Abstract
Short phasic bursts of neuronal activity in dopamine neurons produce rapid and transient increases in extracellular dopamine concentrations throughout the mesocorticolimbic system, which are associated with the initiation of goal-directed behaviors. It is well established that acute exposure to many addictive drugs produce increases in tonic dopamine levels that occur on the order of minutes. However, recent studies suggest that abused drugs similarly enhance phasic dopamine release events that occur on a subsecond time scale. Furthermore, drug experience modulates the synaptic and intrinsic properties of dopamine neurons, which could affect dopamine burst firing and phasic dopamine release. This review will provide a general introduction to the mesolimbic dopamine system, as well as the primary methods used to detect dopamine neurons and dopamine release. We present the role of phasic dopamine release in appetitive behaviors in the context of contemporary theories regarding the function of dopamine. Next we discuss the known drug-induced changes to dopamine neurons and phasic release in both in vitro and in vivo preparations. Finally, we offer a simple model that chronic drug experience attenuates tonic/basal dopamine levels but promotes phasic dopamine release, which may result in aberrant goal-directed behaviors contributing to the development of addiction.
Keywords: dopamine, drug abuse, addiction, voltammetry
The dopamine system: anatomy and detection
The ventral tegmental area (VTA) and the neighboring substantia nigra (SN) are the primary dopamine producing nuclei in the brain [1]. The VTA is thought to play a particularly important role in drug abuse [2]. However, the SN has been studied far less in the context of drug abuse, with the majority of studies of this region focusing on its role in motor control [3]. A large proportion of the neurons whose cell bodies are in the VTA contain dopamine. For example, in the rat, 2/3 of the ~ 14,000 VTA neurons contain tyrosine hydroxylase, the rate-limiting enzyme in dopamine synthesis, and as such are presumably dopaminergic [1]. The non-dopamine producing cells in the VTA are likely GABA- and glutamate-producing; however, there is some debate whether glutamate and dopamine are co-released from the same neurons [4, 5] or whether these neuronal subtypes exist in distinct populations [6]. The dopamine innervation from the VTA onto various nuclei depends upon the target, as dopamine neurons in the VTA comprise ~85% neurons projecting from VTA to the nucleus accumbens (NAcc), ~50% of the neurons projecting to the amygdala, and ~30% of the neurons to the prefrontal cortex (PFC) [1]. As with its projections, the VTA receives input from a diverse array of brain regions including the PFC, NAcc, bed nucleus of the stria terminalis, lateral dorsal tegmentum (LDT), pedunculopontine tegmental nucleus (PPT), amygdala and areas of the hypothalamus [7–10]. Thus, the VTA is a heterogeneous brain region with extensive afferent input and efferent projections.
In electrophysiological studies, dopamine neurons are identified in vivo primarily based on the presence of a triphasic and long-duration action potential waveform [11, 12]. These neurons can (i) be hyperpolarized and quiescent, (ii) fire action potentials in a pacemaker-like fashion (2–10 Hz) or (iii) fire action potentials in bursts up to 15–30 Hz [11]. The pacemaker dopamine neuron firing is thought to give rise to the ‘tonic’ levels of dopamine with concentrations ranging from 5–20 nM [13, 14], while the burst firing is thought to give rise to ‘phasic’ elevated dopamine levels which can reach as high as 1 µM [15, 16]. The burst firing of dopamine neurons requires glutamatergic input, activation of N-methyl-D-aspartate (NMDA) receptors, opening of high-threshold calcium currents, and finally activation of calcium-activated potassium currents to terminate the burst [17]. Furthermore, activation of brainstem nuclei such as the PPT and LDT are involved in dopamine neuron burst generation [17, 18] and dopamine release in the NAcc and striatum [9, 19]. Although robust immunohistochemical methods can identify dopamine, GABA, and glutamate neurons in the VTA [6], electrophysiological identification of neuronal subtypes is problematic [20]. In vivo juxtacellular labeling of recorded VTA neurons in the rat demonstrate that many neurons with a triphasic and long-duration waveform actually are not dopaminergic [21], although it has been suggested that these findings are difficult to interpret because of methodological considerations [12].
In contrast to in vivo recordings, dopamine neurons in brain slice preparations do not spontaneously fire action potentials in bursts, but rather only exhibit pacemaker-like action potential firing [22]. The reported frequency of putative dopamine neuron firing in the slice varies whether one uses extracellular (3–8 Hz) [22], perforated-patch (2–5 Hz) [23], or whole-cell recordings (1–3 Hz) [20, 24]. Regardless of the recording technique, the firing of putative GABA neurons is significantly greater than dopamine neurons and is usually higher than 10 Hz [22]. Although the firing rate can provide a crude segregation of neuronal subtypes in brain slices, a more reliable electrophysiological marker of dopamine neurons was found to be presence of the hyperpolarization-activated, cyclic nucleotide-regulated cation current (Ih) [22]. However, subsequent work found that not all cells with the Ih produced dopamine [20, 25], which may be explained by the species utilized, as the Ih is present in 98% of TH-containing neurons in the mouse VTA [24], but only in ~50% in the rat [20]. Recent studies highlight that the electrophysiological properties and pharmacological manipulations can identify dopamine neuron content in brain slices if the projection target of the neuron is known [26, 27]. Regardless, many of the brain slice electrophysiological studies discussed below have solely used the Ih as a marker of dopamine content.
A number of analytical techniques are utilized to chemically detect dopamine in vivo. Some are best suited to detect tonic changes thought to arise from alterations in pacemaker-like firing, while others are optimized for isolating phasic dopamine release events thought to arise from burst firing [28–30]. One of the most commonly used methods is microdialysis, which has excellent selectivity and sensitivity for analyte detection; however, microdialysis suffers from relatively low temporal resolution (minutes) and is best suited for the quantitative analysis of basal and slowly changing tonic dopamine levels. In contrast, electrochemical techniques offer excellent temporal resolution to isolate phasic release events but offer poor analyte selectivity. These techniques take advantage of the fact that application of a modest potential (~200 mV vs Ag/AgCl) to a suitable electrode is sufficient to drive electrolysis of dopamine due to its oxidation to dopamine-o-quinone. The current produced by the electrolysis can be measured at the electrode and is proportional to the number of molecules undergoing oxidation, and therefore the concentration at the electrode surface. Different command waveforms can be used for the application of the potential to the electrode, the simplest being a continuous, constant potential in (constant-potential) amperometry. This variant has microsecond temporal resolution and is often used to study the kinetics of dopamine release and reuptake; however, it offers little chemical selectivity since any oxidized compound will be detected with constant-potential amperometry recordings, which has deterred researchers from using this technique in behaving animals. A more selective electrochemical method is fast-scan cyclic voltammetry (FSCV), which utilizes a triangle input waveform to separate electrolysis from different analytes into temporally-resolved peaks in the output current. Since the voltage is swept to an oxidizing potential and back, current is generated (in opposite directions) during oxidation and reduction processes, whereby producing two electrochemical peaks for a given compound making chemical resolution more robust. FSCV can be employed to record dopamine release in awake-behaving rodents [28–30], and is capable of detecting changes in dopamine levels that occur in the range of 0.1 to 100 s [31].
The role of dopamine in appetitive behaviors: pharmacology, electrophysiology, and genetic techniques
Since the identification of dopamine over 50 years ago [32], a number of theories have been developed to explain the role of dopamine in behavior. We will briefly discuss some of the prominent theories and the supporting evidence to provide a framework for understanding the current views of dopamine function. We would like to preface that these theories are not necessarily mutually exclusive, but rather they provide different perspectives on the role of dopamine in behavior. More in depth discussion on theories of dopamine function can be found elsewhere [33–38].
The most well-established and uncontroversial theory of dopamine function is that dopamine release is involved with sensorimotor behaviors [33, 35]. Dopamine plays a key role in motor tasks, as this is the primary deficit observed with those suffering from Parkinson’s Disease (PD), a disease that leads to the selective degeneration of dopamine neurons [39]. However, it should be noted that symptoms of PD do not typically appear until a majority of SN dopamine neurons and terminals have degenerated [3, 40]. Furthermore, mice that are deficient in dopamine production are catatonic and require supplements for survival and normal motor behaviors [41].
In addition to enabling normal motor activity, many lines of evidence support a critical role of dopamine in motivation [33, 35]. For example, manipulations that impair dopamine signaling in the NAcc and are without effect on motor behavior have been found to shift food consumption from a preferred food option that required lever pressing for receipt toward a less palatable food option that was freely available [42–45]. In another behavioral assay that examined effort and motivation, rats were given an option in a T-maze to obtain a lower food reward with no obstacle or a higher food reward that required climbing over a barrier. Systemic dopamine receptor antagonism [46, 47] or local dopamine depletions in the NAcc [48] shifted the response from the high reward side to the low reward side. Importantly, rats still preferred the high reward side when the barrier was removed under conditions when dopamine signaling was impaired, which suggests that these manipulations were not a result of a learning deficit [46–48]. Motivation is also assessed in operant tasks under progressive ratio (PR) reinforcement schedules. Under PR reinforcement paradigms, the operant requirement (the number of lever presses) increases on subsequent trials until the ‘break-point’, which is the number of lever presses for reinforcer delivery on the last completed trial and is a measure motivation [49]. Inhibiting dopamine signaling in the NAcc reduces the break-point for natural reinforcers [49–51]. Conversely, enhancing dopamine signaling in the NAcc by local amphetamine injections [52] or in mice with impaired dopamine transporter function [53] increases the break-point for natural reinforcers. Together, these studies highlight that dopamine, especially within the NAcc, may function to overcome the motivational costs required for completing tasks requiring a high level of effort [35, 54].
The ‘incentive-salience’ hypothesis of dopamine builds upon the general motivational hypothesis discussed above [33]. In short, incentive-salience is the neural representation of motivational value generated in response to a reward-related stimulus. This motivational representation is dynamic and can be applied to internally generated or externally experienced reward-related stimuli to give the stimulus incentive value, which can take control of behavior. In this hypothesis, it is thought that dopamine modulates the incentive value of such reward-related stimuli [33]. This hypothesis separates ‘liking’ of rewards, as measured by hedonic responses, from ‘wanting’ of the reward, as measured by motivational metrics [33]. Specifically, a variety of insults to the function of the dopamine system do not affect taste-reactivity or ‘liking’ [55–57]. Conversely, enhancing dopamine levels in dopamine transporter knock-down mice increases the ‘wanting’ for natural reinforcers as evidenced by increased break-points under PR reinforcement schedules [53] and by running faster to receive a reward [58]. These general findings have been mirrored in human studies where dopamine levels correspond to self-reports of ‘wanting’ and not to ‘liking’ [59, 60].
Studies employing electrophysiological recordings of dopamine neurons in awake-behaving animals provide evidence that dopamine can encode a ‘prediction-error’ signal in the brain [37]. In primates and rats, it was found that dopamine neurons increase in firing to the receipt of a reward, but after training dopamine neurons instead fire to cues that predict the availability of the reward [61, 62]. Interestingly, when a predicted reward is omitted, the firing of dopamine neurons is depressed [62]. Together, this evidence suggests that dopamine neuron firing signals the scalar discrepancy between the actual reward obtain and that predicted [62]. In support, dopamine neuron firing correlates with the probability of reward availability [63], as well as the magnitude of the reward [64]. Interestingly, the behavior of the phasic dopamine activation under these and other reward-related paradigms maps extraordinarily well onto a teaching signal proposed in the theoretical learning models in the field of reinforcement learning [38, 62, 65]. However, some have argued that the phasic dopamine response in these tasks occurs too fast for any cortical-mediated computation to occur [34, 66], suggesting that it contributes to a simple, low-computation process, consistent with (model-free) reinforcement learning models [67]. What becomes evident after examining these theories is that the dopamine system is associated with a diverse array of behaviors, which illustrates that dopamine may subserve various functions depending upon the location, context, and duration of its release [37]. Based upon the cellular effects of dopamine, it is thought that dopamine inhibits weak inputs and augments strong inputs to striatal neurons [68], which may also explain why dopamine is critically involved with many behaviors. The theories we have presented regarding the role of dopamine in behaviors was intended to provide a general framework for conceptualizing dopamine function. With this foundation, we will now explore the work that has specifically examined phasic dopamine release in the NAcc during appetitive behaviors.
The role of phasic dopamine release in appetitive behaviors: electrochemistry
The prominent theories of dopamine function developed primarily from the findings of pharmacological, genetic, and electrophysiological experimental techniques. However, these techniques do not provide direct information on dopamine release in forebrain terminal regions during discrete behavioral events on a physiological time scale. Pharmacological and genetic manipulations can produce long-lasting or permanent changes, which prevent using these techniques for isolating behavioral effects, related to dopamine changes, on a subsecond level. While electrophysiological recordings have excellent temporal resolution, it is not a perfect proxy of dopamine concentration since models of release processes incorporate several non-linear functions [69]. Moreover, since there are currently no reliable electrophysiological criteria for sorting VTA neurons by their targets, these data cannot inform us on transmission in specific terminal structures. Therefore, voltammetric approaches, such as FSCV, offer an unparalleled capacity to quantitate phasic changes in dopamine concentration occurring on a physiological time scale. These techniques have provided further insights into dopamine’s role in the brain during behavior that are complementary to pharmacological, genetic, and electrophysiological methods. Below we will discuss the findings regarding phasic dopamine release in the NAcc using FSCV in drug-free appetitive behaviors.
Presentation of novel sensory stimuli activates the mesocorticolimbic system. Specifically, electrophysiological recordings in both rats and primates indicate that putative dopamine neurons increase their firing rate in response to tactile stimulation [70], presentation of an auditory stimulus [70], or an unexpected delivery of sucrose [62]. In studies utilizing microdialysis, increases in dopamine overflow are observed after handling [71], and during sexual behaviors [72]. Using FSCV recordings in the NAcc, it was demonstrated that the number of spontaneous transient dopamine release events are enhanced six-fold in response to the presentation of a conspecific [73, 74]. However, the effect on transient dopamine events was significantly attenuated with repeated conspecific presentations, presumably correlating with the reduced novelty and habituation towards the conspecific [73].
Although the frequency of dopamine transients increases during conspecific presentation, it is difficult to associate dopamine release to any one specific behavior [73]. Subsequent studies examined phasic dopamine release in response to more easily controlled experimental conditions, such as with the self-administration of sucrose [75]. Using FSCV it was found that dopamine levels increase in response to the presentation of a cue predicting sucrose availability, and that the peak in the rise of dopamine coincided with the lever press for sucrose [75]. Control experiments found that unreinforced cue presentations did not affect dopamine levels in naïve rats, suggesting that the phasic NAcc dopamine release observed in this task was dependent upon a learned association [75]. Further highlighting a role of phasic dopamine release in learned behaviors, FSCV recordings in the NAcc were made from rats undergoing Pavlovian conditioning where a conditioned stimulus (CS+) reliably predicts reinforcer delivery (unconditioned stimulus, US) [76–78]. Early in training, phasic dopamine responses are observed primarily to the reward retrieval (US). After rats learn the CS-US association, dopamine is released to the presentation of the (CS), while the response to the US is attenuated. However, a stimulus that did not predict reward availability (CS-) also increased dopamine release to the presentation and offset of the CS-, suggesting some generalization between the conditioned stimuli [76]. Together, these studies using a between-animal design suggest that there is a transfer of the phasic dopamine response from the US to the CS [76, 77] that reflects the electrophysiological recordings in similar paradigms [36, 61]. An important future FSCV experiment would be to utilize a within-animal design so that the time-course of the transfer from the US to the CS could be accurately determined. It should be noted that phasic dopamine release is observed to both the US and CS in rats [76], but dopamine neurons tend to fire only to either the US or the CS in primates [36]. This discrepancy could reflect differences in the species studies, the training paradigm utilized, or functional differences between dopamine neuron firing and release. It should be noted that despite the caveats raised above in relating in vivo electrophysiological data to dopamine release, all of these results in rats obtained using FSCV are extremely consistent with the electrophysiological studies performed in behaving monkeys and rats.
Some studies have examined the role of phasic dopamine release during intra-cranial self-stimulation (ICSS) procedures, where learning to lever press for a highly reinforcing electrical stimulation can be assessed within an animal in a single session [38, 79]. A recent report found that the magnitude of dopamine released in the NAcc to cues predicting ICSS availability was correlated with the learning to lever press for electrical stimulation [79]. Specifically, cue-evoked dopamine responses increased in magnitude during acquisition, disappeared during extinction, and reappeared upon reinstatement [79]. These results are exciting since they correlate dopamine responses with learning an operant task. Somewhat analogous to natural rewards, ICSS is dopamine-dependent [80], although maintained levels of dopamine release to the self-stimulation are not required for operant responding in ICSS paradigms [81]. However, caution should be exercised when extending these results to all aspects of natural reinforcerment because ICSS removes the sensory component of reward processing. To summarize, phasic dopamine release using FSCV has been assessed in many appetitive behaviors, and it is apparent that an increase in the number of phasic dopamine events in the NAcc is associated with novelty and unexpected rewards [73, 74, 76]. Experiments employing operant tasks also highlight that cues predicting reinforcer availability elevate dopamine release in the NAcc [75, 79], where the peak dopamine response corresponds to the operant action [75, 82]. Studies involving Pavlovian conditioning also suggest that phasic dopamine is released primarily to the US early in training and to the CS after extensive training [76, 77]. Thus, these findings provide evidence of the involvement of phasic dopamine release in motor actions, motivation, modulating incentive value of reward-related stimuli, and learning. However, further experiments with multiple reward magnitudes will be required to determine if phasic dopamine release can also function as a prediction-error signal [36]. We will now discuss the effect of addictive drugs on the dopamine system, highlight how drugs alter phasic dopamine release, and suggest how these changes could modulate behavior.
The effect of abused substances on dopamine neurons: firing rate and tonic release
In order to understand the role of dopamine in drug abuse, it is important to first discuss how drugs affect the dopamine system acutely and after multiple drug exposures. Studies employing microdialysis techniques demonstrate that non-contingent administration of abused drugs such as alcohol, nicotine, opiates, psychostimulants, and cannabinoids increase dopamine levels in the NAcc [83, 84], while non-habit forming drugs do not affect dopamine overflow [83]. The cellular mechanism by which addictive drugs increase dopamine levels depends upon the cellular targets of the drug studied. Psychostimulants such as amphetamine and cocaine enhance dopamine overflow by affecting dopamine clearance from the extracellular space [85, 86]. Opiates activate dopamine neurons through inhibiting local GABA input [87, 88]. Similar to opiates, ethanol reduces the firing of VTA GABA neurons [89], but also directly modulates the excitability of dopamine neurons [90–92]. Additionally, ethanol affects both excitatory and inhibitory neurotransmission in the VTA [93, 94]. Nicotine activates and desensitizes dopamine neurons and inhibitory inputs to dopamine neurons in the VTA [95, 96], but prolonged nicotinic receptor activation is thought to cause a net excitatory effect on the dopamine system that may involve effects on presynaptic glutamate release [95, 97]. Regardless of the cellular mechanism, in vivo and in vitro recordings of dopamine neurons demonstrate that non-contingent peripheral administration of alcohol [90], nicotine [96, 98], opiates [87, 88], and cannabinoids [99, 100] increase dopamine neuron firing. Furthermore, nicotine [98], opiates [87], and cannabinoids [99] all increase the burst firing of dopamine neurons, which is thought to give rise to phasic dopamine release events [13]. Conversely, dopamine neuron firing is attenuated after administration of cocaine [101, 102] and amphetamine [103] in anesthetized animals and brain slices, due to the autoinhibitory effects of dopamine at high concentrations after psychostimulant exposure [101].
While the acute effects of drugs are well studied, the effect of multiple drug exposures on the dopamine system is far more complicated in part because of differences arising from the drug studied, how the drug is administered (dose, frequency, and route), and the duration after drug experience. Although there is some debate, many studies support the notion that multiple drug exposures lead to an impaired function of the dopamine system [104], though the timing of this effect can vary. For example, there is a transient (<10 days) increase in dopamine neuron activity in rats that received multiple non-contingent cocaine injections [105] or were trained to self-administer cocaine [106]. However, multiple non-contingent cocaine injections decrease dopamine neuron population activity after 3–5 wks of withdrawal [107], and also reduce dopamine overflow starting 24 hrs after the last drug exposure and last for at least 10 days [108–110]. Similar to extended withdrawal after chronic cocaine treatment, the activity of dopamine neurons and tonic/basal dopamine levels are reduced after chronic treatment of amphetamine [110], nicotine [107, 111], ethanol [107, 110, 112, 113], and morphine [110, 114, 115], but see [116]. Interestingly, chronic treatment with cannabinoids attenuated basal burst firing and the cannabinoid-mediated increase in firing rates in SN dopamine neurons, but was without effect on VTA dopamine neurons [117]. A subsequent drug exposure after withdrawal from chronic drug experience has been shown to return dopamine neuron firing rates and dopamine release to and above basal levels with ethanol [118], amphetamine [119], and morphine [114], but see [116]. Collectively, these studies suggest that drugs acutely active the dopamine system, but chronic drug exposure dampens basal/tonic dopamine levels.
The effect of abused substances on dopamine neurons: synaptic plasticity and burst firing
As discussed above, dopamine burst firing requires glutamatergic input, NMDA receptor activation, opening of high-threshold calcium currents, and finally activation of calcium-activated potassium currents to terminate the burst [17]. Thus, it follows that changes in synaptic inputs, calcium currents, or calcium-activated potassium currents could alter burst patterns of firing in dopamine neurons. Although incomplete, a growing body of evidence suggests that abused drugs can modify the synaptic inputs onto dopamine neurons as well as the currents found in dopamine neurons important for burst generation. For example, many studies have now shown that a single non-contingent injection of cocaine increases the ratio of a-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor currents to NMDA receptor currents (AMPA/NMDA) on VTA dopamine neurons [120–125]. This effect is observed 24 hours after the cocaine injection, and persists for up to 5 days [124]. The AMPA/NMDA ratio is thought to be a reliable measure of excitatory synaptic strength, where increases have been associated with enhanced AMPA receptor function [120, 124, 126]. In agreement with enhanced excitatory synaptic function on dopamine neurons, a single cocaine injection also increased the frequency and amplitude of miniature excitatory post-synaptic currents [124]. Furthermore, increases in the AMPA/NMDA ratio are associated with an impaired ability to generate long-term potentiation (LTP), a cellular mechanism that strengthens excitatory synapses and is thought to be important in learning and memory [124, 127]. Thus, an enhanced AMPA/NMDA ratio is likely occluding the ability to elicit LTP because the excitatory synapse cannot be further strengthened [124, 127]. However, for the purpose of this discussion, we highlight that such augmentations in the AMPA/NMDA ratio are enhancing excitatory synaptic strength that could promote burst generation in dopamine neurons.
While much of the work examining synaptic alterations on dopamine neurons has focused on the effects of cocaine, similar increases in the AMPA/NMDA ratio on VTA dopamine neurons have been observed 24 hrs after a single injection of amphetamine, nicotine, or morphine [123]. Subsequent work demonstrated that these synaptic changes are rapid, as an enhanced AMPA/NMDA ratio is observed 2–3 hrs after injection of cocaine and amphetamine [128, 129]. Interestingly, in vitro exposure to cocaine transiently increases NMDA receptor currents [130] and increases the AMPA/NMDA ratio after 3–5 hrs [128], although this may not extend to all psychostimulants [129].
Many studies examining drug-mediated changes on VTA dopamine neuron synaptic plasticity utilized non-contingent drug-administration, though recent studies have now examined these synaptic changes under conditions where rodents self-administer the drug. For example, an increase in VTA AMPA receptor levels is observed in rats that have self-administered nicotine [131]. Interestingly, food, sucrose, and cocaine self-administration increase the AMPA/NMDA ratio immediately after training, but only rats that self-administered cocaine (and not yoked controls) exhibited a persistent increase in the AMPA/NMDA ratio [132]. This study highlights how the changes on VTA dopamine neurons will depend upon the method of drug administration, as chronic peripheral injection of cocaine transiently (< 10 days) affects the AMPA/NMDA ratio [121], though these changes are longer lasting (> 21 days) in self-administering rodents [132]. Originally, it was hypothesized that these changes in glutamate receptor function are responsible for behavioral sensitization, which is an enhanced motor response to a subsequent drug exposure) [124]. Instead, further studies found that the AMPA/NMDA ratio is not associated with the development of behavioral sensitization [121, 125], but rather may be important for initiating long-lasting changes, which promote addiction-like behaviors [132, 133].
Recently, addictive drugs have been shown to affect inhibitory synaptic inputs on VTA dopamine neurons. Multiple injections of cocaine [122] and a single injection of morphine [134] reduce the inhibitory input on VTA dopamine neurons. These effects are not unitary across drugs, as a single injection of ethanol was found to increase inhibitory input on VTA dopamine neurons [135]. However, withdrawal after chronic ethanol exposure promotes burst firing of dopamine neurons by inhibiting the function of calcium-activated potassium currents [136]. These studies highlight that exposure to addictive substances can strengthen excitatory synaptic input, reduce inhibitory synaptic input, and alter the function of ion currents in VTA dopamine neurons. While these drug-induced adaptations have not been characterized or identified for all addictive substances, we suggest that these changes will increase the burst firing of VTA dopamine neurons, since this firing pattern is dependent upon glutamatergic input, NMDA receptor activation, and calcium-activated potassium currents [17]. In support, dopamine burst firing is elevated in an anesthetized preparation using rats that readily self-administer cocaine [137], or in rats during early withdrawal after cocaine self-administration [106]. We therefore would expect that behaviorally relevant stimuli would enhance dopamine burst firing to a greater level after experience with drugs. Empirical data of dopamine neuron firing patterns in awake-behaving rodents after drug exposure is lacking, though recent studies have begun to examine these questions by examining phasic dopamine release, thought to be dependent upon burst firing [13], with FSCV in rodents during drug-related behaviors.
The effect of abused substances on phasic dopamine release
FSCV has been utilized to examine phasic dopamine release in a variety of model systems, though it is important to note that drug-mediated effects on dopamine release can result from direct effects on dopamine neuron excitability or from changes in dopamine uptake. Numerous studies have examined the effect of ethanol on dopamine release in striatal brain slices from drug-naïve rats, and it was shown that moderate doses were without effect on dopamine uptake [138–140]. In contrast, chronic ethanol vapor exposed rats exhibited enhanced dopamine uptake in vitro, which was thought to be a compensatory mechanism resulting from the elevated dopamine levels due to the prolonged ethanol treatment [141]. In awake, behaving rodents, acute peripheral injections of ethanol at doses that increase tonic dopamine neuron firing were found to attenuate electrically stimulated dopamine release [142]. This ethanol-mediated reduction of phasic dopamine release was thought to result from enhanced tonic dopamine levels that impaired phasic dopamine release due to depletion of releasable dopamine and activation of release-regulating autoreceptors [142]. However, intravenous ethanol infusions sometimes increased the frequency of spontaneous phasic dopamine transients in awake-behaving rats [143]. Similar effects have also been observed with cannabinoid receptor activation, where intravenous infusions of cannabinoids reduced electrically stimulated dopamine release, but increased the frequency and amplitude of spontaneous phasic dopamine release events [144]. These findings with ethanol and cannabinoid administration highlight that it can be difficult to parsimoniously use the findings from in vitro preparations and artificial electrical stimulations to predict the net effect in awake, behaving rodents. Reductionalist preparations and electrical stimulations are better suited to examine specific aspects of dopamine transmission, such as the involvement of specific ion channels, changes in release kinetics and the quantity of dopamine release, which can more difficult to accurately ascertain using in vivo preparations. Regardless, these findings highlight that ethanol and cannabinoids produce changes in phasic dopamine release.
A number of studies have examined the effect of nicotine on phasic dopamine release in both in vitro and in vivo preparations. In contrast to ethanol, acute in vivo nicotine exposure enhances dopamine uptake in the striatum [145]. Nicotine exerts frequency-dependent effects on phasically stimulated dopamine release in vitro, where at low firing rates dopamine release is attenuated, but at high firing bursts nicotine enhances dopamine release [146, 147]. Intravenous infusions of nicotine were also found to increase the frequency and amplitude of spontaneous phasic dopamine release events [143]. In agreement with the findings from other abused substances, intravenous infusions of cocaine also increase spontaneous phasic dopamine release events in the NAcc [31, 143, 148–150]. Interestingly, endogenous cannabinoids modulate the cocaine-, nicotine-, and ethanol-mediated increases in phasic dopamine release, as the effects of drugs on phasic dopamine release are attenuated by systemic cannabinoid receptor antagonism [143]. While the locus of this effect is yet to be determined, it is speculated that it is within the VTA, where cannabinoid receptor activation reduces GABA release on VTA dopamine neurons [151]. Together, these findings suggest that abused drugs may exert similar effects on phasic dopamine release even though their respective cellular effects are quite distinct. Future studies are required to systematically examine drug-specific effects on phasic dopamine release. Below we discuss the effects of cocaine on phasic dopamine release, as this has been the most thoroughly studied addictive substance.
Early microdialysis studies found that abused drugs have regionally distinct effects on tonic dopamine levels with the greatest enhancement of dopamine levels found in the ventral striatum [83, 84]. A recent report identified regional differences on cocaine-mediated effects on phasic dopamine release with larger effects in the NAcc shell subregion compared to the NAcc core [148]. It was suggested that the preferential effect on dopamine release in the NAcc shell by cocaine could be critically important for the primary reinforcing effects of drug [148]. Similar to differential effects of contingent and non-contingent drug administration on synaptic plasticity on VTA dopamine neurons [132], the effect of cocaine infusions on phasic dopamine release can depend upon the contingency of the administration [149]. No changes in dopamine levels are observed within 10 s of a non-contingent cocaine administration to awake, drug-naïve rats. However, phasic dopamine events are increased during this time frame with contingent cocaine administration, highlighting that these early dopamine release events (< 10 s after drug delivery) may be important for learned associations, and are not a result of the pharmacological actions of cocaine [149]. Identical to natural reinforcers [75, 79], cues that predict cocaine availability are able to elicit phasic dopamine release that persist even when the drug is not administered [150, 152]. Interestingly, the rise in dopamine levels is associated with the initiation of approach to lever press for cocaine [149, 150, 152], which is thought to be causal since stimulation of dopamine neurons was found to promote this behavior [152]. The field of FSCV recordings in behaving rodents during drug-related behaviors is nascent, and many questions regarding the prolonged effects on drugs on phasic dopamine release remain unanswered. One limitation present in many behavioral studies using FSCV arises from the usage of acute glass-insulated microelectrodes, which need to be physically inserted on each recording day. Due to this approach with acute recordings, FSCV recordings are likely in different locations across days, and successfully inserting electrodes becomes more difficult after multiple recordings sessions [30]. We have developed chronically implanted microelectrodes that permits for stable FSCV recordings over multiple days and is well-suited to address changes in phasic dopamine release over long-lasting behavioral paradigms (manuscript in preparation). Future studies employing chronically implanted FSCV electrodes will be able to test for changes in the pattern of phasic dopamine release during the transition to compulsive drug taking in rodent models of addiction.
Mesocorticolimbic function in human addicts
The development of human imaging techniques has provided insights into functional changes within the brain that occur in human addicts, which support many of the effects observed in rodents. Many human addiction studies have utilized functional magnetic resonance imaging (fMRI) or positron emission tomography (PET) [153]; however, it is important to note that the fMRI and PET identify changes on the order of seconds to minutes, while FSCV can identify phasic dopamine changes on a subsecond time scale in rodents [28, 154]. fMRI identifies changes in blood oxygen levels, with a time resolution of a few seconds, thought to represent changes in neural activity. PET can be used to make more specific measurements of neurotransmission by monitoring radiotracers that selectively bind to proteins. For example, radiolabeled dopamine-receptor ligands can be monitored in the brain, and their concentration decreases as they are displaced from receptors following release of endogenous dopamine. However, the temporal resolution for PET is in minutes and only increases, not decreases, in dopamine concentration are detected [155].
Only recently with fMRI was it shown that the VTA is activated by rewarding events in humans [156], which mirrors the electrophysiological recordings of dopamine neurons from primates and rodents [61, 62]. Paralleling rodent work [83], exposure to psychostimulants increased dopamine levels in the human striatum that was associated with the reinforcing effects of the drug [157–159]. Furthermore, decreased striatal dopamine responses were reported in detoxified cocaine abusers [160], consistent with the lower dopamine population activity and dopamine overflow in rodents after chronic drug exposure [107–110]. The level of radiolabeled dopamine-receptor ligands under basal conditions has also proved useful in assessing difference in neural function between individuals. Human abusers of alcohol [161], cocaine [162], heroin [163], and methamphetamine [164] have lower levels of dopamine receptor binding compared to non-abusers, which has led to the hypothesis that low numbers of dopamine receptors, whether due to genetics or previous drug exposure, can make an individual more susceptible to drug abuse [153–155]. Moreover, individuals that are resistant to drug use (close relatives of addicts that do not abuse drugs) exhibit increased dopamine receptor binding, suggesting that higher levels of dopamine receptors could protect against the development of addiction [165]. While alterations in the level of radiotracer binding may reflect changes in receptor affinity rather than actual receptor number, this metric is incredibly robust, indicating important underlying physiological differences. In summary, human imaging studies have provided evidence that drugs acutely increase dopamine levels and that chronic drug exposure impairs the function of the dopamine system; however, improving the temporal resolution in current imaging techniques will allow for a more direct examination of phasic dopamine signals in addicts.
The role of phasic dopamine release in drug abuse
Briefly, we will summarize what we have discussed in this review and offer our model regarding the role of phasic dopamine release in the development of drug abuse, which is also schematically presented in Fig. (1). Dopamine neurons can fire in a regular pacemaker-like fashion, which is thought to give rise to the tonic levels of dopamine [13, 14]. Alternatively, dopamine neurons can fire in bursts, which is thought to produce phasic dopamine release events [15, 16]. In a drug-naïve state there will be a tonic dopamine tone arising from pacemaker-like dopamine neuron firing. Behaviorally-relevant novel stimuli will increase dopamine burst firing [70] and phasic dopamine release [73]. However, presentation of stimuli associated with drug intake, such as drug paraphernalia or cues predicting drug availability, will not affect dopamine neuron firing or release because these stimuli are not salient in a drug-naïve state.
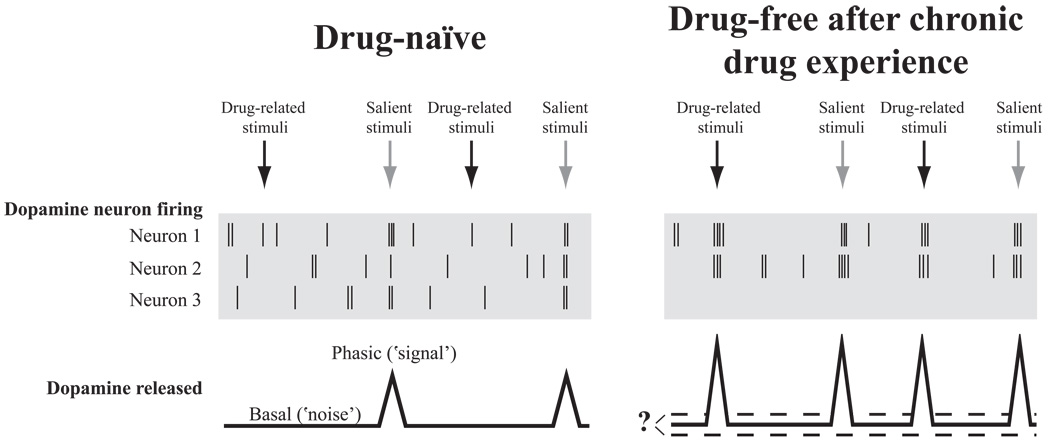
Figure 1.
Schematic representation of changes to the dopamine system after chronic drug experience. (Left) Illustration of the firing patterns of three hypothetical dopamine neurons from a drug-naïve individual where each vertical line represents an action potential. For clarity, there is no distinction between transient increases in firing rate and burst firing, as both are represented by a cluster of action potentials. The specific firing patterns responsible for phasic dopamine release are unknown, but phasic release likely results from coordinated activity of dopamine neurons firing in single-spikes and/or bursts. Notice that behaviorally relevant salient stimuli elicits coordinated activity of dopamine neurons that translates into a phasic increase in dopamine release that could occur in brain regions receiving VTA input, such as the NAcc. However, presentation of drug-related stimuli are without effect on dopamine neuron firing and release in the drug-naïve condition. (Right) After withdrawal from chronic drug treatment, dopamine neuron firing rate and population activity can be reduced, which is represented by fewer spontaneous action potentials and the lack of activity in Neuron 3. While the effect on basal dopamine levels remains controversial, many studies demonstrate instrinsic and synaptic changes on dopamine neurons that could promote the efficacy of glutamatergic inputs on dopamine neurons. We hypothesize that these intrinsic and synaptic changes will increase dopamine neuron firing and phasic release in response to drug-related stimuli. We propose that this increased ‘signal to noise’ of phasic dopamine release to basal dopamine levels contributes to the aberrent processing of drug-related stimuli, which in turn can promote drug seeking.
After an association is learned between a reinforcer and a cue predicting reinforcer availability, the cue in turn becomes salient and can elicit phasic dopamine release for natural [75, 76, 79], and drug reinforcers [149]. Interestingly, cues predicting drug availability appear to be more resistant to extinction than cues predicting non-drug reinforcer availability [79, 150], which suggests that additional changes occur in the dopamine system due to the drug experience. In support, learning an association between cues and natural reinforcers transiently affects the synaptic properties of dopamine neurons [78, 132], while drug experience promotes long-lasting changes to the intrinsic and synaptic properties of dopamine neurons [124, 132, 136]. We posit that these prolonged cellular adaptations in dopamine neurons after drug experience will function to strengthen previously learned associations and promote dopamine burst firing and phasic release in response to previously weak or neutral stimuli. In addition, withdrawal after chronic drug exposure attenuates tonic dopamine levels and dopamine neuron population activity [107–115]. Together, this suggests that chronic drug exposure will enhance the ‘signal to noise’ of phasic dopamine release events, where the phasic ‘signals’ become more salient due to the attenuated ‘noise’ of tonic background dopamine levels. Because phasic dopamine release is associated with initiating goal-directed behaviors [75, 149, 152], it follows that the enhanced ‘signal to noise’ of phasic dopamine signaling will promote drug seeking in response to drug-related stimuli, which is important in the development of addiction. A corollary of this hypothesis is that chronic drug exposure will also affect the processing of cue-related associations that do not involve drugs. In support, amphetamine treatment promotes habit formation after reinforcer devaluation [166], and enhances both inhibitory and excitatory Pavlovian associations [167, 168]. Furthermore, human addicts and healthy subjects with reduced dopaminergic function exhibit impaired decision-making abilities [169–171], highlighting that dysregulation of the dopamine system can alter cognition and behavior. Therefore, addiction can be debilitating for individuals because chronic drug experience not only promotes drug seeking, but also affects proper decision-making.
Others have suggested that dysfunctions in the dopamine system are involved with addiction [104, 172]. However, we extend upon these hypotheses and posit that chronic drug exposure enhances phasic dopamine release, attenuates basal tonic dopamine levels, and promotes aberrant stimulus-reinforcer associations that are important in the development of addiction. Our model fits within the framework of most contemporary theories of dopamine function. Specifically, the increase in the ‘signal to noise’ of phasic dopamine signaling could be interpreted as an enhanced motivation to pursue drugs [35], or may reflect a potentiation in the incentive-value of drug-related stimuli [33], or could provide a ‘prediction-error’ teaching signal that reinforces certain behaviors [36]. However, we believe that the suggested augmented phasic dopamine release after drug experience is likely not involved in learning per se [34, 38], as the stimulus-reinforcer associations have already been made. While changes in the dopamine system are associated with learning [76, 78, 79], it can be difficult to ascribe a causal relationship between dopamine and learning [33]. Regardless, our model proposes that an enhanced ‘signal to noise’ in phasic dopamine release after chronic drug experience will promote drug seeking and aberrant behaviors related to cue-stimuli associations. Future studies will be needed to test the predictions in our model. However, recent improvements in FSCV recording strategies now permit voltammetric recordings over months, which will be an invaluable experimental technique to specifically examine the role of phasic dopamine release in the development of addiction-related behaviors.
Learning Objectives
The basic anatomy of the VTA dopamine system and common methods to detect dopamine neurons and dopamine release.
The evidence supporting contemporary theories of dopamine function.
The role of phasic dopamine release in appetitive behaviors.
The acute and prolonged effect of abused drugs on dopamine neuron properties, dopamine neuron firing and dopamine release.
The effect of abused drugs on phasic dopamine release.
Future Research Questions
Do dopamine neuron firing patterns change in awake, behaving rodents after abstinence from chronic drug exposure?
Do the patterns and amplitude of phasic dopamine release change after abstinence from chronic drug exposure?
Is non-drug related learning affected in a phasic dopamine-dependent manner after chronic drug exposure?
Are there changes in the pattern of phasic dopamine release during the transition from casual to compulsive drug use?
Acknowledgments
This work was completed while under the support of National Institute of Health funds: T32-AA009455 (MJW), F32-DA024540 (JJC), R21-DA021793 (PEMP), and R01-MH079292 (PEMP).
References
- 1.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982 Jul-Dec;9(1–6):321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 2.Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003 Jul;168(1–2):44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- 3.Riederer P, Wuketich S. Time course of nigrostriatal degeneration in parkinson's disease. A detailed study of influential factors in human brain amine analysis. Journal of neural transmission. 1976;38(3–4):277–301. doi: 10.1007/BF01249445. [DOI] [PubMed] [Google Scholar]
- 4.Kawano M, Kawasaki A, Sakata-Haga H, Fukui Y, Kawano H, Nogami H, et al. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J Comp Neurol. 2006 Oct 10;498(5):581–592. doi: 10.1002/cne.21054. [DOI] [PubMed] [Google Scholar]
- 5.Lapish CC, Kroener S, Durstewitz D, Lavin A, Seamans JK. The ability of the mesocortical dopamine system to operate in distinct temporal modes. Psychopharmacology (Berl) 2007 Apr;191(3):609–625. doi: 10.1007/s00213-006-0527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007 Jan;25(1):106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol. 2005 Mar 7;483(2):217–235. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- 8.Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979 Sep 1;187(1):117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- 9.Forster GL, Blaha CD. Pedunculopontine tegmental stimulation evokes striatal dopamine efflux by activation of acetylcholine and glutamate receptors in the midbrain and pons of the rat. Eur J Neurosci. 2003 Feb;17(4):751–762. doi: 10.1046/j.1460-9568.2003.02511.x. [DOI] [PubMed] [Google Scholar]
- 10.Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007 May 23;27(21):5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983 Oct;10(2):301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- 12.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007 May;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nature neuroscience. 2003 Sep;6(9):968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 14.Parsons LH, Justice JB., Jr Extracellular concentration and in vivo recovery of dopamine in the nucleus accumbens using microdialysis. J Neurochem. 1992 Jan;58(1):212–218. doi: 10.1111/j.1471-4159.1992.tb09298.x. [DOI] [PubMed] [Google Scholar]
- 15.Garris PA, Christensen JR, Rebec GV, Wightman RM. Real-time measurement of electrically evoked extracellular dopamine in the striatum of freely moving rats. J Neurochem. 1997 Jan;68(1):152–161. doi: 10.1046/j.1471-4159.1997.68010152.x. [DOI] [PubMed] [Google Scholar]
- 16.Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988 Jan;24(1):19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- 17.Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997 Dec;25(3):312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- 18.Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc Natl Acad Sci U S A. 2006 Mar 28;103(13):5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 2000 Oct;12(10):3596–3604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- 20.Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006 Dec 15;577(Pt 3):907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004 Mar 26;303(5666):2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- 22.Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989 Oct;9(10):3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuhoff H, Neu A, Liss B, Roeper J. I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci. 2002 Feb 15;22(4):1290–1302. doi: 10.1523/JNEUROSCI.22-04-01290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008 Apr 15;586(8):2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cameron DL, Wessendorf MW, Williams JT. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience. 1997 Mar;77(1):155–166. doi: 10.1016/s0306-4522(96)00444-7. [DOI] [PubMed] [Google Scholar]
- 26.Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008 Mar 13;57(5):760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci. 2008 Sep 3;28(36):8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heien ML, Wightman RM. Phasic dopamine signaling during behavior, reward, and disease states. CNS Neurol Disord Drug Targets. 2006 Feb;5(1):99–108. doi: 10.2174/187152706784111605. [DOI] [PubMed] [Google Scholar]
- 29.Robinson DL, Venton BJ, Heien ML, Wightman RM. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clinical chemistry. 2003 Oct;49(10):1763–1773. doi: 10.1373/49.10.1763. [DOI] [PubMed] [Google Scholar]
- 30.Phillips PE, Robinson DL, Stuber GD, Carelli RM, Wightman RM. Real-time measurements of phasic changes in extracellular dopamine concentration in freely moving rats by fast-scan cyclic voltammetry. Methods in molecular medicine. 2003;79:443–464. doi: 10.1385/1-59259-358-5:443. [DOI] [PubMed] [Google Scholar]
- 31.Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, et al. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci U S A. 2005 Jul 19;102(29):10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlsson A, Lindqvist M, Magnusson T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957 Nov 30;180(4596):1200. doi: 10.1038/1801200a0. [DOI] [PubMed] [Google Scholar]
- 33.Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007 Apr;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 34.Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nat Rev Neurosci. 2006 Dec;7(12):967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- 35.Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002 Dec 2;137(1–2):3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- 36.Schultz W. Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol. 1997 Apr;7(2):191–197. doi: 10.1016/s0959-4388(97)80007-4. [DOI] [PubMed] [Google Scholar]
- 37.Schultz W. Multiple dopamine functions at different time courses. Annual review of neuroscience. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 38.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004 Jun;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 39.Bergstrom BP, Garris PA. "Passive stabilization" of striatal extracellular dopamine across the lesion spectrum encompassing the presymptomatic phase of Parkinson's disease: a voltammetric study in the 6-OHDA-lesioned rat. J Neurochem. 2003 Dec;87(5):1224–1236. doi: 10.1046/j.1471-4159.2003.02104.x. [DOI] [PubMed] [Google Scholar]
- 40.Bezard E, Dovero S, Prunier C, Ravenscroft P, Chalon S, Guilloteau D, et al. Relationship between the appearance of symptoms and the level of nigrostriatal degeneration in a progressive 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson's disease. J Neurosci. 2001 Sep 1;21(17):6853–6861. doi: 10.1523/JNEUROSCI.21-17-06853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995 Dec 29;83(7):1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 42.Cousins MS, Salamone JD. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacology, biochemistry, and behavior. 1994 Sep;49(1):85–91. doi: 10.1016/0091-3057(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 43.Cousins MS, Sokolowski JD, Salamone JD. Different effects of nucleus accumbens and ventrolateral striatal dopamine depletions on instrumental response selection in the rat. Pharmacology, biochemistry, and behavior. 1993 Dec;46(4):943–951. doi: 10.1016/0091-3057(93)90226-j. [DOI] [PubMed] [Google Scholar]
- 44.Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacology, biochemistry, and behavior. 2001 Jul-Aug;69(3–4):373–382. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- 45.Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology (Berl) 1991;104(4):515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- 46.Denk F, Walton ME, Jennings KA, Sharp T, Rushworth MF, Bannerman DM. Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort. Psychopharmacology (Berl) 2005 May;179(3):587–596. doi: 10.1007/s00213-004-2059-4. [DOI] [PubMed] [Google Scholar]
- 47.Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994 Dec 15;65(2):221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 48.Cousins MS, Atherton A, Turner L, Salamone JD. Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behav Brain Res. 1996 Jan;74(1–2):189–197. doi: 10.1016/0166-4328(95)00151-4. [DOI] [PubMed] [Google Scholar]
- 49.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of neuroscience methods. 1996 May;66(1):1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 50.Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacology, biochemistry, and behavior. 1998 Dec;61(4):341–348. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- 51.Hamill S, Trevitt JT, Nowend KL, Carlson BB, Salamone JD. Nucleus accumbens dopamine depletions and time-constrained progressive ratio performance: effects of different ratio requirements. Pharmacology, biochemistry, and behavior. 1999 Sep;64(1):21–27. doi: 10.1016/s0091-3057(99)00092-1. [DOI] [PubMed] [Google Scholar]
- 52.Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behavioral neuroscience. 2003 Apr;117(2):202–211. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]
- 53.Cagniard B, Balsam PD, Brunner D, Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006 Jul;31(7):1362–1370. doi: 10.1038/sj.npp.1300966. [DOI] [PubMed] [Google Scholar]
- 54.Phillips PE, Walton ME, Jhou TC. Calculating utility: preclinical evidence for cost-benefit analysis by mesolimbic dopamine. Psychopharmacology (Berl) 2007 Apr;191(3):483–495. doi: 10.1007/s00213-006-0626-6. [DOI] [PubMed] [Google Scholar]
- 55.Berridge KC, Venier IL, Robinson TE. Taste reactivity analysis of 6-hydroxydopamine-induced aphagia: implications for arousal and anhedonia hypotheses of dopamine function. Behavioral neuroscience. 1989 Feb;103(1):36–45. doi: 10.1037//0735-7044.103.1.36. [DOI] [PubMed] [Google Scholar]
- 56.Kaczmarek HJ, Kiefer SW. Microinjections of dopaminergic agents in the nucleus accumbens affect ethanol consumption but not palatability. Pharmacology, biochemistry, and behavior. 2000 Jun;66(2):307–312. doi: 10.1016/s0091-3057(00)00182-9. [DOI] [PubMed] [Google Scholar]
- 57.Pecina S, Berridge KC, Parker LA. Pimozide does not shift palatability: separation of anhedonia from sensorimotor suppression by taste reactivity. Pharmacology, biochemistry, and behavior. 1997 Nov;58(3):801–811. doi: 10.1016/s0091-3057(97)00044-0. [DOI] [PubMed] [Google Scholar]
- 58.Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher "wanting" but not "liking" for sweet rewards. J Neurosci. 2003 Oct 15;23(28):9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evans AH, Pavese N, Lawrence AD, Tai YF, Appel S, Doder M, et al. Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Annals of neurology. 2006 May;59(5):852–858. doi: 10.1002/ana.20822. [DOI] [PubMed] [Google Scholar]
- 60.Leyton M, Casey KF, Delaney JS, Kolivakis T, Benkelfat C. Cocaine craving, euphoria, and self-administration: a preliminary study of the effect of catecholamine precursor depletion. Behavioral neuroscience. 2005 Dec;119(6):1619–1627. doi: 10.1037/0735-7044.119.6.1619. [DOI] [PubMed] [Google Scholar]
- 61.Pan WX, Schmidt R, Wickens JR, Hyland BI. Dopamine cells respond to predicted events during classical conditioning: evidence for eligibility traces in the reward-learning network. J Neurosci. 2005 Jun 29;25(26):6235–6242. doi: 10.1523/JNEUROSCI.1478-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997 Mar 14;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 63.Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003 Mar 21;299(5614):1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 64.Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005 Mar 11;307(5715):1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- 65.Waelti P, Dickinson A, Schultz W. Dopamine responses comply with basic assumptions of formal learning theory. Nature. 2001 Jul 5;412(6842):43–48. doi: 10.1038/35083500. [DOI] [PubMed] [Google Scholar]
- 66.Comoli E, Coizet V, Boyes J, Bolam JP, Canteras NS, Quirk RH, et al. A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nature neuroscience. 2003 Sep;6(9):974–980. doi: 10.1038/nn1113. [DOI] [PubMed] [Google Scholar]
- 67.Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nature neuroscience. 2005 Dec;8(12):1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- 68.Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annual review of neuroscience. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- 69.Montague PR, McClure SM, Baldwin PR, Phillips PE, Budygin EA, Stuber GD, et al. Dynamic gain control of dopamine delivery in freely moving animals. J Neurosci. 2004 Feb 18;24(7):1754–1759. doi: 10.1523/JNEUROSCI.4279-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freeman AS, Bunney BS. Activity of A9 and A10 dopaminergic neurons in unrestrained rats: further characterization and effects of apomorphine and cholecystokinin. Brain research. 1987 Mar 3;405(1):46–55. doi: 10.1016/0006-8993(87)90988-7. [DOI] [PubMed] [Google Scholar]
- 71.Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem. 1999 Mar;72(3):1088–1094. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- 72.Fiorino DF, Coury A, Phillips AG. Dynamic changes in nucleus accumbens dopamine efflux during the Coolidge effect in male rats. J Neurosci. 1997 Jun 15;17(12):4849–4855. doi: 10.1523/JNEUROSCI.17-12-04849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robinson DL, Heien ML, Wightman RM. Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J Neurosci. 2002 Dec 1;22(23):10477–10486. doi: 10.1523/JNEUROSCI.22-23-10477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robinson DL, Phillips PE, Budygin EA, Trafton BJ, Garris PA, Wightman RM. Sub-second changes in accumbal dopamine during sexual behavior in male rats. Neuroreport. 2001 Aug 8;12(11):2549–2552. doi: 10.1097/00001756-200108080-00051. [DOI] [PubMed] [Google Scholar]
- 75.Roitman MF, Stuber GD, Phillips PE, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004 Feb 11;24(6):1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nature neuroscience. 2007 Aug;10(8):1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- 77.Sunsay C, Rebec GV. Real-time dopamine efflux in the nucleus accumbens core during Pavlovian conditioning. Behavioral neuroscience. 2008 Apr;122(2):358–367. doi: 10.1037/0735-7044.122.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, et al. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008 Sep 19;321(5896):1690–1692. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Owesson-White CA, Cheer JF, Beyene M, Carelli RM, Wightman RM. Dynamic changes in accumbens dopamine correlate with learning during intracranial self-stimulation. Proc Natl Acad Sci U S A. 2008 Aug 19;105(33):11957–11962. doi: 10.1073/pnas.0803896105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.You ZB, Chen YQ, Wise RA. Dopamine and glutamate release in the nucleus accumbens and ventral tegmental area of rat following lateral hypothalamic self-stimulation. Neuroscience. 2001;107(4):629–639. doi: 10.1016/s0306-4522(01)00379-7. [DOI] [PubMed] [Google Scholar]
- 81.Garris PA, Kilpatrick M, Bunin MA, Michael D, Walker QD, Wightman RM. Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature. 1999 Mar 4;398(6722):67–69. doi: 10.1038/18019. [DOI] [PubMed] [Google Scholar]
- 82.Cheer JF, Heien ML, Garris PA, Carelli RM, Wightman RM. Simultaneous dopamine and single-unit recordings reveal accumbens GABAergic responses: implications for intracranial self-stimulation. Proc Natl Acad Sci U S A. 2005 Dec 27;102(52):19150–19155. doi: 10.1073/pnas.0509607102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997 Jun 27;276(5321):2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- 85.Kuhr WG, Ewing AG, Near JA, Wightman RM. Amphetamine attenuates the stimulated release of dopamine in vivo. The Journal of pharmacology and experimental therapeutics. 1985 Feb;232(2):388–394. [PubMed] [Google Scholar]
- 86.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987 Sep 4;237(4819):1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 87.Iwatsubo K, Clouet DH. Effects of morphine and haloperidol on the electrical activity of rat nigrostriatal neurons. The Journal of pharmacology and experimental therapeutics. 1977 Aug;202(2):429–436. [PubMed] [Google Scholar]
- 88.Melis M, Gessa GL, Diana M. Different mechanisms for dopaminergic excitation induced by opiates and cannabinoids in the rat midbrain. Progress in neuro-psychopharmacology & biological psychiatry. 2000 Aug;24(6):993–1006. doi: 10.1016/s0278-5846(00)00119-6. [DOI] [PubMed] [Google Scholar]
- 89.Gallegos RA, Lee RS, Criado JR, Henriksen SJ, Steffensen SC. Adaptive responses of gamma-aminobutyric acid neurons in the ventral tegmental area to chronic ethanol. The Journal of pharmacology and experimental therapeutics. 1999 Dec;291(3):1045–1053. [PubMed] [Google Scholar]
- 90.Brodie MS, Appel SB. Dopaminergic neurons in the ventral tegmental area of C57BL/6J and DBA/2J mice differ in sensitivity to ethanol excitation. Alcoholism, clinical and experimental research. 2000 Jul;24(7):1120–1124. [PubMed] [Google Scholar]
- 91.Koyama S, Brodie MS, Appel SB. Ethanol inhibition of m-current and ethanol-induced direct excitation of ventral tegmental area dopamine neurons. Journal of neurophysiology. 2007 Mar;97(3):1977–1985. doi: 10.1152/jn.00270.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. Journal of neurophysiology. 2006 Feb;95(2):619–626. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Ethanol enhances GABAergic transmission onto dopamine neurons in the ventral tegmental area of the rat. Alcoholism, clinical and experimental research. 2008 Jun;32(6):1040–1048. doi: 10.1111/j.1530-0277.2008.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xiao C, Shao XM, Olive MF, Griffin WC, 3rd, Li KY, Krnjevic K, et al. Ethanol Facilitates Glutamatergic Transmission to Dopamine Neurons in the Ventral Tegmental Area. Neuropsychopharmacology. 2008 Jul 2; doi: 10.1038/npp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002 Mar 14;33(6):905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 96.Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997 Nov 27;390(6658):401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- 97.Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000 Aug;27(2):349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 98.Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux JP, et al. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006 Jun 15;50(6):911–921. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 99.French ED, Dillon K, Wu X. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport. 1997 Feb 10;8(3):649–652. doi: 10.1097/00001756-199702100-00014. [DOI] [PubMed] [Google Scholar]
- 100.Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. European journal of pharmacology. 1998 Jan 2;341(1):39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- 101.Einhorn LC, Johansen PA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: studies in the ventral tegmental area. J Neurosci. 1988 Jan;8(1):100–112. doi: 10.1523/JNEUROSCI.08-01-00100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lacey MG, Mercuri NB, North RA. Actions of cocaine on rat dopaminergic neurones in vitro. British journal of pharmacology. 1990 Apr;99(4):731–735. doi: 10.1111/j.1476-5381.1990.tb12998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sanghera MK, Trulson ME, German DC. Electrophysiological properties of mouse dopamine neurons: in vivo and in vitro studies. Neuroscience. 1984 Jul;12(3):793–801. doi: 10.1016/0306-4522(84)90171-4. [DOI] [PubMed] [Google Scholar]
- 104.Koob GF, Le Moal M. Addiction and the brain antireward system. Annual review of psychology. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 105.Henry DJ, Greene MA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: repeated administration. The Journal of pharmacology and experimental therapeutics. 1989 Dec;251(3):833–839. [PubMed] [Google Scholar]
- 106.Marinelli M, Cooper DC, Baker LK, White FJ. Impulse activity of midbrain dopamine neurons modulates drug-seeking behavior. Psychopharmacology (Berl) 2003 Jul;168(1–2):84–98. doi: 10.1007/s00213-003-1491-1. [DOI] [PubMed] [Google Scholar]
- 107.Shen RY, Choong KC, Thompson AC. Long-term reduction in ventral tegmental area dopamine neuron population activity following repeated stimulant or ethanol treatment. Biological psychiatry. 2007 Jan 1;61(1):93–100. doi: 10.1016/j.biopsych.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 108.Parsons LH, Smith AD, Justice JB., Jr . Synapse. 1. Vol. 9. New York, NY: 1991. Sep, Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine; pp. 60–65. [DOI] [PubMed] [Google Scholar]
- 109.Robertson MW, Leslie CA, Bennett JP., Jr Apparent synaptic dopamine deficiency induced by withdrawal from chronic cocaine treatment. Brain research. 1991 Jan 11;538(2):337–339. doi: 10.1016/0006-8993(91)90451-z. [DOI] [PubMed] [Google Scholar]
- 110.Rossetti ZL, Hmaidan Y, Gessa GL. Marked inhibition of mesolimbic dopamine release: a common feature of ethanol, morphine, cocaine and amphetamine abstinence in rats. European journal of pharmacology. 1992 Oct 20;221(2–3):227–234. doi: 10.1016/0014-2999(92)90706-a. [DOI] [PubMed] [Google Scholar]
- 111.Liu ZH, Jin WQ. Decrease of ventral tegmental area dopamine neuronal activity in nicotine withdrawal rats. Neuroreport. 2004 Jun 28;15(9):1479–1481. doi: 10.1097/01.wnr.0000126218.25235.b6. [DOI] [PubMed] [Google Scholar]
- 112.Bailey CP, Manley SJ, Watson WP, Wonnacott S, Molleman A, Little HJ. Chronic ethanol administration alters activity in ventral tegmental area neurons after cessation of withdrawal hyperexcitability. Brain research. 1998 Aug 24;803(1–2):144–152. doi: 10.1016/s0006-8993(98)00654-4. [DOI] [PubMed] [Google Scholar]
- 113.Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7966–7969. doi: 10.1073/pnas.90.17.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Diana M, Muntoni AL, Pistis M, Melis M, Gessa GL. Lasting reduction in mesolimbic dopamine neuronal activity after morphine withdrawal. Eur J Neurosci. 1999 Mar;11(3):1037–1041. doi: 10.1046/j.1460-9568.1999.00488.x. [DOI] [PubMed] [Google Scholar]
- 115.Diana M, Pistis M, Muntoni A, Gessa G. Profound decrease of mesolimbic dopaminergic neuronal activity in morphine withdrawn rats. The Journal of pharmacology and experimental therapeutics. 1995 Feb;272(2):781–785. [PubMed] [Google Scholar]
- 116.Georges F, Le Moine C, Aston-Jones G. No effect of morphine on ventral tegmental dopamine neurons during withdrawal. J Neurosci. 2006 May 24;26(21):5720–5726. doi: 10.1523/JNEUROSCI.5032-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu X, French ED. Effects of chronic delta9-tetrahydrocannabinol on rat midbrain dopamine neurons: an electrophysiological assessment. Neuropharmacology. 2000 Jan 28;39(3):391–398. doi: 10.1016/s0028-3908(99)00140-9. [DOI] [PubMed] [Google Scholar]
- 118.Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, et al. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996 May 15;16(10):3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robinson TE, Jurson PA, Bennett JA, Bentgen KM. Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: a microdialysis study in freely moving rats. Brain research. 1988 Oct 18;462(2):211–222. doi: 10.1016/0006-8993(88)90549-5. [DOI] [PubMed] [Google Scholar]
- 120.Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nature neuroscience. 2006 May;9(5):636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 121.Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004 Aug 25;24(34):7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005 Oct 13;437(7061):1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003 Feb 20;37(4):577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 124.Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001 May 31;411(6837):583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 125.Wanat MJ, Bonci A. Synapse. 10. Vol. 62. New York, NY: 2008. Oct, Dose-dependent changes in the synaptic strength on dopamine neurons and locomotor activity after cocaine exposure; pp. 790–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gao C, Wolf ME. Dopamine alters AMPA receptor synaptic expression and subunit composition in dopamine neurons of the ventral tegmental area cultured with prefrontal cortex neurons. J Neurosci. 2007 Dec 26;27(52):14275–14285. doi: 10.1523/JNEUROSCI.2925-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hyman SE, Malenka RC, Nestler EJ. Neural Mechanisms of Addiction: The Role of Reward-Related Learning and Memory. Annual review of neuroscience. 2006 Apr 20; doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 128.Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008 Sep 10;28(37):9092–9100. doi: 10.1523/JNEUROSCI.1001-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Faleiro LJ, Jones S, Kauer JA. Rapid synaptic plasticity of glutamatergic synapses on dopamine neurons in the ventral tegmental area in response to acute amphetamine injection. Neuropsychopharmacology. 2004 Dec;29(12):2115–2125. doi: 10.1038/sj.npp.1300495. [DOI] [PubMed] [Google Scholar]
- 130.Schilstrom B, Yaka R, Argilli E, Suvarna N, Schumann J, Chen BT, et al. Cocaine enhances NMDA receptor-mediated currents in ventral tegmental area cells via dopamine D5 receptor-dependent redistribution of NMDA receptors. J Neurosci. 2006 Aug 16;26(33):8549–8558. doi: 10.1523/JNEUROSCI.5179-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang F, Chen H, Steketee JD, Sharp BM. Upregulation of ionotropic glutamate receptor subunits within specific mesocorticolimbic regions during chronic nicotine self-administration. Neuropsychopharmacology. 2007 Jan;32(1):103–109. doi: 10.1038/sj.npp.1301033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, et al. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008 Jul 31;59(2):288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, et al. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008 Aug 14;59(3):497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 134.Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007 Apr 26;446(7139):1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- 135.Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002 Mar 15;22(6):2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM, Bonci A. Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. Journal of neurophysiology. 2007 Oct;98(4):2297–2310. doi: 10.1152/jn.00824.2007. [DOI] [PubMed] [Google Scholar]
- 137.Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci. 2000 Dec 1;20(23):8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Budygin EA, Phillips PE, Wightman RM, Jones SR. Synapse. 2. Vol. 42. New York, NY: 2001. Nov, Terminal effects of ethanol on dopamine dynamics in rat nucleus accumbens: an in vitro voltammetric study; pp. 77–79. [DOI] [PubMed] [Google Scholar]
- 139.Mathews TA, John CE, Lapa GB, Budygin EA, Jones SR. Synapse. 4. Vol. 60. New York, NY: 2006. Sep 15, No role of the dopamine transporter in acute ethanol effects on striatal dopamine dynamics; pp. 288–294. [DOI] [PubMed] [Google Scholar]
- 140.Samson HH, Hodge CW, Erickson HL, Niehus JS, Gerhardt GA, Kalivas PW, et al. Alcohol. 5. Vol. 14. Fayetteville, NY: 1997. Sep-Oct, The effects of local application of ethanol in the n. accumbens on dopamine overflow and clearance; pp. 485–492. [DOI] [PubMed] [Google Scholar]
- 141.Budygin EA, Oleson EB, Mathews TA, Lack AK, Diaz MR, McCool BA, et al. Effects of chronic alcohol exposure on dopamine uptake in rat nucleus accumbens and caudate putamen. Psychopharmacology (Berl) 2007 Sep;193(4):495–501. doi: 10.1007/s00213-007-0812-1. [DOI] [PubMed] [Google Scholar]
- 142.Budygin EA, Phillips PE, Robinson DL, Kennedy AP, Gainetdinov RR, Wightman RM. Effect of acute ethanol on striatal dopamine neurotransmission in ambulatory rats. The Journal of pharmacology and experimental therapeutics. 2001 Apr;297(1):27–34. [PubMed] [Google Scholar]
- 143.Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, et al. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007 Jan 24;27(4):791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004 May 5;24(18):4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Middleton LS, Cass WA, Dwoskin LP. Nicotinic receptor modulation of dopamine transporter function in rat striatum and medial prefrontal cortex. The Journal of pharmacology and experimental therapeutics. 2004 Jan;308(1):367–377. doi: 10.1124/jpet.103.055335. [DOI] [PubMed] [Google Scholar]
- 146.Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nature neuroscience. 2004 Jun;7(6):583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- 147.Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nature neuroscience. 2004 Jun;7(6):581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- 148.Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008 Aug 27;28(35):8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stuber GD, Roitman MF, Phillips PE, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology. 2005 May;30(5):853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- 150.Stuber GD, Wightman RM, Carelli RM. Extinction of cocaine self-administration reveals functionally and temporally distinct dopaminergic signals in the nucleus accumbens. Neuron. 2005 May 19;46(4):661–669. doi: 10.1016/j.neuron.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 151.Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004 Dec 8;24(49):11070–11078. doi: 10.1523/JNEUROSCI.3695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003 Apr 10;422(6932):614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 153.Fowler JS, Volkow ND, Kassed CA, Chang L. Imaging the addicted human brain. Science & practice perspectives / a publication of the National Institute on Drug Abuse, National Institutes of Health. 2007 Apr;3(2):4–16. doi: 10.1151/spp07324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47 Suppl 1:3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 155.Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Molecular psychiatry. 2004 Jun;9(6):557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- 156.D’Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD responses reflecting dopaminergic signals in the human ventral tegmental area. Science. 2008 Feb 29;319(5867):1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- 157.Drevets WC, Gautier C, Price JC, Kupfer DJ, Kinahan PE, Grace AA, et al. Amphetamine-induced dopamine release in human ventral striatum correlates with euphoria. Biological psychiatry. 2001 Jan 15;49(2):81–96. doi: 10.1016/s0006-3223(00)01038-6. [DOI] [PubMed] [Google Scholar]
- 158.Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, et al. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997 Apr 24;386(6627):827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- 159.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C, et al. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. The Journal of pharmacology and experimental therapeutics. 1999 Oct;291(1):409–415. [PubMed] [Google Scholar]
- 160.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997 Apr 24;386(6627):830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 161.Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, et al. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcoholism, clinical and experimental research. 1996 Dec;20(9):1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- 162.Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, et al. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. The American journal of psychiatry. 1990 Jun;147(6):719–724. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- 163.Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, et al. Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology. 1997 Feb;16(2):174–182. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- 164.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. The American journal of psychiatry. 2001 Dec;158(12):2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 165.Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, et al. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Archives of general psychiatry. 2006 Sep;63(9):999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- 166.Nelson A, Killcross S. Amphetamine exposure enhances habit formation. J Neurosci. 2006 Apr 5;26(14):3805–3812. doi: 10.1523/JNEUROSCI.4305-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Harmer CJ, Phillips GD. Enhanced appetitive conditioning following repeated pretreatment with d-amphetamine. Behavioural pharmacology. 1998 Jul;9(4):299–308. [PubMed] [Google Scholar]
- 168.Harmer CJ, Phillips GD. Enhanced conditioned inhibition following repeated pretreatment with d-amphetamine. Psychopharmacology (Berl) 1999 Feb;142(2):120–131. doi: 10.1007/s002130050870. [DOI] [PubMed] [Google Scholar]
- 169.Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39(4):376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 170.Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl) 2008 Apr;197(3):421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sevy S, Hassoun Y, Bechara A, Yechiam E, Napolitano B, Burdick K, et al. Emotion-based decision-making in healthy subjects: short-term effects of reducing dopamine levels. Psychopharmacology (Berl) 2006 Oct;188(2):228–235. doi: 10.1007/s00213-006-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hyman SE. Addiction: a disease of learning and memory. The American journal of psychiatry. 2005 Aug;162(8):1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]