Abstract
Background
The lack of biomarkers that are predictive of safety is a critical gap in the development of microbicides. The present experiments were designed to evaluate the predictive value of in vitro models of microbicide safety.
Methods
Changes in the epithelial barrier were evaluated by measuring transepithelial electrical resistance (TER) after exposure of human epithelial cells to candidate microbicides in a dual-chamber system. The significance of observed changes was addressed by challenging cultures with human immuodeficiency virus (HIV) and measuring the ability of virus to cross the epithelium and infect target T cells cultured in the lower chamber.
Results
Exposure to nonoxynol-9 (N-9) or cellulose sulfate (CS), but not 9-[2-(phosphonomethoxy)propyl]adenine (also referred to as tenofovir) or PRO2000, resulted in a rapid and sustained reduction in TER and a marked increase in HIV infection of T cells cultured in the lower chamber. Moreover, CS triggered nuclear factor κB activation in peripheral blood mononuclear cells and increased HIV replication in chronically infected U1 cells.
Conclusions
Epithelial barrier disruption and enhanced viral replication may have contributed to the increased risk of HIV acquisition observed in phase 3 trials of N-9 and CS. Expansion of in vitro safety testing to include these models would provide a more stringent preclinical assessment of microbicide safety and may prove to be more predictive of clinical outcomes.
The growing human immunodeficiency virus (HIV) pandemic, which disproportionately affects women, demands novel prevention strategies that obviate the need for condom negotiation. Topical microbicides offer such a strategy. However, the lack of biomarkers that are predictive of safety and efficacy has thwarted the development of microbicides. Clinical trial failures with nonoxynol-9 (N-9), C31G (Savvy), Carraguard, and cellulose sulfate (CS) highlight the inadequacies of current assays [1–4]. Particularly concerning was the unanticipated finding of increased acquisition of HIV infection in 1 of the 2 phase 3 clinical trials of CS, which resulted in the premature closure of both studies [2].
Preclinical safety studies have primarily relied on in vitro measurements of cell viability and the effect on lactobacilli [5], the rabbit vaginal irritation model, and limited macaque studies [6]. Outcomes measured in phase 1 clinical trials include signs of irritation, colposcopic abnormalities, measurement of a limited array of inflammatory cytokines, and semiquantitative vaginal cultures for select bacteria [1, 7]. However, these approaches have failed to predict the clinical outcomes in large-scale efficacy trials.
The multilayered squamous epithelium in the vagina and ectocervix and the single-layer columnar epithelium of the endocervix provide the first lines of defense against HIV. Disruption of this barrier facilitates the migration of HIV into the laminae propriae, where HIV target cells are more abundant. The importance of this barrier is highlighted by macaque studies demonstrating enhanced simian immunodeficiency virus vaginal transmission if animals are treated with medroxyprogesterone, which thins the vaginal epithelium [8]. Moreover, cervical ectopy, in which columnar epithelium extends beyond the external cervical os onto the vaginal surface, is associated with increased susceptibility to HIV type 1 (HIV-1) [9, 10].
We took advantage of a dual-chamber culture system to evaluate the effect of microbicides on the epithelial barrier by measuring changes in transepithelial electrical resistance (TER), expression of tight and adherens junctional proteins [11], and the subsequent ability of HIV to traverse this barrier and infect target cells cultured in the lower chamber. We also examined the inflammatory response to the drugs in culture, including their ability to induce HIV replication in chronically infected U1 cells. To validate these models, we evaluated 2 drugs that have been associated with an increased risk of HIV acquisition, N-9 and CS, and 2 drugs that are currently in advanced clinical studies, 9-[2-(phosphonomethoxy)propyl]adenine (PMPA; also referred to as tenofovir) and PRO2000.
METHODS
Cultures
CaSki (cervical) and HEC-1-A (uterine) epithelial cells were obtained from the American Tissue Culture Collection and cultured as described elsewhere [12, 13]. Jurkat-Tat-CCR5 cells (JT-CCR5) were provided by Q. Sattentau (Sir William Dunn School of Pathology, University of Oxford, Oxford, United Kingdom) [14]. Reconstituted human vaginal tissue (VLC-100) was purchased from MatTek. Chronically infected U1 cells were obtained from the AIDS Research and Reference Reagent Program (ARRRP), Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health [15]. Peripheral blood mononuclear cells (PBMCs) were isolated from HIV-seronegative leukocyte-enriched preparations (New York Blood Bank) by means of ficoll-hypaque density gradient centrifugation and were cultured in Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal bovine serum, 2 mmol/L L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Reagents
N-9 was purchased from Sigma. PMPA was a gift from Gilead Sciences. CS (lots 148656 and 8097) was provided by the Program for the Topical Prevention for Contraception and Development (Rush University, Chicago, Illinois), and commercial CS sodium salt was purchased from Acros Organics (lot A0244748). PRO2000 was provided by Indevus Pharmaceuticals. Working stocks of unformulated microbicides were resuspended in phosphate-buffered saline (10 mg/mL), stored at 4°C, and mixed either with medium or with pooled human seminal fluid (SeF) immediately before each experiment. SeF was processed from semen obtained from healthy volunteers who provided informed consent after institutional review board approval, as described elsewhere [16].
Dual-chamber cultures
HEC-1-A cells were plated on 0.4-μm-pore polyester inserts in 24-well plates (Corning) at a density of ~0.5 × 105 to 1 × 105 cells/insert in complete medium. Medium was changed every 2 to 3 days, and TER was monitored daily using a Millicell-ERS voltohmmeter (Millipore) and corrected for background. When TER reached a plateau (4 or 5 days), the apical surface was exposed to the indicated concentrations of each microbicide diluted in medium or to medium alone for 18 h or daily for 2 h each day. Alternatively, cells were treated with microbicides and pooled human SeF or with SeF alone (final concentration of SeF, 25% [vol/vol]). Drugs were removed at the indicated times after exposure, and cells washed thrice with serum-free medium and overlaid with 200 μL of complete medium apically. TER was monitored beginning 24 h after removal of drug and then daily. After the last microbicide exposure, cells were washed and challenged apically with HIV-1Ba-L (40 ng of p24 antigen) in complete medium. Immediately after virus challenge, 1 × 105 JT-CCR5 cells were added to basolateral chambers, and cultures were maintained for an additional 5 days. Apical and basolateral supernatants were collected at different times and tested for p24 content [14].
Confocal microscopy
MatTek tissue was stained for 30 min with EZ-Link sulfosuccinimidobiotin (1:1000 dilution; Pierce), which reacts with primary amines on cell-surface proteins, and then fixed with 4% paraformaldehyde solution (Electron Microscopy). The biotinylated cells were reacted with Alexa Fluor 546–conjugated or Alexa Fluor 350–conjugated streptavidin antibody (1:1000 dilution, S11225 and S11249; Invitrogen). Nuclei were detected by staining with 4′,6-diamidino-phenylindole dihydrochloride (Invitrogen). To visualize junctional proteins or HIV-1 p24, cells were permeabilized with 1% Triton-X after fixation and incubated with the appropriate primary antibodies (zona occludens 1 [ZO-1], 1:500 dilution, 90–1200 [Invitrogen]; desmoglein, 1:500 dilution, 27B2 [Invitrogen]; nectin, 1: 500 dilution, H-62:sc-28639 and CK6:sc-21722 [Santa Cruz Biotechnology]; syndecans, 1:200 dilution, 34164B-A38 [Abcam]; HIV-1 p24, 1:200 dilution, 183-H12-5C [ARRRP]) and subsequently with an Alexa Fluor 350–conjugated, Alexa Fluor 488–conjugated, or Alexa Fluor 555–conjugated anti-mouse secondary antibody (1:1000 dilution; Invitrogen). Images were examined using a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss) fitted with a Plan-Apochromat 100×/1.4 oil-immersion objective and captured in an optical slice of 1.0 μm with appropriate filters. Three-dimensional images were generated using Velocity 4 confocal software (Improvision).
RNA extraction and real-time quantitative-competitive reverse-transcription polymerase chain reaction
Total RNA was extracted, cDNA was synthesized, and reverse-transcription polymerase chain reaction amplification was performed as described elsewhere [17]. Commercially available probes for human interleukin 6 (IL-6) (Hs00985639_m1), E-cadherin (Hs00170423_m1), ZO-1 (Hs00268480_m1), desmoglein (Hs00170047_m1), and the ribosomal large protein subunit (RPLPO) (4333761F) housekeeping gene were obtained from ABI. Quantification was normalized against the number of RPLPO transcripts in the same RNA extracts.
Cytotoxicity and cytokine and chemokine response to microbicides
Cell proliferation was assessed using the CellTiter 96 cell-proliferation assay (Promega). CaSki cells were exposed to microbicide at 100 μg/mL for 2 h daily for 5 consecutive days. Culture supernatants were collected immediately before each exposure and stored at −80°C after addition of complete protease inhibitor cocktail (Roche). Interleukin 1α (IL-1α), interleukin 1β, IL-6, interleukin 8, interferon α2, and RANTES (regulated on activation, normal T cell expressed and secreted) concentrations were determined using multiplex proteome array beads (Chemicon International), as described elsewhere [18]. Transforming growth factor β, secretory leukocyte protease inhibitor (SLPI), and interleukin 1 receptor antagonist were measured by enzyme-linked immunosorbent assay (R&D Systems).
Activation of HIV-1 replication in U1 cells
U1 cells were seeded at 1 × 105 cells/well in round-bottomed 96-well micro-plates and exposed to microbicides at 100 μg/mL for 18 h, to medium alone, or to tumor necrosis factor α (TNF-α) at 100 U/mL (R&D Systems), a concentration previously shown to induce transactivation and HIV release from U1 cells [19]. After removal of drug, cells were washed thrice and cultured in fresh medium for an additional 48 h. Cell-free supernatants were collected and HIV-1 was quantified by determining p24 content.
Nuclear factor κB transcription factor assay
CaSki cells, U1 cells, or PBMCs were exposed to microbicides at 100 μg/mL for 18 h. After removal of drug by washing thrice with serum-free medium, nuclear extracts were prepared, and nuclear protein was quantified [17]. Nuclear extracts were stored at −80°C, and nuclear factor κB (NF-κB) p65 levels were quantified as described elsewhere [17].
RESULTS
Drop in TER triggered by exposure to N-9 and CS
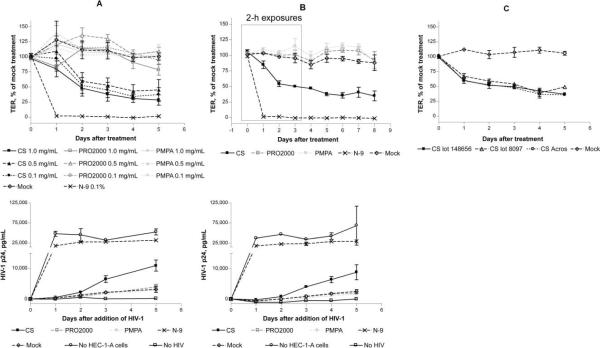
HEC-1-A cells were used to assess the effect of microbicides on the mucosal epithelial barrier by monitoring TER as a marker of epithelial integrity. TER values across polarized HEC-1-A cells reached a steady state of ~350 Ω · cm2 after 4 or 5 days and formed a relatively impervious barrier to HIV migration (figure 1). Polarized cells were exposed apically to a single dose (18 h) (figure 1A) or to repeated doses (2 h daily for 4 consecutive days) (figure 1B) of microbicides (or medium), and changes in TER were monitored at the indicated times. A single 18-h exposure to N-9 completely disrupted tight junctions, with TER reaching background levels (figure 1A). Both single and repeated exposures to CS (0.1, 0.5, and 1.0 mg/mL) triggered a drop in TER of ~50%, which persisted following drug removal (figure 1A and 1B). Similar results were obtained with 2 separate lots of CS and with commercially available CS (figure 1C). In contrast, no drop in TER was observed after exposure to PRO2000 or PMPA at the same concentrations. However, exposure to 1.0 mg/mL PRO2000 did result in a transient fall in TER, with a second drop 4 to 5 days after treatment (figure 1A).
Figure 1.
Induction of a reduction in transepithelial electrical resistance (TER) and facilitation of human immunodeficiency virus type 1 (HIV-1) translocation and infection of target cells by treatment with nonoxynol-9 (N-9) and cellulose sulfate (CS). HEC-1-A cells were cultured in transwells (0.5 × 105 to 1 × 105 cells/insert), and TER was monitored daily. After the TER reached a plateau (4 or 5 days), cells were exposed to the indicated microbicides—0.1% N-9 (vol/vol) or 0.1–1.0 mg/mL CS, PRO2000, or 9-[2-(phosphonomethoxy)propyl]adenine (PMPA)—for 18 h (A) or 2 h daily for 4 days (B). The effect of different CS stocks on TER was also evaluated after 18 h of treatment at 100 μg/mL (C). After removal of microbicides by washing thrice, HIV-1Ba-L (40 ng of p24 antigen) and JT-CCR5 cells (1 × 105 cells/well) were added to the upper and lower chambers, respectively. The TER was monitored 24 h after drug removal and then daily (upper panels). Supernatants were collected from the basolateral chambers at the indicated times after the addition of HIV-1 and tested for p24 content by enzyme-linked immunosorbent assay (lower panels). Results are means ± standard deviations relative to values for untreated controls from at least 2 independent experiments in which each condition was tested in duplicate.
Enhanced HIV transmission results from TER decrease
To determine whether the reduction in TER resulted in a change in epithelial permeability that was sufficient for HIV migration, microbicide-exposed HEC-1-A cells were challenged apically with HIV-1Ba-L, and transmigration was monitored by quantifying the HIV infection of JT-CCR5 cells added to the basolateral chamber. The imperviousness of the epithelial barrier to HIV was assessed by including mock-treated cultures (negative control) and maximal HIV migration of HIV across the insert pores by including transwells in which no cells were seeded (positive control). After application of N-9, HIV freely traversed the transwell membrane, resulting in p24 levels comparable to that for the positive control (no HEC-1-A cells). Exposure to CS also resulted in increased HIV replication, with p24 levels >2-fold higher than that in mock-treated cultures. Similar results were obtained with all 3 stocks of CS (data not shown). In contrast, no increase in HIV infection was observed after exposure to PRO2000 or PMPA, even at concentrations as high as 1.0 mg/mL (figure 1A and 1B).
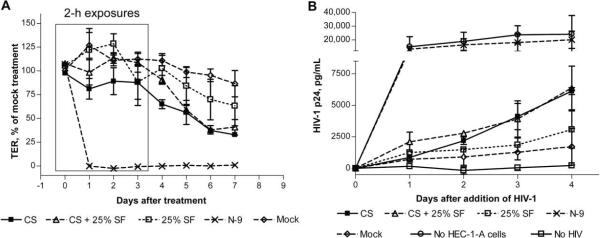
Persistence of the disruption in the epithelial barrier in the setting of SeF
To determine whether the response to microbicides could be modified by SeF, the experimental conditions were modified to mimic what might happen clinically, where the epithelium would be exposed both to microbicides and to SeF. Little or no cytotoxicity was observed when HEC-1-A cells were exposed to 25% SeF for up to 6 h; however, overnight exposure resulted in a modest reduction in cell viability. Thus, HEC-1-A cells were treated for 2 h daily for 4 consecutive days with microbicides and SeF (25% vol/vol) or SeF alone, washed, and then exposed apically to 40 ng of HIV-1Ba-L. SeF itself caused a 25% drop in TER (figure 2A) and resulted in a 2-fold increase in HIV infection of T cells cultured in the lower compartment (figure 2B). The drop in TER and increase in HIV migration in response to N-9 and CS persisted when cells were exposed to the microbicides in the presence of SeF, indicating that SeF failed to protect the epithelium from the deleterious effects of the compounds.
Figure 2.
Persistence of the cellulose sulfate (CS)–induced reduction in transepithelial electrical resistance (TER) and of the increase in human immunodeficiency virus type 1 (HIV-1) migration in the presence of human seminal fluid (SeF). HEC-1-A cells were cultured in transwells (0.5 × 105 to 1 × 105 cells/insert), and TER was monitored daily. After the TER reached a plateau (4 or 5 days), cells were treated for 2 h daily for 4 days with pooled SeF or with 200 μg/mL CS (final concentration, 100 μg/mL) in the presence of SeF at a final concentration of 25% (vol/vol). Immediately after the final treatment, the cultures were washed, and HIV-1Ba-L (40 ng of p24 antigen) and JT-CCR5 cells (1 × 105 cells/well) were added to the upper and lower chambers, respectively. The TER was monitored 24 h after the initial treatment and then daily (A). Culture supernatants were collected from basolateral chambers at the indicated times after the addition of HIV-1 and tested for p24 content by enzyme-linked immunosorbent assay (B). Results are means ± standard deviations relative to values for untreated controls from at least 2 independent experiments in which each condition was tested in duplicate.
Down-modulation of junctional proteins triggered by CS
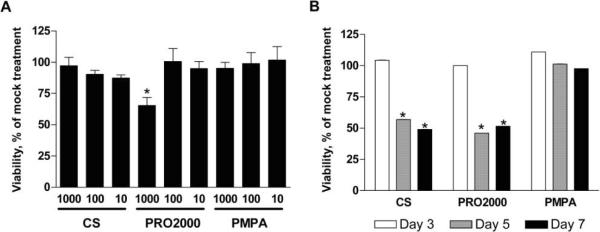
The observation that N-9 induced a persistent drop in TER is consistent with its cytotoxicity [20]. However, the sustained drop in TER in response to CS was unanticipated. The TER decline was not related to toxicity, because no loss in cell viability was observed after an 18-h exposure of cells to CS (or PMPA), even at concentrations as high as 1.0 mg/mL (figure 3A). In addition, little cytotoxicity was observed following repeated exposure of HEC-1-A cells to microbicides (data not shown). However, repeated exposure to CS and PRO2000 did induce a 50% reduction in CaSki cell viability (figure 3B).
Figure 3.
Toxicity of microbicide candidates. HEC-1-A cells were cultured in 96-well microtiter plates and exposed to the indicated concentrations (in micrograms per milliliter) of microbicides for 18 h (A). To address the effects of chronic exposure, CaSki cells were treated with microbicides at 100 μg/mL for 2 h daily for 7 consecutive days and assayed 24 h after 3, 5, and 7 exposures (B). After removal of compound, cell proliferation was assessed using the CellTiter 96 cell-proliferation assay (Promega). Results are expressed as the percentage viability relative to that of mock-treated controls and are means ± standard deviations from 2 independent experiments in which each condition was tested in triplicate. Asterisks indicate statistically significant differences (P < .05) relative to mock-treated cells, as determined by the Student t test.
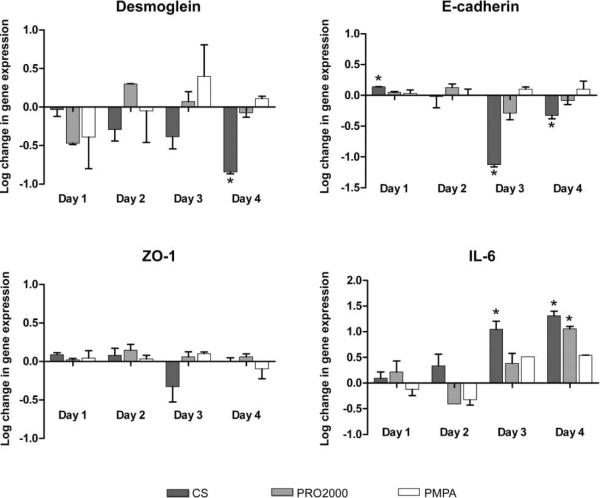
To evaluate whether CS down-regulated junctional protein expression, HEC-1-A cells were treated with microbicides (2 h daily for 4 consecutive days), and, 24 h after each dose, cells were harvested and RNA was extracted for quantitative-competitive reverse-transcription polymerase chain reaction. CS, but not PMPA or PRO2000, induced almost a 1 log reduction relative to values for mock-treated cells in the expression of E-cadherin and desmoglein, with the down-modulation peaking after the third or fourth doses, consistent with a cumulative effect (figure 4). There was also a modest decline in ZO-1 gene expression. Conversely, CS induced at least a 1 log increase in IL-6 gene expression. A significant increase in IL-6 gene expression was also observed following the fourth exposure of cells to PRO2000.
Figure 4.
Down-regulation of junctional proteins and up-regulation of interleukin 6 (IL-6) triggered by repeated exposure to cellulose sulfate (CS). Confluent monolayers of HEC-1-A cells were exposed to candidate microbicides for 2 h daily for 4 days in 24-well plates. After each treatment, cells were washed and incubated for 24 h before harvest and RNA isolation. Desmoglein, E-cadherin, zona occludens 1 (ZO-1), and IL-6 expression was measured by quantitative-competitive reverse-transcription polymerase chain reaction. Results are presented as the log change in gene expression and were normalized using the ribosomal large protein subunit (RPLPO) housekeeping gene as an endogenous control. Each panel shows the means ± standard deviations from 3 independent experiments in which each condition was tested in duplicate. Asterisks indicate statistically significant differences (P < .01 ) relative to mock-treated cells, as determined by 1-way analysis of variance with the Bonferroni correction. PMPA, 9-[2-(phosphonomethoxy)propyl]adenine.
Modest inflammatory response in epithelial cells triggered by CS and PRO2000
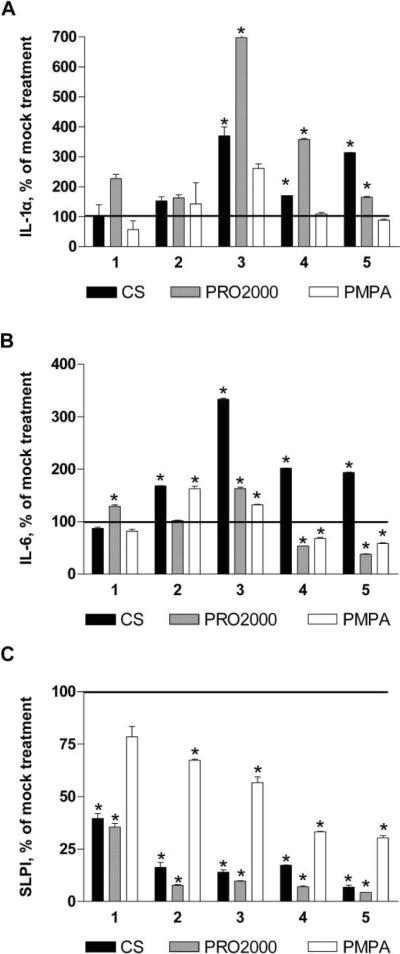
Although the increase in epithelial permeability could facilitate HIV acquisition, induction of an inflammatory response could enhance infection by recruiting or activating HIV target cells or by augmenting HIV transcription. We previously examined the response to CS and PRO2000 in immortalized endocervical cells, focusing primarily on IL-1α and SLPI, but we did not examine the NF-κB response [21]. We expanded these experiments and evaluated the response to CS, PRO2000, and PMPA. Pilot experiments demonstrated that CaSki cells produced more cytokines than did HEC-1-A cells; thus, we focused on CaSki cells. CS and PRO2000 triggered significant increases in IL-1α and IL-6 release, with the most pronounced IL-6 release occurring after the third exposure to CS (figure 5A and 5B). This is consistent with the augmented gene expression observed in HEC-1-A cells (figure 4). A modest reduction in IL-6 release was observed after the fourth and fifth doses of PRO2000 and PMPA. All 3 drugs triggered a loss in SLPI, although the response to PMPA was relatively modest (figure 5C). None of the other chemokines or cytokines measured was significantly altered (relative to mock treatment) after exposure to any of the microbicides.
Figure 5.
Modest inflammation due to cellulose sulfate (CS) and PRO2000. CaSki cells were treated with the indicated microbicides at 100 μg/mL for 2 h daily for 5 consecutive days. Culture supernatants were collected 24 h after the indicated number of treatments, and levels of interleukin 1α (IL-1α) (A), interleukin 6 (IL-6) (B), and secretory leukocyte protease inhibitor (SLPI) (C) were determined as described in Methods. Results are presented as the percentage change relative to mock-treated cells and are means ± standard deviations from 2 independent experiments in which each condition was tested in triplicate. Asterisks indicate statistically significant differences (P < .05) relative to mock-treated cells, as determined by the Student t test.
Activation of NF-κB and promotion of HIV replication by CS
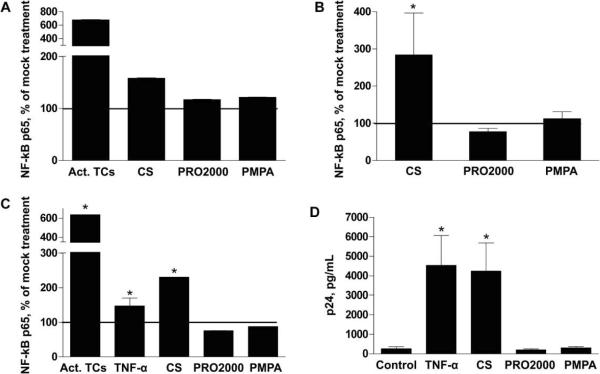
The observation that CS triggers an increase in IL-1α and IL-6 release and decreases SLPI release suggests that CS might activate NF-κB. This transcription factor plays a major role in the regulation of HIV-1 gene expression by binding to 2 sites on the HIV long terminal repeat enhancer region to initiate transcription of the integrated HIV genome [22]. To investigate this, CaSki cells, U1 cells, and PBMCs were treated with microbicides for 18 h, and nuclear extracts were then prepared and analyzed for NF-κB p65. Activated Jurkat T cells served as a positive control for the CaSki cell and PBMC experiments, and TNF-a treatment served as a positive control for activating HIV replication in U1 cells. CS exposure triggered significant activation of NF-κB in PBMCs and U1 cells, and there was a trend toward an increase in NF-κB activation in CaSki cells (figure 6A–6C). In contrast, neither PRO2000 nor PMPA had any significant effect on NF-κB activation in any cell type. Notably, there was a marked enhancement of HIV release after a single 18-h treatment of U1 cells with CS, consistent with observed NF-κB activation (figure 6D). No significant increase in HIV-1 p24 levels was detected after exposure of U1 cells to culture supernatants obtained from CaSki cells treated with microbicides (compared with mock treatment), indicating that the modest cytokine response alone is not sufficient to activate the viral long terminal repeat.
Figure 6.
Nuclear factor κB (NF-κB) activation in CaSki cells, U1 cells, and peripheral blood mononuclear cells (PBMCs) and increased human immunodeficiency virus (HIV) release from U1 cells due to exposure to cellulose sulfate (CS). CaSki cells (A), PBMCs (B), and U1 cells (C) were exposed to each microbicide at 100 μg/mL for 18 h, and then nuclear extracts were prepared and analyzed for NF-κB p65 levels. Activated Jurkat T cells (act. TCs) were included as a positive control. Results are presented as the percentage increase in NF-κB p65 levels relative to those of mock-treated cells and are means ± standard deviations from 2 independent experiments in which each condition was tested in triplicate. After an 18-h exposure of U1 cells to microbicides, the compounds were removed by washing, and cells were cultured in complete medium for an additional 48 h. HIV release was assessed by measuring p24 content in culture supernatants by enzyme-linked immunosorbent assay (D). Tumor necrosis factor α (TNF-α) was included as a positive control, and results are means ± standard deviations from 2 independent experiments in which each condition was tested in triplicate. Asterisks indicate a significant increase in NF-κB p65 and HIV-1 p24 levels (P < .01), as determined by the Student t test.
Further demonstration of epithelial disruption by confocal microscopy
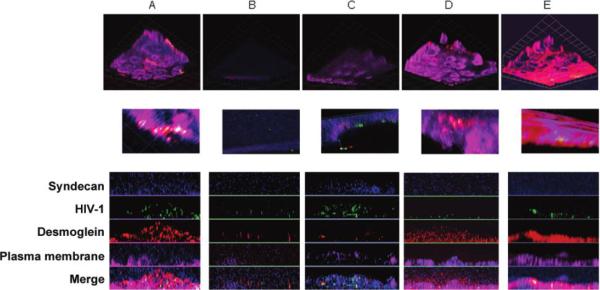
Reconstituted vaginal tissue was treated with each microbicide or medium for 18 h before challenge with HIV, and the cultures were maintained for 7 days, consistent with the dual-chamber model experiments (figures 1 and 2). Tissue integrity and the presence of HIV particles were monitored by confocal microscopy. Syndecan proteoglycans were stained because a recent study demonstrated that HIV gp120 binds to heparan sulfate glycosaminoglycan side chains of syndecan to enhance the in trans infectivity of virus [23]. In mock-treated tissue, the epithelium remained intact, and few HIV particles (stained for p24 [green]) were observed colocalized with syndecan (blue) and with the adherens junction protein, desmoglein (red) (figure 7A). In contrast, the epithelium was completely disrupted after exposure to N-9 (figure 7B). Further magnification revealed free HIV particles (green). Disruption of cellular architecture in tissue treated with CS was also demonstrated with a loss in desmoglein staining (red) and an increase in free HIV (green) (figure 7C). In contrast, after exposure to PRO2000 or PMPA the epithelium resembled the mock-treated tissue, with a few HIV particles again observed colocalized with syndecan (turquoise) and desmoglein (yellow) (figure 7D and 7E). Similar confocal images were obtained with HEC-1-A cells cultured in the dual-chamber model (data not shown).
Figure 7.
Epithelial disruption and increase in human immunodeficiency virus type 1 (HIV-1) due to nonoxynol-9 (N-9) and cellulose sulfate (CS), as detected by confocal imaging in reconstituted vaginal tissue. Reconstituted vaginal tissue from MatTek was exposed to culture medium (A), 0.1% N-9 (B), 100 μg/mL CS (C), 100 μg/mL PRO2000 (D), or 100 μg/mL 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) (E) for 18 h. After removal of compounds, HIV-1Ba-L was added to the apical chambers, and cultures were maintained for 7 days. Cultures were washed, fixed, and stained for cellular membrane (magenta), HIV-1 p24 (green), syndecan (blue), and desmoglein (red), as described in Methods. Images were captured using an optical slice of 1.0 μm with appropriate filters, and 3-dimensional reconstructions were generated and are shown in the upper and middle (higher magnification) panels. Corresponding z-stack images of each color channel are shown in the lower panels. Grid length corresponds to 9.2 μm.
DISCUSSION
The dual-chamber culture model and the experiments with monocytic cells (U1 cells and PBMCs) uncovered unanticipated adverse effects associated with CS that, in hindsight, might have predicted the clinical trial outcomes. After the failure of the N-9 clinical trial, subsequent studies suggested that the increased risk of HIV acquisition could be attributed to a proinflammatory response. This resulted in the addition of cytokine measurements to phase 1 clinical studies [24]. CS gel underwent extensive phase 1 evaluations that included such measurements, and no significant increases in levels of proinflammatory cytokines were observed [7, 25–29]. The results of the present in vitro experiments are consistent, as we observed only a modest increase in inflammatory cytokines in epithelial cell culture supernatants, and these had no significant effect on HIV replication in U1 cells. However, direct exposure of U1 cells to CS at 100 μg/mL, a concentration likely to be present in the genital tract after gel application [30], resulted in a significant increase in HIV replication, which was associated with NF-κB activation. Significant NF-κB activation was also observed after exposure of PBMCs to CS. Whether these in vitro findings contributed to the clinical trial outcomes is not known. CS could have increased HIV replication in incoming infected PBMCs in semen, thereby increasing the risk of transmission. Notably, a recent study also demonstrated that low doses of CS (<1 μg/mL) significantly increased HIV replication in vitro, although the mechanisms were not elucidated [31].
The dual-chamber culture model results suggest a completely different mechanism by which microbicides might inadvertently increase HIV acquisition. Consistent with the results of studies demonstrating that HIV crosses an intact mucosal epithelium poorly [23, 32], we found that polarized HEC-1-A cells provide a relatively impervious barrier to HIV migration. Disruption of this barrier after N-9 exposure was predicted by its known surfactant properties. However, the results obtained with CS were surprising. Notably, no significant or persistent drop in TER was observed after exposure to PRO2000, indicating that this is not a property shared by all sulfated or sulfonated polymers. Differences in chemical composition and molecular weight between CS and PRO2000 may have contributed to the differential response. CS has a peak molecular mass of 2300 kDa, whereas PRO2000 has a peak molecular mass of only 5 kDa [21].
The sustained drop in TER (figure 1), which persisted even after removal of the drug, is consistent with the observed down-regulation of junctional protein gene expression (figure 4). Regulation of junctional proteins is incompletely understood, and there is a paucity of data focusing on genital tract epithelium. Junctional complexes contain a large number of cytoplasmic proteins, which serve as adaptors that link the integral membrane proteins to the actin cytoskeleton and play roles in transcription, polarity, and other signaling functions. It is possible that CS-induced epithelial disruption and activation of NF-κB are linked through signaling pathways. Consistent with this notion is the finding that junctional adhesion molecules are redistributed away from junctions in response to inflammation [33].
The results of ongoing studies in mice are consistent with the results obtained in the dual-chamber culture model here. We and others have previously shown that exposure to N-9, but not PRO2000, increases the susceptibility of mice to herpes simplex virus (HSV) infection [18, 34]. More recently, we found that the increase in susceptibility to HSV infection is associated with the disruption of epithelial tight junctions (S.S.W., N.C., E.F., P.M.M.M., M.J.K., and B.C.H., unpublished data). Notably, CS gel also increased the susceptibility of mice to genital herpes in a pilot experiment—8 of 10 mice developed genital herpes when challenged with a low dose of HSV type 2 twelve hours after the seventh daily dose of CS, compared with 4 of 10 mice treated with hydroxyethylcellulose (P < .05). Further studies of CS gel are planned in collaboration with CONRAD.
These experiments were conducted with active drugs and should be expanded to evaluate formulations, which could also affect the epithelial barrier because of osmolarity or the effects of other excipients [35]. These findings suggest that phase 1 trials should be expanded to include an assessment of the effect that microbicides have on epithelial integrity by obtaining biopsy specimens. This is important for gels, as well as for ring formulations. Comparable experiments should also be conducted to address the effect of rectally applied microbicides, for which the columnar epithelium may be more vulnerable.
The findings of the current models are consistent with the safety observed in the recently completed HIV Prevention Trials Network 035 trial, in which a 30% reduction in HIV incidence was observed in women who applied 0.5% PRO2000 gel [36]. However, it is important to note that a modest inflammatory response, a reduction in SLPI level, and a transient drop in TER were observed with higher doses of PRO2000. It is possible that this contributed to the finding that the 2% PRO2000 gel had little or no chance of showing efficacy and the discontinuation of the 2% gel arm of the Microbicide Development Programme trial (MDP 301). Importantly, the current results predict that PMPA will not increase HIV acquisition through disruption of the epithelium or activation of NF-κB pathways. The results of ongoing clinical trials will need to be translated back to the bench to optimize these and other biomarkers of safety.
Acknowledgments
We thank Robert Anderson and Don Waller (Program for the Topical Prevention for Contraception and Development, Rush University, Chicago, Illinois) for the gift of cellulose sulfate, Al Profy (Indevus Pharmaceuticals) for PRO2000, and James Rooney (Gilead Sciences) for PMPA. We also thank Tara Ford and Julie Petrie for their assistance, the Microscopy Core Facilities at Albert Einstein College of Medicine (Bronx, New York) and Mount Sinai School of Medicine (New York, New York) for their support, and the Center for AIDS Research, Albert Einstein College of Medicine.
Financial support: National Institutes of Health (grants AI065309, AI079763, and UL1RR025750).
Footnotes
Potential conflicts of interest: none reported.
Presented in part: 15th Conference on Retroviruses and Opportunistic Infections (CROI 2008), Boston, 3–6 February 2008; Microbicides 2008, New Delhi, 24–27 February 2008.
References
- 1.Van Damme L, Chandeying V, Ramjee G, et al. Safety of multiple daily applications of COL-1492, a nonoxynol-9 vaginal gel, among female sex workers. COL-1492 Phase II Study Group. AIDS. 2000;14:85–8. doi: 10.1097/00002030-200001070-00010. [DOI] [PubMed] [Google Scholar]
- 2.Van Damme L, Govinden R, Mirembe FM, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359:463–72. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 3.Feldblum PJ, Adeiga A, Bakare R, et al. SAVVY vaginal gel (C31G) for prevention of HIV infection: a randomized controlled trial in Nigeria. PLoS ONE. 2008;3:e1474. doi: 10.1371/journal.pone.0001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turville SG, Aravantinou M, Miller T, et al. Efficacy of Carraguard-based microbicides in vivo despite variable in vitro activity. PLoS ONE. 2008;3:e3162. doi: 10.1371/journal.pone.0003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lackman-Smith C, Osterling C, Luckenbaugh K, et al. Development of a comprehensive human immunodeficiency virus type 1 screening algorithm for discovery and preclinical testing of topical microbicides. Antimicrob Agents Chemother. 2008;52:1768–81. doi: 10.1128/AAC.01328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patton DL, Sweeney YC, Cummings PK, Meyn L, Rabe LK, Hillier SL. Safety and efficacy evaluations for vaginal and rectal use of BufferGel in the macaque model. Sex Transm Dis. 2004;31:290–6. doi: 10.1097/01.olq.0000124614.91448.d4. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz JL, Mauck C, Lai JJ, et al. Fourteen-day safety and acceptability study of 6% cellulose sulfate gel: a randomized double-blind phase I safety study. Contraception. 2006;74:133–40. doi: 10.1016/j.contraception.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Marx PA, Spira AI, Gettie A, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–9. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 9.Myer L, Wright TC, Jr, Denny L, Kuhn L. Nested case-control study of cervical mucosal lesions, ectopy, and incident HIV infection among women in Cape Town, South Africa. Sex Transm Dis. 2006;33:683–7. doi: 10.1097/01.olq.0000216026.67352.f9. [DOI] [PubMed] [Google Scholar]
- 10.Moss GB, Clemetson D, D'Costa L, et al. Association of cervical ectopy with heterosexual transmission of human immunodeficiency virus: results of a study of couples in Nairobi, Kenya. J Infect Dis. 1991;164:588–91. doi: 10.1093/infdis/164.3.588. [DOI] [PubMed] [Google Scholar]
- 11.Dezzutti CS, James VN, Ramos A, et al. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob Agents Chemother. 2004;48:3834–44. doi: 10.1128/AAC.48.10.3834-3844.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pattillo RA, Hussa RO, Story MT, Ruckert AC, Shalaby MR, Mattingly RF. Tumor antigen and human chorionic gonadotropin in CaSki cells: a new epidermoid cervical cancer cell line. Science. 1977;196:1456–8. doi: 10.1126/science.867042. [DOI] [PubMed] [Google Scholar]
- 13.Kuramoto H, Tamura S, Notake Y. Establishment of a cell line of human endometrial adenocarcinoma in vitro. Am J Obstet Gynecol. 1972;114:1012–9. doi: 10.1016/0002-9378(72)90861-7. [DOI] [PubMed] [Google Scholar]
- 14.Mesquita PM, Wilson SS, Manlow P, et al. Candidate microbicide PPCM blocks human immunodeficiency virus type 1 infection in cell and tissue cultures and prevents genital herpes in a murine model. J Virol. 2008;82:6576–84. doi: 10.1128/JVI.00335-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folks TM, Justement J, Kinter A, Dinarello CA, Fauci AS. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987;238:800–2. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- 16.Patel S, Hazrati E, Cheshenko N, et al. Seminal plasma reduces the effectiveness of topical polyanionic microbicides. J Infect Dis. 2007;196:1394–402. doi: 10.1086/522606. [DOI] [PubMed] [Google Scholar]
- 17.Fakioglu E, Wilson SS, Mesquita PM, et al. Herpes simplex virus down-regulates secretory leukocyte protease inhibitor: a novel immune evasion mechanism. J Virol. 2008;82:9337–44. doi: 10.1128/JVI.00603-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galen BT, Martin AP, Hazrati E, et al. A comprehensive murine model to evaluate topical vaginal microbicides: mucosal inflammation and susceptibility to genital herpes as surrogate markers of safety. J Infect Dis. 2007;195:1332–9. doi: 10.1086/513279. [DOI] [PubMed] [Google Scholar]
- 19.Poli G, Kinter AL, Justement JS, Bressler P, Kehrl JH, Fauci AS. Transforming growth factor beta suppresses human immunodeficiency virus expression and replication in infected cells of the monocyte/macrophage lineage. J Exp Med. 1991;173:589–97. doi: 10.1084/jem.173.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herold BC, Kirkpatrick R, Marcellino D, et al. Bile salts: natural detergents for the prevention of sexually transmitted diseases. Antimicrob Agents Chemother. 1999;43:745–51. doi: 10.1128/aac.43.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheshenko N, Keller MJ, MasCasullo V, et al. Candidate topical microbicides bind herpes simplex virus glycoprotein B and prevent viral entry and cell-to-cell spread. Antimicrob Agents Chemother. 2004;48:2025–36. doi: 10.1128/AAC.48.6.2025-2036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen CA, Sodroski JG, Haseltine WA. The location of cis-acting regulatory sequences in the human T cell lymphotropic virus type III (HTLV-III/LAV) long terminal repeat. Cell. 1985;41:813–23. doi: 10.1016/s0092-8674(85)80062-3. [DOI] [PubMed] [Google Scholar]
- 23.Bobardt MD, Chatterji U, Selvarajah S, et al. Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells. J Virol. 2007;81:395–405. doi: 10.1128/JVI.01303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fichorova RN, Tucker LD, Anderson DJ. The molecular basis of nonoxynol-9–induced vaginal inflammation and its possible relevance to human immunodeficiency virus type 1 transmission. J Infect Dis. 2001;184:418–28. doi: 10.1086/322047. [DOI] [PubMed] [Google Scholar]
- 25.Doh AS, Ngoh N, Roddy R, Lai JJ, Linton K, Mauck C. Safety and acceptability of 6% cellulose sulfate vaginal gel applied four times per day for 14 days. Contraception. 2007;76:245–9. doi: 10.1016/j.contraception.2007.05.083. [DOI] [PubMed] [Google Scholar]
- 26.El-Sadr WM, Mayer KH, Maslankowski L, et al. Safety and acceptability of cellulose sulfate as a vaginal microbicide in HIV-infected women. AIDS. 2006;20:1109–16. doi: 10.1097/01.aids.0000226950.72223.5f. [DOI] [PubMed] [Google Scholar]
- 27.Malonza IM, Mirembe F, Nakabiito C, et al. Expanded phase I safety and acceptability study of 6% cellulose sulfate vaginal gel. AIDS. 2005;19:2157–63. doi: 10.1097/01.aids.0000194797.59046.8f. [DOI] [PubMed] [Google Scholar]
- 28.Mauck C, Weiner DH, Ballagh S, et al. Single and multiple exposure tolerance study of cellulose sulfate gel: a phase I safety and colposcopy study. Contraception. 2001;64:383–91. doi: 10.1016/s0010-7824(01)00271-2. [DOI] [PubMed] [Google Scholar]
- 29.Mauck C, Frezieres R, Walsh T, Robergeau K, Callahan M. Cellulose sulfate: tolerance and acceptability of penile application. Contraception. 2001;64:377–81. doi: 10.1016/s0010-7824(01)00270-0. [DOI] [PubMed] [Google Scholar]
- 30.Keller MJ, Zerhouni-Layachi B, Cheshenko N, et al. PRO 2000 gel inhibits HIV and herpes simplex virus infection following vaginal application: a double-blind placebo-controlled trial. J Infect Dis. 2006;193:27–35. doi: 10.1086/498533. [DOI] [PubMed] [Google Scholar]
- 31.Tao W, Richards C, Hamer D. Enhancement of HIV infection by cellulose sulfate. AIDS Res Hum Retroviruses. 2008;24:925–9. doi: 10.1089/aid.2008.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouschbacher M, Bomsel M, Verronese E, et al. Early events in HIV transmission through a human reconstructed vaginal mucosa. AIDS. 2008;22:1257–66. doi: 10.1097/QAD.0b013e3282f736f4. [DOI] [PubMed] [Google Scholar]
- 33.Weber C, Fraemohs L, Dejana E. The role of junctional adhesion molecules in vascular inflammation. Nat Rev Immunol. 2007;7:467–77. doi: 10.1038/nri2096. [DOI] [PubMed] [Google Scholar]
- 34.Cone RA, Hoen T, Wong X, Abusuwwa R, Anderson DJ, Moench TR. Vaginal microbicides: detecting toxicities in vivo that paradoxically increase pathogen transmission. BMC Infect Dis. 2006;6:90. doi: 10.1186/1471-2334-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuchs EJ, Lee LA, Torbenson MS, et al. Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: potential implication for HIV transmission. J Infect Dis. 2007;195:703–10. doi: 10.1086/511279. [DOI] [PubMed] [Google Scholar]
- 36.Karim SA, Coletti A, Richardson B, et al. Safety and effectiveness of vaginal microbicides BufferGel and 0.5% PRO 2000/5 gel for the prevention of HIV infection in women: results of the HPTN 035 trial [abstract 48LB]. Program and abstracts of the 16th Conference on Retroviruses and Opportunistic Infections; Montreal. 2009. [Google Scholar]