INTRODUCTION
Mood disorders are highly prevalent in women throughout their lifetime (Pearlstein et al. 1997), being about twice as prevalent in women than men (Alexander et al. 2007; Halbreich and Kahn 2001; Kessler et al. 1994; Pearlstein et al. 1997). One such mood disorder that affects females exclusively is premenstrual dysphoric disorder (PMDD). This disorder is characterized by premenstrual emotional and physical symptoms severe enough to interfere with function during the last week of the luteal phase of the menstrual cycle, but remit with the onset of menses. PMDD afflicts 5 – 7% of women in their reproductive years (Pearlstein and Steiner 2008), and although the symptoms of PMDD are of shorter duration than those of other depressive disorders, the impact of PMDD symptoms on quality of life during the premenstrual luteal phase is equivalent to that seen with major depressive disorder (MDD), posttraumatic stress disorder, and panic disorder (Freeman and Sondheimer 2003).
The underlying pathophysiologic mechanisms contributing to PMDD remain elusive. The lack of a consensus on the biological basis of PMDD and the lack of efficacy of selective serotonin reuptake inhibitors in up to 40% of PMDD women (Halbreich et al. 2006; Steiner and Soares 2008) speaks to the heterogeneity of PMDD and suggests that there may be clinically distinct subgroups of PMDD women with differing pathogeneses (Klatzkin et al. 2006a; 2006b).
One such subgroup of PMDD women may be those with a history of MDD. A history of MDD has been reported in 30–70% of women with PMDD (Cohen et al. 2002; Pearlstein et al. 1990; Yonkers 1997). Not only are PMDD women more likely to have experienced a prior episode of MDD (Harrison et al. 1989; Pearlstein et al. 1990), but they are also more likely to develop a future episode of MDD than are non-PMDD women (Hartlage et al. 2001; Roca et al. 1999). The high comorbidity of PMDD with histories of MDD has fueled debates in the literature regarding the nosology of the two disorders. For example, it has been suggested that 1) MDD may play a role in the etiology of PMDD (Kendler et al. 1998); 2) PMDD is in fact a distinct entity when compared to other depressive disorders such as MDD (Endicott et al. 1999); and 3) a history of MDD may have special biological relevance for PMDD symptomatology and responses to stress (Klatzkin et al. 2006b). Studies aimed at addressing the phenotypic similarities and differences seen in women with PMDD relative to women with histories of MDD may shed light on the extent of overlap in pathogenic pathways and, consequently, inform treatment for a large proportion of women with PMDD who are otherwise nonresponsive to current FDA-approved treatments (Halbreich 2008).
Since both PMDD and depressive disorders are associated with increased daily stress (Girdler et al. 1993; 1998), and are exacerbated by stressful life events (Kendler et al. 2004; Paddison et al. 1990), a pathogenic role of stress responsive dysregulation has been implicated in both disorders (Cohen et al. 2002; Endicott 1993; Harrison et al. 1989; Kendler et al. 1998; Pearlstein et al. 1990; Yonkers 1997). Experimental studies examining stress responses in PMDD women have been scant, and results mixed. However, when considered together, the majority of evidence points toward decreased activation of the hypothalamic pituitary adrenal (HPA) and the sympathetic nervous system (SNS) axes in PMDD women relative to non-PMDD controls. For example, in both the follicular and luteal phases of the menstrual cycle, PMDD women show blunted heart rate (HR), blood pressure (BP), and cardiac output reactivity to a variety of laboratory psychological stressors relative to non-PMDD women (Girdler et al. 1993), suggesting blunted SNS activation to stressors. Similarly, PMDD women exhibit lower baseline and stress-induced β-endorphin levels (Chuong et al. 1985; Facchinetti et al. 1994; Giannini et al. 1990; Straneva et al. 2002), and blunted adrenocorticotropic hormone (ACTH) and cortisol responses to serotonergic challenges (Bancroft et al. 1991; Su et al. 1997), and lower cortisol levels during mental stress (Girdler et al., 1998), suggesting blunted HPA-axis activation.
That PMDD and MDD represent two distinctly different depressive disorders is supported by evidence suggesting differential dysregulation in the stress axes in women with PMDD versus women with current or prior depressive disorders. In contrast to the profile seen in PMDD, the overwhelming evidence in patients with current depression is for a hyperactive HPA-axis reflected in heightened baseline levels of corticotrophin releasing hormone (CRH), cortisol, β-endorphin, and ACTH (Carroll et al. 2007; Catalan et al. 1998; Galard et al. 2002; Gold et al. 1986a; 1986b; 2005; Goodwin et al. 1993; Gotthardt et al. 1995; Heuser et al. 1996; Juruena et al. 2006; Krittayaphong et al. 1996; Wong et al. 2000; Young et al. 2001; 2000b). Upregulation of the SNS is also present in patients with current depression, reflected in increased baseline and stress-induced norepinephrine (NE), SBP, and HR compared to controls (Carney et al. 1999; 1988; Gold et al. 2005; Hamer et al. 2007; Lake et al. 1982; Lechin et al. 1995; Udupa et al. 2007; Veith et al. 1994; Volkers et al. 2003; Wong et al. 2000). With regard to SNS and HPA-axis functioning in euthymic individuals with a history of MDD, most studies, though not all (Ahrens et al. 2008), report hyperactivity of both stress axes (Broadley et al. 2005; Davydov et al. 2007; Kathol 1985; Young et al. 2000a), indicating persistent dysregulation beyond the remission of the depressive disorder.
Another clinically significant aspect of both PMDD and MDD that may have relevance for the nosology of these mood disorders are the physical, or somatic symptoms that are common to both. Somatic symptoms such as breast tenderness, bloating, and joint or muscle pain are important features of PMDD and MDD and contribute to overall dysfunction (Steiner et al. 2001). Although laboratory-based methods of assessing pain sensitivity are positively related to clinical pain (Edwards and Fillingim 1999; Fillingim et al. 1999), there are few studies assessing pain sensitivity in PMDD (Fillingim et al. 1995; Kuczmierczyk and Adams 1986; Kuczmierczyk et al. 1986; Straneva et al. 2002) and MDD patients (Klauenberg et al. 2008; Bar et al. 2005; 2006; Dickens et al. 2003; Lautenbacher et al. 1994; 1999; Schwier et al. 2009; Terhaar et al. 2009).
Prior studies from our laboratory have shown that women with PMDD exhibit lower ischemic pain threshold and tolerance compared with controls in both the follicular and luteal phases of the menstrual cycle (Fillingim et al. 1995; Straneva et al. 2002), and others have shown that PMDD women endorse higher pain intensity ratings in response to pressure pain irrespective of menstrual cycle phase (Kuczmierczyk and Adams 1986; Kuczmierczyk et al. 1986). These results, taken together, suggest that PMDD is associated with enhanced pain sensitivity. In contrast, a systematic review and meta-analysis of the literature in MDD concluded that pain threshold was higher in individuals with current MDD compared to healthy controls (Dickens et al. 2003). More recent studies have assessed both threshold and tolerance to multiple experimental pain stimuli in depressed patients, and although findings have been mixed, taken together they indicate reduced sensitivity to experimental pain in MDD (Bar et al. 2005; 2006; Klauenberg et al. 2008; Lautenbacher et al. 1994; 1999; Schwier et al. 2009; Sindrup and Jensen 1999; Terhaar et al. 2009;). To the extent that pain sensitivity is modulated by stress responsive systems (e.g. stress-induced analgesia) (al’Absi et al. 2002; Girdler et al. 2005; Maixner 1991; Mechlin et al. 2005), diagnosis-related differences in stress responses may play a role in the experience of pain in both PMDD and MDD patients.
Consequently, the primary purpose of the current study was to examine evidence for common versus different phenotypic profiles related to SNS, HPA and pain measures in women with PMDD and women with histories of MDD, and whether a history of MDD holds special relevance for PMDD women with respect to stress and pain profiles. Although a diagnosis of current MDD may be superimposed on the diagnosis of PMDD (American Psychiatric Association [DSM-IV], 1994), this comorbidity is less common than is a history of MDD in PMDD samples. Hence, our focus was on histories of MDD, and not current MDD in women with PMDD. While the present investigation is exploratory in nature, it is the first, to our knowledge, to approach this issue experimentally with the inclusion of four groups of women: 1) PMDD women with histories of MDD; 2) PMDD women with no history of MDD; 3) non-PMDD women with histories of MDD; and 4) non-PMDD women with no history of MDD. Diagnosis-related differences in phenotypes would be supported by statistical main effects of prior MDD or PMDD in the absence of PMDD × Prior MDD interactions. On the other hand, if histories of MDD hold special relevance for the SNS, HPA-axis, and/or pain sensitivity measures in PMDD women (or if the effects of PMDD and prior MDD were additive), then we would anticipate PMDD × Prior MDD interactions in these measures.
METHODS
Participants
The sample of 54 women (19–51 years of age) who comprise this report represent a sub-sample of participants recruited for a larger, ongoing investigation. The participants in this substudy were specifically recruited via newspaper, radio, or posted advertisements to examine the influence of histories of MDD and PMDD diagnosis on pain sensitivity and stress responses. While it was not necessary to engage in selective recruitment efforts targeting PMDD women with histories of MDD, in order to recruit non-PMDD controls with prior MDD, a proportion of our advertisements targeted women with a prior MD episode. Since the ongoing parent project is not focused on histories of MDD, the participants comprising this report represent the final sample of participants for the substudy related to PMDD and histories of MDD and are composed of four groups: 1) Non- PMDD women with no prior MDD (N = 18); 2) non-PMDD women with prior MDD (N = 9); 3) PMDD women with no prior MDD (N = 17); 4) PMDD women with prior MDD (N = 10).
Participants with a current Axis I psychiatric disorder were excluded (based on interview, see below) but referred for treatment. Also excluded was any woman who was pregnant or breastfeeding, had irregular menstrual cycles, was taking prescription medication (including oral contraceptives and psychotropics), had a cardiovascular disorder, a history of or a current chronic or acute pain condition, an endocrine disorder including diabetes or thyroid disorder, or other chronic medical illness. A diagnosis of MDD was based on a structured clinical interview (see below) with the requirement of one year in full remission. Participants in the prior MDD group were required to have had at least one prior episode of MDD, though they may also have had histories of minor depression, dysthymia, or adjustment disorder with depressed mood. The never depressed groups were free of any lifetime depressive illness, including minor depression and adjustment disorder. The protocol was approved by the institution’s Institutional Review Board, and all participants provided informed, written consent before participating. Participants received $100 compensation.
Procedures
Screening and Enrollment
After an initial phone-screening interview, each participant was scheduled for an enrollment session. During this session, informed consent was obtained, a series of stethoscopic blood pressures was taken, and participants underwent a diagnostic interview using the MINI International Neuropsychiatric Interview for Axis I disorders (Sheehan et al. 1998). Also, because one goal of the ongoing parent project is to examine the influence of abuse histories on pain sensitivity and stress responses, a validated structured interview to assess previous abuse experiences (Leserman et al. 1997) was also administered. Once determined to be eligible, participants were instructed on the Daily Record of Severity of Problems (DRSP) form (Endicott et al. 2006), which they were asked to fill out daily for 2–3 consecutive menstrual cycles in order to confirm the diagnosis of PMDD. Upon completion of the DRSP ratings, participants underwent a second screening visit where they were instructed on the use of home ovulation testing kits that were used to target the testing session during the late luteal (symptomatic) phase.
Confirming PMDD Diagnosis
The DRSP (Endicott et al. 2006) was used to confirm PMDD and non-PMDD status for all participants. The DRSP consists of 24 symptoms (depressed, hopeless, worthless or guilty, anxious, mood swings, more sensitive, angry or irritable, conflict, less interest, difficulty concentrating, fatigue, increased appetite or overate, food cravings, slept more, trouble sleeping, overwhelmed, out of control, breast tenderness, breast swelling or bloating, headache, joint or muscle pain, less productivity or efficiency due to above problems, interference with hobbies or social activities due to above problems, and interference with relationships due to the above problems), rated on a severity scale from 1 (not present) to 6 (extreme). In order to discourage retrospective reporting, completed forms were mailed back weekly. To classify participants with PMDD, each met the following criteria (Endicott et al. 2006): 1) During the seven day window prior to menses, a score of 4 (moderate severity) or greater was required on at least two days on at least five of the items assessing mood state listed above; 2) During the seven day window prior to menses, a score of at least 4 (moderate) or greater was required on at least two days for at least one of the items assessing functional impairment listed above; 3) At least a 30% increase in total symptom severity score was required (see Data Analysis) during the seven days preceding menses compared with follicular phase days 4–10; 4) criteria 1–4 were met on two menstrual cycles.
Non-PMDD women met the following criteria: 1) no more than mild emotional symptoms occurring during the premenstrual days; 2) no evidence for functional impairment associated with emotional symptoms; 3) these criteria were met on two menstrual cycles.
Test Session
Each of the participants were tested once during the luteal phase of the menstrual cycle, 5–12 days after home urine testing revealed the luteinizing hormone surge that preceded ovulation (corresponding to days 18–25 of an idealized 28 day cycle). Cycle phase was subsequently confirmed to be ovulatory based upon serum progesterone. We tested in the luteal (symptomatic) phase only, since there is little consistent evidence that menstrual cycle phase influences blood pressure (BP) or heart rate (HR) in healthy controls (Stoney et al. 1990; Weidner and Helmig 1990) or BP, HR, and neuroendocrine differences between PMDD and non-PMDD groups at rest or during stress (Girdler et al. 1993; 1998; Straneva et al. 2002; Su et al. 1997).
All laboratory testing began between the hours of 7:00am and 9:30am. The laboratory visit lasted approximately three hours and thirty minutes and followed a fixed sequence. The order of testing was as follows: 1) Instrumentation for BP monitoring (Suntech 4240 Exercise BP monitor) and stethoscopic BP assessments to ensure reliable cuff placement and microphone position; 2) I.V. setup and recovery; 3) Baseline 1 Rest; 4) Beck Depression Inventory and Spielberger State Anxiety Inventory; 5) Pain Testing; 6) Recovery; 7) Baseline 2 Rest; 8) Trier Social Stress Test. These events are described more fully below.
I.V. Setup
A research nurse inserted a butterfly needle into a forearm vein. A non-heparinized, multi-stop-cock system was employed, which allowed the nurse to draw blood samples without the added stress involved in multiple venipunctures. Participants recovered for 15 minutes following IV placement.
Baseline 1 Rest
Quiet rest ensued for a subsequent 10 minutes. BP and HR measures were taken at minutes 1, 3, 6, and 9, and averaged. Blood was sampled at min 10 for norepinephrine (NE), β-endorphin, cortisol, and for progesterone.
Pain Testing Procedures
Participants were exposed to two pain tests. One of two task orders was used, counterbalancing order across PMDD and prior MDD groups.
The Submaximal Effort Tourniquet Procedure
In this procedure, as described previously (Maixner et al. 1990), a tourniquet cuff was positioned on the participant’s arm and the arm placed to the side. Before inflating the tourniquet cuff to 200 mm Hg (Hokanson E20 Rapid Cuff Inflator), the participant’s arm was raised for 30 seconds to promote venous drainage, and then the cuff was inflated, the experimenter’s stopwatch started, and the arm returned to the side. To promote forearm ischemia, participants engaged in 20 handgrip exercises at 30% of their maximum force with an intersqueeze interval of 2 seconds. Participants were instructed to indicate when the sensations in their arm first became painful (pain threshold) and when they were no longer willing or able to tolerate the pain (pain tolerance). A maximum time limit of 20 minutes was enforced, though participants were not informed of this limit.
Hand Cold Pressor
The apparatus for the cold pressor consisted of a container filled with ice and water that was maintained at 4°C as recorded immediately before initiating the test. The use of a water circulator prevented the water from warming near the participant’s hand. At the onset of the test, participants were instructed to submerge their hand to the marked line on their wrist and to remain still. Participants were instructed to indicate to the experimenter when the sensations in their hand first became painful (pain threshold) and to also indicate when they were no longer willing or able to tolerate the pain by saying “stop” (pain tolerance). A maximum time limit of 5 minutes was imposed, though participants were not informed of this limit.
Pain intensity and unpleasantness ratings were obtained for both the cold pressor and tourniquet ischemic pain tasks. Participants were instructed that at the point of tolerance for each pain task, they would be asked to rate the intensity and unpleasantness of their pain using separate visual analog scales (0 to 100). Thus, immediately before deflating the tourniquet cuff and immediately before removal of the hand from the ice bath, the experimenter held up one visual analog scale for intensity rating, with the 100-cm line anchored by the words “not at all intense” and “the most intense pain imaginable.” Next, the experimenter held up the scale for unpleasantness, with the 100-cm line anchored by the words “not at all unpleasant” and “the most unpleasant pain imaginable.”
Recovery
A five minute recovery followed each pain task.
Baseline 2 Rest
Following the recovery period, quiet rest ensued for an additional 10 minutes, serving as a baseline from which to calculate cardiovascular reactivity. BP and HR measures were taken at minutes 1, 3, 6, and 9 and averaged. Blood was sampled at min 10 for baseline levels of NE, β-endorphin, and cortisol.
The Trier Social Stress Test (TSST)
A modified version of the TSST (Kirschbaum et al. 1993) was employed (modified to include serial addition as opposed to serial subtraction). The TSST is a stress test which reliably induces large and consistent HPA-axis and cardiovascular responses (Kirschbaum et al. 1993; 1995a; 1995b). The TSST involves four components: 1) Pre-Task Instructions (5 min) during which time participants are introduced to the ‘selection committee’ who will later listen to their job talk. Participants are also given the instructions for the mental arithmetic task. The duration of the instruction period averaged 5 minutes; 2) Speech Preparation Period (5 min): Participants were told that they should imagine that they are applying for their ideal job and were then left alone for 5 minutes to prepare their talk describing why they would be the ideal candidate for the position; 3) Job Speech (5 min): immediately following the preparation period, the selection committee returned to the testing room and asked the participant to deliver her talk describing to the committee why she would be the perfect applicant for the position. If the participant finished before 5 minutes, the committee responded in a standardized way, with prepared questions to ensure that the participant spoke for the entire period; and 4) Paced Auditory Serial Addition Test (PASAT; (Gronwall 1977)) (8.5 min) involves the tape recorded presentation of numbers from 1 – 9. Participants are to add each number presented on the tape to the immediately preceding number and to state the answer aloud. There are four series of numbers, with progressively shorter inter-digit intervals (2.4, 2.0, 1.6, and 1.2 seconds). The experimenter remained in the room to monitor performance.
Task Assessments
Task assessment questionnaires were administered after the cessation of each pain task, as well as at the end of the TSST. Using a visual analog scale, the participants rated: 1) how difficult they found the task; 2) how tense they were during the task; 3) how well they were able to concentrate during the task; and 4) how much effort they put into the task.
Cardiovascular and Neuroendocrine Sampling During TSST
All measurements took place while participants were in a comfortable seated position. BP and HR measures were taken at minutes 1, 3, and 5 of the Speech Preparation Period, minutes 1, 3, and 5 of the Job Speech, and minutes 2, 4, 6, and 8 of Serial Addition and averaged to constitute task levels. NE was sampled at the end of minute 2 of Speech and minute 2 of Serial Addition since catecholamines peak within the first minutes of stress and have a short half-life (3 min) (Dimsdale and Ziegler 1991).
Our markers for assessing the HPA-axis were cortisol and β-endorphin, and these levels were assessed at minute 10 of Baseline 2 Rest. We were unable to assess these factors in response to stress since, due to logistical reasons, our laboratory study protocol was restricted to early morning hours when the diurnal effects on the HPA-axis are significant and prevent valid assessment of cortisol and β-endorphin stress reactivity (Clow et al. 2004; Cohen et al. 2006; Restituto et al. 2008). Thus, we restricted our analyses involving HPA-axis measures to Baseline rest concentrations only.
Rationale and Selection of Biomarkers
The cardiovascular biomarkers chosen in the present study to reflect the SNS axis were SBP, DBP, and HR (though HR is also determined by parasympathetic cardiac drive), since previous research has shown them to be sensitive to diagnosis related differences involving both PMDD and MDD (Carney et al. 1999; 1988; Hamer et al. 2007; Lechin et al. 1995; Girdler et al. 1993; 2007; Udupa et al. 2007; Volkers et al. 2003). Plasma NE was selected as the neuroendocrine biomarker of SNS activity since it is reflective of sympathetic nerve activity (Goldstein et al., 1983) and has consistently differentiated PMDD and MDD patients from controls (Girdler et al., 1998; Gold et al. 2005; Lake et al. 1982; Veith et al. 1994; Wong et al. 2000). Cortisol was selected as the biomarker of basal HPA-axis activity since it has been shown to differentiate PMDD and MDD patients from controls (Bancroft et al. 1991; Carroll et al. 2007; Catalan et al. 1998; Galard et al. 2002; Girdler at al., 1998; Gold et al. 1986a; 1986b; 2005; Goodwin et al. 1993; Gotthardt et al. 1995; Heuser et al. 1996; Juruena et al. 2006; Su et al. 1997; Wong et al. 2000; Young et al. 2001; 2000b), while β-endorphin was selected as an additional HPA-axis biomarker since it is released from both the hypothalamus and the pituitary gland and plays a key role in the suppression of pain sensations (Berne and Levy, 1998), it has been shown to be correlated with sensitivity to experimental pain (al’Absi et al.,2002; Girdler et al., 2005; Mechlin et al., 2005; Straneva et al., 2002), and it differs as a function of both PMDD and MDD diagnoses (Chuong et al. 1985; Facchinetti et al. 1994; Giannini et al. 1990; Goodwin et al., 1993; Krittayaphong et al., 1996; Straneva et al. 2002; Young et al., 2000b),
Measurements
Blood Pressure and Heart Rate
The Suntech Exercise BP monitor, Model 4240 (SunTech Medical Instruments, Inc., Raleigh, NC) provided automated measurement of BP and HR during the sessions. The Suntech Exercise BP monitor uses the auscultatory technique, with R-wave Gating. This BP monitor is accurate within +/− 2 mmHg between 0 mmHg and 300 mmHg. Prior to initiating the baseline rest period, five standard stethoscopic blood pressures were taken simultaneously with the automated pressures in order to ensure correct microphone placement and cuff positioning.
Plasma Norepinephrine
Norepinephrine concentrations were determined using the ELISA (Enzyme-Linked ImmunoSorbent Assay) method, and employing a kit from ALPC Diagnostics. The sensitivity of the assay is 0.9 pg/mL, and the intra- and inter-assay coefficients were 5% and 6.1% respectively.
Plasma cortisol and serum progesterone
Plasma cortisol and serum progesterone were determined using radioimmunoassay techniques commercially available from ICN Pharmaceuticals, INC. The specificity of the antiserum for progesterone is very high, showing only 0.05–2.5% cross-reactivity with other steroid compounds, and the sensitivity of the assay is 0.01 ng/mL. The intra- and inter-assay coefficients of variation from the progesterone assay were approximately 5.2% and 10.9%, respectively. Luteal phase progesterone levels <3 mg/ml were considered reflective of an anovulatory cycle. For cortisol, the sensitivity of the assay is 0.07 μg/dL and the specificity is high, showing 0.05–2.2% cross-reactivity with similar compounds, except prednisolone, where 94% cross-reactivity is obtained. The intra- and inter-assay coefficients of variation from the cortisol assay were approximately 3% and 9.8%, respectively.
Plasma β-endorphin
Plasma β-endorphin levels in EDTA plasma were determined using the ELISA method, and employing a kit from MD Biosciences. The intra- and inter-assay coefficients of variation from the assay were approximately 6% and 12%, respectively, and the assay sensitivity was 0.18 μg/ml.
DATA ANALYSIS
Demographics
Group differences in demographic factors, state anxiety and depression scores assessed during the laboratory protocol, and baseline SNS and HPA-axis factors were examined using a 2 (PMDD: yes vs. no) × 2 (Prior MDD: yes vs. no) ANOVA or chi square analyses. In order to assess whether the proportion of PMDD women as well as the proportion of women with a history of MDD differed by minority race, a 2 (PMDD) × 2 (Prior MDD) × 2 (Race: Non-Hispanic Whites vs. Minorities: African American, Hispanic, Asian, or Multi-racial) chi square analysis was utilized. Similarly, a 2 (PMDD) × 2 (Prior MDD) × 2 (Abuse: yes vs. no) chi square analysis was performed to determine whether the proportion of women with an abuse history differed by PMDD or Prior MDD status. Because we detected marginally greater abuse rates in non-PMDD women with prior MDD relative to non-PMDD women with no prior MDD (see Table 1), all analyses employed abuse histories (Yes = 1, No = 0) as a covariate. Although the covariate of abuse history was non-significant for all analyses (all ps > .05), it was retained nonetheless as a more conservative analytic approach. Finally, for women with prior MDD, PMDD and non-PMDD groups were compared on months since last major depressive episode and number of prior MDD episodes with a one way ANOVA. Where significant interactions emerged, simple effects analyses conducted separately in MDD or PMDD groups were conducted to explore the source of the interaction.
Table 1.
Mean (±SEM) Demographic Factors as a Function of PMDD Status and Prior MDD
| Non-PMDD | PMDD | |||
|---|---|---|---|---|
| No Prior MDD (n = 18) | Prior MDD (n = 9) | No Prior MDD (n = 17) | Prior MDD (n = 10) | |
| Age | 33.1 (2.0) | 31.1 (2.9) | 34.5 (2.1) | 33.9 (2.7) |
| Body Mass Index | 24.1 (1.3) | 25.6 (1.9) | 25.5 (1.4) | 23.7 (1.8) |
| A Beck Depression Inventory | 2.1 (1.2) | 3.4 (1.7) | 7.6 (1.3) | 7.6 (16) |
| State Anxiety | 27.5 (2.1) | 29.7 (2.9) | 31.7 (2.2) | 32.0 (2.8) |
| No. Minority Race (%) | 8 (44%) | 1 (11%) | 7 (41%) | 4 (40%) |
| B No. Abuse (%) | 5 (28%) | 6 (67%) | 9 (53%) | 4 (40%) |
| Prior Episodes of MDD | NA | 1.9 (.27) | NA | 1.4 (.26) |
| Months in Remission from MDD | NA | 59.5 (23.6) | NA | 87.6 (22.4) |
PMDD > Non-PMDD; p < .05
Prior MDD > No Prior MDD in Non-PMDD women only; p = .05
Daily Symptom Ratings
For each of the 24 DRSP symptoms, a follicular (days 4 through 10) and luteal (days -7 through -1 before menses) phase average was calculated for cycle one and cycle two, and cycle one and cycle two were averaged to create an overall follicular and an overall luteal phase average. Next, each symptom was placed into one of five core symptom categories (Endicott et al. 2006): 1. Somatic (fatigue, breast tenderness, breast swelling or bloating, headache, joint or muscle pain); 2. Depression: (depressed, hopeless, worthless or guilty, slept more, trouble sleeping, overwhelmed); 3. Anger/Irritability: (anger or irritability, conflict); 4. Anxiety: (anxiety); and 5. Impairment: (less productivity or efficiency, interference with hobbies or social activities, interference with relationships), and averaged to yield one total follicular and luteal score for each of the five core categories.
Women were assessed for differences in the five core symptom categories using a 2 (PMDD) × 2 (Prior MDD) × 2 (Menstrual Cycle Phase) repeated measures ANOVA with Menstrual Cycle Phase as the repeated factor. Where significant interactions emerged, simple effects analyses conducted separately in MDD or PMDD groups were conducted to explore the source of the interaction.
Hypothalamic Pituitary Adrenal (HPA)-Axis and Sympathetic Nervous System (SNS)
Group differences in baseline cortisol and β-endorphin were analyzed using a 2 (PMDD) × 2 (Prior MDD) ANOVA. Next, cardiovascular and NE stress responsivity based on PMDD and Prior MDD status were examined by calculating delta scores (Stress task – Baseline) and performing a 2 (PMDD) × 2 (Prior MDD) × 2 (Stress Task: Speech vs. Math) repeated measures ANOVA with stress task as the repeated factor.
Pain Sensitivity for Cold Pressor and Tourniquet Ischemic Tasks
To determine diagnosis-related differences in pain sensitivity, a 2 (PMDD) × 2 (Prior MDD) × 2 (Period: Threshold vs. Tolerance) repeated measures ANOVA with Period as the repeated factor was performed separately for the ischemic and cold pressor pain task since these pain tasks differ in their endogenous pain regulatory mechanisms (e.g. opioid versus non-opioid, respectively) (Frid et al., 1979; Girdler et al., 2005; Schull et al., 1981). Pain intensity and unpleasantness ratings from 0–100 given immediately following voluntary tolerance for each pain task were analyzed using a 2 (PMDD) × 2 (Prior MDD) ANOVA. Where significant interactions emerged, simple effects analyses were conducted separately in MDD or PMDD groups to examine the source of the interaction.
Task Assessments
The participants’ experiences of each pain and stress task (difficulty, tension, inability to concentrate, effort) as measured by the task assessment questionnaire was analyzed by PMDD and Prior MDD status using a 2 (PMDD) × 2 (Prior MDD) ANOVA. The analyses were performed separately for each pain and stress task and for each of the 4 items on the task assessment. Higher scores indicate greater difficulty, tension, inability to concentrate, and effort.
RESULTS
Demographics
As seen in Table 1 there were no group differences in age, BMI, state anxiety, or minority race (ps > .05). PMDD women had higher BDI scores than non-PMDD women (F(4, 53) = 11.5, p < .01), results that would be expected during the symptomatic luteal phase of the menstrual cycle when the inventory was administered. In addition, a marginally greater proportion of non-PMDD women with prior MDD had an abuse history relative to non-PMDD women with no prior MDD (χ2 = 3.8, p = .05). Lastly, in analyses performed in women with prior MDD only, PMDD and non-PMDD women did not differ in number of prior episodes of MDD or months in remission from MDD (all ps >.05).
Daily Symptom Ratings
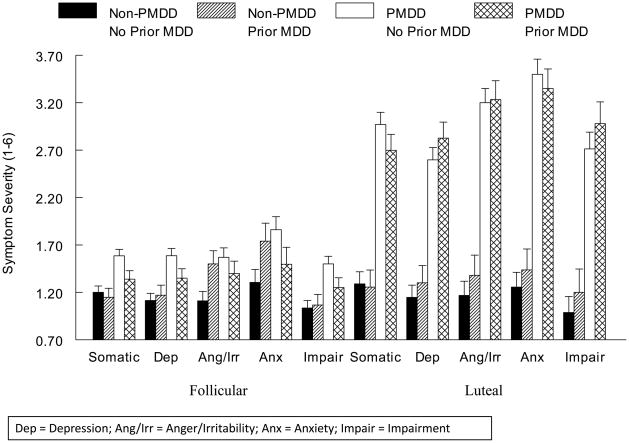
As expected, for non-PMDD women, no menstrual cycle-related differences in any core symptom category were present (all ps > .05), while for PMDD women, symptom severity was significantly greater in the luteal phase than the follicular phase for each core symptom category (all ps < .0001) (Figure 1). While in general, women with PMDD reported greater severity of somatic symptoms (F(1, 49) = 100.3, p < .0001), depression (F(1, 49) = 82.3, p < .0001), anger/irritability (F(1, 49) = 76.8, p < .0001), anxiety (F(1, 49) = 56.9, p < .0001), and impairment (F(1, 49) = 68.0, p < .0001) than non-PMDD women, PMDD × Menstrual Cycle Phase interactions were present for all symptom categories [somatic (F(1, 49) = 66.4, p < .0001), depression (F(1, 49) = 57.4, p < .0001), anger/irritability (F(1, 49) = 94.1, p < .0001), anxiety (F(1, 49) = 111.7 p < .0001), and impairment (F(1, 49) = 49.5, p < .0001)], reflecting the significantly greater PMDD-related differences for somatic symptoms, depression, and impairment in the luteal phase than in the follicular phase. For the symptoms of anger/irritability (F(4, 53) = 117.5, p < .0001) and anxiety (F(4, 53) = 125.7, p < .0001), however, the PMDD-related differences were isolated to the luteal phase only.
Figure 1.
Mean (± SEM) Daily Mood Ratings Core Symptom Categories as a Function of PMDD and Prior MDD Status
Hypothalamic Pituitary Adrenal Axis Biomarkers
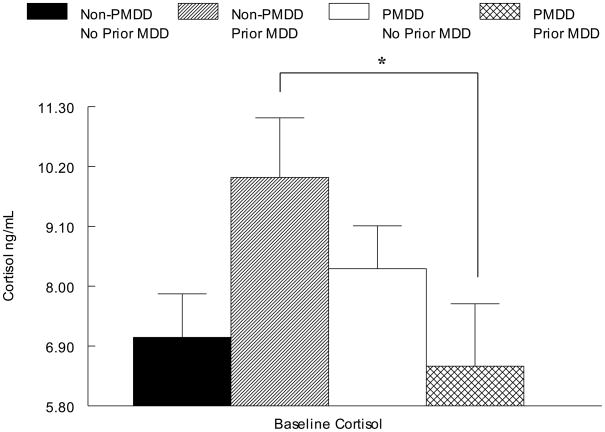
As seen in Table 2 and Figure 2, the omnibus ANOVA revealed a PMDD × Prior MDD interaction for cortisol (F(4, 50) = 5.8, p < .05). The source of this interaction was assessed with a simple effects analysis conducted separately in each MDD group, indicating that PMDD women had lower baseline cortisol concentrations than non-PMDD women, but only if a history of MDD was present (F(2, 16) = 4.5, p = .05). In contrast, there were no diagnosis-related differences in baseline β-endorphin (all ps > .05) (Table 2).
Table 2.
Mean (±SEM) Baseline SNS and HPA-axis Factors as a Function of PMDD Status and Prior MDD
| Non-PMDD | PMDD | |||
|---|---|---|---|---|
| No Prior MDD (n = 18) | Prior MDD (n = 9) | No Prior MDD (n = 17) | Prior MDD (n = 10) | |
| Systolic Blood Pressure | 110.0 (2.8) | 109.5 (4.2) | 111.9 (2.9) | 112.8 (3.8) |
| Diastolic Blood Pressure | 65.2 (2.1) | 68 (3.1) | 70.3 (2.1) | 67.7 (2.7) |
| Heart Rate | 66.4 (2.6) | 68.2 (3.7) | 67.0 (2.7) | 65.9 (3.5) |
| A Norepinephrine (pg/mL) | 399.9 (39.0) | 272.8 (51.6) | 358.4 (39.5) | 389.2 (57.8) |
| B Cortisol (ng/mL) | 7.1 (. 80) | 10.0 (1.1) | 8.3 (.79) | 6.5 (1.1) |
| B-endorphin (ng/mL) | .059 (.008) | .064 (.01) | .078 (.007) | .072 (.01) |
No Prior MDD > Prior MDD only in non-PMDD; p = .06
PMDD < non-PMDD only in Prior MDD; p < .05
Figure 2.
Mean (± SEM) Cortisol as a Function of PMDD and Prior MDD Status
Sympathetic Nervous System Biomarkers
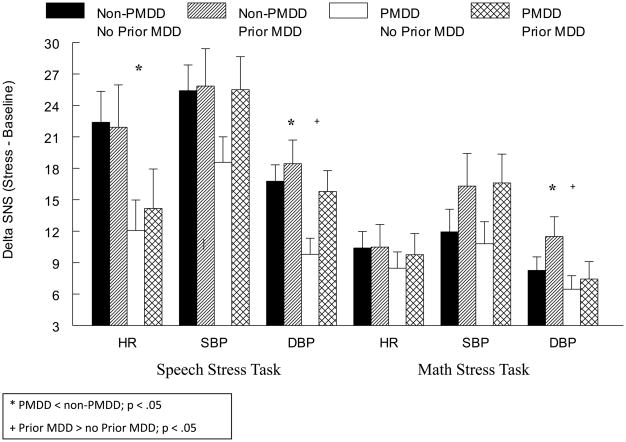
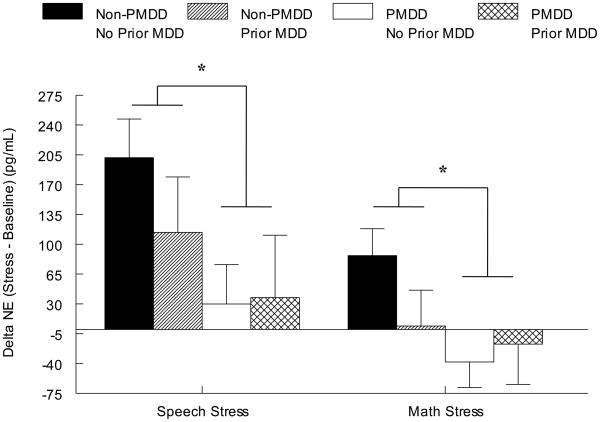
Despite no diagnosis-related differences for HR, SBP, DBP, or NE at baseline rest (Table 2), the omnibus ANOVA indicated a main effect of PMDD diagnosis for DBP (F(1, 47) = 8.1, p < .01) and NE (F(1, 38) = 6.0, p < .05) reactivity to stress since PMDD women had blunted reactivity relative to non-PMDD women, regardless of MDD histories (Figures 3 and 4). The omnibus test also revealed a PMDD × Stress Task interaction for HR (F(1, 48) = 10.8, p < .01). Subsequent simple effects analyses conducted separately by task revealed that regardless of MDD histories, PMDD women had blunted HR responses to the speech task relative to non-PMDD women (F(4, 52) = 7.0, p < .05). Furthermore, the omnibus ANOVA also indicated that women with a history of MDD, regardless of PMDD status, displayed greater DBP stress reactivity than women with no history of MDD during both stressors (F(1, 47) = 4.7, p < .05). No diagnosis-related differences in the SBP response to stress were present (all ps > .05).
Figure 3.
Mean (± SEM) Change in Sympathetic Nervous System Factors from Baseline to Speech Stress as a Function of PMDD and Prior MDD Status
Figure 4.
Mean (± SEM) Change in Norepinephrine from Baseline to Speech Stress as a Function of PMDD and Prior MDD Status
Speech and Math Task Assessments
A seen in Table 3, the omnibus tests revealed that PMDD women, regardless of MDD history, reported more difficulty (F(3, 52) = 6.3, p < .05), more tension (F(4, 52) = 9.5, p < .01), and a greater inability to concentrate (F(4, 52) = 15.5, p < .001) during speech stress than non-PMDD women. No diagnosis-related differences for self-reported effort were present for the speech stressor (all ps > .05).
Table 3.
Mean (±SEM) Stress Task Assessments as a Function of PMDD Status and Prior MDD
| Non-PMDD | PMDD | ||||
|---|---|---|---|---|---|
| No Prior MDD (n = 18) | Prior MDD (n = 9) | No Prior MDD (n = 17) | Prior MDD (n = 10) | ||
| Speech Stress Task | A Difficulty | 5.7 (.6) | 4.8 (.9) | 6.0 (.6) | 8.1 (.8) |
| A Tension | 5.3 (.6) | 4.3 (.9) | 6.3 (.6) | 7.8 (.8) | |
| A Inability to Concentrate | 3.8 (.6) | 3.5 (.8) | 6.0 (.6) | 7.0 (.8) | |
| Effort | 3.6 (.2) | 3.4 (.2) | 3.5 (.2) | 3.2 (.2) | |
| Math Stress Task | B Difficulty | 6.5 (.6) | 8.8 (.8) | 7.5 (.5) | 8.0 (.7) |
| Tension | 6.0 (.6) | 7.7 (.8) | 7.5 (.6) | 6.9 (.8) | |
| C Inability to Concentrate | 5.7 (.6) | 5.1 (.8) | 7.6 (.6) | 7.2 (.8) | |
| Effort | 7.8 (.5) | 7.7 (.7) | 8.6 (.5) | 8.5 (.7) | |
PMDD > Non-PMDD; p < .05;
Prior MDD > No Prior MDD; p <.05
PMDD > Non-PMDD; p < .01
For math stress, the omnibus ANOVA revealed that PMDD women, regardless of MDD history, reported greater inability to concentrate than non-PMDD women (F(4, 52) = 7.9, p < .01), while women with prior MDD, regardless of PMDD, reported greater difficulty than women with no prior MDD (F(4, 52) = 4.5, p < .05) (Table 3). No diagnosis-related differences for self-reported tension or effort were present for the math stressor (all ps > .05).
Pain Sensitivity to Cold Pressor and Tourniquet ischemic Tasks
Cold Pressor Task
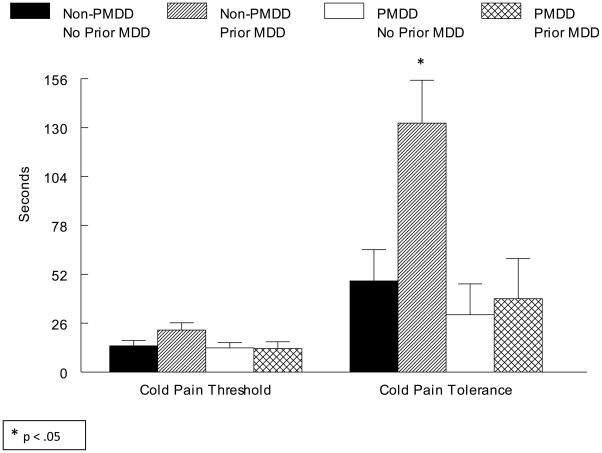
For the cold pressor task, the omnibus ANOVA revealed a PMDD × Prior MDD interaction (F(1, 48) = 4.0, p = .05). Subsequent simple effects analyses conducted separately in PMDD and non-PMDD women revealed that only in non-PMDD women were women with prior MDD less sensitive to cold pressor pain than women with no prior MDD (F(1, 23) = 4.6, p < .05; see Figure 5).
Figure 5.
Mean (± SEM) Cold Pressor Pain Threshold and Tolerance as a Function of PMDD and Prior MDD Status
Tourniquet Ischemic Task
Although the same pattern of effects was present for tourniquet ischemic pain sensitivity (i.e., less sensitivity in non-PMDD women with prior MDD), the PMDD × Prior MDD interaction failed to reach conventional levels of statistical significance.
Pain Intensity and Unpleasantness Ratings and Task Assessments
As summarized in Table 4, the omnibus ANOVA revealed a PMDD × Prior MDD interaction for cold pressor unpleasantness (F(4, 53) = 4.1, p < .05). Subsequent simple effects analyses conducted separately in those with versus without a history of MDD indicated that only in women with a history of MDD did PMDD women report greater cold pressor pain unpleasantness than non-PMDD women (F(2, 18) = 11.1, p < .01). No significant differences in the task assessments (difficulty, tension, inability to concentrate, and effort) were found for the cold pressor task (ps > .05).
Table 4.
Mean (±SEM) Pain Task Assessments as a Function of PMDD Status and Prior MDD
| Non-PMDD | PMDD | ||||
|---|---|---|---|---|---|
| No Prior MDD (n = 18) | Prior MDD (n = 9) | No Prior MDD (n = 17) | Prior MDD (n = 10) | ||
| Cold Pressor Pain Task | Intensity | 52.2 (4.7) | 36.3 (6.7) | 48.1 (4.8) | 43.0 (6.2) |
| A Unpleasantness | 53.9 (4.9) | 37.8 (6.9) | 48.8 (5.0) | 56.9 (6.5) | |
| Difficulty | 4.3 (.8) | 4.5 (1.2) | 4.4 (.8) | 5.5 (1.1) | |
| Tension | 4.6 (.8) | 6.0 (1.1) | 5.0 (.8) | 5.3 (1.0) | |
| Inability to Concentrate | 3.6 (.9) | 4.3 (1.2) | 4.9 (.9) | 5.2 (1.1) | |
| Effort | 6.3 (.7) | 6.5 (1.0) | 6.5 (.7) | 7.8 (.9) | |
| Tourniquet Ischemic Pain Task | B Intensity | 36.8 (3.9) | 26.9 (5.5) | 36.3 (4.0) | 27.4 (5.1) |
| Unpleasantness | 42.6 (4.2) | 35.0 (5.9) | 38.6 (4.3) | 34.9 (5.5) | |
| Difficulty | 3.5 (.6) | 3.1 (.8) | 2.2 (.6) | 2.6 (.8) | |
| Tension | 3.6 (.7) | 3.6 (.9) | 3.7 (.7) | 3.8 (.9) | |
| Inability to Concentrate | 2.5 (.6) | 1.1 (.8) | 2.6 (.6) | 3.0 (.8) | |
| A Effort | 6.2 (.8) | 5.3 (1.1) | 5.1 (.8) | 8.6 (1.0) | |
PMDD > non-PMDD only in Prior MDD; p < .01
Prior MDD < No Prior MDD; p < .05
For the tourniquet ischemic task, the omnibus ANOVA revealed that women with a history of MDD reported less pain intensity than did women with no history of MDD, regardless of PMDD status (F(4, 53) = 4.1, p< .05), although no group differences were found for tourniquet ischemic unpleasantness (ps > .05). The omnibus test also revealed a PMDD × Prior MDD interaction for reported effort during the tourniquet ischemic task emerged (F(4, 53) = 5.4, p < .05). Subsequent simple effects analyses conducted separately as a function of MDD diagnosis indicated that only in women with prior MDD did PMDD women report putting forth more effort than non-PMDD women (F(2, 18) = 5.7, p < .05). No group differences were present for difficulty, tension, and inability to concentrate (all ps > .05).
DISCUSSION
The results of the present investigation suggest unique phenotypic differences associated with PMDD and a history of MDD with respect to cardiovascular and neuroendocrine stress-responsive factors. At the same time, however, our results also indicate that histories of MDD may hold special relevance for PMDD women, at least with respect to the HPA-axis and pain sensitivity.
The chief finding of our study that supports a consistent phenotypic difference between PMDD and MDD diagnoses was the observation that PMDD women, regardless of whether they have a history of MDD, show blunted stress reactivity in biomarkers reflecting sympathetic nervous system (SNS) activation relative to non-PMDD women. This was true for BP, HR and plasma NE and is consistent with other prior reports in PMDD samples (Girdler et al. 1993; 1998), but adds to that prior literature by controlling for histories of MDD.
In contrast, after controlling for histories of MDD, we did not find evidence for heightened sensitivity to experimental pain as reflected in pain threshold or tolerance levels that has previously been reported in PMDD cohorts (Fillingim et al. 1995; Kuczmierczyk and Adams 1986; Kuczmierczyk et al. 1986; Straneva et al. 2002), though we did find that in women with no prior MDD history, PMDD women reported greater pain unpleasantness than non-PMDD women. On the other hand, the most robust evidence for diagnosis related differences in pain sensitivity was seen in non-PMDD women with histories of MDD who differed from all other women in their greater cold pressor threshold and tolerance levels. Moreover, all women with prior MDD, regardless of the PMDD diagnosis, exhibited lower pain intensity ratings to the cold pressor test as well. Given that up to 37% of non-PMDD women would be expected to have a history of MDD (Weiss et al. 1999), the absence of controlling for histories of MDD in all prior studies of PMDD may have contributed to the findings for decreased experimental pain sensitivity in non-PMDD women relative to PMDD women (Fillingim et al. 1995; Kuczmierczyk and Adams 1986; Kuczmierczyk et al. 1986; Straneva et al. 2002).
It is worth underscoring that one remarkable feature of our findings is that, on average, the non-PMDD women with prior MDD, who showed the markedly elevated cold pressor pain threshold and tolerance levels, had been in full remission from their last MDD episode for nearly five years. While other studies, including a recent meta-analysis, have reported decreased pain sensitivity in patients with current MDD (Bar et al. 2005; 2006; Lautenbacher et al. 1994; 1999; Sindrup and Jensen 1999), our results add to two other reports in the literature documenting the persistence of this hypoalgesia in patients in remission from their MDD. Specifically, Bar and colleagues (2003) reported increased thermal pain threshold and tolerance in euthymic women with a history MDD compared to controls, and a recent study from our laboratory showed that women with a history of mood disorders were less sensitive to tourniquet ischemic pain than women with no history of a mood disorder (Klatzkin et al. 2007). Thus, the results of the current investigation add to the evidence that histories of MDD are associated with persistent alterations in pain sensitivity in women, particularly women without comorbid PMDD. Taken together, our preliminary findings suggest that altered sensitivity to experimental pain is more likely a phenotypic feature of histories of MDD than it is of PMDD.
Given the well documented evidence that, relative to men, women have increased clinical pain (Unruh 1996) and also show decreased experimental pain tolerance (Riley et al. 1998; Sheffield et al. 2000), it may seem paradoxical that women with a history of MDD, who also have increased clinical pain (Bromberger et al. 2005), show decreases sensitivity to experimental pain stimuli. Lautenbacher and Krieg (1994) have addressed this paradox by hypothesizing that diminished processing of painful stimuli could be responsible for both phenomena. The authors argue that reduced processing of nociceptive stimuli at both spinal and subcortical stages may cause hypoalgesia to phasic experimental pain, and at the same time cause hyperalgesia to endogenous clinical pain due to deficient activation of inhibitory systems. This theory is supported by Bar et al. (2005) who found increased pain sensitivity to ischemic pain (deep somatic pain), but decreased pain sensitivity to heat and electric pain (phasic surface pain) in patients with current MDD compared to controls. Although Lautenbacher et al. (1999) failed to find a significant correlation between clinical pain complaints and pain threshold in depressed patients, this does not rule out the possibility that alterations in central and peripheral pain processing contribute to both phenomena (i.e. increased clinical pain but decreased sensitivity to acute experimental pain). Thus, our results indicating that women with a history of MDD continue to display dysfunctions in pain regulation are consistent with the vast majority of the literature on experimental pain sensitivity in MDD and may well relate to the increased clinical pain reported in the disorder.
In addition to evidence for persistent disturbance in pain sensitivity associated with histories of MDD, and consistent with the literature in patients with current MDD who show heightened sympathetic reactivity to stress (Carney et al. 1999; Hamer et al. 2007; Lechin et al. 1995), our study found that women with histories of MDD, regardless of PMDD status, showed heightened DBP reactivity to mental stressors relative to never depressed women. While others have shown increased activation of the HPA-axis in euthymic individuals with histories of depression (Kathol 1985; Pintor et al. 2007; Trestman et al. 1991; Young et al. 2000a), findings that are consistent with our observation for elevated basal cortisol levels in non-PMDD women with prior MDD, this is the first study to our knowledge to examine cardiovascular reactivity to stress as a function of histories of MDD.
The potential clinical relevance of the disturbances seen in both the SNS and HPA-axis biomarkers employed in the present study comes from the growing evidence from both animal and human studies that chronic or severe stress exposure can result in persistent alterations in neurobiological systems that are stress responsive, including the SNS and HPA-axes (Bremner and Vermetten, 2001; Coplan et al., 1996; Heim et al., 2001; Ladd et al., 1996; Lindley et al., 2004). PMDD is more recently conceptualized as a stress-related disorder, owing both to greater traumatic stress rates in PMDD populations (Girdler et al., 2003; 2004; Golding et al., 2000; Perkonnig et al., 2004; Wittchen et al., 2003) as well as greater chronic psychosocial stress exposure (Girdler 1993; 1998). Not only may MDD itself be considered a chronic stressor but there is robust clinical and experimental evidence that environmental stressors serve as a pathogenic trigger to develop MDD (Kendler et al., 2004). While the phenotypic differences seen in SNS stress reactivity in the present study between women with PMDD and those with histories of MDD cannot be explained at present, the pattern of blunted SNS reactivity in the PMDD cohort is consistent with animal and human studies suggesting that long-term activation of the stress axes (due to repeated or severe stress) can manifest in hypo-responsiveness of the system to subsequent stressors (DeBellis and Thomas, 2003; Lindley et al., 2004; Resnick et al., 2005; Rosler, 1994; Young and Breslau, 2004), especially in the face of on-going chronic stress (Heim et al., 2001).
Consistent with the concept of allostatic load (McEwen and Stellar, 1993), we hypothesize that in women, genetically vulnerable to develop PMDD (Kendler et al., 1998), the SNS-axis will show cumulative long-term effects of the systems’ attempts to adapt to chronic/severe stress, and that this will be manifested in blunted SNS reactivity to subsequent stressors. In contrast, our results suggest that in women genetically vulnerable to develop MDD (Kendler et al., 2001; 2006; Sullivan et al., 2000), hyper-reactivity of the stress axes may be sustained beyond remission of the depressive episode as suggested by other research showing persistent HPA- axis activation in euthymic individuals with histories of MDD (Kathol 1985; Young et al. 2000a). Exaggerated reactivity to stress is also consistent with enhanced allostatic load (McEwen and Stellar, 1993), possibly resulting from chronic or severe stress exposure in those vulnerable to MDD.
The persistent disturbance in not only SNS and HPA-axis function, but also in pain sensitivity, that we noted in women free of current MDD but who had histories of MDD raises the possibility that these alterations may reflect a trait, as opposed to state, characteristic of MDD and thus underlie the neurobiological vulnerability to develop of MDD. This is consistent with the suggestion that persistent dysregulation in stress responses to even mild stressors may form the basis for the development of mood disorders (Heim et al. 2001), though longitudinal studies would be needed to address this issue.
While this study reports evidence to suggest that both PMDD and histories of MDD are associated with independent stress-related phenotypic profiles, we also obtained evidence that histories of MDD may hold special relevance for PMDD women. For example, a history of MDD was associated with diametrically opposite effects on basal cortisol concentrations in PMDD and non-PMDD women since PMDD women with prior MDD had lower cortisol concentrations relative to all other groups and statistically lower than the non-PMDD women with similar MDD histories. While blunted HPA-axis activation has been previously documented in PMDD (Chuong et al. 1985; Facchinetti et al. 1994; Giannini et al. 1990; Girdler and Klatzkin 2006; Girdler et al. 2007; 1998; Roca et al. 2003; Straneva et al. 2002), since those prior studies did not control for prior MDD, the effect may well have been driven by greater rates of prior MDD in PMDD samples. That the HPA-axis is differentially affected by histories of MDD in PMDD women also comes from other studies of the HPA-axis modulator, allopregnanolone. Allopregnanolone is a GABAergic neuroactive steroid that negatively modulates the HPA-axis to restore homeostasis following stress (Bitran et al. 1999; 2000; Brot et al. 1997; Brussaard et al. 1999; Guo et al. 1995; Morrow et al. 1987). Our prior research has shown that allopregnanolone reactivity to stress predicts PMDD symptoms, but only in PMDD women with a history of MDD and not in PMDD with no history of MDD (Klatzkin et al. 2006b). Thus, there may be a special relevance of prior MDD related to the regulation of the HPA-axis and symptomatology in PMDD.
Hypocortisolemia may also have special clinical relevance for PMDD patients as recent evidence has indicated that blunted HPA axis function is associated with a number of stress-related bodily disorders (Heim et al. 2000). Other evidence that histories of MDD differentially impacted PMDD women comes from our results showing that only in women with a history of MDD did PMDD women report greater unpleasantness during the cold pressor task and more effort during the tourniquet ischemic task than non-PMDD women. Since PMDD is a disorder characterized by heightened mood symptoms during the luteal phase of the menstrual cycle when the laboratory study protocol took place, the greater negative ratings by PMDD women with histories of MDD during the pain and stress tasks, that were not seen in non-PMDD with prior MDD, may reflect an additive effect of PMDD and a prior history of MDD. Thus, with respect to both HPA-axis activation and stress-induced mood states, a history of MDD may provide a “context of vulnerability” for women with PMDD.
However, it must be acknowledged that β-endorphin concentrations, also reflecting HPA-axis activity (Tsigos and Chrousos 2002), did not differ as a function of either PMDD or prior MDD status. Results pertaining to diagnosis-related differences in β-endorphin concentrations in MDD (Hegadoren et al. 2009) or PMDD (Bloch et al. 1998; Chuong et al. 1985; Facchinetti et al. 1994; Giannini et al. 1990; Hamilton and Gallant 1988; Rapkin et al. 1996) samples have been mixed. While the absence of parallel findings for basal cortisol and β-endorphin concentrations in the current study cannot be explained, since β-endorphin is released from the anterior pituitary while cortisol is released from the adrenal cortex, it is possible that the absence of parallel findings in our study reflects the differential regulation of the HPA-axis, including its feedback mechanisms, by histories of MDD in PMDD and non-PMDD women. While the elevated cortisol concentrations in non-PMDD women with prior MDD is consistent with the vast majority of studies in patients with current MDD (Galard et al. 2002; Goodwin et al. 1993; Gotthardt et al. 1995; Young et al. 2000b) and is consistent with enhanced central drive of the HPA axis, the lower cortisol concentrations in PMDD women with prior MDD (also consistent with the vast majority of studies in PMDD women with unknown MDD histories; (Girdler et al. 1998; Straneva et al. 2002) may reflect diminished central drive of the axis and/or enhanced negative feedback by cortisol on both the hypothalamus and the anterior pituitary. In the absence of adrenocorticotropin (ACTH) concentrations this must remain a speculative hypothesis but, regardless of mechanism, the results do underscore existing evidence for persistent alteration in the regulation of the HPA-axis in women with histories of MDD (Heim et al. 2008; Karp et al. 2005; Kunzel et al. 2003; Plotsky et al. 1998) and add to that literature by suggesting that the alteration in the axis is influenced differently in women who have current comorbid PMDD.
While our study has many notable strengths, including the use of strict diagnostic criteria based on structured interview and daily prospective ratings to establish the MDD and PMDD diagnoses, respectively, as well as a comprehensive assessment of stress-relevant biomarkers and pain response measures in order to address an innovative question related to the unique versus shared phenotypic profiles of PMDD and MDD, it is not without its limitations. A clear limitation to the present study relates to the small sample size in each of the PMDD × MDD cells. This limited our ability to conduct correlational analyses examining the relationship of stress-responsive measures to either pain sensitivity and/or symptom severity. Given the exploratory nature of our study, in combination with the small cell sizes, we also did not adjust for the number of different statistical tests and this must too be viewed as a limitation. As such, the present results should be considered preliminary though these results should serve a heuristic value for future studies designed to pursue such questions.
Another limitation to the study design concerns the fixed order of the pain and mental stress tests. It could be argued that diagnosis-related differences in pain responses carried over to influence diagnosis-related differences in responses to the mental stressors. While this cannot be ruled out, it is unlikely because: 1) only non-PMDD women with prior MDD differed in their pain sensitivity, yet all women with prior MDD (PMDD and non-PMDD) showed greater BP responses to stress; 2) prior research from our laboratory in healthy non-PMDD and never depressed individuals did counter-balance the order of pain testing and mental stress testing using identical pain and stress tests employed in the present study, and we found no evidence that order of events influenced responses to either the pain test or the mental stress test (Mechlin et al. 2005) and; 3) 15 minutes of recovery and rest was interspersed between the two conditions.
Finally, it could be argued that the heterogeneity of our sample with respect to histories of sexual or physical abuse is a limitation of our study. While it must be acknowledged that the inclusion of women with abuse histories was to meet the aims of the larger, on-going parent investigation, their inclusion also increases the generalizability of our findings to both PMDD samples, since histories of abuse are reported in 40 – 80% of PMDD populations (Girdler et al. 2003; 2004; 2007; Paddison et al. 1990) and to women with histories of MDD, since a history of abuse is a known risk factor for the development of MDD (Weiss et al. 1999). Moreover, even in non-PMDD and non-MDD samples, rates of abuse for women in the United States are sobering. Population-based national surveys have indicated that 13%–27% of women are sexually abused as children (Badgley 1984; Finkelhor et al. 1990; MacMillan et al. 1997). When adult sexual abuse, battering, and other forms of physical abuse are included, more than one third of women from the general population have had these experiences (Resnick et al. 1993). Consistent with the evidence linking histories of abuse to the development of MDD, we did find that non-PMDD women with prior MDD were more likely to have an abuse history than non-PMDD women with no prior MDD. However, this proportional difference is unlikely to explain the MDD-related differences in the biological measures in our study since non-PMDD women with prior MDD exhibited elevated cortisol and no difference in NE responses – a pattern opposite to that typically associated with abuse histories (i.e., lower cortisol or blunted cortisol response to challenge and elevated NE) (Bremner and Vermetten 2001; Bremner et al. 1996; Heim et al. 2001; Newport et al. 2004; Resnick et al. 1995; Stein et al. 1997). Moreover, we statistically controlled for histories of abuse in all analyses. Thus, while our sample is heterogeneous with respect to histories of abuse, we believe it is representative of the population of women with mood disorders.
In summary, our results suggest that both PMDD and histories of MDD are associated with unique phenotypic profiles with respect to stress-responsive measures and pain sensitivity, though histories of MDD may also have special relevance to PMDD women. If these results are confirmed, longitudinal studies designed to assess the predictive ability of persistent disturbance in stress reactivity or pain sensitivity and subsequent risk for recurrence of depressive episodes in women with a history of depression would be indicated and would inform the design of future biobehavioral intervention studies.
Acknowledgments
This research was support by NIH grants MH051246, MH080837, and MH079532, CTRC grant RR00046, and CTSA grant UL1RR025747.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM - IV. Washington, DC: 1994. [Google Scholar]
- Ahrens T, Deuschle M, Krumm B, van der Pompe G, den Boer JA, Lederbogen F. Pituitary-adrenal and sympathetic nervous system responses to stress in women remitted from recurrent major depression. Psychosom Med. 2008;70:461–467. doi: 10.1097/PSY.0b013e31816b1aaa. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Petersen KL, Wittmers LE. Adrenocortical and hemodynamic predictors of pain perception in men and women. Pain. 2002;96:197–204. doi: 10.1016/s0304-3959(01)00447-x. [DOI] [PubMed] [Google Scholar]
- Alexander JL, Dennerstein L, Kotz K, Richardson G. Women, anxiety and mood: a review of nomenclature, comorbidity and epidemiology. Expert Rev Neurother. 2007;7:S45–58. doi: 10.1586/14737175.7.11s.S45. [DOI] [PubMed] [Google Scholar]
- Badgley R. Sexual offences against children/report of the Committee on Sexual Offences Against Children and Youths. Ministry of Supply and Services; Ottawa, Canada: 1984. [Google Scholar]
- Bancroft J, Cook A, Davidson D, Bennie J, Goodwin G. Blunting of neuroendocrine responses to infusion of L-tryptophan in women with perimenstrual mood change. Psychol Med. 1991;21:305–312. doi: 10.1017/s0033291700020407. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Brehm S, Boettger MK, Boettger S, Wagner G, Sauer H. Pain perception in major depression depends on pain modality. Pain. 2005;117:97–103. doi: 10.1016/j.pain.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Brehm S, Boettger MK, Wagner G, Boettger S, Sauer H. Decreased sensitivity to experimental pain in adjustment disorder. Eur J Pain. 2006;10:467–471. doi: 10.1016/j.ejpain.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Bar KJ, Greiner W, Letsch A, Kobele R, Sauer H. Influence of gender and hemispheric lateralization on heat pain perception in major depression. J Psychiatr Res. 2003;37:345–353. doi: 10.1016/s0022-3956(03)00051-7. [DOI] [PubMed] [Google Scholar]
- Berne R, Levy M. Physiology. 4. Mosby Inc; 1998. pp. 939–946. [Google Scholar]
- Bitran D, Dugan M, Renda P, Ellis R, Foley M. Anxiolytic effects of the neuroactive steroid pregnanolone (3 alpha-OH-5 beta-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 1999;850:217–224. doi: 10.1016/s0006-8993(99)02150-2. [DOI] [PubMed] [Google Scholar]
- Bitran D, Klibansky DA, Martin GA. The neurosteroid pregnanolone prevents the anxiogenic-like effect of inescapable shock in the rat. Psychopharmacology (Berl) 2000;151:31–37. doi: 10.1007/s002130000472. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Su TP, Tobin MB, Rubinow DR. Pituitary-adrenal hormones and testosterone across the menstrual cycle in women with premenstrual syndrome and controls. Biol Psychiatry. 1998;43:897–903. doi: 10.1016/s0006-3223(98)00403-x. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E. Stress and development: behavioral and biological consequences. Development and psychopathology. 2001;13:473–489. doi: 10.1017/s0954579401003042. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Charney DS, Southwick SM. Neural mechanisms in dissociative amnesia for childhood abuse: relevance to the current controversy surrounding the “false memory syndrome”. Am J Psychiatry. 1996;153:71–82. doi: 10.1176/ajp.153.7.71. [DOI] [PubMed] [Google Scholar]
- Broadley AJ, Frenneaux MP, Moskvina V, Jones CJ, Korszun A. Baroreflex sensitivity is reduced in depression. Psychosom Med. 2005;67:648–651. doi: 10.1097/01.psy.0000170829.91643.24. [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Kravitz HM, Wei HL, Brown C, Youk AO, Cordal A, Powell LH, Matthews KA. History of depression and women’s current health and functioning during midlife. Gen Hosp Psychiatry. 2005;27:200–208. doi: 10.1016/j.genhosppsych.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Brot MD, Akwa Y, Purdy RH, Koob GF, Britton KT. The anxiolytic-like effects of the neurosteroid allopregnanolone: interactions with GABA(A) receptors. Eur J Pharmacol. 1997;325:1–7. doi: 10.1016/s0014-2999(97)00096-4. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Devay P, Leyting-Vermeulen JL, Kits KS. Changes in properties and neurosteroid regulation of GABAergic synapses in the supraoptic nucleus during the mammalian female reproductive cycle. J Physiol. 1999;516 (Pt 2):513–524. doi: 10.1111/j.1469-7793.1999.0513v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Veith RC, Cryer PE, Skala JA, Lynch T, Jaffe AS. Major depression, heart rate, and plasma norepinephrine in patients with coronary heart disease. Biol Psychiatry. 1999;45:458–463. doi: 10.1016/s0006-3223(98)00049-3. [DOI] [PubMed] [Google Scholar]
- Carney RM, Rich MW, teVelde A, Saini J, Clark K, Freedland KE. The relationship between heart rate, heart rate variability and depression in patients with coronary artery disease. J Psychosom Res. 1988;32:159–164. doi: 10.1016/0022-3999(88)90050-5. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Cassidy F, Naftolowitz D, Tatham NE, Wilson WH, Iranmanesh A, Liu PY, Veldhuis JD. Pathophysiology of hypercortisolism in depression. Acta Psychiatr Scand Suppl. 2007:90–103. doi: 10.1111/j.1600-0447.2007.00967.x. [DOI] [PubMed] [Google Scholar]
- Catalan R, Gallart JM, Castellanos JM, Galard R. Plasma corticotropin-releasing factor in depressive disorders. Biol Psychiatry. 1998;44:15–20. doi: 10.1016/s0006-3223(97)00539-8. [DOI] [PubMed] [Google Scholar]
- Chuong CJ, Coulam CB, Kao PC, Bergstralh EJ, Go VL. Neuropeptide levels in premenstrual syndrome. Fertil Steril. 1985;44:760–765. doi: 10.1016/s0015-0282(16)49034-5. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cohen LS, Soares CN, Otto MW, Sweeney BH, Liberman RF, Harlow BL. Prevalence and predictors of premenstrual dysphoric disorder (PMDD) in older premenopausal women. The Harvard Study of Moods and Cycles. J Affect Disord. 2002;70:125–132. doi: 10.1016/s0165-0327(01)00458-x. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB. Persistent elevations of cerebrospinal fluid concentrations of corticotrophin-releasing factor in adult nonhuman primates exposed to early-life stressors: Implications for the pathophysiology of mood and anxiety disordes. Proc Natl Acad Sci. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydov DM, Shapiro D, Cook IA, Goldstein I. Baroreflex mechanisms in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:164–177. doi: 10.1016/j.pnpbp.2006.08.015. [DOI] [PubMed] [Google Scholar]
- DeBellis MD, Thomas LA. Biologic findings of post-traumatic stress disorder and child maltreatment. Current Psychiatry Reports. 2003;5:108–117. doi: 10.1007/s11920-003-0027-z. [DOI] [PubMed] [Google Scholar]
- Dickens C, McGowan L, Dale S. Impact of depression on experimental pain perception: a systematic review of the literature with meta-analysis. Psychosom Med. 2003;65:369–375. doi: 10.1097/01.psy.0000041622.69462.06. [DOI] [PubMed] [Google Scholar]
- Dimsdale JE, Ziegler MG. What do plasma and urinary measures of catecholamines tell us about human response to stressors? Circulation. 1991;83:II36–42. [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB. Ethnic differences in thermal pain responses. Psychosom Med. 1999;61:346–354. doi: 10.1097/00006842-199905000-00014. [DOI] [PubMed] [Google Scholar]
- Endicott J. The menstrual cycle and mood disorders. J Affect Disord. 1993;29:193–200. doi: 10.1016/0165-0327(93)90033-g. [DOI] [PubMed] [Google Scholar]
- Endicott J, Amsterdam J, Eriksson E, Frank E, Freeman E, Hirschfeld R, Ling F, Parry B, Pearlstein T, Rosenbaum J, Rubinow D, Schmidt P, Severino S, Steiner M, Stewart DE, Thys-Jacobs S. Is premenstrual dysphoric disorder a distinct clinical entity? J Womens Health Gend Based Med. 1999;8:663–679. doi: 10.1089/jwh.1.1999.8.663. [DOI] [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health. 2006;9:41–49. doi: 10.1007/s00737-005-0103-y. [DOI] [PubMed] [Google Scholar]
- Facchinetti F, Fioroni L, Martignoni E, Sances G, Costa A, Genazzani AR. Changes of opioid modulation of the hypothalamo-pituitary-adrenal axis in patients with severe premenstrual syndrome. Psychosom Med. 1994;56:418–422. doi: 10.1097/00006842-199409000-00006. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Edwards RR, Powell T. The relationship of sex and clinical pain to experimental pain responses. Pain. 1999;83:419–425. doi: 10.1016/S0304-3959(99)00128-1. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Girdler SS, Booker D, Light KC, Harris MB, Maixner W. Pain sensitivity in women with premenstrual dysphoric disorder: A preliminary report. Journal of Womens Health. 1995;4:367–374. [Google Scholar]
- Finkelhor D, Hotaling G, Lewis IA, Smith C. Sexual abuse in a national survey of adult men and women: prevalence, characteristics, and risk factors. Child abuse & neglect. 1990;14:19–28. doi: 10.1016/0145-2134(90)90077-7. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sondheimer SJ. Premenstrual Dysphoric Disorder: Recognition and Treatment. Prim Care Companion J Clin Psychiatry. 2003;5:30–39. doi: 10.4088/pcc.v05n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frid M, Singer G, Rana C. Interactions between personal expectations and naloxone: effects on tolerance to ischemic pain. Psychopharmacology (Berl) 1979;65:225–231. doi: 10.1007/BF00492208. [DOI] [PubMed] [Google Scholar]
- Galard R, Catalan R, Castellanos JM, Gallart JM. Plasma corticotropin-releasing factor in depressed patients before and after the dexamethasone suppression test. Biol Psychiatry. 2002;51:463–468. doi: 10.1016/s0006-3223(01)01273-2. [DOI] [PubMed] [Google Scholar]
- Giannini AJ, Martin DM, Turner CE. Beta-endorphin decline in late luteal phase. 1990. [DOI] [PubMed] [Google Scholar]
- Girdler S, Klatzkin R. Biological Responses to Stress in Premenstrual Dysphoric Disorder: Influence of Histories of Abuse and Depression. Gynaecology Forum. 2006;11:27–32. [Google Scholar]
- Girdler SS, Leserman J, Bunevicius R, Klatzkin R, Pedersen CA, Light KC. Persistent alterations in biological profiles in women with abuse histories: influence of premenstrual dysphoric disorder. Health Psychol. 2007;26:201–213. doi: 10.1037/0278-6133.26.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler SS, Maixner W, Naftel HA, Stewart PW, Moretz RL, Light KC. Cigarette smoking, stress-induced analgesia and pain perception in men and women. Pain. 2005;114:372–385. doi: 10.1016/j.pain.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Pedersen CA, Stern RA, Light KC. Menstrual cycle and premenstrual syndrome: modifiers of cardiovascular reactivity in women. Health Psychol. 1993;12:180–192. doi: 10.1037//0278-6133.12.3.180. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Pedersen CA, Straneva PA, Leserman J, Stanwyck CL, Benjamin S, Light KC. Dysregulation of cardiovascular and neuroendocrine responses to stress in premenstrual dysphoric disorder. Psychiatry Res. 1998;81:163–178. doi: 10.1016/s0165-1781(98)00074-2. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Sherwood A, Hinderliter AL, Leserman J, Costello NL, Straneva PA, Pedersen CA, Light KC. Biological correlates of abuse in women with premenstrual dysphoric disorder and healthy controls. Psychosom Med. 2003;65:849–856. doi: 10.1097/01.psy.0000088593.38201.cd. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Thompson KS, Light KC, Leserman J, Pedersen CA, Prange AJ., Jr Historical sexual abuse and current thyroid axis profiles in women with premenstrual dysphoric disorder. Psychosom Med. 2004;66:403–410. doi: 10.1097/01.psy.0000127690.38525.ab. [DOI] [PubMed] [Google Scholar]
- Gold PW, Calabrese JR, Kling MA, Avgerinos P, Khan I, Gallucci WT, Tomai TP, Chrousos GP. Abnormal ACTH and cortisol responses to ovine corticotropin releasing factor in patients with primary affective disorder. Prog Neuropsychopharmacol Biol Psychiatry. 1986a;10:57–65. doi: 10.1016/0278-5846(86)90044-8. [DOI] [PubMed] [Google Scholar]
- Gold PW, Loriaux DL, Roy A, Kling MA, Calabrese JR, Kellner CH, Nieman LK, Post RM, Pickar D, Gallucci W, et al. Responses to corticotropin-releasing hormone in the hypercortisolism of depression and Cushing’s disease. Pathophysiologic and diagnostic implications. N Engl J Med. 1986b;314:1329–1335. doi: 10.1056/NEJM198605223142101. [DOI] [PubMed] [Google Scholar]
- Gold PW, Wong ML, Goldstein DS, Gold HK, Ronsaville DS, Esler M, Alesci S, Masood A, Licinio J, Geracioti TD, Jr, Perini G, DeBellis MD, Holmes C, Vgontzas AN, Charney DS, Chrousos GP, McCann SM, Kling MA. Cardiac implications of increased arterial entry and reversible 24-h central and peripheral norepinephrine levels in melancholia. Proc Natl Acad Sci U S A. 2005;102:8303–8308. doi: 10.1073/pnas.0503069102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding JM, Taylor DL, Menard L, King MJ. Prevalence of sexual abuse history in a sample of women seeking treatment for premenstrual syndrome. J Psychosom Obstet Gynecol. 2000;21:69–80. doi: 10.3109/01674820009075612. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, McCarty R, Polinsky RJ, Kopin IJ. Relationship between plasma norepinephrine and sympathetic neural activity. Hypertension. 1983;5:552–559. doi: 10.1161/01.hyp.5.4.552. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Austin MP, Curran SM, Ross M, Murray C, Prentice N, Ebmeier KP, Bennie J, Carroll S, Dick H, et al. The elevation of plasma beta-endorphin levels in major depression. J Affect Disord. 1993;29:281–289. doi: 10.1016/0165-0327(93)90018-f. [DOI] [PubMed] [Google Scholar]
- Gotthardt U, Schweiger U, Fahrenberg J, Lauer CJ, Holsboer F, Heuser I. Cortisol, ACTH, and cardiovascular response to a cognitive challenge paradigm in aging and depression. Am J Physiol. 1995;268:R865–873. doi: 10.1152/ajpregu.1995.268.4.R865. [DOI] [PubMed] [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Guo AL, Petraglia F, Criscuolo M, Ficarra G, Nappi RE, Palumbo MA, Trentini GP, Purdy RH, Genazzani AR. Evidence for a role of neurosteroids in modulation of diurnal changes and acute stress-induced corticosterone secretion in rats. Gynecol Endocrinol. 1995;9:1–7. doi: 10.3109/09513599509160184. [DOI] [PubMed] [Google Scholar]
- Halbreich U. Selective serotonin reuptake inhibitors and initial oral contraceptives for the treatment of PMDD: effective but not enough. CNS Spectr. 2008;13:566–572. doi: 10.1017/s1092852900016849. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Kahn LS. Role of estrogen in the aetiology and treatment of mood disorders. CNS Drugs. 2001;15:797–817. doi: 10.2165/00023210-200115100-00005. [DOI] [PubMed] [Google Scholar]
- Halbreich U, O’Brien PM, Eriksson E, Backstrom T, Yonkers KA, Freeman EW. Are there differential symptom profiles that improve in response to different pharmacological treatments of premenstrual syndrome/premenstrual dysphoric disorder? CNS Drugs. 2006;20:523–547. doi: 10.2165/00023210-200620070-00001. [DOI] [PubMed] [Google Scholar]
- Hamer M, Tanaka G, Okamura H, Tsuda A, Steptoe A. The effects of depressive symptoms on cardiovascular and catecholamine responses to the induction of depressive mood. Biol Psychol. 2007;74:20–25. doi: 10.1016/j.biopsycho.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Hamilton JA, Gallant S. Premenstrual symptom changes and plasma beta-endorphin/beta-lipotropin throughout the menstrual cycle. Psychoneuroendocrinology. 1988;13:505–514. doi: 10.1016/0306-4530(88)90036-4. [DOI] [PubMed] [Google Scholar]
- Harrison WM, Endicott J, Nee J, Glick H, Rabkin JG. Characteristics of women seeking treatment for premenstrual syndrome. Psychosomatics. 1989;30:405–411. doi: 10.1016/S0033-3182(89)72246-5. [DOI] [PubMed] [Google Scholar]
- Hartlage SA, Arduino KE, Gehlert S. Premenstrual dysphoric disorder and risk for major depressive disorder: a preliminary study. J Clin Psychol. 2001;57:1571–1578. doi: 10.1002/jclp.1119. [DOI] [PubMed] [Google Scholar]
- Hegadoren KM, O’Donnell T, Lanius R, Coupland NJ, Lacaze-Masmonteil N. The role of beta-endorphin in the pathophysiology of major depression. Neuropeptides. 2009 doi: 10.1016/j.npep.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biol Psychiatry. 2008;63:398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heuser IJ, Schweiger U, Gotthardt U, Schmider J, Lammers CH, Dettling M, Yassouridis A, Holsboer F. Pituitary-adrenal-system regulation and psychopathology during amitriptyline treatment in elderly depressed patients and normal comparison participants. Am J Psychiatry. 1996;153:93–99. doi: 10.1176/ajp.153.1.93. [DOI] [PubMed] [Google Scholar]
- Juruena MF, Cleare AJ, Papadopoulos AS, Poon L, Lightman S, Pariante CM. Different responses to dexamethasone and prednisolone in the same depressed patients. Psychopharmacology (Berl) 2006;189:225–235. doi: 10.1007/s00213-006-0555-4. [DOI] [PubMed] [Google Scholar]
- Karp JF, Scott J, Houck P, Reynolds CF, 3rd, Kupfer DJ, Frank E. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66:591–597. doi: 10.4088/jcp.v66n0508. [DOI] [PubMed] [Google Scholar]
- Kathol RG. Persistent elevation of urinary free cortisol and loss of circannual periodicity in recovered depressive patients. A trait finding. J Affect Disord. 1985;8:137–145. doi: 10.1016/0165-0327(85)90036-9. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Neale MC, Prescott CA. Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychol Med. 2001;31:605–616. doi: 10.1017/s0033291701003907. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006;163:109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Corey LA, Neale MC. Longitudinal population-based twin study of retrospectively reported premenstrual symptoms and lifetime major depression. Am J Psychiatry. 1998;155:1234–1240. doi: 10.1176/ajp.155.9.1234. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Prescott CA. Childhood sexual abuse, stressful life events and risk for major depression in women. Psychol Med. 2004;34:1475–1482. doi: 10.1017/s003329170400265x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and participantive responses to acute psychological stress. Psychosom Med. 1995a;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Prussner JC, Stone AA, Federenko I, Gaab J, Lintz D, Schommer N, Hellhammer DH. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med. 1995b;57:468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Klauenberg S, Maier C, Assion HJ, Hoffmann A, Krumova EK, Magerl W, Scherens A, Treede RD, Juckel G. Depression and changed pain perception: hints for a central disinhibition mechanism. Pain. 2008;140:332–343. doi: 10.1016/j.pain.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Mechlin B, Bunevicius R, Girdler SS. Race and histories of mood disorders modulate experimental pain tolerance in women. J Pain. 2007;8:861–868. doi: 10.1016/j.jpain.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzkin RR, Morrow AL, Light KC, Pedersen CA, Girdler SS. Associations of histories of depression and PMDD diagnosis with allopregnanolone concentrations following the oral administration of micronized progesterone. Psychoneuroendocrinology. 2006a;31:1208–1219. doi: 10.1016/j.psyneuen.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Klatzkin RR, Morrow AL, Light KC, Pedersen CA, Girdler SS. Histories of depression, allopregnanolone responses to stress, and premenstrual symptoms in women. Biol Psychol. 2006b;71:2–11. doi: 10.1016/j.biopsycho.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Krittayaphong R, Light KC, Golden RN, Finkel JB, Sheps DS. Relationship among depression scores, beta-endorphin, and angina pectoris during exercise in patients with coronary artery disease. Clin J Pain. 1996;12:126–133. doi: 10.1097/00002508-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Kuczmierczyk AR, Adams HE. Autonomic arousal and pain sensitivity in women with premenstrual syndrome at different phases of the menstrual cycle. J Psychosom Res. 1986;30:421–428. doi: 10.1016/0022-3999(86)90081-4. [DOI] [PubMed] [Google Scholar]
- Kuczmierczyk AR, Adams HE, Calhoun KS, Naor S, Giombetti R, Cattalani M, McCann P. Pain responsivity in women with premenstrual syndrome across the menstrual cycle. Percept Mot Skills. 1986;63:387–393. doi: 10.2466/pms.1986.63.2.387. [DOI] [PubMed] [Google Scholar]
- Kunzel HE, Binder EB, Nickel T, Ising M, Fuchs B, Majer M, Pfennig A, Ernst G, Kern N, Schmid DA, Uhr M, Holsboer F, Modell S. Pharmacological and nonpharmacological factors influencing hypothalamic-pituitary-adrenocortical axis reactivity in acutely depressed psychiatric in-patients, measured by the Dex-CRH test. Neuropsychopharmacology. 2003;28:2169–2178. doi: 10.1038/sj.npp.1300280. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Owens MJ, Nemeroff CB. Persistent changes in corticotrophin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology. 1996;137:1212–1218. doi: 10.1210/endo.137.4.8625891. [DOI] [PubMed] [Google Scholar]
- Lake CR, Pickar D, Ziegler MG, Lipper S, Slater S, Murphy DL. High plasma norepinephrine levels in patients with major affective disorder. Am J Psychiatry. 1982;139:1315–1318. doi: 10.1176/ajp.139.10.1315. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Krieg JC. Pain perception in psychiatric disorders: a review of the literature. J Psychiatr Res. 1994;28:109–122. doi: 10.1016/0022-3956(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Roscher S, Strian D, Fassbender K, Krumrey K, Krieg JC. Pain perception in depression: relationships to symptomatology and naloxone-sensitive mechanisms. Psychosom Med. 1994;56:345–352. doi: 10.1097/00006842-199407000-00010. [DOI] [PubMed] [Google Scholar]
- Lautenbacher S, Spernal J, Schreiber W, Krieg JC. Relationship between clinical pain complaints and pain sensitivity in patients with depression and panic disorder. Psychosom Med. 1999;61:822–827. doi: 10.1097/00006842-199911000-00015. [DOI] [PubMed] [Google Scholar]
- Lechin F, van der Dijs B, Orozco B, Lechin ME, Baez S, Lechin AE, Rada I, Acosta E, Arocha L, Jimenez V, et al. Plasma neurotransmitters, blood pressure, and heart rate during supine-resting, orthostasis, and moderate exercise conditions in major depressed patients. Biol Psychiatry. 1995;38:166–173. doi: 10.1016/0006-3223(94)00258-5. [DOI] [PubMed] [Google Scholar]
- Leserman J, Li Z, Drossman DA, Toomey TC, Nachman G, Glogau L. Impact of sexual and physical abuse dimensions on health status: development of an abuse severity measure. Psychosom Med. 1997;59:152–160. doi: 10.1097/00006842-199703000-00007. [DOI] [PubMed] [Google Scholar]
- Lindley SE, Carlson EB, Benoit M. Basal and dexamethasone suppressed salivary cortisol concentrations in a community sample of patients with posttraumatic stress disorder. Biological Psychiatry. 2004;55:940–945. doi: 10.1016/j.biopsych.2003.12.021. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Fleming JE, Trocme N, Boyle MH, Wong M, Racine YA, Beardslee WR, Offord DR. Prevalence of child physical and sexual abuse in the community. Results from the Ontario Health Supplement. Jama. 1997;278:131–135. [PubMed] [Google Scholar]
- Maixner W. Interactions between cardiovascular and pain modulatory systems: physiological and pathophysiological implications. Journal of Cardiovascular Electrophysiology. 1991;2:S3–12. [Google Scholar]
- Maixner W, Gracely RH, Zuniga JR, Humphrey CB, Bloodworth GR. Cardiovascular and sensory responses to forearm ischemia and dynamic hand exercise. Am J Physiol. 1990;259:R1156–1163. doi: 10.1152/ajpregu.1990.259.6.R1156. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153:2093–2101. [PubMed] [Google Scholar]
- Mechlin MB, Maixner W, Light KC, Fisher JM, Girdler SS. African Americans show alterations in endogenous pain regulatory mechanisms and reduced pain tolerance to experimental pain procedures. Psychosom Med. 2005;67:948–956. doi: 10.1097/01.psy.0000188466.14546.68. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–485. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Newport DJ, Heim C, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal responses to standard and low-dose dexamethasone suppression tests in adult survivors of child abuse. Biol Psychiatry. 2004;55:10–20. doi: 10.1016/s0006-3223(03)00692-9. [DOI] [PubMed] [Google Scholar]
- Paddison PL, Gise LH, Lebovits A, Strain JJ, Cirasole DM, Levine JP. Sexual abuse and premenstrual syndrome: comparison between a lower and higher socioeconomic group. Psychosomatics. 1990;31:265–272. doi: 10.1016/S0033-3182(90)72162-7. [DOI] [PubMed] [Google Scholar]
- Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocrinol Metab Clin North Am. 1997;26:279–294. doi: 10.1016/s0889-8529(05)70247-4. [DOI] [PubMed] [Google Scholar]
- Pearlstein T, Steiner M. Premenstrual dysphoric disorder: burden of illness and treatment update. J Psychiatry Neurosci. 2008;33:291–301. [PMC free article] [PubMed] [Google Scholar]
- Pearlstein TB, Frank E, Rivera-Tovar A, Thoft JS, Jacobs E, Mieczkowski TA. Prevalence of axis I and axis II disorders in women with late luteal phase dysphoric disorder. J Affect Disord. 1990;20:129–134. doi: 10.1016/0165-0327(90)90126-s. [DOI] [PubMed] [Google Scholar]
- Perkonigg A, Yonkers KA, Pfister H, Lieb R, Wittchen HU. Risk factors for premenstrual dysphoric disorder in a community sample of young women: The role of traumatic events and posttraumatic stress disorder. J Clinical Psychiatry. 2004;65:1314–1322. doi: 10.4088/jcp.v65n1004. [DOI] [PubMed] [Google Scholar]
- Pintor L, Torres X, Navarro V, Martinez de Osaba MA, Matrai S, Gasto C. Corticotropin-releasing factor test in melancholic patients in depressed state versus recovery: a comparative study. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1027–1033. doi: 10.1016/j.pnpbp.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatr Clin North Am. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Shoupe D, Reading A, Daneshgar KK, Goldman L, Bohn Y, Brann DW, Mahesh VB. Decreased central opioid activity in premenstrual syndrome: luteinizing hormone response to naloxone. Journal of the Society for Gynecologic Investigation. 1996;3:93–98. doi: 10.1016/1071-5576(95)00045-3. [DOI] [PubMed] [Google Scholar]
- Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. Journal of consulting and clinical psychology. 1993;61:984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- Resnick HS, Yehuda R, Pitman RK, Foy DW. Effect of previous trauma on acute plasma cortisol level following rape. Am J Psychiatry. 1995;152:1675–1677. doi: 10.1176/ajp.152.11.1675. [DOI] [PubMed] [Google Scholar]
- Restituto P, Galofre JC, Gil MJ, Mugueta C, Santos S, Monreal JI, Varo N. Advantage of salivary cortisol measurements in the diagnosis of glucocorticoid related disorders. Clin Biochem. 2008;41:688–692. doi: 10.1016/j.clinbiochem.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Riley JL, 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- Roca CA, Schmidt PJ, Altemus M, Deuster P, Danaceau MA, Putnam K, Rubinow DR. Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. J Clin Endocrinol Metab. 2003;88:3057–3063. doi: 10.1210/jc.2002-021570. [DOI] [PubMed] [Google Scholar]
- Roca CA, Schmidt PJ, Rubinow DR. A follow-up study of premenstrual syndrome. J Clin Psychiatry. 1999;60:763–766. doi: 10.4088/jcp.v60n1108. [DOI] [PubMed] [Google Scholar]
- Rosler A. Editorial: Long-term effects of childhood sexual abuse on the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 1994;78:247–248. doi: 10.1210/jcem.78.2.8106607. [DOI] [PubMed] [Google Scholar]
- Schull J, Kaplan H, O’Brien C. Naloxone can alter experimental pain and mood in humans. Physiological Psychology. 1981;9:245–251. [Google Scholar]
- Schwier C, Kliem A, Boettger MK, Bär KJ. Increased cold-pain thresholds in major depression. J Pain. 2009 doi: 10.1016/j.jpain.2009.07.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Sheffield D, Biles PL, Orom H, Maixner W, Sheps DS. Race and sex differences in cutaneous pain perception. Psychosom Med. 2000;62:517–523. doi: 10.1097/00006842-200007000-00010. [DOI] [PubMed] [Google Scholar]
- Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83:389–400. doi: 10.1016/S0304-3959(99)00154-2. [DOI] [PubMed] [Google Scholar]
- Stein MB, Yehuda R, Koverola C, Hanna C. Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biol Psychiatry. 1997;42:680–686. doi: 10.1016/s0006-3223(96)00489-1. [DOI] [PubMed] [Google Scholar]
- Steiner M, Romano SJ, Babcock S, Dillon J, Shuler C, Berger C, Carter D, Reid R, Stewart D, Steinberg S, Judge R. The efficacy of fluoxetine in improving physical symptoms associated with premenstrual dysphoric disorder. Bjog. 2001;108:462–468. doi: 10.1111/j.1471-0528.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- Steiner M, Soares CN. Psychotropic Therapies. Informa Healthcare; London (UK): 2008. [Google Scholar]
- Stoney CM, Owens JF, Matthews KA, Davis MC, Caggiula A. Influences of the normal menstrual cycle on physiologic functioning during behavioral stress. Psychophysiology. 1990;27:125–135. doi: 10.1111/j.1469-8986.1990.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Straneva PA, Maixner W, Light KC, Pedersen CA, Costello NL, Girdler SS. Menstrual cycle, beta-endorphins, and pain sensitivity in premenstrual dysphoric disorder. Health Psychol. 2002;21:358–367. [PubMed] [Google Scholar]
- Su TP, Schmidt PJ, Danaceau M, Murphy DL, Rubinow DR. Effect of menstrual cycle phase on neuroendocrine and behavioral responses to the serotonin agonist m-chlorophenylpiperazine in women with premenstrual syndrome and controls. J Clin Endocrinol Metab. 1997;82:1220–1228. doi: 10.1210/jcem.82.4.3905. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Terhaar J, Boettger MK, Schwier C, Wagner G, Israel AK, Bär KJ. Increased sensitivity to heat pain after sad mood induction in female patients with major depression. Eur J Pain. 2009;2009 doi: 10.1016/j.ejpain.2009.09.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Trestman RL, Coccaro EF, Bernstein D, Lawrence T, Gabriel SM, Horvath TB, Siever LJ. Cortisol responses to mental arithmetic in acute and remitted depression. Biol Psychiatry. 1991;29:1051–1054. doi: 10.1016/0006-3223(91)90361-o. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Udupa K, Sathyaprabha TN, Thirthalli J, Kishore KR, Lavekar GS, Raju TR, Gangadhar BN. Alteration of cardiac autonomic functions in patients with major depression: a study using heart rate variability measures. J Affect Disord. 2007;100:137–141. doi: 10.1016/j.jad.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- Veith RC, Lewis N, Linares OA, Barnes RF, Raskind MA, Villacres EC, Murburg MM, Ashleigh EA, Castillo S, Peskind ER, et al. Sympathetic nervous system activity in major depression. Basal and desipramine-induced alterations in plasma norepinephrine kinetics. Arch Gen Psychiatry. 1994;51:411–422. doi: 10.1001/archpsyc.1994.03950050071008. [DOI] [PubMed] [Google Scholar]
- Volkers AC, Tulen JH, van den Broek WW, Bruijn JA, Passchier J, Pepplinkhuizen L. Motor activity and autonomic cardiac functioning in major depressive disorder. J Affect Disord. 2003;76:23–30. doi: 10.1016/s0165-0327(02)00066-6. [DOI] [PubMed] [Google Scholar]
- Weidner G, Helmig L. Cardiovascular stress reactivity and mood during the menstrual cycle. Women Health. 1990;16:5–21. doi: 10.1300/J013v16n03_02. [DOI] [PubMed] [Google Scholar]
- Weiss EL, Longhurst JG, Mazure CM. Childhood sexual abuse as a risk factor for depression in women: psychosocial and neurobiological correlates. Am J Psychiatry. 1999;156:816–828. doi: 10.1176/ajp.156.6.816. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Perkonigg A, Pfister H. Trauma and PTSD – An overlooked pathogenic pathway for premenstrual dysphoric disorder? Arch of Womens Ment Health. 2003;6:293–297. doi: 10.1007/s00737-003-0028-2. [DOI] [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TD, Jr, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci U S A. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA. The association between premenstrual dysphoric disorder and other mood disorders. J Clin Psychiatry 58 Suppl. 1997;15:19–25. [PubMed] [Google Scholar]
- Young EA, Aggen SH, Prescott CA, Kendler KS. Similarity in saliva cortisol measures in monozygotic twins and the influence of past major depression. Biol Psychiatry. 2000a;48:70–74. doi: 10.1016/s0006-3223(00)00842-8. [DOI] [PubMed] [Google Scholar]
- Young EA, Breslau N. Cortisol and catecholamines in posttraumatic stress disorder. Arch Gen Psychiatry. 2004;61:394–401. doi: 10.1001/archpsyc.61.4.394. [DOI] [PubMed] [Google Scholar]
- Young EA, Carlson NE, Brown MB. Twenty-four-hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology. 2001;25:267–276. doi: 10.1016/S0893-133X(00)00236-0. [DOI] [PubMed] [Google Scholar]
- Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Hormonal evidence for altered responsiveness to social stress in major depression. Neuropsychopharmacology. 2000b;23:411–418. doi: 10.1016/S0893-133X(00)00129-9. [DOI] [PubMed] [Google Scholar]