Abstract
Body weight regulation is one of the most important homeostatic mechanisms. In recent years many molecular players involved in the energy balance were identified. Although the discovery of leptin almost 15 years ago sparked a great enthusiasm that we will soon understand molecular pathways regulating body weight homeostasis, these expectations turned out to be premature. We know that main site of body weight homeostasis is the hypothalamus with four primary regions – arcuate, paraventricular and ventromedial nuclei, and lateral hypothalamus. Downstream from leptin, the most important signalling peptides are melanocortin, CART, neuropeptide Y and AgRP. Beside those, many other signalling pathways that include signalling by adipokines such as resistin and adiponectin, endocannabinoids in hypothalamus, glucocorticoids from adrenal glands, sex steroid hormones from the gonads and several peptides/hormones secreted by gastrointestinal tract are involved in the body weight homeostasis, and the real challenge for the future is how to understand the complicated interplay between all these molecules. This seems to be a daunting task, but with the new discoveries and with the use of the new molecular tools a rapid progress is being made. The real challenge for the future nevertheless remains how to interfere with these processes and how to help people with body weight problems that are not caused simply by sedentary life style, but also by the genetic make-up of these individuals.
Keywords: Body weight regulation, hypothalamus, leptin
Introduction
Almost 15 years ago a hormone leptin was identified, which is now known to be the key regulator of body weight homeostasis in the mammalian body.1 The existence of such hormone was proposed much earlier, in mid fifties, when two strains of spontaneously mutated mice were identified. Both strains were unable to regulate their body weight and ultimately reached a weight about three times larger than normal mice. Parabiosis experiments with these two strains of mice led to suggestions that one strain lacks a signal, produced by the periphery reporting into the brain the status of energy stores, and another strain lack the receptor for this signal.2 These two strains were designated ob/ob and db/db mice, and the prediction about their underlying cause for obesity was confirmed in 1994 by the discovery of leptin, a hormone produced by white adipose cells. Leptin is a protein, consisting of 167 amino acids with molecular weight 16 kDa.1, 3 Name leptin derives from Greek Leptos meaning lean, as this hormone has a strong anorexigenic effect and thus reduces energy stores. In ob/ob mice, as predicted from the parabiosis experiments, leptin is mutated and could thus no longer serve as a messenger between the adipose tissue and the brain. Soon after discovery of leptin, its receptor was also cloned and it was confirmed that leptin receptor was indeed mutated in db/db mice.4 Leptin is a peptide molecule, produced in white adipose tissue and released into the bloodstream. Concentration of leptin in mammals is in direct correlation with the amount of white adipose tissue -more adipose tissue we have, more leptin is circulating in our blood.5
The role of leptin in humans was confirmed few years after its discovery with identification of patients with mutations in either leptin6, 7 or its receptor.8,9 In these patients, deficiency of leptin or its receptor caused a morbid obesity, and subsequent studies have shown that in patients with leptin deficiency, treatment with recombinant leptin is highly successful.10, 11 However, it also became clear soon that in most cases of human obesity, patients have increased rather than decreased levels of leptin, thus suggesting that leptin responsiveness is affected in the majority of human cases of obesity.12
In addition to leptin, white adipose tissue secretes at least two other peptide hormones involved in body weight homeostasis, resistin and adiponectin.13 Adiponectin is produced by white adipose cells while resistin is produced by macrophages, residing inside the white adipose tissue. Both hormones are thought to have very important roles in modulating insulin sensitivity in peripheral tissues and are thus closely connected with the development of type II diabetes in obesity.14, 15 However, the central role in body weight regulation for both adiponectin and resistin is still controversial as some studies have demonstrated central effects on body weight homeostasis while other studies question such effects of both hormones.16,17
Leptin targets
Studies since the discovery of leptin have focused on downstream targets of leptin in the hypothalamus, a primary site of leptin action. Since leptin is a large peptide molecule, it cannot diffuse freely into the brain but must be actively transported across the blood – brain barrier. One of the few places where large molecules are transported across this barrier is a median eminence, a section of the brain at the bottom of the third ventricle.18, 19 Immediately above the median eminence lays an arcuate hypothalamic nucleus, which is today considered to be the most important target of leptin (Fig. 1). In the arcuate nucleus, many leptin receptors are present, and several neurons in this nucleus are highly responsive to leptin.20, 21 These neurons are usually divided into two groups – POMC/CART neurons and NPY/AgRP neurons. As implicated from the name, the former neurons produce two anorexigenic peptides melanocortin (one of the peptide products of proopiomelanocortin (POMC) protein precursor) and Cocaine and Amphetamine Related Transcript (CART), while later neurons produce neuropeptide Y (NPY) and agouti related peptide (AgRP). All these four peptides are now thought to be the most important players downstream from leptin. The most prominent connections of these neurons lead to the paraventricular hypothalamic nucleus, which is considered to be the main secondary target of leptin (Fig. 1, 2).22–27
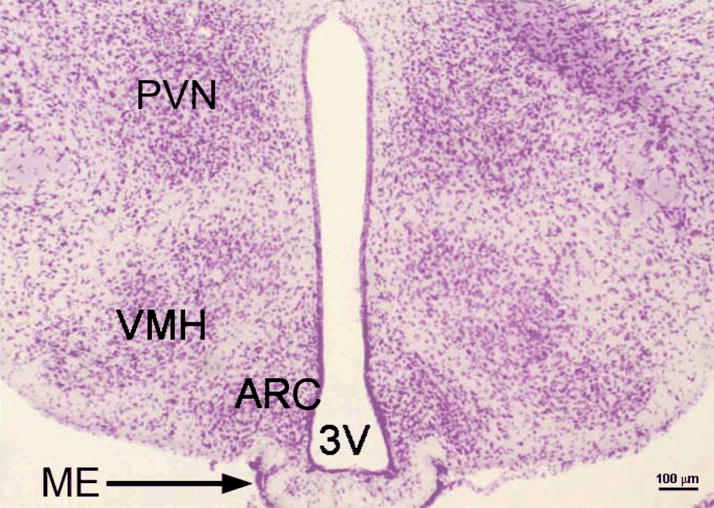
Figure 1.
Coronal section of murine hypothalamus. Paraformaldehyde fixed adult mouse brain was cut on vibrating microtome to 80 μm thickness and stained with cresyl violet dye for 5 minutes. ARC – arcuate nucleus, PVN – paraventricular nucleus, VMH – ventromedialnucleus, 3V – third ventricle, ME – median eminence. . (Photomicrograph owned by the author).
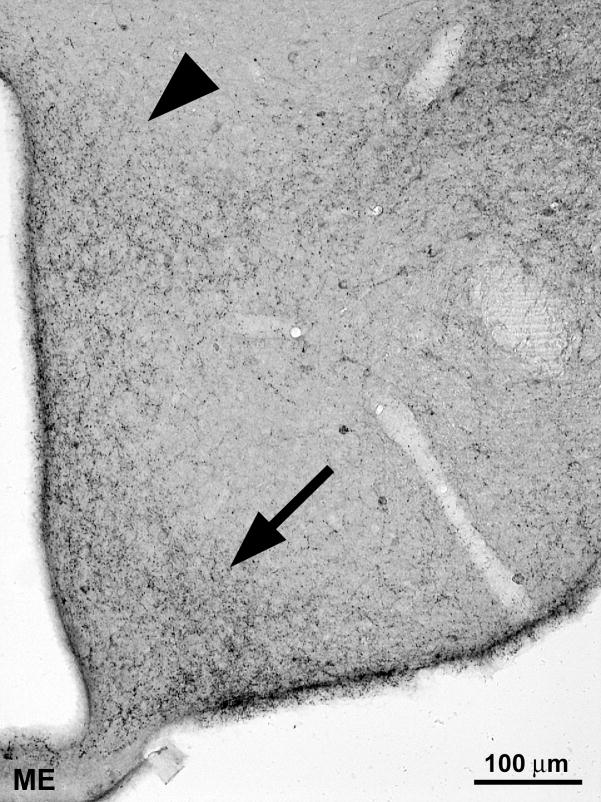
Figure 2.
Immunocytochemical staining for neuropeptide Y in the arcuate (arrow) and paraventricular (arrowhead) nucleus (ME – median eminence). Immunocytochemistry was performed on 80 μm thick floating sections of paraformaldehyde fixed mouse brain using specific antibodies against neuropeptide Y and biotin-streptavidin labelling. (Photomicrograph owned by the author).
Leptin has strong effects on both groups of neurons. Increase in leptin concentration leads to the activation of POMC/CART neurons and these peptides through their receptors convey the signals into the other areas of the brain.5, 28 Increased expression of both POMC and CART leads to reduced appetite and consequently reduced energy intake, as well as an increase in the energy expenditure.24, 26, 29, 30 Conversely, low levels of leptin are a signal for NPY/AgRP neurons which respond with increase in production of both NPY and AgRP, what ultimately leads to increase in the appetite. While NPY directly stimulates food intake through Y1 and Y5 receptors, AgRP acts as an antagonist of melanocortin, binding to melanocortin receptor and preventing action of melanocortin in suppressing appetite.24, 31–34 Furthermore, when stimulated by lack of leptin, NPY/AgRP neurons also secrete an inhibitory neurotransmitter gamma-amino butyric acid (GABA) which seem to constrain the activity of the nearby POMC/CART neurons, thus further preventing anorexigenic effect of these neurons.35 This implies that mechanisms preventing energy loss are stronger than mechanisms preventing excess energy storage. This observation therefore supports the hypothesis that during evolution humans developed more powerful mechanisms for storing the energy than mechanisms to prevent excess energy storage. Several thousand or even million years ago times of abundance were relatively scarce, so it was more important to develop efficient mechanisms for energy storage, and individuals with better developed mechanism for efficient use and storage of energy had an evolutionary advantage. Today, however, this became a disadvantage as our physical activity is usually low while plenty of highly caloric food is widely available, and this led to the epidemic of obesity we witness today.
Beside POMC/NPY system, endocannabionids have important function in regulation of feeding behavior in mammals. The hypothalamic content of endocannabinoids such as anandimide and 2-arachidonoyol glycerol rise during fasting and treatement of animals with 2-arachidonoyol glycerol induce feeding behavior.36 Leptin response in the brain is believed to be partially modulated also by endocannabinoids as leptin could reduce levels of endocannabionoids in the hypothalamus and thus exert its anorexigenic effect also through this pathway.37
Additional role for insulin
Beside leptin, there is another hormone whose existence has been known for long time, and acts as a messenger between periphery and the central nervous system. Insulin, produced by the pancreas, has been known for many years as the hormone responsible for the regulation of glucose homeostasis. It is a peptide dimer with molecular weight of 6 kDa. Two peptide chains (21 and 30 amino acids long) are linked together by two disulfide bridges. Insulin is released from pancreatic beta cells in response to the elevated blood glucose levels and also by some signals from the intestine that are released during feeding.38 However, in addition to its role in promoting glucose storage in the peripheral tissues (muscles, liver and fat), insulin also acts in the brain as an anorexigenic signal. Similarly to leptin, insulin enters the brain via median eminence and exerts its action in the arcuate nucleus, mainly by stimulating POMC/CART neurons.39 As in peripheral tissues, insulin acts also in the brain through insulin receptor with tyrosine kinase activity. Specificity of insulin action is thought to be achieved by differential recruitment of modulator proteins called insulin receptor substrates (IRS). In the brain, the main IRS responsible for action of insulin appears to be IRS2, which is strongly expressed in the arcuate nucleus.39, 40
Glucocorticoid hormones
Glucocorticoid hormones belong to the family of steroid hormones that are produced by several enzymatic reactions from the precursor cholesterol. In humans, the most important glucocorticoid hormone is cortisol, while in rodents, corticosteron is predominant form of glucocorticoids. Both hormones are produced by the adrenal glands.41 Glucocorticoids are important stress modulators, responsible for preparing organism for fight or flight response in dangerous situations. However, they have many other roles in the organism, and one of these is also regulation of feeding behaviour.42, 43 This is partially connected with their role as stress hormones as glucocorticoids function in prolonged stress situation is also to stimulate intake of extra energy, needed to combat a stress.44 However, it is known that glucocorticoids also have a role in physiological (non-stress) regulation of feeding. Secretion of glucocorticoids is diurnal with highest blood levels before the beginning of active part of the diurnal cycle (morning in humans, evening in nocturnal animals).41 This rise in the levels of glucocorticoids has important role in stimulating appetite in the morning to supply the body with calories for active part of the day. Interestingly, glucocroticoids appear to act in close connections with insulin. While glucocorticoids alone could stimulate general food intake in laboratory rats or mice, it is only in concert with insulin that glucocorticoids stimulate specific desire for highly caloric foods such as fat and sucrose, which are needed in stress situation when body needs quick supply of additional energy to respond with fight or flight.44, 45
Gastrointestinal input
In addition to the inputs from insulin and leptin, which are thought to be the most important regulators of long term body weight homeostasis, arcuate nucleus also receives and responds to signals from the gastrointestinal tracts which are released during different phases of digestion. Two of the most important such signals are thought to be ghrelin and peptide YY3-36 (PYY).46, 47 Ghrelin, 28 amino acids long peptide, is released from the endocrine cells in the stomach wall. Relaxed wall of empty stomach is the major signal for the release of ghrelin and thus, ghrelin blood levels are the highest when stomach gets emptied before the next meal. Ghrelin is excreted from the stomach cells and is carried by blood to the hypothalamus, where its receptors are present on NPY/AgRP neurons in the arcuate nucleus. Binding of ghrelin to its receptor strongly stimulates activity of these neurons and thus promoting increase in appetite, and ghrelin is so far the only factor known to directly stimulate the activity of NPY/AgRP neurons. Since levels of ghrelin fall immediately after the meal, ghrelin is thought to be the major mediator of meal regulation, determining the initiation and conclusion of meals.48, 49
PYY, 36 amino acids long peptide, is structurally related to the NPY and is released from the ileum. Its concentrations are the highest between the meals while they normally fall just before the anticipated meals, or when the intestine is empty. Since PYY is structurally related to NPY and can bind to NPY receptors its anorexigenic role seems to be paradoxical. However, it is now thought that PYY selectively binds to the NPY 2 receptors (Y2R), which are present in the arcuate nucleus and exert an auto-inhibitory role on the NPY/AgRP neurons. Since only Y2R receptors (and not Y1 and Y5 which are known to have orexigenic effects) are present in the arcuate nucleus, this explanation seems likely.46, 50 In addition to ghrelin and PYY, several other gastrointestinal hormones, secreted by pancreas, stomach or gut wall, also affect appetite and body weight. These include cholecystokinin (CCK) secreted by duodenum, glucagon like peptide 1 (GLP-1) and oxyntomodulin secreted by intestine wall.51 All three hormones are believed to have central effects in addition to their well established peripheral effects in gastrointestinal tract. They are secreted during feeding and their increased levels act as satiety signals in the brain. 52–57 The role of pancreatic polypeptide (PP) is more controversial as several studies reported that peripheral administration of PP inhibits feeding behaviour while central administration increases appetite and it is not yet known what, if any, are exact roles of this peptide in the brain. 51, 58, 59
Secondary targets in the brain
As mentioned before, strong neuronal connections from the arcuate POMC/CART and NPY/AgRP neurons lead to the paraventricular hypothalamic nucleus (PVN).60–62 This nucleus produce several molecules that are involved in the regulation of energy balance such as corticotrophin releasing hormone (CRH), thyrotropin releasing hormone (TRH), oxytocin and others (Fig. 3).63–65 Older studies with lesions in the PVN provided further evidence for strong involvement of this nucleus in the energy balance regulation as lesions in the PVN produce strong hyperphagia and obesity. 66
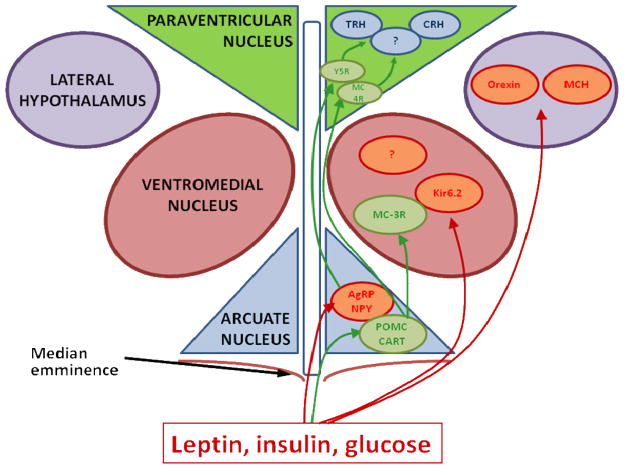
Figure 3.
Schematic presentation of energy homeostasis regulating pathways within the hypothalamus. Positively regulated pathways are marked with green arrows, negatively regulated pathways are marked with red arrows (note that connections to ventromedial nucleus and lateral hypothalamus also go through the median eminence but are drawn on the side for the sake of clarity). Leptin is thought to act primarily on neurons in arcuate nucleus, suppressing expression of neuropeptide Y (NPY) and AgRP and inducing expression of melanocortin (POMC) and CART. These four molecules are transported to the paraventricular nucleus, which is thought to be the main secondary target of leptin and possible targets in paraventricular nucleus include thyrotropin realising hormone (TRH) and corticotrophin realising hormone (CRH). Additionally, leptin, insulin and glucose probably affect ventromedial hypothalamus by altering activity of potassium channels Kir6.2 and melanocortin receptors 3 (MC3-R), and lateral hypothalamus by modulating expression of melanin concentrating hormone (MCH) and orexin. (Original artwork by the author).
In the vicinity of arcuate and paraventricular nuclei are also the ventromedial nucleus (VMH) and lateral hypothalamus, both of which are known to be important in the body weight regulation (Fig. 3). Lateral hypothalamus mainly contains neurons that stimulate food intake and reduce energy balance, and lesions to the lateral hypothalamus result in anorexia and weight loss.28 Two important molecules present in the lateral hypothalamus are orexin and melanin concentrating hormone (MCH). Although the exact role of orexin is not yet determined as it also has important roles in the sleep regulation and its deficiency results in narcolepsy,67 MCH likely have an important physiological role. Central administration or overexpression of this peptide strongly stimulates food intake and deletion of MCH or its receptor causes reduction in body mass and hypermetabolic phenotype,68–70
The role of ventromedial nucleus is less clear. While older studies with lesions in the VMH implicated this region as a satiety centre acting on food intake,71–73 more recent studies also implicated its role in energy consumption.74, 75 Mice lacking SF-1 gene with selectively disrupted VMH cytoarchitecture, and mice lacking melanocortin 3-receptor (MC-3R), strongly expressed in the VMH but not in the PVN or lateral hypothalamus, both exhibit late onset obesity, mainly caused by reduced physical activity and not by hyperphagia.74, 75 Some studies suggest that primary role of the VMH might be in the allocation of the energy resources within the body. According to this hypothesis, VMH would be responsible for sensing the status of energy resources within the brain and respond to these signals by dictating to the periphery whether less energy should be used and more energy made available to the brain.76 This suggestion is supported by the strong expression of ATP dependent Potassium channels Kir6.2 in the VMH, which are thought to be involved in sensing and conferring information about energy requirements.77–79
Body weight regulation in higher brain areas
Although the hypothalamus is clearly the main body weight regulating centre, its influence on behaviour that ultimately leads to decrease or increase in the energy consumption must go through the hierarchically higher brain regions. These signalling pathways are much less understood. One area thought to be involved in the appetite regulation is the nucleus of the solitary tract in the hindbrain.80 This region receives direct inputs from the parasympathetic innervations and responds to the signals related to meal consumption. Namely, during meal ingestion, cholecystokinin release from duodenum and distension of gastric wall directly through parasympathetic innervation activate nucleus of the solitary tract, what is important factor in determining conclusion of the meal. Some studies suggested that leptin influences the nucleus of the solitary tract by making it more sensitive to the regulation by cholecystokinin and gastric distension, thus causing earlier meal termination when sufficient (or excess) energy is stored.80, 81 However, beside the nucleus of the solitary tract, many other areas in the brain must be involved in the regulation of behaviours connected with the energy homeostasis such as increased or decreased physical activity, active food seeking, resting and others, and this will need to be further explored in the future years.
Conclusion
Body weight homeostasis is a process of an outmost importance for the survival of the organism. Therefore, many different mechanisms have developed during the evolution to ensure this homeostasis. Since in nature the more common problem is lack of energy resources rather than their abundance, mechanisms preventing energy loss and favouring energy storage appear to be stronger and more important in the mammalian body. Two major regulators of body weight homeostasis from the periphery are leptin and insulin. Both enter the brain via median eminence and exert their effects mainly in the arcuate hypothalamic nucleus. Four main direct targets of leptin and insulin are melanocortin, CART, neuropeptide Y and Agouti related peptide, of which the first two have strong anorexigenic effects while NPY and AgRP strongly promote feeding behaviour. Downstream from these four targets are many other genes, some in different hypothalamic nuclei, some in other brain regions, that are also involved in the body weight regulation, although we do not yet understand the complicated interplay between all these factors. Beside leptin and insulin, signals directly from the gastrointestinal tract, released during food consumption or fasting periods, as well as other signals such as endocannabinoids, steroid hormones and other adipokines, are also important in the regulation of energy balance. These include ghrelin, released from relaxed stomach wall, which stimulates appetite, PYY released from the ileum between meals, and cholecystokinin, released from extended gastric wall, signalling to the brain that meal should be terminated. Despite almost fifteen years of research since discovery of leptin, we still don’t understand the processes of body weight homeostasis well enough, to be able to interfere with these processes in the patients.
Acknowledgments
Gregor Majdic is supported by grants from ARRS, NIH and ICGEB.
List of abbreviations
- AgRP
agouti related peptide
- ARC
arcuate nucleus
- CART
cocaine and amphetamine related transcript
- CCK
cholecystokinin
- CRH
corticotropin releasing hormone
- Db/db
Leptin receptor deficient mice
- GABA
gamma-amino butyric acid
- GLP-1
glucagon like peptide
- IRS
insulin receptor substrate
- MCH
melanin concentrating hormone
- MC3-R
melanocortin 3 receptor
- ME
median eminence
- NPY
neuropeptide Y
- Ob/ob
Leptin deficient mice
- POMC
proopiomelanocortin
- PP
pancreatic polypeptide
- PVN
paraventricular nucleus
- PYY
peptide YY3-36
- SF-1
steroidogenic factor 1
- TRH
thyrotropin releasing hormone
- VMH
ventromedial hypothalamic nucleus
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Nature. 1994;372:425–32. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Coleman DL. Diabetologia. 1973;9:294–8. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- 3.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Science. 1995;269:540–3. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 4.Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J, et al. Cell. 1995;83:1263–71. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 5.Friedman JM, Halaas JL. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 6.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O’Rahilly S. Nature. 1997;387:903–8. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 7.Farooqi IS. Front Horm Res. 2008;36:1–11. doi: 10.1159/000115333. [DOI] [PubMed] [Google Scholar]
- 8.Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Guy-Grand B. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 9.Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I, Dattani MT, Ercan O, Myhre AG, Retterstol L, Stanhope R, Edge JA, McKenzie S, Lessan N, Ghodsi M, De Rosa V, Perna F, Fontana S, Barroso I, Undlien DE, O’Rahilly S. N Engl J Med. 2007;356:237–47. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. N Engl J Med. 1999;341:879–4. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 11.Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, Licinio J. Proc Natl Acad Sci U S A. 2007;104:18276–9. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 13.Ahima RS, Lazar MA. Mol Endocrinol. 2008;22:1023–31. doi: 10.1210/me.2007-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Michael MD, Kash S, Bensch WR, Monia BP, Murray SF, Otto KA, Syed SK, Bhanot S, Sloop KW, Sullivan JM, Reifel-Miller A. Endocrinology. 2007;148:683–92. doi: 10.1210/en.2006-0708. [DOI] [PubMed] [Google Scholar]
- 15.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Nat Med. 2002;8:731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 16.Qi Y, Takahashi N, Hileman SM, Patel HR, Berg AH, Pajvani UB, Scherer PE, Ahima RS. Nat Med. 2004;10:524–9. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 17.Spranger J, Verma S, Gohring I, Bobbert T, Seifert J, Sindler AL, Pfeiffer A, Hileman SM, Tschop M, Banks WA. Diabetes. 2006;55:141–7. [PubMed] [Google Scholar]
- 18.Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Peptides. 1996;17:305–11. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 19.Banks WA, McLay RN, Kastin AJ, Sarmiento U, Scully S. Am J Physiol. 1999;276:E1099–104. doi: 10.1152/ajpendo.1999.276.6.E1099. [DOI] [PubMed] [Google Scholar]
- 20.Tartaglia LA. J Biol Chem. 1997;272:6093–6. doi: 10.1074/jbc.272.10.6093. [DOI] [PubMed] [Google Scholar]
- 21.van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, Elmquist J, Lowell BB, Barsh GS, de Luca C, Myers MG, Jr, Schwartz GJ, Chua SC., Jr Endocrinology. 2008;149:1773–85. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker RA, Herkenham M. J Comp Neurol. 1995;358:518–30. doi: 10.1002/cne.903580405. [DOI] [PubMed] [Google Scholar]
- 23.Banks AS, Davis SM, Bates SH, Myers MG., Jr J Biol Chem. 2000;275:14563–72. doi: 10.1074/jbc.275.19.14563. [DOI] [PubMed] [Google Scholar]
- 24.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Neuron. 1999;23:775–86. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 25.Elias CF, Kelly JF, Lee CE, Ahima RS, Drucker DJ, Saper CB, Elmquist JK. J Comp Neurol. 2000;423:261–81. [PubMed] [Google Scholar]
- 26.Elias CF, Lee C, Kelly J, Aschkenasi C, Ahima RS, Couceyro PR, Kuhar MJ, Saper CB, Elmquist JK. Neuron. 1998;21:1375–85. doi: 10.1016/s0896-6273(00)80656-x. [DOI] [PubMed] [Google Scholar]
- 27.Menyhert J, Wittmann G, Lechan RM, Keller E, Liposits Z, Fekete C. Endocrinology. 2007;148:4276–81. doi: 10.1210/en.2007-0390. [DOI] [PubMed] [Google Scholar]
- 28.Elmquist JK, Elias CF, Saper CB. Neuron. 1999;22:221–32. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 29.Lambert PD, Couceyro PR, McGirr KM, Dall Vechia SE, Smith Y, Kuhar MJ. Synapse. 1998;29:293–8. doi: 10.1002/(SICI)1098-2396(199808)29:4<293::AID-SYN1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Diabetes. 1997;46:2119–23. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 31.Kanatani A, Mashiko S, Murai N, Sugimoto N, Ito J, Fukuroda T, Fukami T, Morin N, MacNeil DJ, Van der Ploeg LH, Saga Y, Nishimura S, Ihara M. Endocrinology. 2000;141:1011–6. doi: 10.1210/endo.141.3.7387. [DOI] [PubMed] [Google Scholar]
- 32.Dube MG, Xu B, Kalra PS, Sninsky CA, Kalra SP. Brain Res. 1999;816:38–46. doi: 10.1016/s0006-8993(98)00985-8. [DOI] [PubMed] [Google Scholar]
- 33.Marsh DJ, Miura GI, Yagaloff KA, Schwartz MW, Barsh GS, Palmiter RD. Brain Res. 1999;848:66–77. doi: 10.1016/s0006-8993(99)01962-9. [DOI] [PubMed] [Google Scholar]
- 34.Yang YK, Thompson DA, Dickinson CJ, Wilken J, Barsh GS, Kent SB, Gantz I. Mol Endocrinol. 1999;13:148–55. doi: 10.1210/mend.13.1.0223. [DOI] [PubMed] [Google Scholar]
- 35.Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Nature. 2001;411:480–4. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 36.Kirkham TC, Williams CM, Fezza F, Di Marzo V. Br J Pharmacol. 2002;136:550–7. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Marzo V, Goparaju SK, Wang L, Liu J, Batkai S, Jarai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Nature. 2001;410:822–5. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 38.White MF. Science. 2003;302:1710–1. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- 39.Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Diabetes. 2003;52:227–31. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- 40.Lin X, Taguchi A, Park S, Kushner JA, Li F, Li Y, White MF. J Clin Invest. 2004;114:908–16. doi: 10.1172/JCI22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berne RM, Levy MN, Koeppen BM, Stanton BA. Physiology. 2004:883–919. [Google Scholar]
- 42.Akana SF, Cascio CS, Shinsako J, Dallman MF. Am J Physiol. 1985;249:R527–32. doi: 10.1152/ajpregu.1985.249.5.R527. [DOI] [PubMed] [Google Scholar]
- 43.Dallman MF, Akana SF, Pecoraro NC, Warne JP, la Fleur SE, Foster MT. Curr Alzheimer Res. 2007;4:199–204. doi: 10.2174/156720507780362236. [DOI] [PubMed] [Google Scholar]
- 44.Dallman MF, Warne JP, Foster MT, Pecoraro NC. J Physiol. 2007;583:431–6. doi: 10.1113/jphysiol.2007.136051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.la Fleur SE, Akana SF, Manalo SL, Dallman MF. Endocrinology. 2004;145:2174–85. doi: 10.1210/en.2003-1359. [DOI] [PubMed] [Google Scholar]
- 46.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Nature. 2002;418:650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 47.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 48.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. Diabetes. 2001;50:1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 49.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. Nature. 2001;409:194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 50.Lin HC, Chey WY. Regul Pept. 2003;114:131–5. doi: 10.1016/s0167-0115(03)00115-0. [DOI] [PubMed] [Google Scholar]
- 51.Cummings DE, Overduin J. J Clin Invest. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen MA, Ellis SM, Le Roux CW, Batterham RL, Park A, Patterson M, Frost GS, Ghatei MA, Bloom SR. J Clin Endocrinol Metab. 2003;88:4696–701. doi: 10.1210/jc.2003-030421. [DOI] [PubMed] [Google Scholar]
- 53.Dakin CL, Gunn I, Small CJ, Edwards CM, Hay DL, Smith DM, Ghatei MA, Bloom SR. Endocrinology. 2001;142:4244–50. doi: 10.1210/endo.142.10.8430. [DOI] [PubMed] [Google Scholar]
- 54.Gibbs J, Young RC, Smith GP. J Comp Physiol Psychol. 1973;84:488–95. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- 55.Kissileff HR, Pi-Sunyer FX, Thornton J, Smith GP. Am J Clin Nutr. 1981;34:154–60. doi: 10.1093/ajcn/34.2.154. [DOI] [PubMed] [Google Scholar]
- 56.Naslund E, King N, Mansten S, Adner N, Holst JJ, Gutniak M, Hellstrom PM. Br J Nutr. 2004;91:439–46. doi: 10.1079/BJN20031064. [DOI] [PubMed] [Google Scholar]
- 57.Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- 58.Batterham RL, Le Roux CW, Cohen MA, Park AJ, Ellis SM, Patterson M, Frost GS, Ghatei MA, Bloom SR. J Clin Endocrinol Metab. 2003;88:3989–92. doi: 10.1210/jc.2003-030630. [DOI] [PubMed] [Google Scholar]
- 59.Ueno N, Inui A, Iwamoto M, Kaga T, Asakawa A, Okita M, Fujimiya M, Nakajima Y, Ohmoto Y, Ohnaka M, Nakaya Y, Miyazaki JI, Kasuga M. Gastroenterology. 1999;117:1427–32. doi: 10.1016/s0016-5085(99)70293-3. [DOI] [PubMed] [Google Scholar]
- 60.Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. Physiol Behav. 2001;74:683–701. doi: 10.1016/s0031-9384(01)00612-6. [DOI] [PubMed] [Google Scholar]
- 61.Sanchez E, Singru PS, Acharya R, Bodria M, Fekete C, Zavacki AM, Bianco AC, Lechan RM. Endocrinology. 2008 doi: 10.1210/en.2008-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forlano PM, Cone RD. J Comp Neurol. 2007;505:235–48. doi: 10.1002/cne.21447. [DOI] [PubMed] [Google Scholar]
- 63.Blevins JE, Schwartz MW, Baskin DG. Am J Physiol Regul Integr Comp Physiol. 2004;287:R87–96. doi: 10.1152/ajpregu.00604.2003. [DOI] [PubMed] [Google Scholar]
- 64.Lu XY, Barsh GS, Akil H, Watson SJ. J Neurosci. 2003;23:7863–72. doi: 10.1523/JNEUROSCI.23-21-07863.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nillni EA, Vaslet C, Harris M, Hollenberg A, Bjorbak C, Flier JS. J Biol Chem. 2000;275:36124–33. doi: 10.1074/jbc.M003549200. [DOI] [PubMed] [Google Scholar]
- 66.Tokunaga K, Fukushima M, Kemnitz JW, Bray GA. Am J Physiol. 1986;251:R1221–7. doi: 10.1152/ajpregu.1986.251.6.R1221. [DOI] [PubMed] [Google Scholar]
- 67.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 68.Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, Lowell B, Flier JS, Maratos-Flier E. J Clin Invest. 2001;107:379–86. doi: 10.1172/JCI10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. Nature. 1996;380:243–7. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 70.Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. Nature. 1998;396:670–4. doi: 10.1038/25341. [DOI] [PubMed] [Google Scholar]
- 71.Gold RM. J Comp Physiol Psychol. 1970;71:347–56. doi: 10.1037/h0029119. [DOI] [PubMed] [Google Scholar]
- 72.Hetherington AW, Ranson SW. Anatomical record. 1940;78:149–172. [Google Scholar]
- 73.Hetherington AW, Ranson SW. American Journal of Physiology. 1942;136:609–617. [Google Scholar]
- 74.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. Endocrinology. 2000;141:3518–21. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 75.Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL. Endocrinology. 2002;143:607–14. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- 76.Fehm HL, Kern W, Peters A. Prog Brain Res. 2006;153:129–40. doi: 10.1016/S0079-6123(06)53007-9. [DOI] [PubMed] [Google Scholar]
- 77.Evans ML, McCrimmon RJ, Flanagan DE, Keshavarz T, Fan X, McNay EC, Jacob RJ, Sherwin RS. Diabetes. 2004;53:2542–51. doi: 10.2337/diabetes.53.10.2542. [DOI] [PubMed] [Google Scholar]
- 78.Gyte A, Pritchard LE, Jones HB, Brennand JC, White A. J Neuroendocrinol. 2007;19:941–51. doi: 10.1111/j.1365-2826.2007.01607.x. [DOI] [PubMed] [Google Scholar]
- 79.Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, Horiuchi M, Ashcroft F, Minokoshi Y, Roeper J, Seino S. Nat Neurosci. 2001;4:507–12. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- 80.Moran TH, Ladenheim EE, Schwartz GJ. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S39–41. doi: 10.1038/sj.ijo.0801910. [DOI] [PubMed] [Google Scholar]
- 81.Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Am J Physiol. 1999;276:R1545–9. doi: 10.1152/ajpregu.1999.276.5.R1545. [DOI] [PubMed] [Google Scholar]