Abstract
BACKGROUND
Obesity is an established and modifiable risk factor for urinary incontinence, but conclusive evidence for a beneficial effect of weight loss on urinary incontinence is lacking.
METHODS
We randomly assigned 338 overweight and obese women with at least 10 urinary-incontinence episodes per week to an intensive 6-month weight-loss program that included diet, exercise, and behavior modification (226 patients) or to a structured education program (112 patients).
RESULTS
The mean (±SD) age of the participants was 53±11 years. The body-mass index (BMI) (the weight in kilograms divided by the square of the height in meters) and the weekly number of incontinence episodes as recorded in a 7-day diary of voiding were similar in the intervention group and the control group at baseline (BMI, 36±6 and 36±5, respectively; incontinence episodes, 24±18 and 24±16, respectively). The women in the intervention group had a mean weight loss of 8.0% (7.8 kg), as compared with 1.6% (1.5 kg) in the control group (P<0.001). After 6 months, the mean weekly number of incontinence episodes decreased by 47% in the intervention group, as compared with 28% in the control group (P = 0.01). As compared with the control group, the intervention group had a greater decrease in the frequency of stress-incontinence episodes (P = 0.02), but not of urge-incontinence episodes (P = 0.14). A higher proportion of the intervention group than of the control group had a clinically relevant reduction of 70% or more in the frequency of all incontinence episodes (P<0.001), stress-incontinence episodes (P = 0.009), and urge-incontinence episodes (P = 0.04).
CONCLUSIONS
A 6-month behavioral intervention targeting weight loss reduced the frequency of self-reported urinary-incontinence episodes among overweight and obese women as compared with a control group. A decrease in urinary incontinence may be another benefit among the extensive health improvements associated with moderate weight reduction.
Urinary incontinence affects more than 13 million women in the United States and has been associated with profound adverse effects on quality of life1,2; an increased risk of falls, fractures,3 and nursing-home admissions4; and more than $20 billion in estimated annual direct health care costs.5
Observational studies suggest that obesity is a strong risk factor for urinary incontinence,6–9 and preliminary studies suggest that weight loss may have a beneficial effect on urinary incontinence in obese patients.10–14 Reductions in urinary incontinence have been observed in morbidly obese women who have had dramatic weight loss after bariatric surgery.11–13 In a small cohort study of overweight and obese women with incontinence, those who had a weight loss of more than 5% had a reduction of at least 50% in the frequency of incontinence (P = 0.03).14 A 3-month study reported that overweight and obese women randomly assigned to a very-low-calorie liquid diet had a significantly greater decrease in the weekly number of urinary-incontinence episodes than those assigned to no intervention.10
We conducted a randomized, clinical trial, the Program to Reduce Incontinence by Diet and Exercise (PRIDE), to determine whether a behavioral weight-reduction intervention for overweight and obese women with incontinence would result in greater reductions in the frequency of incontinence episodes at 6 months as compared with a control group.
METHODS
PARTICIPANTS
We recruited 338 women between July 2004 and April 2006 in Providence, Rhode Island, and Birmingham, Alabama. Women were eligible for the study if they were at least 30 years of age, had a body-mass index (BMI, the weight in kilograms divided by the square of the height in meters) of 25 to 50, and at baseline reported 10 or more urinary-incontinence episodes in a 7-day diary of voiding. The participants were required to monitor their food intake and physical activity for 1 week, to be able to walk unassisted for two blocks (approximately 270 m) without stopping, and to agree not to initiate new treatments for incontinence or weight reduction for the duration of the study. Previous medical therapy for incontinence or obesity did not affect eligibility. Exclusion criteria included use of medical therapy for incontinence or weight loss within the previous month, current urinary tract infection or four or more urinary tract infections in the previous year, a history of incontinence of neurologic or functional origin (due to factors not involving the lower urinary tract, such as chronic impairment of physical or cognitive functioning), previous surgery for incontinence or urethral surgery, major medical or genitourinary tract conditions, pregnancy or parturition in the previous 6 months, type 1 or type 2 diabetes mellitus requiring medical therapy that increased the risk of hypoglycemia, and uncontrolled hypertension.
The study was approved by the institutional review board at each site, and written informed consent was obtained from all participants before enrollment. Slim-Fast, a meal-replacement product, was donated by the manufacturer, Unilever, which had no role in trial design, data accrual, data analysis, or preparation of the manuscript. The biostatistician authors at the University of California, San Francisco, vouch for the completeness and accuracy of the data.
STUDY DESIGN
Eligible participants were randomly assigned at a 2:1 ratio to an intensive 6-month behavioral weight-loss program or to a structured four-session education program (the control group). Randomization was performed with the use of randomly permuted blocks of three or six, stratified according to clinical center, with random assignment concealed in tamper-proof envelopes. The participants were aware of their treatment assignment, but the staff members who collected the outcome data were not.
The participants completed questionnaires concerning their demographic characteristics, medical and behavioral history, and history of incontinence that were routinely used by the investigators. The participants were weighed to the nearest 0.5 kg on a calibrated digital scale (Tanita BWB 800) while wearing street clothes and without shoes. Height was measured at baseline to the nearest centimeter with the use of a calibrated, wall-mounted stadiometer and a horizontal measuring block.
The participants were trained to complete a 7-day diary of voiding (see the Supplementary Appendix, available with the full text of this article at NEJM.org), and interviewers reviewed the diaries with the participants to answer questions and reconcile inconsistencies.15,16 The participants recorded the time of each void and each incontinence episode. According to the instructions provided, the participants identified each episode as stress incontinence (involuntary loss of urine with coughing, sneezing, straining, or exercise), urge incontinence (loss of urine associated with a strong need or urge to void), or other. For the purposes of analysis, each woman was then classified as having stress-only incontinence, stress-predominant incontinence (i.e., at least two thirds of the total number of episodes were stress episodes), urge-only incontinence, urge-predominant continence (i.e., at least two thirds of the total number of episodes were urge episodes), or mixed incontinence (i.e., at least two types were reported, but no type constituted two thirds of the total number of episodes).
STUDY GROUPS
At randomization, all participants were given a self-help behavioral-treatment booklet with instructions for improving bladder control.17 The booklet provided basic information about incontinence, how to locate pelvic-floor muscles and how to perform daily exercises with them, how to use pelvic-floor muscles to avoid stress incontinence, and how to control urinary urgency, as well as instructions on completing voiding diaries. Incontinence was not discussed further in either the control group or the weight-loss group.
Women assigned to the control group were scheduled to participate in four education sessions at months 1, 2, 3, and 4. During these 1-hour group sessions, which included 10 to 15 women, general information was presented about weight loss, physical activity, and healthful eating habits, according to a structured protocol.
The weight-loss program was designed to produce an average loss of 7 to 9% of initial body weight within the first 6 months of the program and was modeled after that used in the following two large clinical trials: Look AHEAD (Action for Health in Diabetes),18,19 a lifestyle intervention trial intended to achieve and maintain weight loss in patients with diabetes, and the Diabetes Prevention Program.20 The participants in the weight-loss program met weekly for 6 months in groups of 10 to 15 for 1-hour sessions that were led by experts in nutrition, exercise, and behavior change and were based on a structured protocol. The participants were given a standard reduced-calorie diet (1200 to 1500 kcal per day), with a goal of providing no more than 30% of the calories from fat. To improve adherence, the participants were provided with sample meal plans and were given vouchers for a meal-replacement product (Slim-Fast) to be used for two meals a day during months 1 to 4 and for one meal a day thereafter.
The participants were encouraged to gradually increase physical activity (brisk walking or activities of similar intensity) until they were active for at least 200 minutes each week. Behavioral skills, including self-monitoring, stimulus control, and problem-solving, were emphasized.
OUTCOMES
The primary outcome measure was the percentage change in the number of incontinence episodes reported in the 7-day voiding diary at 6 months after randomization.15,16 Secondary outcome measures included the percentage change in the number of episodes of urge and stress incontinence; the proportion of women in whom the frequency of incontinence decreased by at least 50%, 70%, or 100%; and change in a validated measure of participant satisfaction with incontinence treatment (assessed with the use of Likert scales of perceived change in frequency of incontinence, volume of urine loss, the degree to which incontinence is a problem, and satisfaction with the change in incontinence at 6 months).21 In addition, 24-hour involuntary urine loss at baseline and 6 months was determined by a pad test standardized by the International Continence Society. 22 Preweighed urinary-incontinence pads were used for 24 hours and returned by the participants in sealed plastic bags, and the amount of urine lost was measured by weighing the pads.
STATISTICAL ANALYSIS
We estimated that 330 women would need to be enrolled to detect a net reduction in incontinence frequency of six episodes per week after 6 months; this reduction was half the effect seen in a pilot study10 but was large enough to be clinically meaningful. This estimate allowed for a 10% rate of attrition at 6 months and assumed imputation of missing data for 6-month outcomes. In addition, we assumed that correlation of outcomes within the small intervention groups would result in a 25% increase in the required sample size.
We compared the two groups in terms of baseline demographic and clinical characteristics, accounting for potential correlation among the women in each new “wave” (a wave consisted of one control group and two weight-loss groups) who were beginning treatment. Chi-square tests were used to compare the proportion of missing 6-month data according to treatment group.
To assess the effects of treatment on the frequency of incontinence, we used generalized estimating equations with negative binomial models, with adjustment for clinical site and the baseline and 6-month outcomes treated as repeated measures. In a sensitivity analysis, we also used the nonparametric Wilcoxon rank-sum test to compare percentage changes in the frequency of incontinence. The effects of treatment on the percentage change in weight from baseline to 6 months were assessed with the use of linear mixed models adjusted for site.
Attrition in weight-loss studies commonly masks regained weight. To address this potential source of bias, we used multiple-imputation methods to impute missing weight data at 6 months, on the assumption of no change from baseline on average among dropouts. In addition, we imputed missing data on incontinence frequency at 6 months and pad weight for participants in both groups as if they had been assigned to the control group, in which the average weight loss was minimal but some reduction in incontinence frequency was observed. We also performed a complete-case analysis without imputation of missing outcomes.
The proportions of women with reductions of 50%, 70%, and 100% in the frequency of incontinence were compared by generalized estimating equations, with the use of logistic models with robust standard errors. We focused on a 70% reduction in incontinence frequency, because this figure has been reported as a threshold for improvement in patient satisfaction.21
RESULTS
BASELINE CHARACTERISTICS
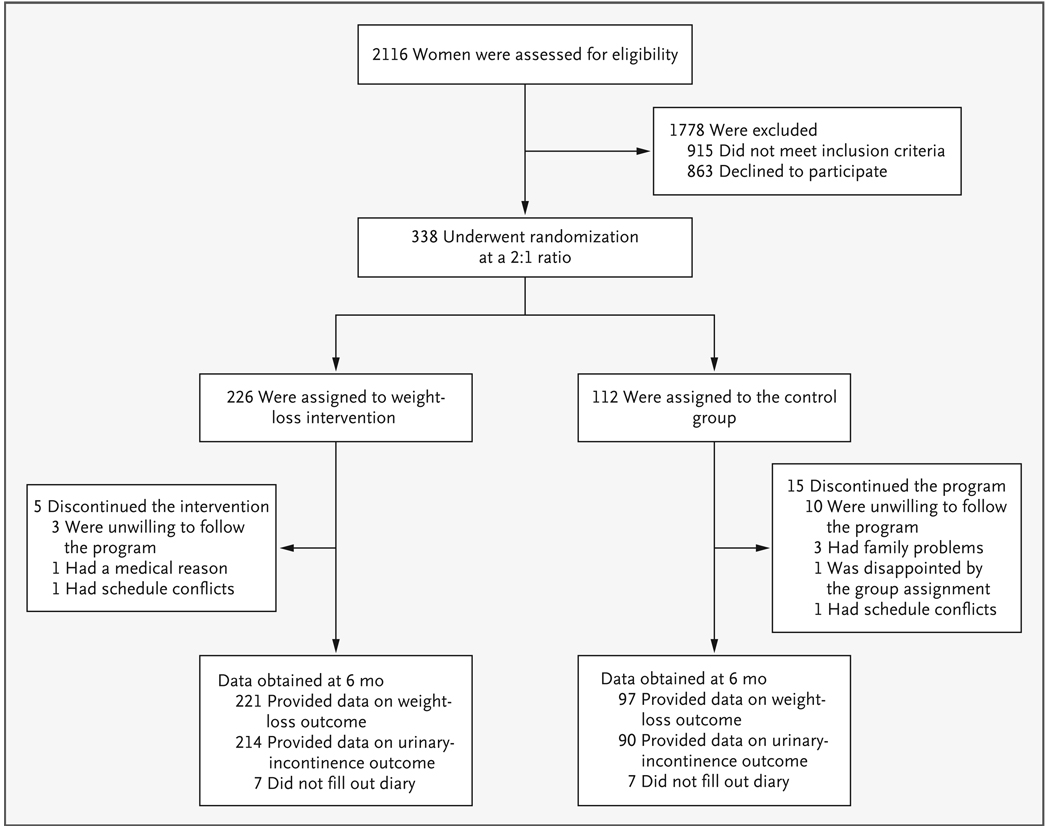
Of the 2116 participants screened by telephone, 1778 were excluded during screening and 338 underwent randomization (Fig. 1). The characteristics of the participants in the weight-loss and control groups were similar at baseline (Table 1).
Figure 1.
Study Participants.
Table 1.
Characteristics of the Participants According to Treatment Group.*
| Characteristic | Total (N = 338) |
Weight-Loss Group (N = 226) |
Control Group (N = 112) |
|---|---|---|---|
| Age — yr | 53±11 | 53±11 | 53±10 |
| Race — no. (%)† | |||
| White | 262 (77.5) | 171 (75.7) | 91 (81.2) |
| Black | 64 (18.9) | 47 (20.8) | 17 (15.2) |
| Other | 12 (3.6) | 8 (3.5) | 4 (3.5) |
| Education beyond high school — no. (%) | 293 (86.7) | 200 (88.5) | 93 (83.0) |
| Relationship status — no. (%) | |||
| Married or living with a partner | 256 (75.7) | 166 (73.5) | 90 (80.4) |
| Single, widowed, or divorced | 82 (24.3) | 60 (26.5) | 22 (19.6) |
| Body-mass index‡ | 36±6 | 36±6 | 36±5 |
| Diabetes — no. (%) | 10 (3.0) | 9 (4.0) | 1 (0.9) |
| Current smoker — no. (%) | 18 (5.3) | 14 (6.2) | 4 (3.6) |
| Current alcohol use — no. (%) | 228 (67.5) | 154 (68.1) | 74 (66.1) |
| Postmenopausal — no./total no. (%) | 177/316 (56.0) | 115/209 (55.0) | 62/107 (57.9) |
| Self-reported health status — no. (%) | |||
| Excellent or very good | 151 (44.7) | 107 (47.3) | 44 (39.3) |
| Good | 150 (44.4) | 99 (43.8) | 51 (45.5) |
| Fair or poor | 37 (10.9) | 20 (8.8) | 17 (15.2) |
| Hysterectomy — no./total no. (%) | 99/337 (29.4) | 70/225 (31.1) | 29/112 (25.9) |
| Parity | 2±1 | 2±1 | 2±1 |
| Type of urinary incontinence — no. (%)§ | |||
| stress only | 18 (5.3) | 8 (3.5) | 10 (8.9) |
| Urge only | 41 (12.1) | 33 (14.6) | 8 (7.1) |
| Stress predominant | 57 (16.9) | 36 (15.9) | 21 (18.8) |
| Urge predominant | 108 (32.0) | 71 (31.4) | 37 (33.0) |
| Mixed incontinence with no predominant type | 114 (33.7) | 78 (34.5) | 36 (32.1) |
| 24-Hr involuntary urine loss — g¶ | 33±55 | 32±55 | 33±48 |
P>0.05 for the comparison between the weight-loss and control groups for all variables listed in the table. Plus–minus values are means ±SD.
Race was self-assessed.
Body-mass index is the weight in kilograms divided by the square of the height in meters.
Type of urinary incontinence was classified according to the participant’s designation of each incontinence episode in a 7-day voiding diary.
Involuntary urine loss was measured by the 24-hour increase in pad weight.
The mean (±SD) age was 53±11 years; 19% were black. The mean BMI (36±6) and the total number of incontinence episodes per week (24±18) (Table 2) were similar in the two groups. At baseline, 297 women had at least one episode of stress incontinence and 320 women had at least one episode of urge incontinence per week. In both groups, urge-related incontinence was more common than stress-related incontinence.
Table 2.
Body Weight and Frequency of Urinary-Incontinence Episodes at Baseline and at 6 Months According to Treatment Group.*
| Outcome | Weight-Loss Group (N = 226) |
Control Group (N = 112) |
P Value |
|---|---|---|---|
| Body weight† | |||
| Baseline — kg | 98±17 | 95±16 | |
| 6 Mo — kg | 90±17 | 94±17 | |
| % Change (95% CI) | −8.0 (−9.0 to −7.0) | −1.6 (−2.7 to −0.4) | <0.001 |
| Urinary-incontinence episodes‡ | |||
| Any incontinence | |||
| Baseline — no./wk | 24±18 | 24±16 | |
| 6 Mo — no./wk | 13±15 | 17±19 | |
| % Change (95% CI) | −47 (−54 to −40) | −28 (−41 to −13) | 0.01 |
| Stress incontinence | |||
| Baseline — no./wk | 9±11 | 10±10 | |
| 6 Mo — no./wk | 4±7 | 7±9 | |
| % Change (95% CI) | −58 (−67 to −46) | −33 (−50 to −9) | 0.02 |
| Urge incontinence | |||
| Baseline — no./wk | 14±14 | 13±15 | |
| 6 Mo — no./wk | 8±11 | 10±15 | |
| % Change (95% CI) | −42 (−51 to −32) | −26 (−44 to −3) | 0.14 |
Plus–minus values are means ±SD and were calculated with the use of multiply imputed data sets for body weight and frequency of urinary-incontinence episodes.
Percentage changes and P values for the comparison between the weight-loss group and the control group were calculated with the use of multiply imputed data sets and mixed linear regression models, with control for clinical site and correlation of outcomes in the intervention groups. The data sets for body weight were based on 221 women in the weight-loss group and 97 in the control group for whom data were available at baseline and 6 months.
Percentage changes and P values for the comparison between the weight-loss and the control groups were calculated with the use of multiply imputed data sets and negative binomial models, with control for clinical site and correlation of outcomes in the intervention group. The data sets for urinary incontinence were based on 214 women in the weight-loss group and 90 in the control group for whom data were available at baseline and 6 months.
FOLLOW-UP
At the 6-month follow-up assessment, 318 women (94.1%) provided weight data (97.8% of the women in the weight-loss group and 86.6% of those in the control group, P<0.001) (Fig. 1), and 304 women (89.9%) completed the 7-day voiding diary (94.7% of those in the weight-loss group and 80.4% of those in the control group, P<0.001). Baseline variables, including age, race, parity, BMI, type of incontinence, frequency of incontinence episodes, and pad weight were not significantly associated with the retention of participants at 6 months.
WEIGHT LOSS
At the 6-month visit, the women in the weight-loss group had a mean loss of 8.0% of body weight from baseline (95% confidence interval [CI], −9.0 to −7.0; mean loss, 7.8 kg), as compared with 1.6% (95% CI, −2.7 to −0.4; mean loss, 1.5 kg) among women in the control group (P<0.001) (Table 2). The results were similar in analyses adjusted for baseline weight and in sensitivity analyses performed with the use of complete-case methods (the mean loss was 8.2% in the weight-loss group and 1.8% in the control group, P<0.001).
URINARY INCONTINENCE
After 6 months, women in the weight-loss group had a mean decrease in the total number of incontinence episodes per week of 47.4% (95% CI, −54.0 to −39.9), as compared with 28.1% in the control group (95% CI, −40.9 to −12.6; P = 0.01) (Table 2). The results were similar in analyses performed with the use of complete-case methods (the mean decrease in the total number of incontinence episodes per week was 49.1% in the intervention group and 34.0% in the control group, P = 0.01) and nonparametric tests (P = 0.003 by the Wilcoxon rank-sum test). The reduction in the number of urinary-incontinence episodes from baseline was attributable primarily to a reduction in episodes of stress incontinence (a decrease of 57.6% in the intervention group as compared with 32.7% in the control group, P = 0.02; P<0.001 by the Wilcoxon rank-sum test). Although the average decrease in the frequency of episodes of urge incontinence was larger in the weight-loss group than in the control group (42.4% vs. 26.0%), this difference was not statistically significant (P = 0.14; P = 0.16 by the Wilcoxon rank-sum test). The effect of treatment on the total number of incontinence episodes per week was similar among subgroups classified at baseline as having stress or stress-predominant incontinence, urge or urge-predominant incontinence, or the mixed type of incontinence (P = 0.75 by a test for heterogeneity).
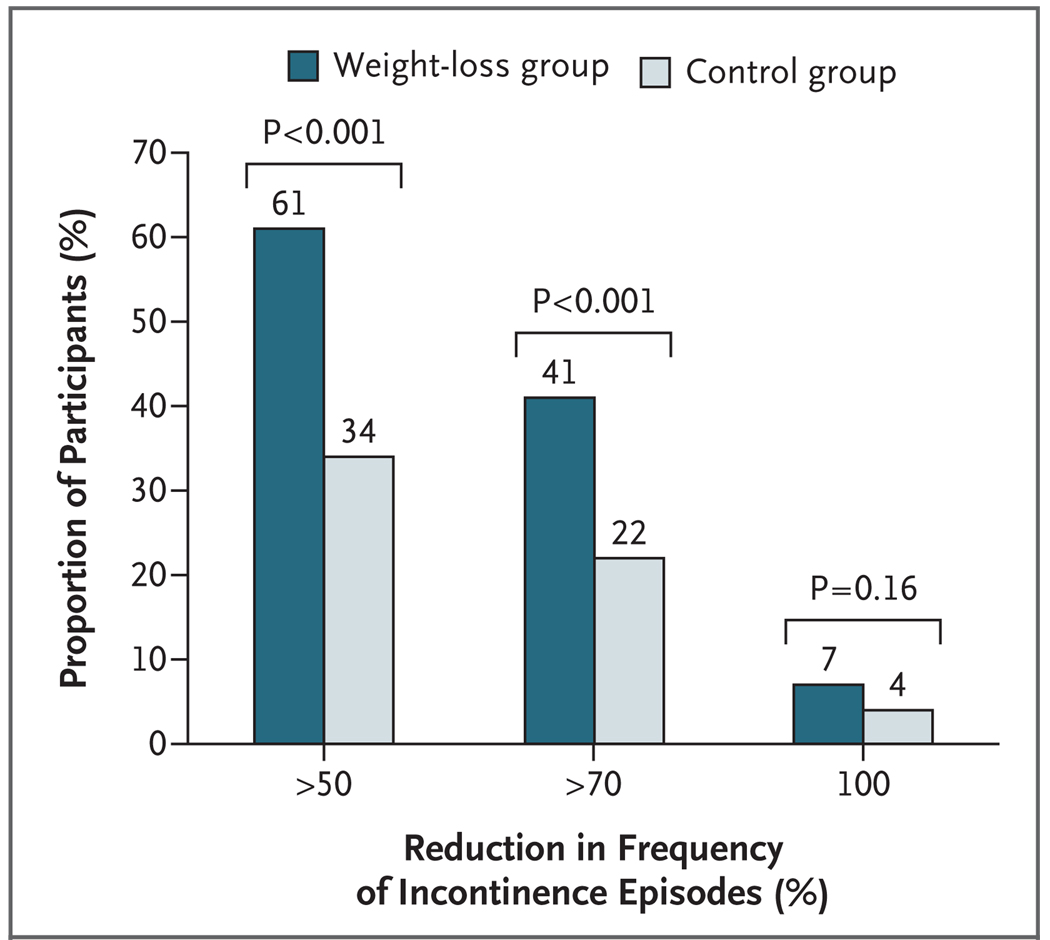
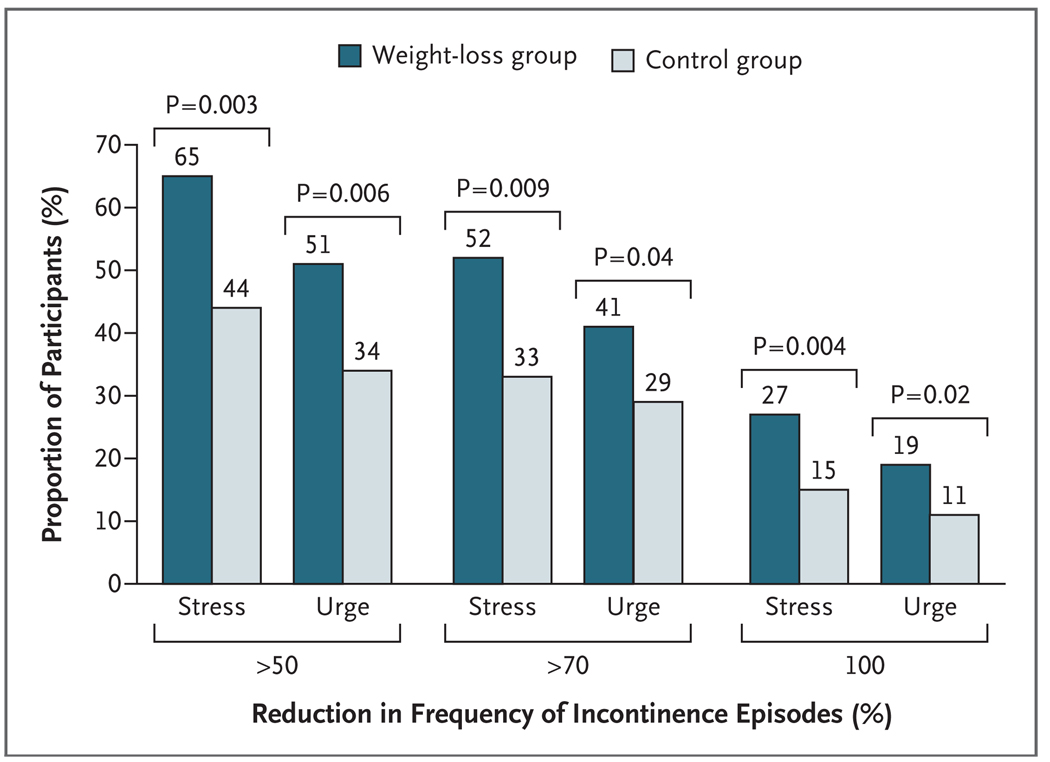
A higher proportion of women in the weight-loss group than in the control group had a reduction of at least 70% in the total number of incontinence episodes per week (P<0.001) (Fig. 2). This pattern was also observed for both stress incontinence and urge incontinence (P = 0.009 and P = 0.04, respectively) (Fig. 3). The results were similar after analysis by complete-case methods.
Figure 2.
Proportion of Participants with Reductions in the Frequency of Any Incontinence Episode at 6 Months.
Figure 3.
Proportion of Participants with Reductions in the Frequency of Episodes of Stress Incontinence and of Urge Incontinence at 6 Months.
No differences were reported between the intervention and control groups in the use of behavioral techniques presented in the self-help incontinence booklet. About one third of the women in both groups reported using urge-suppression techniques or doing pelvic-floor exercises at least weekly, and three quarters of the women found the booklet helpful (P>0.20 for all comparisons). In exploratory analyses, we assessed potential correlates of a decrease in urinary incontinence in the control group. We found moderate associations with weight loss and physical exercise (P = 0.11 and P = 0.05, respectively) but no evidence for an effect of pelvic-floor exercises.
There was no significant change from baseline in either group and no difference between treatment groups in daytime or nighttime voiding frequency. Involuntary urine loss during a 24-hour period, as measured by an increase in pad weight, decreased significantly from baseline, by 45% in the weight-loss group and by 34% in the control group, but the difference between the groups was not statistically significant (P = 0.23).
SATISFACTION WITH AND PERCEPTION OF TREATMENT
As compared with women in the control group, women in the weight-loss group perceived a greater decrease in the frequency of urinary-incontinence episodes and a lower volume of urine loss. They also regarded incontinence as less of a problem and reported higher satisfaction with the change in their incontinence at 6 months (P<0.001 for all comparisons) (Table 3).
Table 3.
Perceptions of Change in Urinary Incontinence at 6 Months as Compared with Baseline According to Treatment Group.*
| Participants’ Perception | Weight-Loss Group (N = 219) |
Control Group (N = 94) |
|---|---|---|
| no. (%) | ||
| Less frequent incontinence episodes | 160 (73.1) | 50 (53.2) |
| Smaller volume of urine loss | 125 (57.1) | 35 (37.2) |
| Incontinence somewhat or much less of a problem |
166 (75.8) | 51 (54.3) |
| Moderately or very satisfied with change in incontinence |
166 (75.8) | 44 (46.8) |
P<0.001 for all comparisons between the weight-loss group and the control group.
DISCUSSION
Among overweight and obese women with urinary incontinence, the comprehensive behavioral weight-loss program in this study resulted in a significantly greater reduction in the frequency of self-reported urinary-incontinence episodes at 6 months as compared with the structured education program. A higher proportion of women in the weight-loss group than in the control group reported a clinically meaningful reduction of at least 70% in the total weekly number of episodes of any incontinence, stress incontinence, and urge incontinence. In addition, the women in the weight-loss group perceived greater improvement in their incontinence and were more satisfied with their improvement.
The 8% reduction in weight achieved in this study slightly exceeded the 6-month weight loss among participants in the lifestyle-intervention subgroup of the Diabetes Prevention Program (7%)18 and approximated the 1-year weight losses in the Look AHEAD trial,23 on which the current intervention was modeled. These trials suggest that behavioral weight-loss programs can consistently produce initial weight losses of this magnitude.
Both stress incontinence and urge incontinence were reduced more in the weight-loss group than in the control group, but the difference between the groups was significant only for stress incontinence. However, there was no interaction between treatment and type of incontinence, a result suggesting that the difference in treatment effects between the subgroup of women with urge incontinence and the subgroup with stress incontinence may have been due to chance. In addition, the proportion of women reporting a clinically meaningful decrease in the number of incontinence episodes per week of 70% or more was greater in the intervention group than the control group for all incontinence episodes, urge-incontinence episodes, and stress-incontinence episodes. This result suggests that overweight or obese women with stress, urge, or mixed incontinence may benefit from weight loss.
Previous studies that have reported significant reductions in incontinence after weight reduction provide information on possible mechanisms by which reduction in incontinence occurs.10,11,14 It has been hypothesized that obesity may contribute to urinary incontinence because of the increase in intraabdominal pressure due to central adiposity, which in turn increases bladder pressure and urethral mobility, exacerbating stress incontinence and possibly urge incontinence.10,11,24,25 Weight reduction may reduce forces on the bladder and pelvic floor, thus reducing incontinence. Positive effects of the weight-loss intervention on incontinence may also have resulted from changes in dietary intake and physical activity.
The frequency of incontinence episodes decreased by about 28% in the control group. This reduction is consistent with reports from trials of other interventions for incontinence and is probably due to regression to the mean and heightened awareness of bladder habits among participants, which may result from completing voiding diaries and questionnaires. The booklet describing behavioral approaches to the control of incontinence has been shown to be effective26,27 and, in combination with four group-education sessions about diet and exercise, may have contributed to improvement in the control group.
The primary outcome measure in our study was change in self-reported incontinence episodes as recorded in the 7-day voiding diary. This is the most common outcome measure in non-surgical intervention trials for urinary incontinence. The participants were trained in diary recording, and each diary was reviewed for completeness by trained research staff. We did not find a parallel difference between treatment groups in 24-hour changes in pad weight. Other trials conducted after our study have also reported a lack of correlation between changes in pad weight and diary-recorded frequency of incontinence, subjective measures of the severity of incontinence, and incontinence-specific quality of life, possibly because these techniques measure different domains of incontinence.28,29 The generalizability of our findings might be limited by the facts that the participants were selected for their potential to adhere to the behavioral weight-loss intervention and that participants with certain medical conditions were excluded. Since the participants were not blinded to their treatment assignment, differential reporting between the randomized groups cannot be excluded. The reductions in the frequency of incontinence in the control group were partially explained by a moderate effect of weight loss and physical activity, but we observed no evidence for a benefit from pelvic-floor exercises. Future studies might examine specifically whether weight loss combined with other incontinence interventions, such as pelvic-floor exercises, would be beneficial.
Previous studies have indicated that behavioral weight-loss interventions can decrease the risk of type 2 diabetes18,30 and hypertension,31 improve control of hypertension31,32 and hyperlipidemia, 32 and improve mood and quality of life.33–35 Our results suggest that a decrease in urinary incontinence may be another benefit among the health improvements associated with moderate weight loss and support consideration of weight reduction as a first-line treatment for overweight and obese women with incontinence.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (U01 DK067860, U01 DK067861, and U01 DK067862) and from the Office of Research on Women’s Health.
Dr. Subak reports serving on an advisory board for Pfizer and receiving grant support from Pfizer; Dr. Grady, receiving grant support from Bionovo; Dr. Kusek, owning stock in Eli Lilly, Pfizer, and deCODE Genetics; and Dr. Burgio, serving on an advisory board for Pfizer, receiving grant support from Pfizer, and receiving advisory-board fees from Astellas and GlaxoSmithKline.
The views expressed are those of the authors and do not necessarily represent the official views of the NIDDK or the National Institutes of Health.
APPENDIX
The investigators in the Program to Reduce Incontinence by Diet and Exercise (PRIDE) are as follows: University of Alabama at Birmingham — F. Franklin (principal investigator), H.E. Richter (coinvestigator), K.L. Burgio (coinvestigator), L. Abdo, C. Bragg, K. Carter, J. Dunlap, S. Gilbert, S. Hannum, A. Hubbell, K. Marshall, L. Pair, P. Pierce, C. Smith, S. Thompson, J. Turman, A. Wrenn; Miriam Hospital — R. Wing (principal investigator), A. Gorin (coinvestigator), D. Myers (coinvestigator), T. Monk, R. Ata, M. Butryn, P. Coward, L. Gay, J. Hecht, A. Lepore-Ally, H. Niemeier, Y. Nillni, A. Pinto, D. Ranslow-Robles, N. Robinson, D. Sepinwall, M.E. Hahn, V.W. Sung, V. Winn, N. Zobel; University of Arkansas for Medical Sciences — D. West (investigator); University of Pennsylvania — G. Foster (consultant); University of California, San Francisco (coordinating center) — D. Grady (principal investigator), L. Subak (co-principal investigator), J. Macer, A. Chang, J. Creasman, J. Quan, E. Vittinghoff, J. Yang; NIDDK — J.W. Kusek, L.M. Nyberg (project officers); Data and Safety Monitoring Board: University of Utah Health Sciences Center — I. Nygaard (chair); Children’s Hospital, Boston — L. Kalish; University of California, San Diego — C. Nager; Medical University of South Carolina — P.M. O’Neil; Johns Hopkins School of Medicine — C.S. Rand; University of Virginia Health Systems — W.D. Steers.
Footnotes
The investigators of the Program to Reduce Incontinence by Diet and Exercise (PRIDE) are listed in the Appendix.
No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Grimby A, Milsom I, Molander U, Wiklund I, Ekelund P. The influence of urinary incontinence on the quality of life of elderly women. Age Ageing. 1993;22:82–89. doi: 10.1093/ageing/22.2.82. [DOI] [PubMed] [Google Scholar]
- 2.Hunskaar S, Vinsnes A. The quality of life in women with urinary incontinence as measured by the Sickness Impact Profile. J Am Geriatr Soc. 1991;39:378–382. doi: 10.1111/j.1532-5415.1991.tb02903.x. [Erratum, J Am Geriatr Soc 1992;40: 976-7.] [DOI] [PubMed] [Google Scholar]
- 3.Brown JS, Vittinghoff E, Wyman JF, et al. Urinary incontinence: does it increase risk for falls and fractures? J Am Geriatr Soc. 2000;48:721–725. doi: 10.1111/j.1532-5415.2000.tb04744.x. [DOI] [PubMed] [Google Scholar]
- 4.Thom DH, Haan MN, Van Den Eeden SK. Medically recognized urinary incontinence and risks of hospitalization, nursing home admission and mortality. Age Ageing. 1997;26:367–374. doi: 10.1093/ageing/26.5.367. [DOI] [PubMed] [Google Scholar]
- 5.Wilson L, Brown JS, Shin GP, Luc KO, Subak LL. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98:398–406. doi: 10.1016/s0029-7844(01)01464-8. [DOI] [PubMed] [Google Scholar]
- 6.Danforth KN, Townsend MK, Lifford K, Curhan GC, Resnick NM, Grodstein F. Risk factors for urinary incontinence among middle-aged women. Am J Obstet Gynecol. 2006;194:339–345. doi: 10.1016/j.ajog.2005.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannestad YS, Rortveit G, Daltveit AK, Hunskaar S. Are smoking and other lifestyle factors associated with female urinary incontinence? The Norwegian EPINCONT Study. BJOG. 2003;110:247–254. [PubMed] [Google Scholar]
- 8.Melville JL, Katon W, Delaney K, Newton K. Urinary incontinence in US women: a population-based study. Arch Intern Med. 2005;165:537–542. doi: 10.1001/archinte.165.5.537. [DOI] [PubMed] [Google Scholar]
- 9.Waetjen LE, Liao S, Johnson WO, et al. Factors associated with prevalent and incident urinary incontinence in a cohort of midlife women: a longitudinal analysis of data: study of women’s health across the nation. Am J Epidemiol. 2007;165:309–318. doi: 10.1093/aje/kwk018. [DOI] [PubMed] [Google Scholar]
- 10.Subak LL, Whitcomb E, Shen H, Saxton J, Vittinghoff E, Brown JS. Weight loss: a novel and effective treatment for urinary incontinence. J Urol. 2005;174:190–195. doi: 10.1097/01.ju.0000162056.30326.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bump RC, Sugerman HJ, Fantl JA, Mc-Clish DK. Obesity and lower urinary tract function in women: effect of surgically induced weight loss. Am J Obstet Gynecol. 1992;167:392–397. doi: 10.1016/s0002-9378(11)91418-5. [DOI] [PubMed] [Google Scholar]
- 12.Deitel M, Stone E, Kassam HA, Wilk EJ, Sutherland DJ. Gynecologic-obstetric changes after loss of massive excess weight following bariatric surgery. J Am Coll Nutr. 1988;7:147–153. doi: 10.1080/07315724.1988.10720232. [DOI] [PubMed] [Google Scholar]
- 13.Burgio KL, Richter HE, Clements RH, Redden DT, Goode PS. Changes in urinary and fecal incontinence symptoms with weight loss surgery in morbidly obese women. Obstet Gynecol. 2007;110:1034–1040. doi: 10.1097/01.AOG.0000285483.22898.9c. [DOI] [PubMed] [Google Scholar]
- 14.Subak LL, Johnson C, Whitcomb E, Boban D, Saxton J, Brown JS. Does weight loss improve incontinence in moderately obese women? Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:40–43. doi: 10.1007/s001920200008. [DOI] [PubMed] [Google Scholar]
- 15.Nygaard I, Holcomb R. Reproducibility of the seven-day voiding diary in women with stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:15–17. doi: 10.1007/pl00004021. [DOI] [PubMed] [Google Scholar]
- 16.Wyman JF, Choi SC, Harkins SW, Wilson MS, Fantl JA. The urinary diary in evaluation of incontinent women: a test-retest analysis. Obstet Gynecol. 1988;71:812–817. [PubMed] [Google Scholar]
- 17.Burgio KL, Pearce KL, Lucco AJ. Staying dry: a practical guide to bladder control. Baltimore: Johns Hopkins University Press; 1989. [Google Scholar]
- 18.Ryan DH, Espeland MA, Foster GD, et al. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 19.Wadden TA, West DS, Delahanty L, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgio KL, Goode PS, Richter HE, Locher JL, Roth DL. Global ratings of patient satisfaction and perceptions of improvement with treatment for urinary incontinence: validation of three global patient ratings. Neurourol Urodyn. 2006;25:411–417. doi: 10.1002/nau.20243. [DOI] [PubMed] [Google Scholar]
- 22.Abrams P, Blaivas JG, Stanton SL, Andersen JT. The standardisation of terminology of lower urinary tract function. Scand J Urol Nephrol Suppl. 1988;114:5–19. [PubMed] [Google Scholar]
- 23.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cummings JM, Rodning CB. Urinary stress incontinence among obese women: review of pathophysiology therapy. Int Urogynecol J Pelvic Floor Dysfunct. 2000;11:41–44. doi: 10.1007/s001920050008. [DOI] [PubMed] [Google Scholar]
- 25.Townsend MK, Danforth KN, Rosner B, Curhan GC, Resnick NM, Grodstein F. Body mass index, weight gain, and incident urinary incontinence in middle-aged women. Obstet Gynecol. 2007;110:346–353. doi: 10.1097/01.AOG.0000270121.15510.57. [DOI] [PubMed] [Google Scholar]
- 26.Burgio KL, Goode PS, Locher JL, et al. Behavioral training with and without bio-feedback in the treatment of urge incontinence in older women: a randomized controlled trial. JAMA. 2002;288:2293–2299. doi: 10.1001/jama.288.18.2293. [DOI] [PubMed] [Google Scholar]
- 27.Goode PS, Burgio KL, Locher JL, et al. Effect of behavioral training with or without pelvic floor electrical stimulation on stress incontinence in women: a randomized controlled trial. JAMA. 2003;290:345–352. doi: 10.1001/jama.290.3.345. [DOI] [PubMed] [Google Scholar]
- 28.Albo M, Wruck L, Baker J, et al. The relationships among measures of incontinence severity in women undergoing surgery for stress urinary incontinence. J Urol. 2007;177:1810–1814. doi: 10.1016/j.juro.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 29.Reid FM, Smith AR, Dunn G. Which questionnaire? A psychometric evaluation of three patient-based outcome measures used to assess surgery for stress urinary incontinence. Neurourol Urodyn. 2007;26:123–128. doi: 10.1002/nau.20303. [DOI] [PubMed] [Google Scholar]
- 30.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 31.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 32.Miller ER, III, Erlinger TP, Young DR, et al. Results of the Diet, Exercise, and Weight Loss Intervention Trial (DEW-IT) Hypertension. 2002;40:612–618. doi: 10.1161/01.hyp.0000037217.96002.8e. [DOI] [PubMed] [Google Scholar]
- 33.Blissmer B, Riebe D, Dye G, Ruggiero L, Greene G, Caldwell M. Health-related quality of life following a clinical weight loss intervention among overweight and obese adults: intervention and 24 month follow-up effects. Health Qual Life Outcomes. 2006;4:43. doi: 10.1186/1477-7525-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlsson J, Sjöström L, Sullivan M. Swedish Obese Subjects (SOS) — an intervention study of obesity: two-year follow-up of health-related quality of life (HRQL) and eating behavior after gastric surgery for severe obesity. Int J Obes Relat Metab Disord. 1998;22:113–126. doi: 10.1038/sj.ijo.0800553. [DOI] [PubMed] [Google Scholar]
- 35.Wing RR, Epstein LH, Marcus MD, Kupfer DJ. Mood changes in behavioral weight loss programs. J Psychosom Res. 1984;28:189–196. doi: 10.1016/0022-3999(84)90019-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.