Abstract
Atopic disease is hypothesized to be protective against several malignancies, including childhood/adolescent leukemia. To summarize the available epidemiologic evidence, the authors performed a meta-analysis of associations between atopy/allergies, asthma, eczema, hay fever, and hives and childhood/adolescent leukemia, acute lymphoblastic leukemia (ALL), and acute myeloid leukemia (AML). They searched MEDLINE literature (1952–March 2009) and queried international experts to identify eligible studies. Ten case-control studies were included. Summary odds ratios and 95% confidence intervals were computed via random-effects models. Odds ratios for atopy/allergies were 1.42 (95% confidence interval (CI): 0.60, 3.35) for 3 studies of leukemia overall, 0.69 (95% CI: 0.54, 0.89) for 6 studies of ALL, and 0.87 (95% CI: 0.62, 1.22) for 2 studies of AML, with high levels of heterogeneity detected for leukemia overall and ALL. Inverse associations were observed for ALL and asthma (odds ratio (OR) = 0.79, 95% CI: 0.61, 1.02), eczema (OR = 0.74, 95% CI: 0.58, 0.96), and hay fever (OR = 0.55, 95% CI: 0.46, 0.66) examined separately. Odds ratios for ALL differed by study design, exposure data source, and latency period, indicating that these factors affect study results. These results should be interpreted cautiously given the modest number of studies, substantial heterogeneity, and potential exposure misclassification but are useful in designing future research.
Keywords: asthma; child; dermatitis, atopic; hypersensitivity; leukemia; rhinitis, allergic, seasonal; urticaria
Leukemia is the most common malignancy among persons under 20 years of age; in recent years, the incidence rate in the United States (42 per 1,000,000 person-years) has increased at an average annual rate of 0.7% (95% confidence interval (CI): −0.1, 1.5) (1). Nonetheless, there are few established risk factors for pediatric/adolescent leukemia, including predisposing genetic conditions and in utero or postnatal exposure to therapeutic irradiation or chemotherapeutic agents (2). Atopic disease (i.e., asthma, eczema, hay fever, and hives) has been studied as a risk factor for several malignancies (3), including childhood/adolescent leukemia, and is the subject of the current meta-analysis.
The prevalence of atopic disease increased worldwide during the latter part of the 20th century and continues to increase in some areas (4). Estimated prevalences of asthma, skin allergies, and hay fever among persons under age 18 years in the United States are 9.1%, 10.0%, and 9.7%, respectively (5, 6). Reasons for the observed increase are not entirely known; however, the hygiene hypothesis presents 1 explanation (7). This hypothesis postulates that several factors in developed countries have contributed to the rise in atopic/allergic conditions, including smaller families, improved public sanitation, increased use of antibiotics, low incidences of helminth infestation and fecal-oral transmission of infection, and a stable micro-flora population in the intestines (8).
In the current study, we aimed to systematically summarize the etiologic evidence regarding the atopy-childhood/adolescent leukemia association, identify sources of heterogeneity in the existing literature, and identify gaps in the current state of knowledge. This research is of interest given the above-described secular trends in leukemia incidence and atopy prevalence and the immune system's role in the development of both leukemia and atopic disease.
MATERIALS AND METHODS
Study identification and selection
Eligible studies were those that included persons diagnosed with leukemia before the age of 19 years; representative, healthy control groups; and reports of any atopic disease, allergies, asthma, atopic dermatitis/eczema, allergic rhinitis/hay fever, or urticaria/hives. Numbers of exposed and unexposed cases and controls and/or measures of association describing risk of childhood leukemia must also have been provided for inclusion; crude odds ratios and standard errors were computed from cell counts if not reported. Studies involving inappropriate control groups, such as siblings (who are not independent from cases in atopic status) and hospital- or specialty clinic-based controls (who may have been inadvertently selected on exposure status), and studies with data overlapping those of other studies were excluded.
To identify all eligible studies published prior to April 1, 2009, we searched the MEDLINE database (National Library of Medicine) via PubMed, using a combination of Medical Subject Headings (MeSH) and keywords (((“allergy and immunology”[MeSH] or “hypersensitivity”[MeSH]) or “asthma”[MeSH] or (“eczema”[MeSH] or “dermatitis, atopic”[MeSH]) or “rhinitis, allergic, seasonal”[MeSH] or “urticaria”[MeSH])) and “leukemia”[MeSH]) or (((atopy or atopic or atop*) or (allergy or allergic or allerg*)) and leukemia) and limiting results to children and adolescents under age 19 years and the English language. We also searched the Cochrane Library Database of Systematic Reviews using relevant keywords. (The Excerpta Medica database was not accessed, as it was deemed unlikely to yield additional references.) Abstracts from resulting articles were reviewed by 2 independent reviewers (A. M. L. and A. M. J.) to determine eligibility. In the infrequent event of discrepancies, the 2 reviewers reached consensus through discussion. Reference lists from retrieved articles were manually examined to identify additional studies.
Additionally, we surveyed 36 international experts in pediatric cancer etiology to request any other relevant published or unpublished results, as direct contact with experts has been shown to be an effective method of study ascertainment (9).
Data extraction
Data from eligible studies, including general study characteristics (study design, participant ages, diagnostic dates, number and source of cases and controls, source of exposure data, and matching variables), quality-related factors (listed below), and results (cell counts, odds ratios, and 95% confidence intervals), were abstracted by the 2 reviewers onto a standardized form that we developed and were compared to ensure accuracy. Dichotomous exposures for which data were abstracted included atopy, allergy, asthma, eczema, hay fever, and hives; dichotomous outcomes included childhood leukemia, acute lymphoblastic leukemia (ALL), and acute myeloid leukemia (AML). A dichotomous variable indicating any report of atopy or allergies was created because of the variability in definitions of composite atopy/allergy variables across eligible studies. If more than 1 definition of an exposure was evaluated in a given study, we abstracted results for the most general definition in order to attain comparability across studies (10, 11). If a definition included a latency period, the corresponding results were abstracted preferentially (12). Adjusted estimates were generally selected over unadjusted estimates; however, in 1 study, we abstracted estimates from the most parsimonious model because of the wide spectrum of adjustment factors included in the full multivariate model and the similarity between the 2 estimates (13). Two authors were contacted for study results specific to children/adolescents (14, 15); additional information was received from 1 of these authors (Karin Söderberg, Karolinska Institutet, personal communication, 2009).
Statistical methods
Ten exposure-disease associations were examined, representing all identified associations with 2 or more eligible studies. Stata software (Stata Corporation, College Station, Texas) was used to conduct all analyses and produce forest plots (16). Summary odds ratios and 95% confidence intervals were computed from study-specific odds ratios and corresponding standard errors using DerSimonian and Laird random-effects models (17), since random-effects models incorporate between-study heterogeneity. Fixed-effects summary odds ratios were also calculated for comparison. To quantify the degree of heterogeneity across studies, we generated Higgins’ I2 statistic and 95% confidence intervals, representing the proportion of the variance attributable to between-study variability (18); confidence intervals could not be calculated for statistics with fewer than 2 df.
We analyzed the influence of individual studies, with each study being omitted in turn, to identify studies contributing disproportionately to the observed heterogeneity. To examine potential sources of heterogeneity, we calculated summary odds ratios for ALL across strata of factors selected a priori as potentially related to study quality. (There were not enough studies of leukemia overall or AML for similar stratification.) These factors included study design (nested vs. other case-control), source of controls (population-based vs. other), source of exposure data (medical record vs. parental report), latency period (yes vs. no), response rates for cases and controls (≥80% vs. <80%), and adjustment for potential confounders (yes vs. no for age, sex, race, socioeconomic status (SES), method of infant feeding, and location of residence).
Notably, there were too few studies for a given association to warrant a formal assessment of publication bias; however, the thorough search strategy employed, in combination with the query of experts, should have eliminated most sources of publication bias (except possible English-language bias).
RESULTS
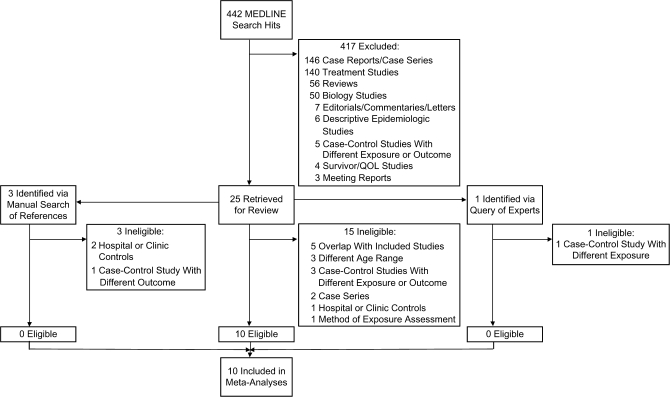
Of the 442 manuscripts identified via the MEDLINE search, 417 were excluded and 25 were retrieved for review; 10 were determined to be eligible upon further review (Figure 1) (10–14, 19–23). No additional eligible studies were found in the Cochrane Database search, manual examination of references, or query of experts.
Figure 1.
Search strategy and study selection process used in a meta-analysis of the association between atopy and leukemia, 1952–March 2009. QOL, quality of life.
Characteristics of the 10 case-control studies meeting the inclusion criteria are provided in Table 1. A majority were published in 2000 or thereafter, and half were conducted in the United States; the remainder originated in Europe and Japan. They collectively included 6,592 childhood/adolescent leukemia cases and 24,171 controls, although different exposure-disease associations were investigated in individual studies. Most studies examined children under age 15 years, with a few exceptions.
Table 1.
Characteristics of 10 Case-Control Studies Included in a Meta-Analysis of the Association Between Atopy and Leukemia, 1952–March 2009
| First Author, Country, and Year (Reference No.) | Study Design | Age Range, years | Source of Cases | Source of Controls | No. of Cases | No. of Controls | Assessment of Exposure | Exposure Variables | Matching/Adjustment Factors |
| Fraumeni, United States, 1964 (19)a | Case-control study (National Cooperative Leukemia Study) | 0–15 | 12 medical centers participating in National Cooperative Leukemia Study | Neighborhood controls | 498 | 498 | Parental interview | Atopic allergy (i.e., asthma, eczema, hay fever) | Matching: neighborhood, age, birth order, family size, race |
| Participation rate: NA | Participation rate: NA | Adjustment: none | |||||||
| Bross, United States, 1972 (20)b | Case-control study (Tri-State Survey) | 1–14 | Cancer registries in the sample areas (New York, Maryland, Minnesota) | Multistage random sample of households in sample areas | 141 | 357 | Parental interview | Allergic diseases (i.e., asthma, hives) | Matching: NA |
| Participation rate: NA | Participation rate: NA | Adjustment: age group (1–4, 5–9, or 10–14 years) | |||||||
| Nishi, Japan, 1989 (13) | Case-control study | 0–14 | All children with immunologically ascertained non-T-cell ALL at 9 hospitals in Hokkaido Prefecture, Japan | Health centers and hospitals in case residential areas, recruited at routine health examinations | 63 ALL | 126 | Maternal interview | Atopic diathesis (i.e., history of asthma or atopic dermatitis) | Matching: age, sex, district of residence at diagnosis |
| Participation rate: 100% | Participation rate: NA | Adjustment: age, sex, district of residence at diagnosis | |||||||
| Wen, United States, 2000 (21) | Case-control study (Children's Cancer Group) | 0–14 | Children's Cancer Group member institutions in the United States | RDD | 1,842 ALL | 1,986 | Maternal telephone interview | Allergic disorders (i.e., asthma, eczema, hay fever, hives, food or drug allergies), asthma, eczema, hay fever, hives | Matching: age (within 25% of age of diagnosis, with a maximum of ±2 years), race (white, black, other), telephone area code and exchange |
| Participation rate: 92% | Participation rate: 76.5% | Adjustment: age, race, telephone area code and exchange, months of breastfeeding, maternal education, maternal race, family income | |||||||
| Schüz, Germany, 2003 (22) | Pooled results from 3 case-control studies | 0–14 | Nationwide German Childhood Cancer Registry (>95% complete) | Local resident registration offices | 1,130 ALL, 164 AML | 2,957 | Self-administered postal questionnaire completed by parents | Atopic disease (i.e., asthma, neurodermatitis, hay fever), asthma, neurodermatitis, hay fever, hives | Matching: gender, date of birth (±1 year), community |
| Participation rate: 78% | Participation rate: 67% | Adjustment: age, year of birth, gender, degree of urbanization (rural, urban, mixed), SES (average, high) | |||||||
| Spector, United States, 2004 (12) | Case-control study | 0–6 | Databases of 4 HMOs in western United States | Databases of 4 HMOs in western United States | 180 ALL | 718 | Subjects’ medical records (diagnosis by a physician) | Allergic conditions (i.e., asthma; eczema; atopy or hives; food, drug, or bee allergy; pollen, dust, or dander allergy), asthma, eczema | Matching: HMO, gender, date of birth (±2 weeks) |
| Participation rate: 100% | Participation rate: 100% | Adjustment: age, gender, race, HMO. Results mutually adjusted for individual allergic conditions. | |||||||
| Jourdan-Da Silva, France, 2004 (11) | Population-based case-control study | 0–14 | National Registry of Childhood Leukemia and Lymphoma in France (all cases aged <15 years) | Randomly selected from general population via RDD on the basis of expected distribution of cases | 408 ALL, 65 AML | 567 | Self-administered questionnaire completed by mothers | Asthma | Matching: age, gender, region of residence at diagnosis |
| Participation rate: 73% | Participation rate: 71% | Adjustment: gender, age, region | |||||||
| Rosenbaum, United States, 2005 (23) | Case-control study (31 counties of New York State) | 0–14 | Institutional tumor registries and departmental records at 4 major medical centers | Randomly selected via Live Birth Certificate Registry (New York State Department of Health) | 255 ALL | 760 | Self-administered postal questionnaire completed by parents | Allergy history (i.e., asthma; eczema; food, drug, or bee allergy; pollen, dust, or cat/dog dander allergy), asthma, eczema | Matching: sex, race (white/nonwhite), birth year |
| Participation rate: 71% | Participation rate: 55% | Adjustment: maternal smoking (yes/no), maternal education (years), breastfed (yes/no), race, birth year | |||||||
| Söderberg, Sweden, 2006 (14)c | Population-based case-control study | 0–18 | All cases in Swedish Cancer Registry | Randomly selected from Swedish nationwide population registry | 875 ALL, 132 AML | 14,865 | Swedish Hospital Discharge Registry | Asthma | Matching: sex, 5-year age group, year of diagnosis |
| Participation rate: 100% | Participation rate: 100% | Adjustment: sex, age, SES | |||||||
| Hughes, United Kingdom, 2007 (10) | Population-based case-control study (United Kingdom Childhood Cancer Study) | 0–14 | United Kingdom residents diagnosed with malignancy | Randomly selected from primary care population registers | 839 (720 ALL, 101 AML) | 1,337 | Primary care records | Allergies (i.e., asthma, eczema, hay fever), asthma, eczema, hay fever | Matching: age, sex, region of residence at diagnosis |
| Participation rate: 92% | Participation rate: 70% | Adjustment: age (single years), sex, region of residence, deprivation index |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; HMO, health maintenance organization; NA, not available; RDD, random digit dialing; SES, socioeconomic status.
Crude odds ratio was calculated from the data provided.
Age-adjusted odds ratio was calculated from data provided in the published article (55).
Results for subjects under age 19 years were obtained from the first author via personal communication.
The distribution of selected quality-related factors is summarized in Table 2. A minority of studies (30%) were nested within a larger cohort, although most used population-based control groups (80%), which should have better represented the cases had they not developed leukemia. Seven studies used parental reports to determine atopic phenotype, while 3 used medical records; none used biomarkers for atopic disease classification. Four studies included a latency period between the onset of atopy and leukemia. Case response rates were 80% or higher in half of the studies, while this high level of participation was reached in only 20% of control groups. Finally, all but 1 study adjusted for age, none adjusted for sibship, and an intermediate number adjusted for sex, race, SES, method of infant feeding, and location of residence, all of which are possible confounders.
Table 2.
Distribution of Factors Related to Quality in the 10 Case-Control Studies Included in a Meta-Analysis of the Association Between Atopy and Leukemia, 1952–March 2009
| Factor | Total |
Leukemia Overall |
Acute Lymphoblastic Leukemia |
Acute Myeloid Leukemia |
||||
| No. | % | No. | % | No. | % | No. | % | |
| Study design | ||||||||
| Nested case-control study | 3 | 30 | 1 | 33 | 3 | 38 | 2 | 50 |
| Population-based controls | 8 | 80 | 2 | 67 | 7 | 88 | 4 | 100 |
| Exposure assessment | ||||||||
| Medical records | 3 | 30 | 1 | 33 | 3 | 38 | 2 | 50 |
| Latency period | 4 | 40 | 2 | 67 | 3 | 38 | 2 | 50 |
| Participation rates | ||||||||
| Cases (≥80%) | 5a | 50 | 1a | 33 | 5 | 63 | 2 | 50 |
| Controls (≥80%) | 2a | 20 | 0a | 0 | 2a | 25 | 1 | 25 |
| Control for confounders | ||||||||
| Age at diagnosis | 9 | 90 | 2 | 67 | 8 | 100 | 4 | 100 |
| Sex | 6 | 60 | 1 | 33 | 6 | 75 | 4 | 100 |
| Race | 3 | 30 | 0 | 0 | 3 | 38 | 0 | 0 |
| Sibship | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Socioeconomic status | 5 | 50 | 1 | 33 | 5 | 63 | 3 | 75 |
| Breastfeeding | 2 | 20 | 0 | 0 | 2 | 25 | 0 | 0 |
| Residence | 5 | 50 | 1 | 33 | 5 | 63 | 3 | 75 |
Participation rates were not available for 2 studies of leukemia overall and 1 study of acute lymphoblastic leukemia.
Table 3 lists excluded case-control studies, reasons for exclusion, and key study results. Primary reasons for exclusion were the use of hospital- or specialty clinic-based control groups, study populations overlapping those of included studies, and inclusion of subjects outside the age range of interest.
Table 3.
Characteristics of Case-Control Studies Excluded From a Meta-Analysis of the Association Between Atopy and Leukemia, 1952–March 2009
| First Author, Country, and Year (Reference No.) | Study Design | Reason for Exclusion | Results |
| Manning, United States, 1957 (56) | Case-control | Specialty clinic-based control group | Suggestion of increased risk of childhood leukemia associated with allergies (i.e., asthma, eczema, hay fever, hives, other) (OR = 2.44, 95% CI: 0.97, 6.14).a |
| Stewart, United Kingdom, 1958 (57) | Case-control | Results presented for childhood cancer cases overall, not for leukemia cases. | No evidence for an association between allergic conditions and childhood malignancy (OR = 1.12, 95% CI: 0.65, 1.94).a |
| Ager, United States, 1965 (58) | Case-control | Very low prevalence of atopic disease reported among cases and controls under age 5 years. This may have been due to misclassification, since exposure was measured first via maternal interview, followed up with medical record verification only for those with a positive maternal report. | No evidence for an association between death from childhood leukemia and asthma, eczema, or hives (for asthma, OR = 0.66, 95% CI: 0.11, 4.03; for eczema, OR = 0.99, 95% CI: 0.14, 7.16; for hives, OR = 0.99, 95% CI: 0.14, 7.16).a |
| Smith, United States, 1973 (55) | Case-control | Study overlapped with Bross (20). Cell counts from this paper were used to calculate overall age-adjusted OR. | Results included in meta-analysis |
| Natarajan, United States, 1973 (59) | Case-control | Study overlapped with Bross (20) | The association between allergic disease (i.e., asthma, hives, eczema) and childhood leukemia was greater among those with maternal exposure to preconception radiation (OR = 4.6, P = 0.0001) than among those with no preconception radiation (OR = 1.9, P = 0.03). |
| Viadana, United States, 1974 (60) | Case-control | Results presented for leukemia cases aged ≥15 years. Unable to locate author to request data for 15- to 18-year-olds. | Not applicable |
| Bross, United States, 1974 (61) | Case-control | Study overlapped with Bross (20) | Association between allergic disease (i.e., asthma, hives, eczema) or bacterial disease (i.e., pneumonia, dysentery, rheumatic fever) and childhood leukemia was greater among those with maternal preconceptional, intrauterine, or postnatal radiation exposure (OR = 4.1, P = 0.0001) than among those with no prior radiation history (OR = 1.6, P = 0.23). |
| Gibson, United States, 1976 (62) | Case-control | Results presented for leukemia cases aged ≥15 years. Unable to locate author to request data for 15- to 18-year-olds. | Not applicable |
| Magnani, Italy, 1990 (63) | Case-control | Hospital-based control group | Inverse association observed between allergic diseases and childhood ALL after adjustment for SES (OR = 0.4, 95% CI: 0.2, 0.8). |
| Zheng, China, 1993 (15) | Case-control | Results presented for leukemia cases aged ≥15 years. Per personal communication with first author, unable to obtain data for 15- to 18-year-olds. | Not applicable |
| Buckley, United States, 1994 (64) | Case-control | Results presented for family history of allergy, not for personal history of allergy. | Family history of allergies (i.e., asthma, hay fever, hives, food or drug allergy) in siblings, parents, and/or grandparents was associated with a modestly increased risk of childhood ALL after adjustment for birth year, race, income, geographic region, and family size (OR = 1.3, P < 0.05). |
| Petridou, Greece, 1997 (65) | Case-control | Hospital-based control group and exposure of hospitalization for allergic disease. | Nonsignificant inverse association observed between hospitalization for allergic disease and childhood leukemia after adjustment for gender, age, location of residence, and other biomedical variables of interest (OR = 0.36, 95% CI: 0.09, 1.43). |
| Kaatsch, Germany, 1998 (66) | 2 case-control studies | Study overlapped with Schüz (22) | Nonsignificant inverse association observed between allergy and childhood leukemia after adjustment for age, sex, place of residence, and SES (OR = 0.90, 95% CI: 0.63, 1.29). |
| Schüz, Germany, 1999 (67) | 2 case-control studies | Study overlapped with Schüz (22) | Inverse association observed between allergy and childhood leukemia after adjustment for gender, date of birth, district of residence, and SES (OR = 0.6, 95% CI: 0.5, 0.8). |
Abbreviations: ALL, acute lymphoblastic leukemia; CI, confidence interval; OR, odds ratio; SES, socioeconomic status.
Crude odds ratio was calculated from the data provided.
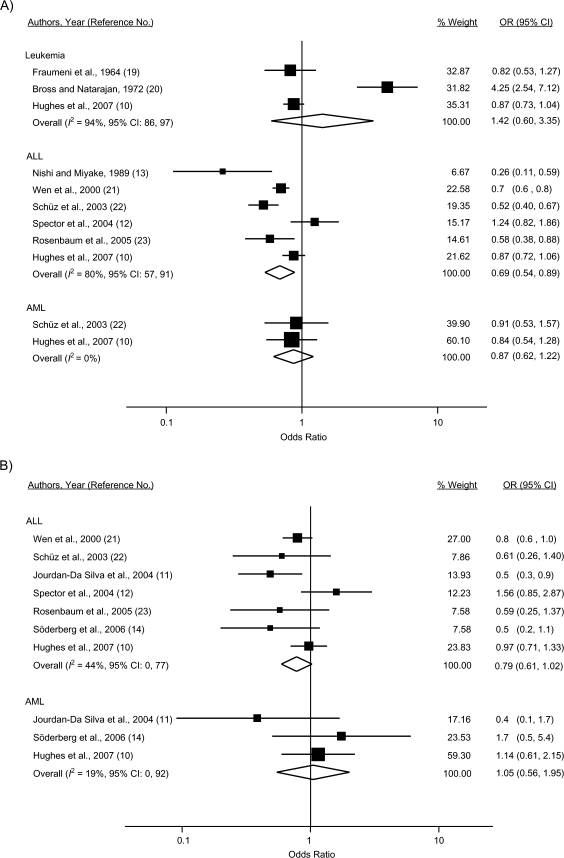
Results of the meta-analysis are depicted in Figure 2. For leukemia overall, the summary odds ratio for any reported atopic disease or allergies was 1.42 (95% CI: 0.60, 3.35), with substantial heterogeneity being present across the 3 studies reporting on this outcome (I2 = 94%). The study by Bross and Natarajan (20) accounted for the observed variability; there was no detectable heterogeneity upon omission of this study (I2 = 0%). Summary odds ratios were not calculated for specific atopic diseases, as these were not assessed in 2 of the 3 studies of leukemia overall.
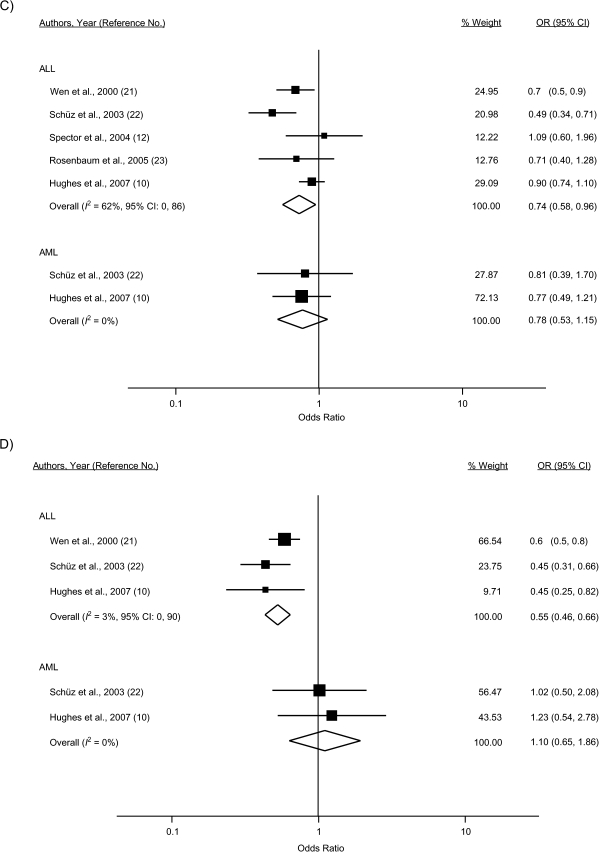
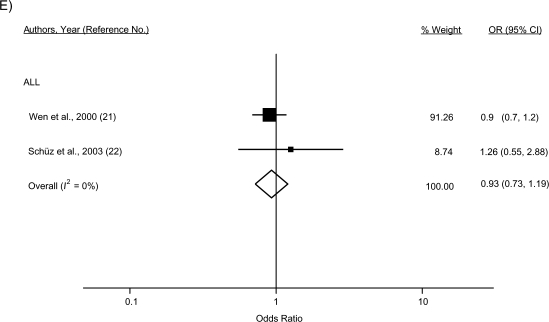
Figure 2.
Study-specific and random-effects summary odds ratios (ORs) for the associations between leukemia overall, acute lymphoblastic leukemia (ALL), and acute myeloid leukemia (AML) and A) atopy or allergies, B) asthma, C) eczema, D) hay fever, and E) hives. The size of each box indicates the relative weight of each study in the meta-analysis; the bars show the 95% confidence intervals (CIs). Higgins’ I2 statistic and 95% CI, a measure of the degree of heterogeneity across studies, is also shown for each summary OR. The 95% CI could not be calculated for I2 with fewer than 2 df. For Fraumeni et al. (19), the crude OR was calculated from data provided in the published article. For Bross and Natarajan (20), the age-adjusted OR was calculated from data provided in the paper by Smith et al. (55). For Söderberg et al. (14), results for children/adolescents under age 19 years were obtained from the first author (Karin Söderberg, Karolinska Institutet, personal communication, 2009).
Thirty-one percent reduced odds of ALL were observed for any atopy/allergies (odds ratio (OR) = 0.69, 95% CI: 0.54, 0.89), although there was evidence of considerable heterogeneity across the 6 included studies (I2 = 80%). The influence analysis did not reveal an obvious outlier among the 6 studies. For atopic diseases examined separately, inverse associations were observed for ALL and asthma (7 studies; OR = 0.79, 95% CI: 0.61, 1.02), eczema (5 studies; OR = 0.74, 95% CI: 0.58, 0.96), and hay fever (3 studies; OR = 0.55, 95% CI: 0.46, 0.66) but not for hives (2 studies; OR = 0.93, 95% CI: 0.73, 1.19).
For AML, a nonsignificant inverse association with any atopy/allergies was calculated (OR = 0.87, 95% CI: 0.62, 1.22) among 2 studies; there was no appreciable heterogeneity detected (I2 = 0%). Notably, these studies, with 164 and 101 AML cases, had less power to detect an association. The summary odds ratios did not indicate evidence of an association for AML and asthma (3 studies; OR = 1.05, 95% CI: 0.56, 1.95), eczema (2 studies; OR = 0.78, 95% CI: 0.53, 1.15), or hay fever (2 studies; OR = 1.10, 95% CI: 0.65, 1.86) assessed individually.
The fixed-effects models produced odds ratios identical or very similar to those generated by random-effects models, with identical or narrower confidence intervals but equivalent interpretations. One possible exception is the fixed-effects odds ratio for atopy/allergies and leukemia overall, which was attenuated in comparison with the random-effects odds ratio (fixed-effects OR = 1.01, 95% CI: 0.86, 1.18). This is not surprising, considering that the high heterogeneity present in this set of studies was attributable to the smallest study with a large, positive association, and this smallest study did not contribute substantially to the fixed-effects odds ratio.
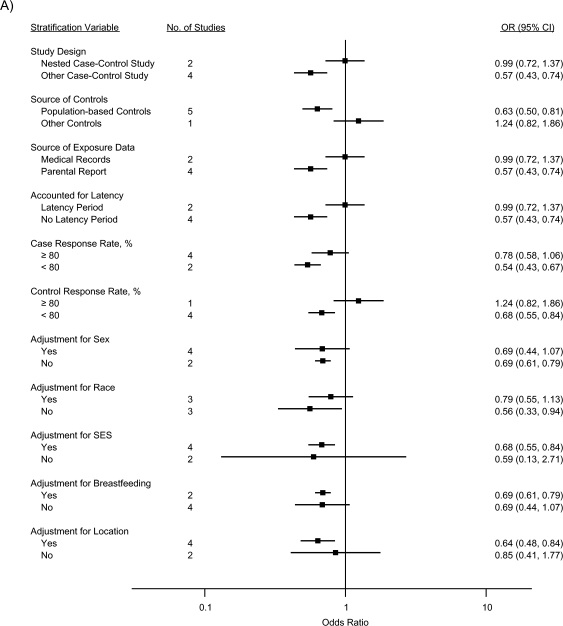
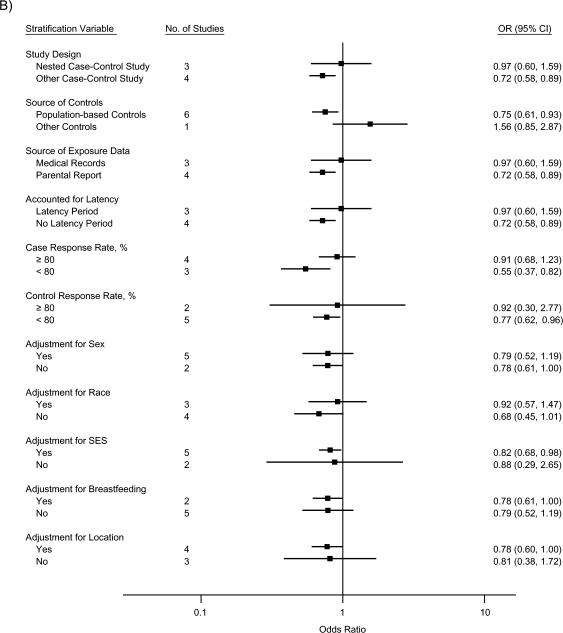
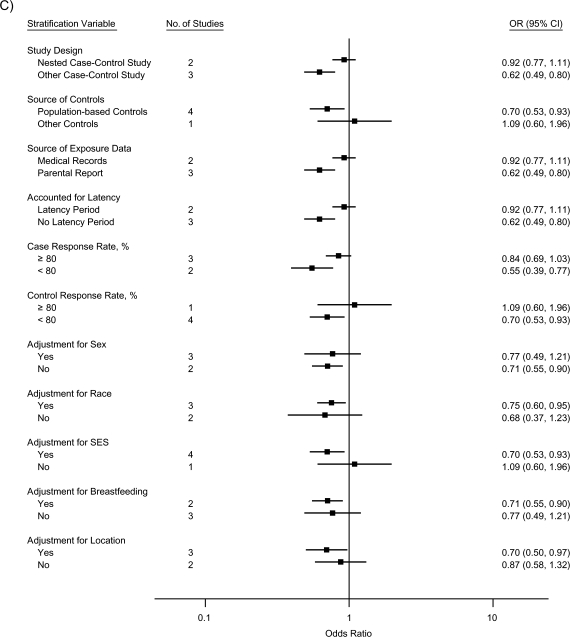
The stratified analyses indicated that the inverse associations for ALL were nearly completely attenuated by a nested case-control study design, use of medical records as the source of exposure data, and inclusion of a latency period, collectively (for atopy/allergies, OR = 0.99, 95% CI: 0.72, 1.37; for asthma, OR = 0.97, 95% CI: 0.60, 1.59; and for eczema, OR = 0.92, 95% CI: 0.77, 1.11); the effect of each could not be evaluated, since the same studies mutually included these factors (Figure 3). Similarly, summary odds ratios for studies with case and control response rates of 80% or higher were closer to the null than those for studies with response rates less than 80%. Controlling for race, SES, and location of residence, respectively, did not produce meaningful differences in summary odds ratios across strata. Adjusting for sex or breastfeeding generated identical or very similar odds ratios across strata, although the precision of the stratum-specific odds ratios differed.
Figure 3.
Random-effects summary odds ratios (ORs) for the association between acute lymphoblastic leukemia and A) atopy or allergies, B) asthma, and C) eczema, according to factors related to study quality. The control response rate was not available for 1 study (13). Bars, 95% confidence interval (CI). SES, socioeconomic status.
DISCUSSION
Results of this meta-analysis indicated an inverse association between atopy/allergies and childhood ALL; the pooled odds of any reported atopy/allergy were 31% lower among cases than among controls and were 21% lower for asthma, 26% lower for eczema, and 45% lower for hay fever. No association was observed for leukemia overall or AML or for any of the specific atopic conditions and AML examined separately, although there were fewer studies of AML and fewer AML cases. The divergent results observed for ALL and AML are of interest, given that atopic status should have been captured similarly across the 2 case groups.
Two conflicting hypotheses have been proposed to explain a causal relation between atopy and cancer. The immune surveillance hypothesis asserts that the immune system recognizes antigens of malignant cells as foreign and mounts a response to them, preventing a majority of potential cancers from developing (24). The presence of an atopic condition is thought to increase the vigilance of the immune system in monitoring for, identifying, and eliminating malignant cells (3, 25). In support of this hypothesis is the observation that immunocompromised persons have a higher incidence of specific malignancies than persons with intact immune systems (26). Notably, immunosupressed persons have an increased risk of non-Hodgkin lymphoma (27), a malignancy with histology identical to that of T-cell ALL (2).
The second hypothesis is that chronic stimulation of the immune system by allergens increases the risk of carcinogenesis (25, 26). A greater number of proliferating cells increases the probability of genetic errors, such as pro-oncogenic mutations, that may not be repaired prior to subsequent divisions (25). This mechanism has been proposed as an explanation for the positive association between autoimmune disease and malignancy (28), for example.
It is not clear whether either of these mechanisms is applicable to childhood/adolescent cancers; however, investigators in the majority of childhood/adolescent leukemia studies reported inverse associations, potentially supporting the immune surveillance hypothesis. Other possible explanations include reverse causality, where the leukemic process induces atopic manifestations, and a common etiology for both atopy and leukemia. There are case reports exemplifying the former explanation (29–31). As an example of the latter, Smith et al. (32) demonstrated that the hygiene hypothesis may also apply to childhood leukemia. In that ecologic study, decreases in the prevalence of hepatitis A infection, a marker for hygiene due to the fecal-oral mode of transmission, were associated with increased ALL risk in the United States and Japan (32). Neither of these is consistent with the observed inverse association, however.
If an inverse relation existed, ALL incidence would be expected to decrease with increasing atopy prevalence. The observed increase in ALL incidence (1) is not inconsistent, however, since ALL is multifactorial, requiring 2 or more “hits” (33), and atopy would constitute a single “hit” (i.e., it is neither sufficient nor necessary). Assuming that the odds ratio for atopy/allergies is 0.69 and the prevalence of atopy in cases is 30%–40%, the attributable fraction for atopy is −14% to −18% (34). It is possible that other putative risk factors with increasing secular trends (e.g., birth weight (35)) may contribute to the increase in ALL and offset any decrease attributable to atopy.
Although no biologic mechanism has been established, the principal factor linking childhood/adolescent leukemia and atopic disease is the rate at which the immune system matures (10). Hypotheses by Greaves (36) and Kinlen (37) suggest an etiologic role for the immune system in the development of childhood leukemia via delayed exposure and abnormal response to early-life infections; no responsible virus or other infectious agent has been identified to date (38, 39). Predominance of T helper 2 (Th2) cells versus T helper 1 (Th1) cells is often described with respect to atopy (40), where each cell type has a specific cytokine profile and associated sequelea. Infants are born with a Th2-dominated immune profile; nonatopic infants gradually migrate to a Th1-dominant process by age 2 years, while infants with a family history of atopy fail to make the Th2-to-Th1 transition (41). Early exposure to infectious agents is thought to stimulate this transition. Two additional T-cell types, T-regulatory and Th17, may also play a role in the complex interplay between atopy, infections, and autoimmune diseases (reviewed by Chang et al. (42)).
There were several notable sources of heterogeneity across the 10 included studies. Results of the stratified analysis indicated that the most important of these were case-control study design, source of exposure data, and/or inclusion of a latency period. Only 3 studies were nested within a larger cohort (10, 12, 14); these types of studies have the advantage of temporality over other case-control designs, in that exposures are measured prior to leukemia diagnosis.
Two aspects of exposure measurement—which data were collected and how they were collected—probably contributed to the observed heterogeneity. As shown in Table 1, definitions for composite atopy/allergy variables differed across studies in that authors included different permutations of asthma, eczema, hay fever, and hives. One study included neurodermatitis instead of eczema (22). In 2 studies, composite variables incorporated food, drug, bee-sting, pollen, dust, or pet-dander allergies, accounting for a large proportion of exposed persons and potentially driving the observed associations (21, 23). Söderberg et al. (14) used the Swedish hospital discharge registry to identify patients discharged for asthma, which is qualitatively different than exposure classifications used in other studies and probably involves substantial underascertainment. Notably, some authors presented definitions that included the presence of symptoms (10) or the use of medications (11). Although they were not used in the current meta-analysis, these definitions, in concert with physician diagnosis, may increase sensitivity and could be considered in future studies.
Three studies used subject medical records for exposure assessment (10, 12, 14), which also has the notable advantage of temporality. Medical records are typically considered the gold standard for medical history data; however, this is only applicable if medical records from all sources are obtainable and complete, which is not always the case (43).
Atopic disease was measured via parental report in the remaining studies (11, 13, 19–23), introducing several potential sources of misclassification and/or recall bias. Subclinical atopy may not be diagnosed by physicians or recognized by parents. For example, it has been estimated that 30%–40% of “healthy” persons are atopic (44), meaning that they produce an immunoglobulin E response to an environmental stimulus. A related issue is that atopic persons experience changes in symptoms over time (45). Conversely, not all cases of asthma, eczema, or hay fever are atopic (46). Parents of cases may be more motivated and more likely to recall exposures than parents of controls (47). It is also possible that early symptoms of leukemia may be mistaken for atopic disease (3). Alternatively, parents of cases may be underreporting allergic diseases if immunosuppressive therapy administered for leukemia reduces allergic symptoms and parents report allergy status after initiation of treatment (22). Parents of controls may report allergic disease arising after a specified reference date, since the reference date does not carry the personal significance of a diagnosis date (22). A sensitivity analysis by Schüz et al. (22), wherein reference dates for controls were reclassified to interview dates, revealed that the observed associations were attenuated somewhat, but they could not be wholly attributed to recall bias.
Results of 1 validation study indicated that parental recall of an asthma diagnosis is high (87% agreement with medical records) (48). A second validation study by Hughes et al. (10) demonstrated that accuracy of maternal recall of asthma was high and approximately equal between cases and controls (sensitivity was 81% in cases vs. 83% in controls), although the level of agreement between maternal recall and medical records was marginally higher among cases (in cases, κ = 0.69; in controls, κ = 0.60). Recall of eczema was somewhat lower (sensitivity was 51% in cases vs. 57% in controls), but agreement with medical records was equivalent between cases and controls (κ = 0.46). Therefore, parental recall of atopic diagnosis is predicted to be similar across cases and controls, although degree of recall will probably vary by condition.
To minimize concerns about misclassification, a latency period should be incorporated between onset of atopic symptoms and leukemia diagnosis. Importantly, only 4 included studies incorporated a latency period, and among those, the length of the latency period varied (3 months (10), 6 months (20), or 1 year (12, 14)).
The age range of included subjects is another potential source of heterogeneity. Most studies examined children/adolescents under age 15 years; however, Bross and Natarajan (20) did not include infants, Spector et al. (12) limited their analysis to children aged 6 years or less, and Söderberg et al. (14) included children and adolescents aged 18 years or less (by request). Age is an important consideration, because the prevalence of atopy increases with age (45). Further, the age-standardized incidence of leukemia is greatest among children under age 5 years, with lower rates among infants and children/adolescents aged 5–19 years (1). Importantly, in 3 studies investigators presented odds ratios by age group; however, categories differed across studies and data could not be reasonably pooled (13, 20, 21).
The principal limitation of any meta-analysis is the great potential for selection bias, encompassing bias related to publication, language, citation, and multiple publication (49). Failure to include all studies of a given association may produce a summary odds ratio that overestimates the true effect (50). To minimize publication bias, we contacted international experts on childhood cancer etiology to inquire about unpublished or unidentified studies in addition to those located in the systematic electronic database search. Additional limitations include the small number of studies for each exposure-disease association and the relatively high level of heterogeneity detected across studies, which may restrict the generalizability of the summary odds ratios produced.
There were also limitations within the individual case-control studies. Recall bias, as discussed above, is a primary concern with retrospective study designs; selection bias is also of concern. Selection bias was probably absent in the studies based on registry or health maintenance organization data (12, 14), but it cannot be ruled out in other studies with lower participation rates, especially since odds ratios for ALL were closer to the null for both cases and controls for response rates of ≥80% versus <80% in the stratified analyses. Also noteworthy is the exclusion of very sick or deceased cases from 1 study, potentially introducing survival bias (11). Further, participants in case-control studies of childhood cancer tend to be of higher SES (51), and SES is associated with both atopy (52) and leukemia (53). Adjustment for SES did not result in disparate stratum-specific odds ratios for ALL for atopy/allergies or asthma, but there was a possible effect for eczema. The potential effects of misclassification and other sources of bias are best evaluated by conducting a formal uncertainty analysis (54).
Although the results of this meta-analysis indicate inverse associations between atopy/allergies and childhood/adolescent ALL, causes of the observed statistical associations must be investigated thoroughly to rule out explanations other than a direct causal relation, such as reverse causality or selection or recall bias. Ideally, future studies would include a prospective design with analysis of biologic specimens to avoid the pitfalls of temporality and misclassification that plague existing studies.
Acknowledgments
Author affiliations: Division of Pediatric Epidemiology and Clinical Research, Department of Pediatrics, Medical School, University of Minnesota, Minneapolis, Minnesota (Amy M. Linabery, Anne M. Jurek, Julie A. Ross); University of Minnesota Masonic Cancer Center, Minneapolis, Minnesota (Anne M. Jurek, Julie A. Ross); and Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota (Sue Duval).
This work was supported by the National Institutes of Health (grant T32CA099936) and the Children's Cancer Research Fund (Minneapolis, Minnesota).
The authors extend a sincere thank-you to all of the researchers who kindly responded to their requests for information, including Mary L. McBride and Drs. Patricia A. Buffler, Greta R. Bunin, Sven Cnattingius, Henrik Hjalgrim, Claire Infante-Rivard, Xiaomei Ma, Martha S. Linet, Patricia A. McKinney, Rachel L. Miller, Elizabeth Milne, Beth A. Mueller, Andrew F. Olshan, Frederica P. Perera, Eleni Petridou, Peggy Reynolds, Paula F. Rosenbaum, Joachim Schüz, Xiao Ou Shu, Karin C. Söderberg, Logan G. Spector, and Wei Zheng.
Conflict of interest: none declared.
Glossary
Abbreviations
- ALL
acute lymphoblastic leukemia
- AML
acute myeloid leukemia
- CI
confidence interval
- MeSH
Medical Subject Headings
- OR
odds ratio
- SES
socioeconomic status
- Th1
T helper 1
- Th2
T helper 2
References
- 1.Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992–2004) Cancer. 2008;112(2):416–432. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Smith MA, Gurney JG, et al., editors. Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program 1975–1995. Bethesda, MD: National Cancer Institute; 1999. (NIH publication no. 99-4649) [Google Scholar]
- 3.Turner MC, Chen Y, Krewski D, et al. An overview of the association between allergy and cancer. Int J Cancer. 2006;118(12):3124–3132. doi: 10.1002/ijc.21752. [DOI] [PubMed] [Google Scholar]
- 4.Asher MI, Montefort S, Björkstén B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537):733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 5.National Center for Health Statistics, Centers for Disease Control and Prevention. Health Data Interactive. Allergic Conditions, Ages 0–17: US, 1999–2007 (Source: NHIS) [electronic database] Hyattsville, MD: National Center for Health Statistics; 2008. ( http://www.cdc.gov/nchs/hdi.htm). (Accessed July 9, 2009) [Google Scholar]
- 6.National Center for Health Statistics, Centers for Disease Control and Prevention. Health Data Interactive. Asthma and Chronic Obstructive Pulmonary Disease: US, 1999–2007 (Source: NHIS) [electronic database] Hyattsville, MD: National Center for Health Statistics; 2008. ( http://www.cdc.gov/nchs/hdi.htm). (Accessed July 9, 2009) [Google Scholar]
- 7.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299(6710):1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol. 2001;1(1):69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 9.McManus RJ, Wilson S, Delaney BC, et al. Review of the usefulness of contacting other experts when conducting a literature search for systematic reviews. BMJ. 1998;317(7172):1562–1563. doi: 10.1136/bmj.317.7172.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes AM, Lightfoot T, Simpson J, et al. Allergy and risk of childhood leukaemia: results from the UKCCS. Int J Cancer. 2007;121(4):819–824. doi: 10.1002/ijc.22702. [DOI] [PubMed] [Google Scholar]
- 11.Jourdan-Da Silva N, Perel Y, Méchinaud F, et al. Infectious diseases in the first year of life, perinatal characteristics and childhood acute leukaemia. Br J Cancer. 2004;90(1):139–145. doi: 10.1038/sj.bjc.6601384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spector L, Groves F, DeStefano F, et al. Medically recorded allergies and the risk of childhood acute lymphoblastic leukaemia. Eur J Cancer. 2004;40(4):579–584. doi: 10.1016/j.ejca.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Nishi M, Miyake H. A case-control study of non-T cell acute lymphoblastic leukaemia of children in Hokkaido, Japan. J Epidemiol Community Health. 1989;43(4):352–355. doi: 10.1136/jech.43.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Söderberg KC, Jonsson F, Winqvist O, et al. Autoimmune diseases, asthma and risk of haematological malignancies: a nationwide case-control study in Sweden. Eur J Cancer. 2006;42(17):3028–3033. doi: 10.1016/j.ejca.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Zheng W, Linet MS, Shu XO, et al. Prior medical conditions and the risk of adult leukemia in Shanghai, People's Republic of China. Cancer Causes Control. 1993;4(4):361–368. doi: 10.1007/BF00051339. [DOI] [PubMed] [Google Scholar]
- 16.Stata Corporation. Stata Statistical Software, Release 10.1. College Station, TX: Stata Corporation LP; 2007. [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Fraumeni JF, Jr, Manning MD, Stark CR. Diseases of hypersensitivity and childhood leukemia. JAMA. 1964;188(5):459. doi: 10.1001/jama.1964.03060310059010. [DOI] [PubMed] [Google Scholar]
- 20.Bross ID, Natarajan N. Leukemia from low-level radiation: identification of susceptible children. N Engl J Med. 1972;287(3):107–110. doi: 10.1056/NEJM197207202870301. [DOI] [PubMed] [Google Scholar]
- 21.Wen W, Shu XO, Linet MS, et al. Allergic disorders and the risk of childhood acute lymphoblastic leukemia (United States) Cancer Causes Control. 2000;11(4):303–307. doi: 10.1023/a:1008958724739. [DOI] [PubMed] [Google Scholar]
- 22.Schüz J, Morgan G, Böhler E, et al. Atopic disease and childhood acute lymphoblastic leukemia. Int J Cancer. 2003;105(2):255–260. doi: 10.1002/ijc.11054. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbaum PF, Buck GM, Brecher ML. Allergy and infectious disease histories and the risk of childhood acute lymphoblastic leukaemia. Paediatr Perinat Epidemiol. 2005;19(2):152–164. doi: 10.1111/j.1365-3016.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- 24.Markiewicz MA, Gajewski TF. The immune system as anti-tumor sentinel: molecular requirements for an anti-tumor immune response. Crit Rev Oncog. 1999;10(3):247–260. [PubMed] [Google Scholar]
- 25.Eriksson NE, Mikoczy Z, Hagmar L. Cancer incidence in 13811 patients skin tested for allergy. J Investig Allergol Clin Immunol. 2005;15(3):161–166. [PubMed] [Google Scholar]
- 26.Penn I. Depressed immunity and the development of cancer. Cancer Detect Prev. 1994;18(4):241–252. [PubMed] [Google Scholar]
- 27.Kersey JH, Shapiro RS, Filipovich AH. Relationship of immunodeficiency to lymphoid malignancy. Pediatr Infect Dis J. 1988;7(5 suppl):S10–S12. [PubMed] [Google Scholar]
- 28.Kinlen LJ. Malignancy in autoimmune diseases. J Autoimmun. 1992;5(suppl A):S363–S371. doi: 10.1016/0896-8411(92)90055-u. [DOI] [PubMed] [Google Scholar]
- 29.Heath JA. Leukaemia presenting as respiratory distress in a child with asthma. J Paediatr Child Health. 2001;37(4):397–399. doi: 10.1046/j.1440-1754.2001.00665.x. [DOI] [PubMed] [Google Scholar]
- 30.Breda L, Di Marzio D, Rollo V, et al. Acute myeloid leukaemia presenting as recurrent generalized urticaria in infancy. Eur J Pediatr. 2008;167(6):697–698. doi: 10.1007/s00431-007-0551-7. [DOI] [PubMed] [Google Scholar]
- 31.Chien AJ, Argenyi ZB, Colven RM, et al. Acute lymphoblastic leukemia presenting with urticarial plaques and hypereosinophilia in a child. J Am Acad Dermatol. 2004;51(5 suppl):S151–S155. doi: 10.1016/j.jaad.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Smith MA, Simon R, Strickler HD, et al. Evidence that childhood acute lymphoblastic leukemia is associated with an infectious agent linked to hygiene conditions. Cancer Causes Control. 1998;9(3):285–298. doi: 10.1023/a:1008873103921. [DOI] [PubMed] [Google Scholar]
- 33.Greaves MF, Maia AT, Wiemels JL, et al. Leukemia in twins: lessons in natural history. Blood. 2003;102(7):2321–2333. doi: 10.1182/blood-2002-12-3817. [DOI] [PubMed] [Google Scholar]
- 34.Greenland S. Applications of stratified analysis methods. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. pp. 283–302. [Google Scholar]
- 35.Caughey RW, Michels KB. Birth weight and childhood leukemia: a meta-analysis and review of the current evidence. Int J Cancer. 2009;124(11):2658–2670. doi: 10.1002/ijc.24225. [DOI] [PubMed] [Google Scholar]
- 36.Greaves MF. Speculations on the cause of childhood acute lymphoblastic leukemia. Leukemia. 1988;2(2):120–125. [PubMed] [Google Scholar]
- 37.Kinlen L. Evidence for an infective cause of childhood leukaemia: comparison of a Scottish new town with nuclear reprocessing sites in Britain. Lancet. 1988;2(8624):1323–1327. doi: 10.1016/s0140-6736(88)90867-7. [DOI] [PubMed] [Google Scholar]
- 38.MacKenzie J, Greaves MF, Eden TO, et al. The putative role of transforming viruses in childhood acute lymphoblastic leukemia. Haematologica. 2006;91(2):240–243. [PubMed] [Google Scholar]
- 39.Vasconcelos GM, Kang M, Pombo-de-Oliveira MS, et al. Adenovirus detection in Guthrie cards from paediatric leukaemia cases and controls. Br J Cancer. 2008;99(10):1668–1672. doi: 10.1038/sj.bjc.6604714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson DS. The Th1 and Th2 concept in atopic allergic disease. Chem Immunol. 2000;78:50–61. doi: 10.1159/000058816. [DOI] [PubMed] [Google Scholar]
- 41.Prescott SL, Macaubas C, Smallacombe T, et al. Reciprocal age-related patterns of allergen-specific T-cell immunity in normal vs. atopic infants. Clin Exp Allergy. 1998;28(suppl 5):39–44. doi: 10.1046/j.1365-2222.1998.028s5039.x. [DOI] [PubMed] [Google Scholar]
- 42.Chang JS, Wiemels JL, Buffler PA. Allergies and childhood leukemia. Blood Cells Mol Dis. 2009;42(2):99–104. doi: 10.1016/j.bcmd.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 43.Seidman DS, Slater PE. Accuracy of the medical interview. Br J Obstet Gynaecol. 1987;94(8):721–723. doi: 10.1111/j.1471-0528.1987.tb03715.x. [DOI] [PubMed] [Google Scholar]
- 44.Wüthrich B. What is atopy? Condition, disease or a syndrome? Curr Probl Dermatol. 1999;28:1–8. doi: 10.1159/000060596. [DOI] [PubMed] [Google Scholar]
- 45.Bottema RW, Reijmerink NE, Koppelman GH, et al. Phenotype definition, age, and gender in the genetics of asthma and atopy. Immunol Allergy Clin North Am. 2005;25(4):621–639. doi: 10.1016/j.iac.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Novak N, Bieber T. Allergic and nonallergic forms of atopic diseases. J Allergy Clin Immunol. 2003;112(2):252–262. doi: 10.1067/mai.2003.1595. [DOI] [PubMed] [Google Scholar]
- 47.Infante-Rivard C, Jacques L. Empirical study of parental recall bias. Am J Epidemiol. 2000;152(5):480–486. doi: 10.1093/aje/152.5.480. [DOI] [PubMed] [Google Scholar]
- 48.Pless CE, Pless IB. How well they remember. The accuracy of parent reports. Arch Pediatr Adolesc Med. 1995;149(5):553–558. doi: 10.1001/archpedi.1995.02170180083016. [DOI] [PubMed] [Google Scholar]
- 49.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Duval SJ, Tweedie RL. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95(449):89–98. [Google Scholar]
- 51.Law GR, Smith AG, Roman E. The importance of full participation: lessons from a national case-control study. Br J Cancer. 2002;86(3):350–355. doi: 10.1038/sj.bjc.6600092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bloom B, Cohen RA, Freeman G. Summary health statistics for U.S. children: National Health Interview Survey, 2007. Vital Health Stat 10. 2009;(239):1–80. [PubMed] [Google Scholar]
- 53.Little J. Epidemiology of Childhood Cancer. Lyon, France: International Agency for Research on Cancer; 1999. [Google Scholar]
- 54.Greenland S, Lash TL. Bias analysis. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. pp. 345–380. [Google Scholar]
- 55.Smith PG, Pike MC, Hamilton LD. Multiple factors in leukaemogenesis. Br Med J. 1973;2(5864):482–483. doi: 10.1136/bmj.2.5864.482-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manning MD, Carroll BE. Some epidemiological aspects of leukemia in children. J Natl Cancer Inst. 1957;19(6):1087–1094. [PubMed] [Google Scholar]
- 57.Stewart A, Webb J, Hewitt D. A survey of childhood malignancies. Br Med J. 1958;1(5086):1495–1508. doi: 10.1136/bmj.1.5086.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ager EA, Schuman LM, Wallace HM, et al. An epidemiological study of childhood leukemia. J Chronic Dis. 1965;18:113–132. doi: 10.1016/0021-9681(65)90096-2. [DOI] [PubMed] [Google Scholar]
- 59.Natarajan N, Bross ID. Preconception radiation and leukemia. J Med. 1973;4(5):276–281. [PubMed] [Google Scholar]
- 60.Viadana E, Bross ID. Use of the medical history to predict the future occurrence of leukemias in adults. Prev Med. 1974;3(1):165–170. doi: 10.1016/0091-7435(74)90072-3. [DOI] [PubMed] [Google Scholar]
- 61.Bross ID, Natarajan N. Risk of leukemia in susceptible children exposed to preconception, in utero and postnatal radiation. Prev Med. 1974;3(3):361–369. doi: 10.1016/0091-7435(74)90048-6. [DOI] [PubMed] [Google Scholar]
- 62.Gibson R, Graham S, Lilienfeld A, et al. Epidemiology of diseases in adult males with leukemia. J Natl Cancer Inst. 1976;56(5):891–898. doi: 10.1093/jnci/56.5.891. [DOI] [PubMed] [Google Scholar]
- 63.Magnani C, Pastore G, Luzzatto L, et al. Parental occupation and other environmental factors in the etiology of leukemias and non-Hodgkin's lymphomas in childhood: a case-control study. Tumori. 1990;76(5):413–419. doi: 10.1177/030089169007600501. [DOI] [PubMed] [Google Scholar]
- 64.Buckley JD, Buckley CM, Ruccione K, et al. Epidemiological characteristics of childhood acute lymphocytic leukemia. Analysis by immunophenotype. The Childrens Cancer Group. Leukemia. 1994;8(5):856–864. [PubMed] [Google Scholar]
- 65.Petridou E, Trichopoulos D, Kalapothaki V, et al. The risk profile of childhood leukaemia in Greece: a nationwide case-control study. Br J Cancer. 1997;76(9):1241–1247. doi: 10.1038/bjc.1997.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaatsch P, Kaletsch U, Meinert R, et al. German case control study on childhood leukaemia—basic considerations, methodology and summary of the results. Klin Padiatr. 1998;210(4):185–191. doi: 10.1055/s-2008-1043877. [DOI] [PubMed] [Google Scholar]
- 67.Schüz J, Kaletsch U, Meinert R, et al. Association of childhood leukaemia with factors related to the immune system. Br J Cancer. 1999;80(3-4):585–590. doi: 10.1038/sj.bjc.6690395. [DOI] [PMC free article] [PubMed] [Google Scholar]