Abstract
The authors examined the associations of toenail selenium levels with blood concentrations of fibrinogen, high-sensitivity C-reactive protein (hs-CRP), and interleukin-6 (IL-6) in an 18-year follow-up study comprising 4,032 Americans aged 20–32 years at baseline (1987) from the Coronary Artery Risk Development in Young Adults (CARDIA) Trace Element Study. Toenail samples were collected in 1987, and selenium concentrations were measured by means of instrumental neutron-activation analysis. Fibrinogen level was analyzed in 1990, 1992, and 2005; hs-CRP was assessed in 1992, 2000, and 2005; and IL-6 was measured in 2005. After adjustment for potential confounders, no statistically significant associations between toenail selenium levels and any of the 3 inflammatory biomarkers were documented. Comparing the highest quintile of toenail selenium level with the lowest, odds ratios for elevated levels of fibrinogen (>460 mg/mL), hs-CRP (>3 μg/mL), and IL-6 (>3.395 pg/mL, 80th percentile) were 1.03 (95% confidence interval (CI): 0.77, 1.38; P for trend = 0.76), 1.02 (95% CI: 0.83, 1.27; P for trend = 0.92), and 0.98 (95% CI: 0.71, 1.36; P for trend = 0.82), respectively. Gender, race/ethnicity, smoking status, and selenium supplementation did not appreciably modify these results. This study found no associations between toenail selenium and inflammation as measured by fibrinogen, hs-CRP, and IL-6.
Keywords: C-reactive protein, fibrinogen, inflammation, interleukin-6, nails, selenium
The recognition of selenium's role in preventing oxidative damage has ignited research into the association between selenium and various chronic inflammatory conditions. Serum selenium level was observed to be inversely associated with rheumatoid factor-negative arthritis in a large case-control study nested in a Finnish cohort (1). Selenium was also found to be protective against other possible inflammation-related diseases, including asthma (2) and pancreatitis (3), in the United Kingdom. In addition, low selenium levels have been found to be associated with some autoimmune conditions, such as autoimmune hepatitis and type 1 diabetes (4, 5). Thus, it has been hypothesized that selenium, incorporated into selenoproteins as an antioxidant, can lower levels of inflammatory biomarkers (6).
However, data directly relating selenium to inflammatory markers or cardiovascular disease risk factors are sparse. Investigators from the Women's Health and Aging Study I (WHAS I) reported an inverse correlation between serum selenium levels and interleukin-6 (IL-6) levels in disabled elderly women (7). A study using data from the Third National Health and Nutrition Examination Survey (NHANES III) also found an inverse association between blood selenium and C-reactive protein (8). In addition, another cross-sectional study found that intake of selenium was associated with lower levels of high-sensitivity C-reactive protein (hs-CRP) in women (9). In contrast, in a randomized trial, Stranges et al. (10) found that selenium supplementation of 200 μg/day increased the risk of type 2 diabetes mellitus, which may be linked to inflammatory markers (11). Further, in a recent large-scale, randomized, placebo-controlled trial, Lippman et al. (12) found no association between selenium supplementation of 200 μg /day and cardiovascular endpoints. Notably, prospective epidemiologic data on the long-term association between selenium and biomarkers of inflammation are lacking.
Toenail selenium level is recognized as a reliable biomarker reflecting relatively long-term selenium status (13, 14). As inflammatory biomarkers, hs-CRP and IL-6 have been widely used, not only in experimental studies but also in epidemiologic studies, for predicting atherosclerosis and cardiovascular disease (15, 16). Fibrinogen is an established clotting factor and is also considered a marker for chronic low-grade inflammation (17, 18). Our purpose in this study was to examine the long-term associations between toenail selenium levels and levels of 3 available inflammatory biomarkers—fibrinogen, hs-CRP, and IL-6—in the Coronary Artery Risk Development in Young Adults (CARDIA) Trace Element Study.
MATERIALS AND METHODS
Study population
The CARDIA cohort was established in 1985, when 5,115 American young adults aged 18–30 years participated in a study of psychological and lifestyle factors that might affect the evolution of coronary artery disease risk. Details of the study design have been published elsewhere (19). Briefly, the cohort was roughly balanced by age (<25 years, ≥25 years), gender, education (≤high school, >high school), and race/ethnicity (African-American, Caucasian) and was recruited from 4 US metropolitan areas (Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California). Six follow-up examinations have been conducted, in 1987, 1990, 1992, 1995, 2000, and 2005. Approximately 70% of participants in the original cohort returned for the sixth visit in 2005, and follow-up rates averaged 94% in each examination cycle. In the present study, 1987 was considered the baseline date, since toenail specimens were collected in that year. A total of 4,624 participants attended the 1987 examination, of which 4,362 (94.3%) provided toenail clippings. In this analysis, we excluded participants who had missing data on toenail weight (n = 75) and those who had an extremely high level of toenail selenium (≥2 μg/g; n = 28), since, based on our laboratory experience, this high concentration is probably due to exogenous contamination. We also excluded participants who had no data on any of the 3 biomarkers of inflammation (n = 222) and those who had arthritis (n = 5). After these baseline exclusions, 4,032 participants were included in this analysis.
All participants gave informed consent. The study design, data collection, and analyses were approved by the institutional review boards of the participating institutions.
Analysis of exposure
Toenail clippings were collected from all 10 toes by the participants themselves with a stainless steel clipper during the clinical examination. All toenail clippings were processed with a washing procedure in a sonicator with deionized water. Selenium levels were analyzed by means of instrumental neutron-activation analysis at the University of Missouri Research Reactor (20). Toenail specimens were treated in random order by the laboratory personnel, who were blinded to other clinical measures. In the present study, the average coefficient of variation in duplicate subsamples for the toenail selenium measurement was 2.45%.
Animal studies suggest that toenail selenium level is best suited for measuring skeletal muscle and heart selenium status. The correlations between toenail selenium and skeletal muscle and heart selenium content were 0.75 and 0.76, respectively (21). In addition, toenail selenium concentration from a single sample represents exposure over a period of 9–12 months (22, 23). The Spearman correlation coefficient for the reproducibility of toenail selenium over a period of 6 years has been shown to be 0.48 (24). In a pilot study we conducted in 2007 among 64 randomly selected CARDIA participants from Chicago field center, the Spearman correlation coefficient for the correlation between toenail selenium concentrations in toenail clippings collected 20 years apart and measured by the same laboratory with the same protocol was 0.56.
Measurements of inflammatory biomarkers
Fibrinogen was measured 3 times, in 1990, 1992, and 2005. In 1990, fibrinogen was assessed via the Clauss method using reagents from the Dade Behring division of Baxter Healthcare Corporation (Dade Behring Inc., Deerfield, Illinois) (25). In 1992 and 2005, fibrinogen antigen was measured using the BN-II nephelometer (N Antiserum to Human Fibrinogen; Dade Behring Inc.) and calibrated with standard normal plasma (SNP reagent; Dade Behring Inc.), which was also selected for consistency with the previously utilized Claus method (18, 26).
Serum hs-CRP was measured in blood samples collected in 1992, 2000, and 2005 using a new enzyme-linked immunosorbent assay method improved with a nephelometry-based high-throughput assay that offers greater sensitivity and reproducibility (27). IL-6 was measured in 2005 only with a high-sensitivity enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, Minnesota) (28).
Assessment of covariates
Data on demographic variables were collected via questionnaire. Smoking status was determined by self-report at each examination and was classified into 3 groups: current, former, and never smokers. Alcohol consumption was assessed with a self-administered questionnaire and as part of a dietary interview at each examination. Weight and height were directly measured while the participant stood in light clothes without shoes. Body mass index (weight (kg)/height (m)2) was classified into 5 groups: <21, 21–22.9, 23–24.9, 25–29.9, and ≥30 (29). At each examination, blood pressure was measured on the right arm with a random-zero sphygmomanometer after the participant had rested for 5 minutes. Systolic and diastolic blood pressures were recorded as phase I and phase V Korotkoff sounds. Physical activity was assessed using the interview-based, validated CARDIA Physical Activity History Questionnaire (30). A score of 100 exercise units is approximately equivalent to participation in vigorous activity for 2–3 hours per week during 6 months of the year. Diet was assessed by means of the validated CARDIA Diet History Questionnaire at the 1985, 1992, and 2005 examinations. We also took data on supplement use into account to assess intakes of related micronutrients and vitamins.
Statistical analysis
Participants were divided into quintiles according to their toenail selenium levels. Group comparisons of characteristics at baseline were performed using analysis of variance, the Kruskal-Wallis test, or the chi-squared test as appropriate.
Binary variables for all 3 inflammatory markers were created. The cutoff values for fibrinogen (>460 mg/L) and hs-CRP (>3 μg/L) were determined on the basis of the literature (31, 32). The generalized estimating equations method with logit linkage was used to calculate multivariable-adjusted prospective odds ratios and 95% confidence intervals for elevated levels of fibrinogen or hs-CRP. Since there is no commonly used cutoff value for IL-6, we defined this binary variable by using its 80th percentile. A logistic regression model was used to estimate odds ratios and 95% confidence intervals for elevated IL-6 level. We considered 3 sequential models in the analysis of each inflammatory marker: Model 1 adjusted for age, gender, race/ethnicity, and study center; model 2 additionally adjusted for education, smoking status, alcohol consumption, physical activity, body mass index, and toenail mass; and model 3 additionally adjusted for baseline dietary intake of vitamin C, folic acid, zinc, vitamin E, and β-carotene. We created an ordinal variable for selenium using the median level of toenail selenium in each quintile for the trend test.
We also considered continuous outcomes for the 3 markers. Box-Cox transformation was used to improve the normality of the distributions of fibrinogen, hs-CRP, and IL-6. Because fibrinogen and hs-CRP were measured multiple times, generalized estimating equations were used to examine the associations between selenium levels and these 2 inflammatory biomarkers; since IL-6 was measured once, the general linear model was used. The sequential modeling strategy described above was used in all models. In addition, we used a nonparametric spline in sensitivity analysis to detect whether there was any “threshold” association.
All analyses were performed using SAS, version 9.1.3 (SAS Institute Inc., Cary, North Carolina). P ≤ 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of the 4,032 study participants are shown in Table 1. The median toenail selenium levels across quintiles were 0.69, 0.78, 0.84, 0.91, and 1.04 μg/g. As compared with participants in the lowest quintile of selenium level, those in the highest quintile were slightly older and were more likely to be female, Caucasian, and non-current smokers. They also had a higher educational level and higher intakes of vitamin C, folic acid, vitamin E, β-carotene, and zinc but had lower alcohol consumption and lower body mass index. Toenail selenium levels varied geographically by study center. Selenium levels were relatively lower in males, African Americans, current smokers, and participants with high alcohol consumption (Table 1).
Table 1.
Baseline Characteristics of American Young Adults According to Quintile of Toenail Selenium Level, CARDIA Trace Element Study, 1987–2005
| Characteristic | Quintile of Toenail Selenium Level |
Total | P Valuea | ||||
| 1 | 2 | 3 | 4 | 5 | |||
| No. of participants | 808 | 806 | 814 | 803 | 801 | 4,032 | |
| Selenium level, μg/g | |||||||
| Median | 0.69 | 0.78 | 0.84 | 0.91 | 1.04 | 0.84 | |
| Range | 0.479–0.737 | 0.738–0.807 | 0.808–0.877 | 0.878–0.960 | 0.961–1.978 | 0.479–1.978 | |
| Mean age, years (SD) | 26.8 (3.6) | 26.8 (3.6) | 27.0 (3.6) | 27.2 (3.7) | 27.2 (3.6) | 27.0 (3.6) | 0.08 |
| Female gender, % | 40.1 | 49.4 | 53.7 | 62.5 | 69.5 | 55.0 | <0.01 |
| Black race/ethnicity, % | 60.9 | 50.1 | 45.6 | 46.3 | 37.0 | 48.0 | <0.01 |
| Mean education, years (SD) | 13.6 (2.2) | 14.1 (2.4) | 14.3 (2.3) | 14.2 (2.4) | 14.5 (2.4) | 14.2 (2.3) | <0.01 |
| Current smoker, % | 45.1 | 30.7 | 27.6 | 22.7 | 17.7 | 28.8 | <0.01 |
| Selenium supplementation, % | 27.0 | 29.0 | 31.8 | 29.0 | 33.6 | 30.1 | 0.03 |
| Alcohol intake, mL/day | |||||||
| Median | 12.2 | 6.8 | 6.1 | 6.1 | 5.1 | 6.4 | <0.01 |
| IQRb | 3.0–27.1 | 0–17.6 | 0–15.6 | 0–16.2 | 0–14.2 | 0–17.6 | |
| Physical activity scorec | |||||||
| Median | 332.0 | 306.5 | 323.5 | 307.0 | 317.0 | 318.0 | 0.32 |
| IQR | 171.0–580.0 | 170.0–528.0 | 174.0–534.0 | 158.0–516.0 | 167.0–507.0 | 168.0–531.0 | |
| Dietary intake, mg/1,000 kcal/day | |||||||
| Vitamin C | |||||||
| Median | 55.1 | 63.1 | 68.2 | 69.8 | 75.0 | 66.4 | <0.01 |
| IQR | 37.7–87.6 | 40.7–98.6 | 45.6–108.5 | 45.4–110.1 | 48.7–121.5 | 42.7–104.1 | |
| Folic acid | |||||||
| Median | 110.7 | 119.3 | 124.2 | 129.1 | 128.4 | 122.5 | <0.01 |
| IQR | 86.5–153.1 | 89.1–178.8 | 95.7–183.3 | 92.8–199.5 | 97.4–221.4 | 91.9–185.8 | |
| Vitamin E | |||||||
| Median | 3.6 | 3.8 | 3.7 | 4.1 | 4.2 | 3.9 | <0.01 |
| IQR | 2.8–4.6 | 3.0–5.3 | 3.0–5.4 | 3.1–6.1 | 3.2–6.4 | 3.0–5.5 | |
| β-carotene | |||||||
| Median | 963.1 | 1,020.9 | 1,094.7 | 1,148.2 | 1,209.1 | 1,070.6 | <0.01 |
| IQR | 619.7–1,496.5 | 654.3–1,714.7 | 727.8–1,816.9 | 710.6–2,123.5 | 733.5–2,137.8 | 687.9–1,805.8 | |
| Zinc (mean) (SD) | 6.8 (3.5) | 7.1 (4.0) | 7.0 (3.3) | 7.6 (8.2) | 7.7 (7.1) | 7.2 (5.6) | <0.01 |
| Mean body mass indexd (SD) | 25.4 (5.2) | 25.3 (5.1) | 25.1 (5.2) | 25.4 (5.8) | 24.6 (5.1) | 25.2 (5.3) | 0.02 |
| Mean toenail mass, mg (SD) | 23.7 (6.8) | 23.5 (6.7) | 23.8 (6.6) | 23.7 (6.3) | 24.0 (6.3) | 23.7 (6.5) | 0.51 |
Abbreviations: CARDIA, Coronary Artery Risk Development in Young Adults; IQR, interquartile range; SD, standard deviation.
P for any difference across the quintiles of toenail selenium level, obtained using analysis of variance, the Kruskal-Wallis test, or the chi-squared test as appropriate.
25th–75th percentiles.
Physical activity was assessed using the CARDIA Physical Activity History Questionnaire (30).
Weight (kg)/height (m)2.
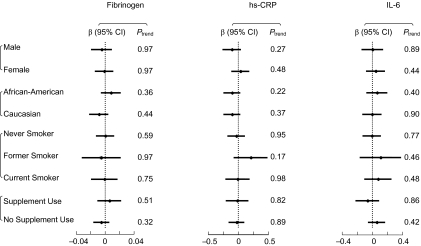
In the logistic regression analysis for binary outcomes of elevated inflammatory marker levels, the multivariable-adjusted odds ratios were 1.03 (95% confidence interval: 0.77, 1.38; P for trend = 0.76) for fibrinogen, 1.02 (95% confidence interval: 0.83, 1.27; P for trend = 0.92) for hs-CRP, and 0.98 (95% confidence interval: 0.71, 1.36; P for trend = 0.82) for IL-6, comparing the highest quintile of selenium level with the lowest (Table 2). Gender, race/ethnicity, smoking status, and supplement use did not appreciably modify the observed null associations (Figure 1). We also considered the 3 markers as continuous outcomes and calculated multivariable-adjusted regression coefficients and their 95% confidence intervals. Consistently, no statistically significant associations were found (data not shown).
Table 2.
Multivariable-Adjusted Odds Ratios for Elevated Levels of Inflammation Biomarkers by Quintile of Toenail Selenium Level, CARDIA Trace Element Study, 1987–2005a
| Inflammation Biomarker | Quintile of Toenail Selenium Level |
P for Trendb | ||||||||
| 1 | 2 |
3 |
4 |
5 |
||||||
| (Referent) | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Fibrinogen (>460 mg/dL) | ||||||||||
| No. of participants | 798 | 799 | 809 | 797 | 798 | |||||
| No. of events | 170 | 163 | 172 | 168 | 168 | |||||
| Model 1c | 1.00 | 1.16 | 0.90, 1.50 | 1.01 | 0.78, 1.31 | 1.02 | 0.77, 1.34 | 1.03 | 0.78, 1.36 | 0.82 |
| Model 2d | 1.00 | 1.29 | 0.99, 1.67 | 1.10 | 0.84, 1.43 | 1.13 | 0.85, 1.51 | 1.02 | 0.76, 1.37 | 0.70 |
| Model 3e | 1.00 | 1.28 | 0.99, 1.67 | 1.12 | 0.85, 1.46 | 1.15 | 0.86, 1.53 | 1.03 | 0.77, 1.38 | 0.76 |
| High-sensitivity C-reactive protein (>3 μg/mL) | ||||||||||
| No. of participants | 770 | 776 | 780 | 762 | 781 | |||||
| No. of events | 339 | 318 | 332 | 318 | 309 | |||||
| Model 1 | 1.00 | 1.11 | 0.92, 1.34 | 1.00 | 0.82, 1.22 | 1.06 | 0.87, 1.30 | 1.14 | 0.93, 1.40 | 0.30 |
| Model 2 | 1.00 | 1.13 | 0.93, 1.38 | 0.97 | 0.79, 1.19 | 1.06 | 0.86, 1.30 | 1.03 | 0.83, 1.28 | 0.99 |
| Model 3 | 1.00 | 1.13 | 0.93, 1.37 | 0.97 | 0.79, 1.18 | 1.04 | 0.85, 1.28 | 1.02 | 0.83, 1.27 | 0.92 |
| Interleukin-6 (>3.395 pg/mL, 80th percentile) | ||||||||||
| No. of participants | 572 | 628 | 613 | 628 | 638 | |||||
| No. of events | 134 | 118 | 137 | 104 | 123 | |||||
| Model 1 | 1.00 | 0.83 | 0.62, 1.11 | 1.10 | 0.82, 1.46 | 0.72 | 0.53, 0.99 | 0.88 | 0.65, 1.21 | 0.34 |
| Model 2 | 1.00 | 0.83 | 0.62, 1.12 | 1.14 | 0.85, 1.54 | 0.75 | 0.54, 1.04 | 0.97 | 0.70, 1.35 | 0.79 |
| Model 3 | 1.00 | 0.84 | 0.62, 1.14 | 1.15 | 0.85, 1.55 | 0.77 | 0.55, 1.06 | 0.98 | 0.71, 1.36 | 0.82 |
Abbreviations: CARDIA, Coronary Artery Risk Development in Young Adults; CI, confidence interval; OR, odds ratio.
Generalized estimating equations with binary outcomes were used for repeated measurements of fibrinogen and high-sensitivity C-reactive protein; a logistic regression model was used for interleukin-6.
An ordinal selenium variable using the median toenail selenium level in each quintile was created for the trend tests.
Adjusted for age, gender (male, female), race/ethnicity (African-American, Caucasian), and study center.
Additionally adjusted for education (≤12, 13–15, or ≥16 years), smoking status (never smoker, former smoker, or current smoker), alcohol consumption (0, 0.1–11.9, 12.0–23.9, or ≥24 mL/day), physical activity score (quintiles), body mass index (weight (kg)/height (m)2; <21, 21–22.9, 23–24.9, 25–29.9, ≥30), and toenail mass (mg; quintiles).
Additionally adjusted for intakes (quintiles) of vitamin C, folic acid, zinc, vitamin E, and β-carotene at baseline.
Figure 1.
Relations between toenail selenium concentration (μg/g) and levels of inflammatory biomarkers (fibrinogen (mg/dL), high-sensitivity C-reactive protein (hs-CRP; μg/mL), and interleukin-6 (IL-6; pg/mL)), determined by comparing the highest quintile of toenail selenium level with the lowest, by gender, race/ethnicity, smoking status, and use of selenium supplements, CARDIA Trace Element Study, 1987–2005. Results were adjusted for the covariates listed for model 3 in Table 2. Bars, 95% confidence interval (CI).
We conducted sensitivity analyses to test the robustness of our findings (data not shown). First, when we excluded participants with self-reported asthma (n = 49), allergic disease (n = 5), and acute pancreatitis (n = 1) at baseline, the results remained the same. Second, when we further excluded those who reported thyroid problems (hyperthyroidism, hypothyroidism, goiter, or other thyroid problems; n = 71), the results were not appreciably changed. Third, the results were similar when we additionally adjusted for intake of long-chain omega-3 polyunsaturated fatty acids in the model; previous studies had suggested a beneficial effect of these fatty acids on inflammation (33) and a possible interaction between these fatty acids and selenium (34). In addition, there was no significant interaction between intake of omega-3 fatty acids or fish and toenail selenium in relation to any of the 3 inflammatory biomarkers. Finally, because we had multiple measurements of fibrinogen and hs-CRP, we examined the associations by separately relating toenail selenium levels to fibrinogen/hs-CRP measured at different time points; the null associations remained consistent, and no secular trend was found.
DISCUSSION
In this large prospective cohort study, we found no significant long-term associations between toenail selenium levels and blood concentrations of fibrinogen, hs-CRP, and IL-6 among healthy American young adults. Gender, race/ethnicity, smoking status, and selenium supplementation did not materially modify these results.
Our findings are unlikely to be explained by low statistical power, because 4,032 participants and the use of continuous outcomes guaranteed approximately 99% power to detect a 0.05-unit change in each inflammatory biomarker when comparing the 2 extreme quintiles of selenium level with a standard deviation of 0.2 and a 2-tailed, Bonferroni correction alpha level of 0.005. Our results are also unlikely to have been substantially biased by confounders, since we used biomarkers for both exposure and outcomes and sequentially adjusted for a number of potential confounders, including major lifestyle, dietary, and nondietary factors. However, the possibility of residual confounding and confounding from unmeasured factors cannot be excluded because of the nature of observational studies. In addition, selenium level was measured in toenails in this study. Toenail selenium is recognized as the best indicator of relatively long-term selenium exposure compared with other biomarkers such as blood selenium. The range of toenail selenium levels in this study was 0.48–1.98 μg/g, which provided wide variation and enabled us to examine any possible association between selenium and inflammatory biomarkers. Moreover, one may observe a null association if the exposure variation falls within a flat portion of a nonlinear relation (i.e., threshold). However, the likelihood of this is low, since there was no “threshold” phenomenon detected in a nonparametric spline analysis. Furthermore, the consistent results across all 3 available inflammatory biomarkers and from sensitivity analyses strengthened our study and increased the robustness of our findings.
In addition to the inherent limitations of observational studies, this study had a few other limitations. First, toenail selenium was measured only at baseline. Thus, we were unable to study the effects of changes in selenium status on inflammatory biomarkers over the 18-year follow-up period. For example, if participants with low selenium levels at baseline increased their intakes of selenium later on, any possible association would be attenuated. However, results were consistent when we further adjusted for, or stratified data according to, selenium supplement use. Further, because of budget and other practical constraints, it is not uncommon for investigators in longitudinal epidemiologic studies to utilize 1 measurement of exposure at baseline with an assumption that this single measurement represents long-term exposure status (35, 36). Second, although good long-term reproducibility of toenail measurements was suggested by 1 published study (24) and by our pilot study, it is possible that random measurement error could prevent the detection of any weak or moderate long-term association. Third, a narrow safety range of selenium intake has been recently suggested (37), while selenium intake is relatively high in the US population (38–40). The possibility cannot be excluded that our null association findings were due to the relatively high selenium intake of this particular population. Selenium intake above a certain level may not increase selenoprotein antioxidant properties (37). Therefore, if the selenium level in the reference group was high enough to provide full antioxidant benefits, further selenium intake might not offer any additional benefits. Finally, because our findings were based on a cohort of American young adults from 4 urban areas, their generalizability may be limited.
In addition, our results were not consistent with findings from the WHAS I (7) and NHANES III (8), which found an inverse association between selenium and IL-6 or C-reactive protein. Notably, these 2 studies measured blood selenium levels, which may reflect a different exposure time frame than toenail selenium. In addition, the WHAS I and NHANES III were cross-sectional studies. The fact that we found no long-term association does not discount the possibility of a short-term relation between selenium and inflammatory biomarkers. Moreover, the inconsistent results may be partially due to the different study populations.
Experimental studies suggest that oxidative stress may promote inflammation (41, 42). Acting as an antioxidant, selenium has been shown to benefit patients with inflammatory diseases (1–4, 43, 44). However, concern has been raised because recent studies have suggested a narrow safety range of selenium intake. A recent large-scale, randomized, placebo-controlled trial found that selenium supplementation did not reduce risk of cardiovascular disease (12). Our study provides further evidence that a high selenium level may not be beneficial for the prevention of cardiovascular disease, even over a long time period. Nevertheless, further studies are warranted to confirm these findings in different populations with different selenium status and to better understand the potential mechanism of action between selenium and inflammatory biomarkers.
In conclusion, this prospective cohort study did not demonstrate any associations between toenail selenium levels and inflammation as measured by fibrinogen, hs-CRP, and IL-6 among apparently healthy American young adults. The null associations remained consistent across subgroups of gender, race/ethnicity, selenium supplement use, and smoking status.
Acknowledgments
Author affiliations: Department of Nutrition, Gillings School of Global Public Health and School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Pengcheng Xun, June Stevens, Ka He); Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Pengcheng Xun, June Stevens, Ka He); Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Kiang Liu, Martha L. Daviglus); Research Reactor Center, University of Missouri-Columbia, Columbia, Missouri (Steven J. Morris); and Department of Epidemiology, School of Public Health, University of Minnesota, Minneapolis, Minnesota (David R. Jacobs, Jr.).
This study was support by National Institutes of Health grants R01HL081572, N01-HC-48047, N01-HC-48048, N01-HC-48049, N01-HC-48050, and N01-HC-95095.
The authors thank Drs. Reis Jared, Ellen Funkhouser, and Deborah Bujnowski for their valuable comments.
Conflict of interest: none declared.
Glossary
Abbreviations
- CARDIA
Coronary Artery Risk Development in Young Adults
- hs-CRP
high-sensitivity C-reactive protein
- IL-6
interleukin-6
- NHANES III
Third National Health and Nutrition Examination Survey
- WHAS I
Women's Health and Aging Study I
References
- 1.Knekt P, Heliövaara M, Aho K, et al. Serum selenium, serum alpha-tocopherol, and the risk of rheumatoid arthritis. Epidemiology. 2000;11(4):402–405. doi: 10.1097/00001648-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Shaheen SO, Sterne JA, Thompson RL, et al. Dietary antioxidants and asthma in adults: population-based case-control study. Am J Respir Crit Care Med. 2001;164(10):1823–1828. doi: 10.1164/ajrccm.164.10.2104061. [DOI] [PubMed] [Google Scholar]
- 3.McCloy R. Chronic pancreatitis at Manchester, UK. Focus on antioxidant therapy. Digestion. 1998;59(suppl 4):36–48. doi: 10.1159/000051441. [DOI] [PubMed] [Google Scholar]
- 4.Pemberton PW, Aboutwerat A, Smith A, et al. Oxidant stress in type I autoimmune hepatitis: the link between necroinflammation and fibrogenesis? Biochim Biophys Acta. 2004;1689(3):182–189. doi: 10.1016/j.bbadis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Ruíz C, Alegría A, Barberá R, et al. Selenium, zinc and copper in plasma of patients with type 1 diabetes mellitus in different metabolic control states. J Trace Elem Med Biol. 1998;12(2):91–95. doi: 10.1016/s0946-672x(98)80031-x. [DOI] [PubMed] [Google Scholar]
- 6.Boosalis MG. The role of selenium in chronic disease. Nutr Clin Pract. 2008;23(2):152–160. doi: 10.1177/0884533608314532. [DOI] [PubMed] [Google Scholar]
- 7.Walston J, Xue Q, Semba RD, et al. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol. 2006;163(1):18–26. doi: 10.1093/aje/kwj007. [DOI] [PubMed] [Google Scholar]
- 8.Ford ES, Liu S, Mannino DM, et al. C-reactive protein concentration and concentrations of blood vitamins, carotenoids, and selenium among United States adults. Eur J Clin Nutr. 2003;57(9):1157–1163. doi: 10.1038/sj.ejcn.1601667. [DOI] [PubMed] [Google Scholar]
- 9.Scheurig AC, Thorand B, Fischer B, et al. Association between the intake of vitamins and trace elements from supplements and C-reactive protein: results of the MONICA/KORA Augsburg Study. Eur J Clin Nutr. 2008;62(1):127–137. doi: 10.1038/sj.ejcn.1602687. [DOI] [PubMed] [Google Scholar]
- 10.Stranges S, Marshall JR, Natarajan R, et al. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147(4):217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 11.Pradhan AD, Manson JE, Rifai N, et al. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 12.Lippman SM, Klein EA, Goodman PJ, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301(1):39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter DJ, Morris JS, Chute CG, et al. Predictors of selenium concentration in human toenails. Am J Epidemiol. 1990;132(1):114–122. doi: 10.1093/oxfordjournals.aje.a115623. [DOI] [PubMed] [Google Scholar]
- 14.Yoshizawa K, Ascherio A, Morris JS, et al. Prospective study of selenium levels in toenails and risk of coronary heart disease in men. Am J Epidemiol. 2003;158(9):852–860. doi: 10.1093/aje/kwg052. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342(12):836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 16.Schieffer B, Selle T, Hilfiker A, et al. Impact of interleukin-6 on plaque development and morphology in experimental atherosclerosis. Circulation. 2004;110(22):3493–3500. doi: 10.1161/01.CIR.0000148135.08582.97. [DOI] [PubMed] [Google Scholar]
- 17.Lee AJ, Smith WC, Lowe GD, et al. Plasma fibrinogen and coronary risk factors: the Scottish Heart Health Study. J Clin Epidemiol. 1990;43(9):913–919. doi: 10.1016/0895-4356(90)90075-z. [DOI] [PubMed] [Google Scholar]
- 18.Thyagarajan B, Jacobs DR, Apostol GG, et al. Plasma fibrinogen and lung function: the CARDIA Study. Int J Epidemiol. 2006;35(4):1001–1008. doi: 10.1093/ije/dyl049. [DOI] [PubMed] [Google Scholar]
- 19.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 20.Morris JS, Stampfer MJ, Willett WC. Dietary selenium in humans: toenails as an indicator. Biol Trace Elem Res. 1983;5(6):529–537. doi: 10.1007/BF02988944. [DOI] [PubMed] [Google Scholar]
- 21.Morris JS, Veatch AE, Spate VL. INAA determination of selenium distribution in castrated and intact dogs [abstract]. Presented at the 11th International Conference on Modern Trends in Activation Analysis. University of Surrey, Guildford, United Kingdom, June 20–25, 2004. [Google Scholar]
- 22.Hulka BS, Wilcosky TC, Griffith JD. Biological Markers in Epidemiology. New York, NY: Oxford University Press; 1990. [Google Scholar]
- 23.Longnecker MP, Stampfer MJ, Morris JS, et al. A 1-y trial of the effect of high-selenium bread on selenium concentrations in blood and toenails. Am J Clin Nutr. 1993;57(3):408–413. doi: 10.1093/ajcn/57.3.408. [DOI] [PubMed] [Google Scholar]
- 24.Garland M, Morris JS, Rosner BA, et al. Toenail trace element levels as biomarkers: reproducibility over a 6-year period. Cancer Epidemiol Biomarkers Prev. 1993;2(5):493–497. [PubMed] [Google Scholar]
- 25.Green D, Ruth KJ, Folsom AR, et al. Hemostatic factors in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Arterioscler Thromb. 1994;14(5):686–693. doi: 10.1161/01.atv.14.5.686. [DOI] [PubMed] [Google Scholar]
- 26.Hozawa A, Jacobs DR, Jr, Steffes MW, et al. Relationships of circulating carotenoid concentrations with several markers of inflammation, oxidative stress, and endothelial dysfunction: the Coronary Artery Risk Development in Young Adults (CARDIA)/Young Adult Longitudinal Trends in Antioxidants (YALTA) Study. Clin Chem. 2007;53(3):447–455. doi: 10.1373/clinchem.2006.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rifai N, Tracy RP, Ridker PM. Clinical efficacy of an automated high-sensitivity C-reactive protein assay. Clin Chem. 1999;45(12):2136–2141. [PubMed] [Google Scholar]
- 28.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 29.He K, Liu K, Daviglus ML, et al. Magnesium intake and incidence of metabolic syndrome among young adults. Circulation. 2006;113(13):1675–1682. doi: 10.1161/CIRCULATIONAHA.105.588327. [DOI] [PubMed] [Google Scholar]
- 30.Pereira MA, FitzerGerald SJ, Gregg EW, et al. A collection of physical activity questionnaires for health-related research. Med Sci Sports Exerc. 1997;29(6 suppl):S1–S205. [PubMed] [Google Scholar]
- 31.Ridker PM, Buring JE, Cook NR, et al. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation. 2003;107(3):391–397. doi: 10.1161/01.cir.0000055014.62083.05. [DOI] [PubMed] [Google Scholar]
- 32.Song IS, Yang WS, Kim SB, et al. Association of plasma fibrinogen concentration with vascular access failure in hemodialysis patients. Nephrol Dial Transplant. 1999;14(1):137–141. doi: 10.1093/ndt/14.1.137. [DOI] [PubMed] [Google Scholar]
- 33.He K, Liu K, Daviglus ML, et al. Intakes of long-chain n-3 polyunsaturated fatty acids and fish in relation to measurements of subclinical atherosclerosis. Am J Clin Nutr. 2008;88(4):1111–1118. doi: 10.1093/ajcn/88.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He K, Liu K, Daviglus ML, et al. Associations of dietary long-chain n-3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2009;103(9):1238–1243. doi: 10.1016/j.amjcard.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daviglus ML, Liu K, Greenland P, et al. Benefit of a favorable cardiovascular risk-factor profile in middle age with respect to Medicare costs. N Engl J Med. 1998;339(16):1122–1129. doi: 10.1056/NEJM199810153391606. [DOI] [PubMed] [Google Scholar]
- 36.Stamler J, Daviglus ML, Garside DB, et al. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA. 2000;284(3):311–318. doi: 10.1001/jama.284.3.311. [DOI] [PubMed] [Google Scholar]
- 37.Institute of Medicine and Panel on Dietary Antioxidants and Related Compounds . Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids: A Report of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. Washington, DC: National Academy Press; 2000. National Academy of Sciences. [Google Scholar]
- 38.Subcommittee on the Tenth Edition of the RDAs . Recommended Dietary Allowances. 10th ed. Washington, DC: National Academy Press; 1989. Food and Nutrition Board, Commission on Life Sciences, National Research Council. [Google Scholar]
- 39.Burk RF. Selenium, an antioxidant nutrient. Nutr Clin Care. 2002;5(2):75–79. doi: 10.1046/j.1523-5408.2002.00006.x. [DOI] [PubMed] [Google Scholar]
- 40.Monsen ER. Dietary reference intakes for the antioxidant nutrients: vitamin C, vitamin E, selenium, and carotenoids. J Am Diet Assoc. 2000;100(6):637–640. doi: 10.1016/S0002-8223(00)00189-9. [DOI] [PubMed] [Google Scholar]
- 41.Lum H, Roebuck KA. Oxidant stress and endothelial cell dysfunction. Am J Physiol Cell Physiol. 2001;280(4):C719–C741. doi: 10.1152/ajpcell.2001.280.4.C719. [DOI] [PubMed] [Google Scholar]
- 42.Mervaala EM, Müller DN, Park JK, et al. Monocyte infiltration and adhesion molecules in a rat model of high human renin hypertension. Hypertension. 1999;33(1):389–395. doi: 10.1161/01.hyp.33.1.389. [DOI] [PubMed] [Google Scholar]
- 43.Czuczejko J, Halota W, Zachara BA, et al. Plasma selenium concentration, glutathione peroxidase and glutathione S-transferase activities in patients with chronic liver diseases [in Polish] Pol Merkur Lekarski. 2002;13(76):312–315. [PubMed] [Google Scholar]
- 44.Czuczejko J, Zachara BA, Staubach-Topczewska E, et al. Selenium, glutathione and glutathione peroxidases in blood of patients with chronic liver diseases. Acta Biochim Pol. 2003;50(4):1147–1154. [PubMed] [Google Scholar]