Abstract
Purpose
To investigate the impact of bladder filling state on dosimetry and determine the best bladder dosimetric parameter in vaginal-cuff brachytherapy.
Materials and Methods
Twenty women received vaginal cylinder high-dose-rate (HDR) brachytherapy with each fraction followed by a planning CT scan on a prospective clinical trial. The bladder was full for fraction 2 and empty for fraction 3. Dose volume histogram (DVH) and dose surface histogram (DSH) values were generated for the bladder, rectum, and urethra. The midline maximum bladder point (MBP) and the midline maximum rectal point (MRP) were recorded. Paired t-tests, Pearson correlations, and regression analyses were performed.
Results
The volume and surface area of bladder irradiated were significantly smaller when the bladder was empty than when full. Of several DVH and DSH parameters evaluated, the bladder D2cc, V50, V70 and SA50 significantly predicted the difference in empty versus full filling states. The V70 and D2cc were significantly correlated with the MBP. Bladder filling did not alter the volume or surface area of rectum irradiated. However, an empty bladder did result in the nearest point of bowel being significantly closer to the vaginal cylinder than when the bladder was full.
Conclusions
In order to minimize radiation dose to the bladder, patients receiving vaginal-cuff HDR brachytherapy should be treated with an empty bladder if feasible. The MBP correlates well with the volumetric assessments of bladder dose and provides a non-invasive method for reporting maximum bladder point dose using 3D imaging. The MBP can therefore be used as a surrogate for complex dosimetry in the clinic.
Keywords: Vaginal cuff brachytherapy, CT bladder dosimetry
Introduction
Vaginal cylinder brachytherapy, either alone or after external-beam radiation therapy (EBRT), can be used in the treatment of selected gynecologic malignancies.1–3 The most common use of vaginal-cylinder brachytherapy is in the primary adjuvant treatment of endometrial cancer.4 Following a total abdominal hysterectomy (TAH) and bilateral salpingo-oophorectomy (BSO), radiotherapy may be indicated, depending upon the surgical stage and histology of the resected tumor.5–7 The use of high-dose-rate (HDR) vaginal-cylinder brachytherapy has been shown to be effective in many previous publications.8–10
The use of vaginal-cuff brachytherapy reduces local recurrence rates but may increase the risk of normal tissue toxicity.11, 12 Therefore it is important to measure the dose received by normal tissues in the pelvis during vaginal-cuff brachytherapy. The international standard of critical structure dose measurement in gynecologic brachytherapy (ICRU 38) has several limitations:13 the dose points are not representative of the volume and surface area of normal tissue irradiated;14–16 the dose points may underestimate the dose received by normal tissues, as other tissue may be closer to the brachytherapy source; and in addition, calculation of the ICRU bladder dose point requires the placement of a urinary catheter. Although urinary catheterization was previously common during low-dose-rate (LDR) vaginal-cylinder brachytherapy, patient discomfort has decreased its routine use during outpatient HDR treatments.
Most physicians prescribe dose at a uniform distance from the cylinder with no dose sculpting to avoid normal tissue. Therefore, the conformation of the normal structures must be changed in order to alter normal tissue doses. The optimal state of bladder filling for patients undergoing vaginal-cuff brachytherapy has been examined by Hoskin et al., who used urinary catheterization to ensure consistent states of bladder filling prior to vaginal-cuff brachytherapy.17 However, alternative less invasive methods of bladder filling and bladder dose estimation are needed for routine daily practice.
3D image–based contouring of the organs at risk (OAR) with computerized dosimetry is one non-invasive method of determining the doses received by the normal tissues during vaginal-cylinder brachytherapy. However, which parameters best represent dose to the bladder is unknown. This prospective trial assesses normal-tissue doses during vaginal-cuff brachytherapy and examines the effect of non-invasive bladder filling on normal-tissue dosimetry using CT imaging.
Materials and Methods
Patients
Between February 2004 and September 2004, 20 women were enrolled in a prospective clinical trial approved by the relevant Institutional Review Boards. Inclusion criteria included previous hysterectomy, histologically verified gynecologic carcinoma, ECOG performance status of ≤2, and age over 18 years. Patients with distant metastases or inoperable disease were excluded. Each patient gave signed informed consent to participate in the study.
Radiotherapy
All patients were treated with HDR brachytherapy cylinder insertions to the vaginal cuff. Each patient received a minimum of 3 HDR treatments. Generally, patients who underwent EBRT to the pelvis received three cylinder insertions and those who did not received five cylinder insertions. The cylinder diameter ranged from 2.0 to 3.5 cm. The widest cylinder that the patient could tolerate was inserted to ensure the optimal coverage of the vaginal surface. The standard doses were 18 Gy in 3 fractions following pelvic EBRT or 30 Gy in 5 fractions for brachytherapy alone, all with dose prescribed at the cylinder surface. Variation from the standard dose was permitted if clinical circumstances indicated. If the patient did not have risk factors for lower vaginal involvement, the upper half of the vagina (as measured by CT) was treated. Treatment was administered using an Iridium 192 HDR afterloader (Nucletron, Veenendaal, The Netherlands).
Bladder filling was varied for each fraction. Fraction one was considered a reference fraction, with the bladder in a variable state of filling determined by patient choice and comfort. One hour prior to fraction two, the patient consumed 32 ounces of water18 and did not empty her bladder before treatment (designated 'full bladder'). Immediately prior to the third fraction, the patient emptied her bladder ('empty bladder'). The cylinder angle was kept as close to horizontal as was physiologically comfortable.
Following the first CT scan, an individual treatment plan was calculated. Brachytherapy was administered once the treatment plan was completed. For subsequent fractions, the patient underwent cylinder insertion, CT planning scan, and brachytherapy treatment without need for generation of a new plan; therefore, for fractions 2 and 3 the cylinder was in place for a shorter time.
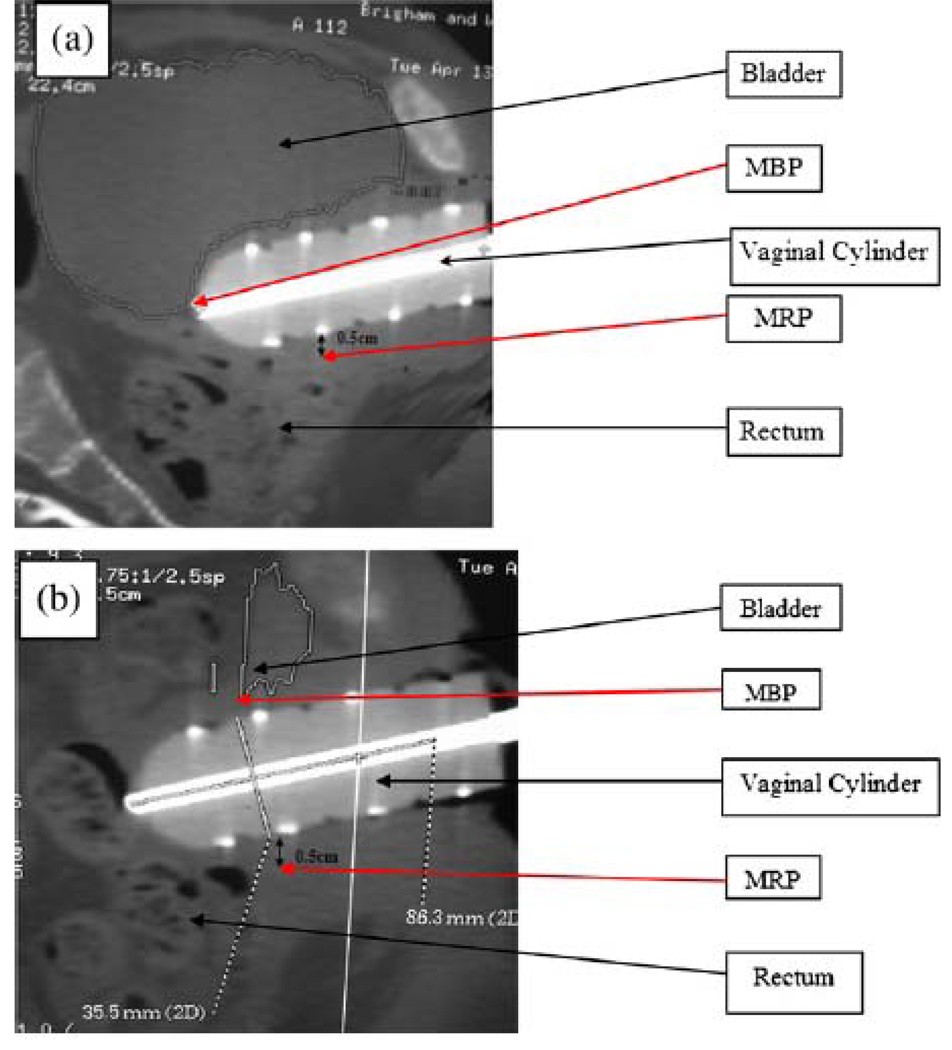
The bladder, rectum, and urethra were contoured in 3 dimensions by one physician (A.S.), and the location of the cylinder was marked (Advantage Sim MD, version 7.0.83, GE Medical Systems, Waukasha, Wisconsin, USA). The closest distance to the bowel was measured from the apex of the vaginal cylinder. A sagittal CT image was printed out using 1:1 magnification before each brachytherapy fraction at the midplane of the vaginal cylinder with the bladder contour visible (see figures 1A and 1B). Following the first CT planning scan, the images were transferred to Plato planning software (Nucletron), and the brachytherapy treatment was planned. The isodose curves at the cylinder midplane were printed out using a 1:1 magnification and superimposed on the sagittal CT image. The MBP was defined as the point of intersection of the bladder contour and the maximum isodose line on a midline reconstructed sagittal CT image. The MBP was reported both as an absolute dose and as a percentage of the prescribed dose (%MBP). The maximum rectal point (MRP) was defined as the dose 0.5 cm from the posterior surface of the vaginal cylinder, as per ICRU 38 guidelines.13 The MRP was reported both as an absolute dose and as a percentage of the prescribed dose (%MRP).
Figure 1.
Figures 1 A and B. Sagittal CT slice through the center of the vaginal cylinder in the same patient with a full bladder (A) and an empty bladder (B) showing the approximate position of the MBP.
The structure contours were read into a separate software package developed at the Brigham and Women’s Hospital19, 20 to generate dose volume histograms (DVH) and dose surface-area histograms (DSH) for the bladder, rectum, and urethra. The accurate transfer of the contours was confirmed using a deformation registration algorithm.19, 20
V100 was defined as the volume of the bladder receiving at least 100% of the dose, and SA100 as the surface area of the bladder receiving at least 100% of the dose. The volume and surface area of bladder receiving at least 100%, 90%, 80%, and 50% and the volume receiving at least 70% of the dose were measured and defined using the above conventions. The volume and surface area of rectum receiving at least 100%, 90%, and 80% and the volume receiving at least 75% and 55% of the dose were measured and defined using the above conventions. These dose-reporting parameters were chosen because similar volumetric parameters have been correlated with late side effects in both the bladder and rectum in prostate cancer,21–23 and assessment of an absolute dose received allows accurate comparison of the difference between a surface area of tissue irradiated and a volume of tissue irradiated. The maximum dose received by 2 cc of tissue (D2cc) was recorded for the bladder and rectum. The D2cc measurement has been determined by the GEC-ESTRO group to be representative of a clinically significant determinant of critical-organ dosimetry in gynecologic cancer15, 16, 24, 25 and has been correlated with late rectal side effects in cervical cancer.26
Statistical Analysis
The study sample size was determined as follows. A small preliminary data sample of 14 repeated measurements from four individual patients revealed an average change (± SD) in bladder size between the empty and full states of 2.5-fold. Using a conservative assumption of the average fold change effect size (± SD) of 2, it was estimated that with 20 study subjects the power to detect at least 2-fold within-subject mean change in bladder size between full and empty using a two-sided paired t-test at a 5% significance level was 90%.
All dosimetric parameters outlined above were summed and the mean calculated. A two-tailed paired t-test was used to compare the difference in bladder and rectal volumes and surface areas by CT scan between the full and empty bladder, and a Bonferroni correction was performed to assess statistical significance. Pearson correlation analysis within each bladder filling state was performed to examine whether there were linear associations between MBP and bladder DVH, DSH, and D2cc as well as MRP and rectal DVH, DSH, and D2cc. Multivariate regression modeling was applied to all parameters; however, the significant correlation between parameters resulted in substantial collinearity and non-convergence of the model. Another multivariate regression was analyzed to examine if DVH and DSH were confounded with D2cc to predict the MBP and MRP; however, no confounding was seen, and therefore the univariate t-test results sufficed.
Results
Twenty patients were enrolled; none had received prior vaginal cuff brachytherapy. Cylinder sizes were 2.0 cm (1), 2.5 cm (1), 3.0 cm (6), and 3.5 cm (12). The median treatment length was 7 cm [range (rg), 4–10 cm]. The median dose per fraction was 6 Gy (rg, 5.75–7.5 Gy). The median number of fractions treated was 3 (rg, 3–5). No patients had a past history of bladder problems, chronic dysuria, or interstitial cystitis. No patients described discomfort from bladder filling over the course of insertion and treatment.
The bladder volume and surface area were both significantly smaller in the empty state than the full state (Table 1). We posited that irradiated volumes under 1.5–2.0 cm3 are clinically negligible;25 the V100, V90, and V80 (and hence the SA100, SA90, and SA80) were therefore not useful parameters to measure, as only 2 patients had values over 2.0 cm3 for V90 and V100 and 5 patients for V80. Therefore, the V70 was taken as the most appropriate volumetric parameter to analyze the highest dose received by the bladder. The bladder V70, V50, SA50, and D2cc were significantly lower for the empty bladder than for the full bladder, with the V50 and SA50 remaining significant after Bonferroni correction (Table 2). For the rectum, the V100, V90, V80, and V75 were not considered useful parameters to assess given the low volumes irradiated. The V55 and D2cc measured a clinically significant volume of rectal tissue; there was no significant difference in these parameters between the full and empty bladder states.
Table 1.
Bladder volume and surface area in full and empty bladder
| Bladder filling |
Mean volume (cm3) |
Range (cm3) |
Mean surface area (cm3) |
Range (cm3) |
|---|---|---|---|---|
| Full* | 278.3 | 48.9–784.2 | 259.9 | 102.4–529.4 |
| Empty | 93.8 | 26.8–228.0 | 146.1 | 84.6–236.7 |
| p | 0.003 | 0.001 |
Patients consumed 32 oz of water 1 h before cylinder insertion.
Table 2.
Significant dosimetric parameters identified on comparison of bladder volume, surface area, and maximal point measurements with full versus empty bladder
| Mean bladder dosimetric value |
Full bladder* (range) |
Empty bladder (range) |
p |
|---|---|---|---|
| V70 (cm3) | 3.70 (0.19–11.39) | 2.26 (0–14.72) | 0.03† |
| V50 (cm3) | 18.47 (3.64–37.89) | 10.52 (0.78–28.91) | 0.003†‡ |
| SA50 (cm2) | 39.27 (9.57–62.99) | 27.21 (5.92–72.14) | 0.007†‡ |
| Dcc(Gy) | 4.56 (3.44–6.30) | 4.06 (2.56–8.27) | 0.03† |
| MBP (Gy) | 5.49 (4–7) | 4.72 (3–8.5) | 0.008† |
| % MBP | 90 (67–100) | 77 (50–113) | 0.005† |
| MRP (Gy) | 4.4 (3.88–5.59) | 4.4 (3.88–5.59) | >0.05 |
| %MRP | 72 (55–76) | 72 (55–76) | >0.05 |
Abbreviations: V70 = volume of bladder receiving ≥70% of dose; V50 = volume of bladder receiving ≥50% of dose; SA50 = surface area of bladder receiving ≥50% of dose; Dcc = maximal dose received by 2 cm3 of tissue; MBP = point of intersection of bladder contour and maximal isodose line on midline reconstructed sagittal image; MRP = dose 0.5 cm from posterior surface of vaginal cylinder.
Patients consumed 32 oz. of water 1 h before cylinder insertion.
Statistically significant p value.
Statistically significant after Bonferroni correction.
The MBP and %MBP were significantly lower in the empty bladder than the full bladder (Table 2). The MRP and %MRP were the same for the full and empty bladder states, as the rectum was a fixed distance (0.5 cm) from the cylinder. When clinically relevant dosimetric parameters were assessed, a positive correlation between the bladder V70 and the bladder D2cc was seen with the MBP in the full and empty bladder states (Table 3). There was no correlation between the MBP and the bladder V50 or the SA50 in the full bladder, and the MBP correlated with the bladder V50 only in the empty bladder. The MRP correlated with the rectal V55 and rectal D2cc in both bladder states. There was no correlation between treatment length or cylinder diameter and the bladder V70, V50, SA50, or D2cc for the full or empty bladder states. The cylinder size showed a positive correlation with the MRP (correlation coefficient 0.83, p<0.0001), but there was no correlation between treatment length and MRP.
Table 3.
Correlations of MBP and MRP with clinically relevant bladder and rectum dosimetric parameters with full and empty bladder
| Full bladder* | Empty bladder | |||
|---|---|---|---|---|
| Mean dosimetric value |
Correlation coefficient |
p | Correlation coefficient |
p |
| Bladder V70 (cm3) | 0.49 | 0.03† | 0.46 | 0.04† |
| Bladder V50 (cm3) | 0.29 | 0.22 | 0.50 | 0.02† |
| Bladder SA50 (cm2) | 0.13 | 0.60 | 0.45 | 0.05 |
| Bladder Dcc (Gy) | 0.66 | 0.002† | 0.65 | 0.002† |
| Rectum V55 (cm3) | 0.51 | 0.02† | 0.45 | 0.05 |
| Rectum Dcc (Gy) | 0.80 | <0.0001† | 0.73 | 0.0002† |
Abbreviations as in Table 2.
Patients consumed 32 oz. of water 1 h before cylinder insertion.
Statistically significant.
The median distance to the nearest point of bowel as determined on the midplane sagittal CT image was 5.75 mm (rg, 2.2–30.7 mm) for an empty bladder and 11.6 mm (rg, 3.3–62.8 mm) for a full bladder (p=0.002). The urethral D1cc received less than 50% of the total dose in all cases. The median urethral V55 was 0.056 cm3 for the full bladder and was 0.061 cm3 for the empty bladder.
Discussion
To our knowledge, this is the first prospective clinical trial investigating normal-tissue dose reporting and the effect of bladder volume using CT-based dosimetry in vaginal-cuff HDR brachytherapy. Overall, the bladder and rectum received low doses of radiation. The volume and surface area of bladder irradiated were smaller when the bladder was empty than when it was full, possibly because a full bladder may drop around the posterior aspect of a vaginal cylinder in a post-hysterectomy patient. Of the various dosimetric parameters assessed, the bladder V50 and D2cc yielded the greatest difference in empty versus full filling states. Bladder filling did not alter the volume or surface area of rectum irradiated. However, an empty bladder did result in the nearest point of bowel being significantly closer to the vaginal cylinder than when the bladder was full. The MBP provided an efficient and accurate estimate of the bladder dose following cylinder insertion and prior to brachytherapy treatment. The MBP correlated well with the volumetric assessments of bladder dose and can therefore be used as a surrogate for complex dosimetry. This trial validated that the ICRU 38 rectal point (the MRP) is also a good surrogate for volumetric assessment of rectal dose in cylinder brachytherapy, as the MRP correlated well with the volumetric assessment of rectal dose.
The use of vaginal-cuff HDR brachytherapy is increasing in the U.S.9 Simulation, via 2D fluoroscopic imaging, is performed at many centers to provide documentation of the cylinder size, bladder and rectum point dose estimates. For HDR administration, standard recommendations include estimation of the bladder dose using a Foley catheter. The introduction of 3D imaging for gynecologic brachytherapy planning has obviated placement of a urinary catheter for bladder dose estimation. The use of CT scanning in vaginal brachytherapy has been reported,17, 27–29 but the volumetric dosimetry to the OAR in HDR cylinder brachytherapy is unknown.
Studies of tandem and ovoid brachytherapy in the treatment of cervical cancer have assessed the conventional ICRU 38 reference points and compared them to CT-based volumetric dose estimations.15, 24, 30 Schoeppel et al. determined that the CT maximum bladder and rectal doses might not be the best index doses to correlate with outcome, because the volumes irradiated are so small, suggesting that volumetric assessments are preferable.15 Pelloski et al. found that the ICRU 38 rectal point in LDR tandem and ovoid brachytherapy is a reasonable surrogate for volumetric assessment of rectal dose received (D2cc).24 However, the ICRU 38 bladder point is not a reasonable surrogate for the bladder D2cc. Our study confirmed that the rectal ICRU 38 dose point (the MRP) is a good surrogate for the rectal D2cc in vaginal-cylinder brachytherapy.
Previous studies of the effect of bladder filling on normal-tissue dosimetry in gynecologic cancer have focused predominantly on patients undergoing brachytherapy with a uterine tandem in an intact uterus.31, 32 These studies required placement of a urinary catheter to facilitate bladder filling and emptying and for ICRU 38 dose point estimation. Sun et al. found a statistically significant decrease in the V50 as contoured on a planning CT when a median of 220 cc of water was instilled into the bladder before intracavitary cervix implant.31 The median bladder-wall dose, analyzed as a volume rather than a surface area, was significantly decreased when the bladder was full. The bladder D5cc was not affected by bladder filling. When considering the variation between our results and those of Sun et al., it is important to consider that patients undergoing intracavitary implants have an intact uterus, which may displace the bladder away from the implant, and also that intracavitary-implant patients have vaginal packing to further displace the posterior bladder wall, which is not possible in vaginal-cylinder brachytherapy. Also, hysterectomy requires removal of paravaginal and parametrial support tissues, which results in the rectum and bladder moving closer to the brachytherapy applicators and therefore the high-dose regions move nearer the sources. This movement was observed to generally be greater in the full bladder than the empty bladder in this study and may account for the significantly higher MBP in the full bladder state. Figure 1 demonstrates this effect, showing the full bladder falling posteriorly over the apex of the cylinder, as was seen consistently among patients. Due to source anisotropy, the cylinder apex is an area of greater dose inhomogeneity and, depending on the tissue thickness at the vaginal cuff, this can result in an MBP over 100% of the prescribed dose despite a prescription point at the cylinder surface.
Hoskin and Vidler examined the effect of bladder filling in patients undergoing vaginal-cylinder brachytherapy.17, 33 Their initial pilot study of 5 patients showed that the instillation of 70 ml of water into the bladder decreased the amount of small bowel in the field but increased the posterior bladder-wall dose.33 However, a subsequent 30-patient study showed that the instillation of 100 ml of water into the bladder decreased the amount of small bowel in the field without significantly increasing the mean maximum bladder dose.17 The study analyzed only the mean maximum bladder dose and did not assess volumetric parameters. Therefore the possible effect of a larger proportion of bladder surface area coming into contact with the cylinder in the larger bladder was not assessed. The higher median bladder volume of 191 cc for the full bladder state in our study may also account for the differences.
Limitations of this study include the fact that the delineation of normal tissue can be difficult using CT imaging. The gold standard for normal-tissue contouring in gynecologic brachytherapy is MRI scanning,16 which is limited by cost and availability. However, contouring the organs at risk using CT imaging has been shown not to be significantly different than MRI imaging.30 CT-based contouring can be subject to inter-observer variability of up to 11%,43 but the variation was minimized in our study by ensuring that one physician contoured all OAR. Intra-observer variability may occur, with up to 3% variability found in CT-based prostate-cancer contouring.44 We did not consent patients for oral contrast and therefore we were not able to differentiate small bowel from large bowel for the bowel point dose estimation. Future studies will address the issue of small-bowel dosing.
Following EBRT and intracavitary brachytherapy implant for treatment of cervix cancer, a linear relationship is seen between the total dose to the bladder and rectum and the incidence of late toxicity.45, 46 The same relationship would be expected following EBRT and vaginal- cylinder brachytherapy. However, it is important to remember that these studies used the ICRU 38 reference points for these associations. Koom et al. have shown a positive correlation between the D2cc dose and the risk of rectal damage seen by sigmoidoscopy in treatment of cervical cancer.26 The use of volumetric dosimetry has not yet been correlated with late complications in the bladder. Further work is needed in CT-based volumetric dosimetry and vaginal-cylinder brachytherapy to determine clinical outcomes related to D2cc measurements. Toxicity was not assessed; prospective follow-up will determine whether the state of bladder filling at the time of cylinder brachytherapy is associated with a risk of late complications. Bowel toxicity would be an important area to assess in view of the decrease in the shortest distance to bowel in the empty bladder state.
Conclusion
This study indicates that the HDR brachytherapy dose to the bladder is lower when the bladder is empty than when it is full. A useful, quick, and easy method of bladder dose determination, the MBP, was established that correlated well with volumetric dose determination. Future prospective studies of vaginal-cuff brachytherapy may consider requiring treatment with an empty bladder in order to minimize dose to the bladder; however, further early and late toxicity data for this approach are necessary.
Acknowledgements
Ms. C. Tanaka for handling regulatory issues; Dr. S. Mutyala and Dr. P. Devlin for patient care.
Footnotes
Results forming part of this manuscript were presented in poster form at the annual meeting of the American Society for Therapeutic Radiology and Oncology, Oct 2005, Denver CO
Conflict of interest notification: Dr Stewart received a resident travel grant from Nucletron in 2005
References
- 1.Chadha M. Gynecologic brachytherapy-II: Intravaginal brachytherapy for carcinoma of the endometrium. Sem Rad Onc. 2002;12:53–61. doi: 10.1053/srao.2002.28665. [DOI] [PubMed] [Google Scholar]
- 2.Southcott BM. Carcinoma of the endometrium. Drugs. 2001;61:1395–1405. doi: 10.2165/00003495-200161100-00003. [DOI] [PubMed] [Google Scholar]
- 3.Ogino I, Kitamura T, Okamoto N, et al. High dose rate intracavitary brachytherapy for recurrent or residual lesions in the vaginal cuff: results in post-hysterectomy patients with carcinoma of the cervix. Int J Gynecol Cancer. 2001;11:61–68. doi: 10.1046/j.1525-1438.2001.011001061.x. [DOI] [PubMed] [Google Scholar]
- 4.Tangjitgamol S, Manusirivithaya S, Lertbutsayanukul C. Adjuvant therapy for early-stage endometrial cancer: a review. Int J Gynecol Cancer. 2007 Sep–Oct;17(5):949–956. doi: 10.1111/j.1525-1438.2007.00860.x. 2007. [DOI] [PubMed] [Google Scholar]
- 5.Alektiar KM, Venkatraman E, Chi DS, et al. Intravaginal brachytherapy alone for intermediate-risk endometrial cancer. Int J Rad Oncol Biol Phys. 2005;62:111–117. doi: 10.1016/j.ijrobp.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JM, Stea B, Hallum AV, et al. High-dose-rate postoperative vaginal cuff irradiation alone for stage IB and IC endometrial cancer. Int J Rad Oncol Biol Phys. 2000;46:417–425. doi: 10.1016/s0360-3016(99)00427-7. [DOI] [PubMed] [Google Scholar]
- 7.Cengiz M, Singh AK, Grigsby PW. Postoperative vaginal brachytherapy alone is the treatment of choice for grade 1–2, stage IC endometrial cancer. Int J Gynecol Cancer. 2005;15:926–931. doi: 10.1111/j.1525-1438.2005.00156.x. [DOI] [PubMed] [Google Scholar]
- 8.Petereit DG, Tannehill SP, Grosen EA, et al. Outpatient vaginal cuff brachytherapy for endometrial cancer. Int J Gynecol Cancer. 1999;9:456–462. doi: 10.1046/j.1525-1438.1999.99061.x. [DOI] [PubMed] [Google Scholar]
- 9.Small WJ, Erickson B, Kwakwa F. American brachytherapy society survey regarding practice patterns of postoperative irradiation for endometrial cancer: Current status of vaginal brachytherapy. Int J Rad Oncol Biol Phys. 2005;63:1502–1507. doi: 10.1016/j.ijrobp.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 10.Fayed A, Mutch DG, Rader JS, et al. Comparison of high dose rate and low dose rate brachytherapy in the treatment of endometrial carcinoma. Int J Rad Oncol Biol Phys. 2007;67:480–484. doi: 10.1016/j.ijrobp.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Moss EL, Stevens A, Gray L, et al. Toxicity, recurrence and survival after adjuvant radiotherapy treatment for FIGO stage I cancer of the endometrium. Clin Onc. 2003;15:250–254. doi: 10.1016/s0936-6555(03)00149-3. [DOI] [PubMed] [Google Scholar]
- 12.Herwig R, Bruns F, Strasser H, et al. Late urologic effects after adjuvant irradiation in stage I endometrial cancer. Urology. 2004;63:354–358. doi: 10.1016/j.urology.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 13.International Commission on Radiation Units and Measurements. Dose and volume specifications for reporting intracavitary therapy in gynecology (report 38) Bethesda MD: International Commission on Radiation Units and Measurements; 1985. [Google Scholar]
- 14.Paley PJ, Goff BA, Minudri R, et al. The prognostic significance of radiation dose and residual tumor in the treatment of barrel-shaped endophytic cervical carcinoma. Gyn Onc. 2000;76:373–379. doi: 10.1006/gyno.1999.5691. [DOI] [PubMed] [Google Scholar]
- 15.Schoeppel SL, LaVigne ML, Martel MK, et al. Three-dimensional treatment planning of intracavitary gynecologic implants: analysis of ten cases and implications for dose specification. Int J Rad Oncol Biol Phys. 1994;28:277–283. doi: 10.1016/0360-3016(94)90168-6. [DOI] [PubMed] [Google Scholar]
- 16.Potter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): Concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Rad and Oncol. 2006;78:67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Hoskin PJ, Vidler K. Vaginal vault brachytherapy: the effect of varying bladder volumes on normal tissue dosimetry. Br J of Radiol. 2000;73:864–866. doi: 10.1259/bjr.73.872.11026862. [DOI] [PubMed] [Google Scholar]
- 18.Radiation Therapy Oncology Group. [accessed 21.4.07; 2006];A phase II study of intensity modulated radiation therapy (IMRT) to the pelvis +/− chemotherapy for post-operative patients with either endometrial or cervical carcinoma RTOG 0418. www.rtog.org.
- 19.Xiong L, Viswanathan A, Stewart A, et al. Deformable structure registration of bladder through surface mapping. Med Phys. 2006;33:1848–1856. doi: 10.1118/1.2198192. [DOI] [PubMed] [Google Scholar]
- 20.Xiong L, Viswanathan A, Stewart A, et al. Bladder dose distribution in cylinder brachytherapy treatment. Med Phys. 2006;33:2074. [Google Scholar]
- 21.Akimoto T, Katoh H, Kitamoto Y, et al. Rectal bleeding after high-dose-rate brachytherapy combined with hypofractionated external-beam radiotherapy for localised prostate cancer: impact of rectal dose in high-dose-rate brachytherapy on occurrence of grade 2 or worse rectal bleeding. Int J Rad Oncol Biol Phys. 2006;65:364–370. doi: 10.1016/j.ijrobp.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Tran A, Wallner K, Merrick G, et al. Rectal fistulas after prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2005;63:150–154. doi: 10.1016/j.ijrobp.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Pinkawa M, Fischedick K, Asadpour B, et al. Low-grade toxicity after conformal radiation therapy for prostate cancer-impact of bladder volume. Int J Rad Oncol Biol Phys. 2006;64:835–841. doi: 10.1016/j.ijrobp.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Pelloski CE, Palmer M, Chronowski GM, et al. Comparison between CT-based volumetric calculations and ICRU reference-point estimates of radiation doses delivered to bladder and rectum during intracavitary radiotherapy for cervical cancer. Int J Rad Oncol Biol Phys. 2005;62:131–137. doi: 10.1016/j.ijrobp.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 25.Wachter-Gerstner N, Wachter S, Reinstadler E, et al. Bladder and rectum dose defined from MRI based treatment planning for cervix cancer brachytherapy: comparison of dose-volume histograms for organ contours and organ wall, comparison with ICRU rectum and bladder reference point. Rad and Oncol. 2003;68:269–276. doi: 10.1016/s0167-8140(03)00189-0. [DOI] [PubMed] [Google Scholar]
- 26.Koom WS, Sohn DK, Kim JY, et al. Computed tomography-based high-dose-rate intracavitary brachytherapy for uterine cervical cancer: Preliminary demonstration of correlation between dose–volume parameters and rectal mucosal changes observed by flexible sigmoidoscopy. Int J Rad Oncol Biol Phys. 2007;68(5):1446–1454. doi: 10.1016/j.ijrobp.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Hoskin PJ, Bownes P, Summers A. The influence of applicator angle on dosimetry in vaginal vault brachytherapy. Br J of Radiol. 2002;75:234–237. doi: 10.1259/bjr.75.891.750234. [DOI] [PubMed] [Google Scholar]
- 28.Garipagaoglu M, Tuncel N, Koseoglu FG, et al. Geometric and dosimetric variations of ICRU bladder and rectum reference points in vaginal cuff brachytherapy using ovoids. Int J Rad Oncol Biol Phys. 2004;58:1607–1615. doi: 10.1016/j.ijrobp.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 29.Kim RY, Pareek P, Duan J, et al. Postoperative intravaginal brachytheapy for endometrial cancer: dosimetric analysis of vaginal colpostats and cylinder applicators. Brachy. 2002;1:138–144. doi: 10.1016/s1538-4721(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 30.Viswanathan AN, Dimopolous J, Kirisits C, et al. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Rad Oncol Biol Phys. 2007;68(2):491–498. doi: 10.1016/j.ijrobp.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Sun LM, Huang HY, Huang EY, et al. A prospective study to assess the bladder distension effects on dosimetry in intracavitary brachytherapy of cervical cancer via computed tomography-assisted techniques. Brachytherapy. 2005;77:77–82. doi: 10.1016/j.radonc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Pilepich MV, Prasad SC, Perez CA. Effect of bladder distension on dosimetry in gynecological implants. Radiology. 1981;140:516–518. doi: 10.1148/radiology.140.2.7255731. [DOI] [PubMed] [Google Scholar]
- 33.Hoskin PJ, Vidler K. The influence of bladder volume on dosimetry during vaginal vault brachytherapy. J Brachyther Int. 1998;14:1–5. [Google Scholar]
- 34.Li S, Boyer A, Lu Y, et al. Analysis of the dose-surface histogram and dose-wall histogram for the rectum and bladder. Med Phys. 1997;24:1107–1116. doi: 10.1118/1.598014. [DOI] [PubMed] [Google Scholar]
- 35.Fenwick JD, Khoo VS, Nahum AE, et al. Correlations between dose-surface histograms and the incidence of long-term rectal bleeding following conformal or conventional radiotherapy treatment of prostate cancer. Int J Rad Oncol Biol Phys. 2001;49:473–480. doi: 10.1016/s0360-3016(00)01496-6. [DOI] [PubMed] [Google Scholar]
- 36.Koper PCM, Heemsbergen WD, Hoogeman MS, et al. Impact of volume and location of irradiated rectum wall on rectal blood loss after radiotherapy. Int J Rad Oncol Biol Phys. 2004;49:685–698. doi: 10.1016/j.ijrobp.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Lu Y, Li S, Spelbring D, et al. Dose-surface histograms as treatment planning tool for prostate conformal radiotherapy. Med Phys. 1995;22:279–284. doi: 10.1118/1.597451. [DOI] [PubMed] [Google Scholar]
- 38.MacKay RI, Hendry JH, Moore CJ, et al. Predicting late rectal complications following prostate conformal radiotherapy using biologically effective doses and normalized dose-volume histograms. Br J of Radiol. 1997;70:517–526. doi: 10.1259/bjr.70.833.9227235. [DOI] [PubMed] [Google Scholar]
- 39.Meijer GJ, van den Brink M, Hoogeman MS, et al. Dose-wall histograms and normalized dose-surface histograms for the rectum: a new method to analyze the dose distribution over the rectum in conformal radiotherapy. Int J Rad Oncol Biol Phys. 1999;45:1073–1080. doi: 10.1016/s0360-3016(99)00270-9. [DOI] [PubMed] [Google Scholar]
- 40.Tucker SL, Dong L, Cheung R, et al. Comparison of rectal dose-wall histogram versus dose-volume histogram for modeling the incidence of late rectal bleeding after radiotherapy. Int J Rad Oncol Biol Phys. 2004;60:1589–1601. doi: 10.1016/j.ijrobp.2004.07.712. [DOI] [PubMed] [Google Scholar]
- 41.Tudor GSJ. Comparison of rectal dose histograms under conditions of nonlinear isodoses. Phys Med Biol. 2007;52:275–289. doi: 10.1088/0031-9155/52/1/018. [DOI] [PubMed] [Google Scholar]
- 42.Ting JY, Wu X, Fiedler JA, et al. Dose-volume histograms for bladder and rectum. Int J Rad Oncol Biol Phys. 1997;38:1105–1111. doi: 10.1016/s0360-3016(97)00312-x. [DOI] [PubMed] [Google Scholar]
- 43.Saarnak AE, Boersma M, van Bunningen BNFM, et al. Inter-observer variation in delineation of bladder and rectum contours for brachytherapy of cervical cancer. Rad and Oncol. 2000;56:37–42. doi: 10.1016/s0167-8140(00)00185-7. [DOI] [PubMed] [Google Scholar]
- 44.Lebesque JV, Bruce AM, Kroes APG, et al. Variation in volumes, dose-volume histograms and estimated normal tissue complication probabilities of rectum and bladder during conformal radiotherapy of T3 prostate cancer. Int J Rad Oncol Biol Phys. 1995;33:1109–1119. doi: 10.1016/0360-3016(95)00253-7. [DOI] [PubMed] [Google Scholar]
- 45.Chen SW, Liang JA, Yang SN, et al. The prediction of late rectal complications following the treatment of uterine cervical cancer by high-dose-rate brachytherapy. Int J Rad Oncol Biol Phys. 2000;47:955–961. doi: 10.1016/s0360-3016(00)00559-9. [DOI] [PubMed] [Google Scholar]
- 46.Montana GS, Fowler WC. Carcinoma of the cervix: Analysis of bladder and rectal radiation dose and complications. Int J Rad Oncol Biol Phys. 1989;16:95–100. doi: 10.1016/0360-3016(89)90015-1. [DOI] [PubMed] [Google Scholar]