003.18.2.333. [DOI] [PubMed] [Google Scholar]
- 16.Espeland MA, Stefanick ML, Kritz-Silverstein D, et al. Effect of postmenopausal hormone therapy on body weight and waist and hip girths. Postmenopausal Estrogen-Progestin Interventions Study Investigators. J Clin Endocrinol Metab. 1997;82(5):1549–1556. doi: 10.1210/jcem.82.5.3925. [DOI] [PubMed] [Google Scholar]
- 17.Kritz-Silverstein D, Barrett-Connor E. Long-term postmenopausal hormone use, obesity, and fat distribution in older women. JAMA. 1996;275(1):46–49. [PubMed] [Google Scholar]
- 18.Chen Z, Bassford T, Green SB, et al. Postmenopausal hormone therapy and body composition—a substudy of the estrogen plus progestin trial of the Women's Health Initiative. Am J Clin Nutr. 2005;82(3):651–656. doi: 10.1093/ajcn.82.3.651. [DOI] [PubMed] [Google Scholar]
- 19.Sowers MF, Kshirsagar A, Crutchfield MM, et al. Joint influence of fat and lean body composition compartments on femoral bone mineral density in premenopausal women. Am J Epidemiol. 1992;136(3):257–265. doi: 10.1093/oxfordjournals.aje.a116491. [DOI] [PubMed] [Google Scholar]
- 20.Khosla S, Atkinson EJ, Riggs BL, et al. Relationship between body composition and bone mass in women. J Bone Miner Res. 1996;11(6):857–863. doi: 10.1002/jbmr.5650110618. [DOI] [PubMed] [Google Scholar]
- 21.Arabi A, Garnero P, Porcher R, et al. Changes in body composition during post-menopausal hormone therapy: a 2 year prospective study. Hum Reprod. 2003;18(8):1747–1752. doi: 10.1093/humrep/deg331. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, Lohman TG, Stini WA, et al. Fat or lean tissue mass: which one is the major determinant of bone mineral mass in healthy postmenopausal women? J Bone Miner Res. 1997;12(1):144–151. doi: 10.1359/jbmr.1997.12.1.144. [DOI] [PubMed] [Google Scholar]
- 23.Compston JE, Bhambhani M, Laskey MA, et al. Body composition and bone mass in post-menopausal women. Clin Endocrinol (Oxf) 1992;37(5):426–431. doi: 10.1111/j.1365-2265.1992.tb02354.x. [DOI] [PubMed] [Google Scholar]
- 24.Reid IR, Ames R, Evans MC, et al. Determinants of total body and regional bone mineral density in normal postmenopausal women—a key role for fat mass. J Clin Endocrinol Metab. 1992;75(1):45–51. doi: 10.1210/jcem.75.1.1619030. [DOI] [PubMed] [Google Scholar]
- 25.Gallicchio L, Visvanathan K, Miller SR, et al. Body mass, estrogen levels, and hot flashes in midlife women. Am J Obstet Gynecol. 2005;193(4):1353–1360. doi: 10.1016/j.ajog.2005.04.001. [DOI] [PubMed] [Google Scholar]