Abstract
Varied and fascinating interactions occur between males and females to lead to the production of progeny. Interactions between the sexes continue even after the act of mating—but at the molecular and cellular level instead of between individual animals. Molecules transferred from males to females during mating (via the seminal fluid) exert potent effects on females’ physiology and (at least in some animals) on behavior. Taking advantage of genetic, genomic, and biochemical tools for Drosophila, we investigate molecular interactions that underlie this form of chemical communication. Recent data show that molecules and cells from both sexes participate in this “ballet,” facilitating the mutually beneficial outcome of increased progeny production. Examples to be presented include the storage and utilization of sperm in the mated female, and a proteolytic pathway that begins in the male but ends in the female and involves both male and female contributions. Despite the joint benefit of increased progeny production, the “interests” of the mating male can differ from those of his mate. Over evolutionary time this disconnect can, in theory, precipitate a “battle” between the sexes, potentially leading to the rapid sequence changes that have been observed for some seminal proteins across species.
Keywords: mating behavior, proteolysis, rapid evolution, seminal proteins, sequence conservation

Mariana Federica Wolfner is Professor of Developmental Biology and Stephen H. Weiss Presidential Fellow in Cornell University's Department of Molecular Biology and Genetics. She received her undergraduate degree in Biology and Chemistry from Cornell, her PhD in Biochemistry from Stanford University, and was a postdoctoral fellow at University of California, San Diego. Her laboratory's research, on the functions, genetics, and evolution of reproductive proteins, focuses on insect seminal proteins and on the proteins that activate development by the fertilized egg. She also is committed to undergraduate and graduate education and mentoring. She serves on several editorial boards and grants panels and has been, or is, an officer, chair or board member of several scientific societies including, recently, the Chair of the Section on Biological Sciences of the American Association for the Advancement of Science (AAAS). In addition to the honor of being a Wilhelmine Key Lecturer, she has been honored by awards for teaching and/or advising and is a Fellow of the AAAS.
Mating occurs after members of the reproductive pair recognize one another and interact through premating phenomena such as courtship. After mating, the members of the couple usually separate physically and, depending on the species, can go their separate ways. But their physiology and, in some species, overt behaviors are profoundly changed by the encounter. What causes these fascinating effects? Tinbergen (1963) proposed that the underpinnings of behaviors can be viewed from several perspectives: causation, development, evolution, and function. Among causations are the neural networks underlying a given behavior and the molecular, social, and environmental cues that lead to the behavior. This article will discuss a group of molecular cues for Drosophila postmating behaviors, as well as some evolutionary and functional perspectives on them. It will describe how, rather than a one-time effect, molecules provided by one member of a mating pair interact with molecules and cells of the other, in a back-and-forth dialogue. Some of these interactions appear to synergize, as in the moves of a couple dancing a ballet, but some appear to have antagonistic effects, suggesting a “battle” between male- and female-derived molecules. Even from an evolutionary perspective, signs of a similar dichotomy emerge. Some molecules that mediate reproductive behaviors show evolutionary characteristics of having been driven by conflicts, or battles, between the interests of animals engaged in reproduction. Yet others that must work together in a pathway must have been evolutionarily constrained by the mechanistic “ballet.” Thus, there is a complexity and nuance to the cues for these reproductive behaviors, integrating both ballet and battle.
Mated females, on which this article focuses, show a variety of changes relative to their unmated counterparts. They store sperm from the mating (Neubaum and Wolfner 1999b; Bloch Qazi et al. 2003) for times that can range from hours to days (most mammals [Suarez 2008]), to weeks (insects such as Drosophila [Kaufmann and Demerec 1942]) to years (Hymenoptera, e.g., [Baer and Boomsma 2006; den Boer et al. 2008] and some reptiles, e.g., [Magnusson 1979]). Storage of sperm is important in reproduction as it allows extended progeny production from a single (or clustered) mating; in some animals, it allows females to produce progeny for years after these encounters. Another important aspect of sperm storage arises in species whose females mate with multiple males. The sperm/ejaculates of these different males can compete within the female for fertilization opportunities (Parker 1970), or the female can exert preference (e.g., cryptic female choice) over which male's sperm fertilize her eggs (Eberhard 1996). Sperm competition and cryptic female choice can have powerful evolutionary consequences. Another set of changes seen in mated females is physiological. For example, uterine muscles contract postmating in many mammals (thought to facilitate the movement of sperm to storage or fertilization sites, e.g., [Langendijk et al. 2005]), and ovulation is triggered by mating in camelids (Chen et al. 1985). In insects, egg production (and egg laying behaviors) is triggered or stimulated by mating (reviewed in [Wolfner et al. 2005]). A third type of change is in the immune system of females. Mated females induce genes of the immune response (Lawniczak and Begun 2004; McGraw et al. 2004; Peng et al. 2005b; Mack et al. 2006), perhaps to protect their reproductive tracts or gametes/embryos from microbial attack, for example, by microbes introduced during mating. Finally, in arthropods at least, mating causes changes in overt behaviors, such as receptivity to remating and/or feeding (e.g., in Drosophila, reviewed in [Kubli 2003; Wolfner et al. 2005]; see also [Carvalho et al. 2006]). For example, mated lepidopteran females cease their production of pheromones that attract mates to them (e.g., Raina et al. 1994), and mated Drosophila females show a rejection response (kicking, extruding their ovipositor [Manning 1967]) if males attempt to mate with them. Feeding behaviors also change: mated Drosophila females eat more than unmated females (Carvalho et al. 2006), and mating stimulates female ticks to take blood meals (Weiss and Kaufman 2004). What sorts of molecules trigger these varied and fascinating changes, and how do they work with the female's molecules and physiology to accomplish this? How do molecules made in one animal (the male) that affect another (the female) evolve? The answers to these questions can impact not only our understanding of reproduction, chemical communication, and evolution but also potentially, practical applications in the regulation of reproduction.
Cues for postmating behaviors are particularly amenable to study in the fruit fly Drosophila melanogaster (In this article, “Drosophila” refers to D. melanogaster, unless otherwise noted). There is a wealth of genetic and molecular tools for this animal, including collections of available mutants, and methods to easily manipulate the expression of any desired gene (e.g., see http://flybase.org/). Recently, the complete genomic sequence of 12 species spanning the genus was released (Clark et al. 2007). Fruit flies are easy to grow in the laboratory. Drosophila mating behaviors are rapid and occur in a reproducible sequence (Hall 1994). This makes it easy for researchers to detect any perturbation caused by the genetic or other manipulations they have carried out with the flies. Drosophila is also a proved excellent model for discovery and analysis of genes of biomedical relevance. Drosophila is also a model insect, and thus, its analysis may inform methods to curtail the reproduction of insects that transmit diseases or are agricultural pests or, alternatively, to protect the reproductive capacity of beneficial insects.
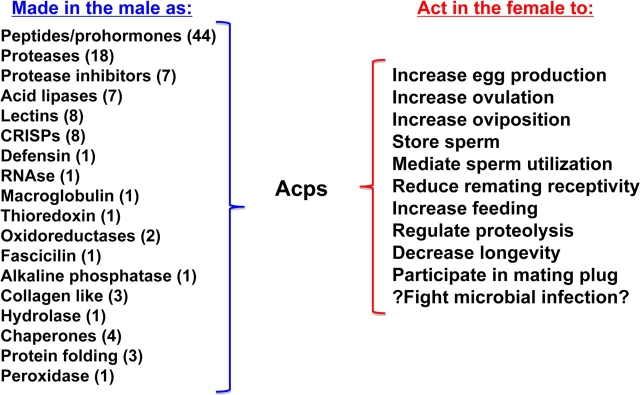
In principle, postmating changes in female Drosophila could be caused by nonmating interactions with males such as during courtship, by physical effects of the act of mating itself, or by molecules (in the seminal fluid or contact pheromones) or cells (e.g., sperm) transferred from the male to the female during mating. A variety of studies have been performed to identify the causes of the postmating changes in females. These include exposing females to males in the absence of mating or allowing females to mate with males that cannot provide sperm or, alternatively, components of seminal fluid made in the male fly's reproductive glands (such as the “Acp” proteins made in his accessory glands). These studies have shown that Acps from males are essential to trigger several postmating changes in the female (reviewed in Wolfner 1997; Chapman 2001; Kubli 2003; Wolfner et al. 2005). Specifically, Acps induce females to reject further mating attempts, to eat more, to increase egg production/laying, and to undertake the storage, retention, and release of sperm (“sperm management”; Figure 1).
Figure 1.
Acps and the postmating responses they regulate. At left are listed the protein categories into which many of the 112 Acps can be assigned; numbers in parentheses are the number of Acps in that category. At right are listed postmating responses that have been shown to be induced by Acps. [The “?” at “Fight microbial infection” refers to the fact that although some Acps have antimicrobial activity in vitro, and Acps can induce expression of antimicrobial peptides in mated females, systemic immunity did not seem to be affected by mating (ability to fight microbes locally in the reproductive tract was not been tested)]. Please see text for references. This figure appears in color in the online version of Journal of Heredity.
To understand how the Acp group of seminal proteins cause postmating behavioral changes in females, we need to know what those proteins are. Acps have been identified by several means. Differential cDNA hybridization, expressed sequence tag screens, and microarrays have identified genes expressed at high levels, or exclusively, in the male accessory gland (Schäfer 1986; DiBenedetto et al. 1987; Swanson et al. 2001; Chintapalli et al. 2007). Those predicted (or known) to encode secreted proteins, which can thus constitute part of the seminal fluid, are considered Acp genes. Proteomic screens have identified additional proteins made in male accessory glands or proteins transferred by males to females (Walker et al. 2006; Findlay et al. 2008). Together, these studies have identified 138 seminal proteins, including 112 predicted Acps (Ravi Ram and Wolfner 2007a), although this is likely an underestimate, since low-abundance transcripts/proteins could have been missed. BLAST searches, 3D-homology modeling, and threading of sequences to known protein structures give clues as to the biochemical function of Acps (Mueller et al. 2004), which have been verified biochemically in test cases. For example, the amino acid sequence of Acp62F threads to that of a secreted protease inhibitor. This threading predicts Acp62F's active site residue as typical of a trypsin inhibitor; in vitro studies have confirmed that Acp62F can inhibit trypsin (Lung et al. 2002). The results of sequence comparisons and structural predictions have shown that Acps fall into a number of protein classes (Figure 1). Most of these classes are found in seminal proteins across animals. Forty percent of Acps appear to be peptide hormones or prohormones (precursors of peptide hormones), likely to be triggers of physiological or behavioral changes in females. A quarter of Acps are proteolysis regulators, and several Acps are proteins in families predicted to bind to sperm based on studies in mammals (lectins, CRISPs; e.g., Ignotz et al. 2001; Busso et al. 2003; Gwathmey et al. 2006; Cohen et al. 2008; Da Ros et al. 2008). Additional Acps are in protein classes expected to exert protective effects on females’ reproductive tracts or the gametes and zygotes within them. Proteins in this class include predicted antimicrobial peptides and antioxidants (Lung et al. 2001; Mueller et al. 2004; Mueller et al. 2007). Additional seminal proteins include members of the odorant-binding protein class (Findlay et al. 2008). Antibody and protein-labeling studies have confirmed transfer of 52% of predicted Acps to females during mating (Monsma and Wolfner 1988; Heifetz et al. 2000; Ottiger et al. 2000; Ravi Ram et al. 2005; Findlay et al. 2008). Lack of evidence for transfer of the others may be due to sensitivity of detection and/or lack of antibody reagents.
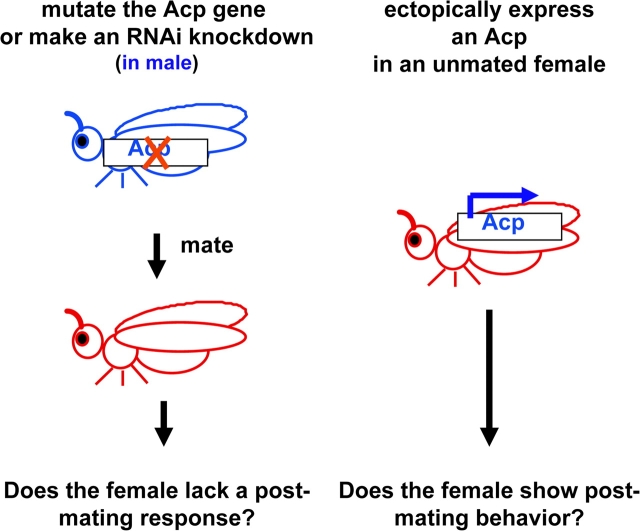
Although such sequence-based studies can predict biochemical functions for many Acps, to understand their true biological functions, one needs to use a genetic approach. Two complementary approaches are used (Figure 2). First, males can be generated that lack a given Acp. This can be done either by generating a mutation in the Acp gene or by RNA interference (RNAi). In the latter approach, transgenic males are generated that transcribe both strands of the Acp gene or express a hairpin RNA double stranded for the Acp gene. This can “knock down” the Acp protein's expression by ∼97% (Ravi Ram et al. 2006; Dietzl et al. 2007). Females are mated to males that lack individual Acps (or all Acps) and tested for postmating behaviors. Any behavior not shown by these females must have required the Acp(s) that was removed (Herndon and Wolfner 1995; Neubaum and Wolfner 1999a; Chapman et al. 2003; Liu and Kubli 2003; Wigby and Chapman 2005; Carvalho et al. 2006; Ravi Ram et al. 2006; Ravi Ram and Wolfner 2007b; Mueller et al. 2008; Wong et al. 2008a). In a converse approach, unmated females can be engineered to express a single Acp (or, in a nongenetic approach, injected with the pure Acp). If these females show any behaviors that are normally only seen postmating, it suggests that the tested Acp might play a role in inducing such behaviors in a normal mating (Chen et al. 1988; Aigaki et al. 1991; Lung et al. 2002; Carvalho et al. 2006; Mueller et al. 2007). However, caution must be exercised in interpreting results of ectopic expression. Phenotypes can reflect effects of the presence of higher levels, or unusual placement, of the Acp relative to those after a mating, and lack of phenotype could simply reflect the need for the Acp to be transferred with other male contributions during mating.
Figure 2.
Genetic tests to determine biological functions of Acps. The functions of Acps can be determined by knocking out their production in males (by RNAi or mutation) and then determining which postmating responses in those males’ mates are lacking. Alternatively, unmated females can be engineered to express individual Acps. If those females show responses like those of mated females, it suggests, though does not prove, that the ectopically expressed Acp may induce that response in a mating. This figure appears in color in the online version of Journal of Heredity.
Approximately 40 Acps have been tested by these methods, and additional Acps have been tested for allelic associations with sperm competition parameters (Clark et al. 1995; Fiumera et al. 2005, 2007). As described below, functions have been found for many of these Acps. In particular, one Acp (the sex peptide [SP]) causes both the increased feeding and decreased mating-receptivity behaviors. The SP and 2 other Acps induce aspects of egg-laying behavior. At least 6 Acps are involved in the storage, retention, and regulated release of sperm (sperm management). Other Acps affect nonbehavioral phenomena in mated females including proteolysis (2 Acps) and longevity/toxicity (4 Acps, including the SP). Several Acps induce the production of antimicrobial peptides (McGraw et al. 2004; Peng et al. 2005b; Mack et al. 2006), although studies of systemic immunity do not find a change in the ability of females to fight off infection by injected bacteria (local immunity in the reproductive tract has not been tested) (Fedorka et al. 2007; Wigby et al. 2008). An additional 3 Acps on their own can fight systemic infection on ectopic expression (Mueller et al. 2007), although whether this relates to an ability to guard against microbial infection after mating (e.g., in the reproductive tract) is unknown. Several additional Acps have been suggested, from association studies, to play roles in sperm competition.
Mutational, RNAi, ectopic expression, and injection experiments have shown that the 36–amino acid SP (Acp70A) induces mated females to feed, to reject mating attempts by subsequent males, and to increase their oogenic rate; it also decreases their longevity (Chen et al. 1988; Aigaki et al. 1991; Chapman et al. 2003; Liu and Kubli 2003; Wigby and Chapman 2005; Carvalho et al. 2006). Its mode of action is not yet known, but it is known to enter the circulatory system of the mated female (Pilpel et al. 2008) (as do about two-thirds of the Acps tested; they cross a special part of the reproductive tract wall to do this) (Monsma et al. 1990; Lung and Wolfner 1999; Ravi Ram et al. 2005). From the circulatory system, the SP has access to the neural–endocrine system and could thus exert its effects on behavior via any of those targets. In fact, it has been shown to increase synthesis of juvenile hormone B3 (in in vitro experiments) (Moshitzky et al. 1996) and to bind to multiple targets in the nervous system (Ottiger et al. 2000). Recently, a G-protein–coupled receptor for SP has been reported (Yapici et al. 2008). This receptor, SPR, is detected in the sperm storage organs and also in the nervous system. Genetic experiments have shown that SPR's function is needed in particular neurons (those that express the sex-determining protein Fruitless) in order for the SP to affect behavior. This is the first receptor for any Acp, and its future study will be important in determining how the SP causes its effects. SP's action has one additional feature. Peng et al. (2005a) have shown that the SP binds to sperm stored within the mated female; this association allows the SP to persist within the mated female for longer than most Acps. The stored SP is gradually cleaved from the sperm, releasing the SP, and allowing it to enter the circulatory system. This storage and release of SP allows postmating behavioral changes to persist for many days after mating.
Other Acps are needed to increase the production and ovulation of eggs by the female and the transit and storage of sperm in the mated female's reproductive tract (Neubaum and Wolfner 1999a; Heifetz et al. 2000; Ravi Ram and Wolfner 2007b). As the actions of these Acps are becoming understood, it is increasingly clear that these actions occur in a sequence of events that involves a molecular or physiological cross-talk (or ballet) between male and female molecules and cells. This ballet, in which one partner takes a step, then the other, then the first, etc. toward a shared goal, will be discussed in the next sections.
Ballet
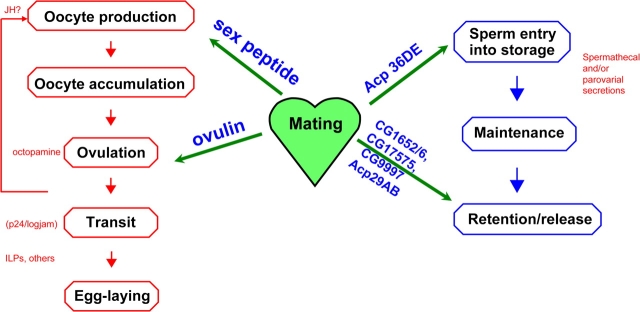
Crosstalk occurs between female and male during sperm management. Like other behaviors, sperm management involves a series of sequential steps (Figure 3) (Bloch Qazi et al. 2003). Sperm must reach and enter storage, they must be retained there but also be released at a rate appropriate to fertilize eggs, and they must be maintained as viable while in storage. Drosophila females store sperm in 2 types of sperm storage organ (the coiled seminal receptacle and a pair of spermathecae). They store ∼1000 sperm (approximately a quarter of what they receive from their mate) for up to ∼2 weeks (Kaufmann and Demerec 1942; Gilbert 1981). Sperm are used very efficiently in D. melanogaster; approximately half of the sperm that are stored eventually fertilize eggs.
Figure 3.
Stepwise pathways, Acps, and female products regulate egg production and sperm storage. On the left side are the separate steps in the egg production process. Several female molecules have been associated with these steps, such as those shown here in red (see text for references). On the right are the separate steps in sperm storage. Spermathecal and parovarial secretions (red) have been shown to be needed for storage of sperm, although this has not been narrowed to specific molecules. In the center of the figure are shown, in blue, Acps that have been associated with specific steps in the egg production process or sperm storage. Please see text for references. This figure appears in color in the online version of Journal of Heredity.
The reproductive tract of an unmated Drosophila female presents barriers to the storage of sperm. The tract is tightly closed and contains a flap of tissue that covers the openings to the sperm-storage organs (Adams and Wolfner 2007). Both of these situations, particularly in light of the large size of Drosophila sperm (e.g., Pitnick et al. 1995), could in principle make it impossible for sperm to reach and/or enter storage. Mating causes conformational changes in the female's reproductive tract that appear likely to facilitate the entry of sperm into storage (Adams and Wolfner 2007). Specifically, the lumen of the reproductive tract opens, and the flap that covers the opening to the sperm storage organs is pulled aside by the conformational change. As with other aspects of reproductive behavior, these conformational changes occur in a reproducible sequence, in this case composed of 10 sequential and distinct stages.
The conformational changes must be the consequences of changes in the contraction and relaxation of the reproductive tract musculature. Two types of data suggest that these mating-induced changes occur by modulating molecules that already preexist in females; in other words, that the female reproductive tract is “poised” to respond to Acps. First, transcriptome studies of whole females and of the lower reproductive tract show only small-magnitude changes during the time that the conformational changes are occurring (McGraw et al. 2004; Mack et al. 2006). Second, the reproductive tract is innervated, and vesicles can be seen along its length in mature but unmated female Drosophila (Heifetz and Wolfner 2004). These vesicles contain a variety of neuromodulators whose release, in theory, could prompt changes in muscle contractile state. Mating causes regulated release and reuptake of the contents of these vesicles, and Acps mediate some of the vesicle release. The lower reproductive tract (the part that undergoes the conformational changes just described) shows increased vesicle release at 20–90 min postmating, during the time when this region is undergoing the shape changes. Future studies are needed to identify which molecules are released and whether and how they prompt the muscle contractions/relaxations.
Acps are necessary for the conformational changes in the lower reproductive tract and for release of some vesicle contents (Heifetz and Wolfner 2004; Adams and Wolfner 2007). If a female is mated to a male lacking Acps, her reproductive tract fails to proceed through the conformational stages, arresting at a very early conformation. (Interestingly, sperm themselves are not required to prompt the conformational changes that accompany their storage; in the absence of sperm, the reproductive tract proceeds through its normal sequence of stages, at the normal rate.) Acps also regulate the release of some of the vesicles along the reproductive tract, including in the lower reproductive tract, in this case. It is tempting to hypothesize that they regulate the muscle contraction this way.
As noted above, 6 Acps thus far have been discovered to be essential for aspects of sperm management (Neubaum and Wolfner 1999a; Ravi Ram and Wolfner 2007b; Wong et al. 2008a). Of the 6 Acps, only the glycoprotein Acp36DE is necessary for the first step (sperm entry into storage); the other 5 Acps control the later steps of sperm retention or release. Consistent with the model that the conformational changes noted above facilitate or allow sperm storage, Acp36DE is necessary for progression of the conformational changes (Avila FW and Wolfner MF, submitted). Future studies will address whether Acp36DE exerts its function on sperm storage by regulating vesicle release in the lower reproductive tract.
Studies of Acp action in sperm management have begun to uncover an interdependent pathway of interactions between females and males. This ballet begins when male molecules (Acps) regulate the release of vesicles in the female's reproductive tract (Heifetz and Wolfner 2004). The female reproductive tract presumably responds by muscle contractions/relaxations that cause conformational changes that expose the openings to the sperm storage organs (Adams and Wolfner 2007). This in turn allows the male's sperm to enter storage. Later, once sperm are in storage, contributions from the female (likely, secretions of the spermathecae and parovaria [Anderson 1945; Allen and Spradling 2008; Prokupek et al. 2008]) are required for sperm to fertilize eggs (it is as yet not known whether those molecules act to keep stored sperm alive and capable of fertilizing eggs or whether they modulate the release or retention of sperm). Male-derived Acps are also necessary at this stage, including the Acps needed to release sperm from storage (CG17575, CG9997, CG1652, CG1656 [Ravi Ram and Wolfner 2007b]) and Acp29AB, which is required for retention of sperm in storage (Wong et al. 2008a). Preliminary studies suggest that the 4 sperm-release Acps function within a single interdependent pathway, controlling each other's transfer to or stability in females, or ability to associate with sperm (Ravi Ram K and Wolfner MF, submitted). Future studies are required to characterize this pathway and how it interfaces with the female factors needed for the maintenance and release/retention of stored sperm.
Another example of a molecular ballet between females and males occurs in the regulation of egg-laying behavior in mated female Drosophila. As with sperm management, egg-laying behavior involves a sequence of distinct steps (Figure 3) (Heifetz et al. 2000; Bloch Qazi et al. 2003): eggs are made in the ovary, ovulated, moved through the oviducts to the uterus where they will be fertilized, and then deposited onto the substratum. Analogous to sperm management behaviors, female-derived and male-derived molecules regulate steps in this behavioral sequence, although how they interact with one another is not yet known; it is an area of active investigation. A partial list of female-derived molecules that participate in this sequence is: receptors for Acps (to date, only one, the SP receptor mentioned above, has been reported [Yapici et al. 2008]), juvenile hormone (a hormone that is needed for oogenesis [Riddiford 1993]), octopamine (which is needed for ovulation [Han et al. 1998; Monastirioti 2003; Cole et al. 2005]), logjam (a vesicle transport proteins in the p24 family that is required for movement of eggs down the reproductive tract [Carney and Taylor 2003]), and insulin-like peptides (which are part of the sensing system by which a female chooses egg-deposition sites) (Yang et al. 2008). Male-derived molecules include 2 Acps that have been shown to regulate particular steps in the pathway (Herndon and Wolfner 1995; Heifetz et al. 2000; Chapman et al. 2003; Liu and Kubli 2003) and a third Acp, a peptide, whose specific action is not yet known (Ravi Ram and Wolfner 2007b). The SP increases the rate of oogenesis (Soller et al. 1997, 1999). Because SP can elevate levels of the juvenile hormone B3 in Drosophila tissues in vitro, it is tempting to speculate that at least some of SP's stimulation of oogenesis derives from this effect (Moshitzky et al. 1996). The prohormone ovulin regulates the ovulation step (release of eggs from the ovary) (Monsma and Wolfner 1988; Heifetz et al. 2000). It is not yet known how it does so. After mating, ovulin targets to the base of the ovary (Heifetz et al. 2000), where it could potentially act directly to promote ovulation, perhaps by relaxing the musculature in this region to allow the large egg to move out of the ovary. Ovulin also enters the circulatory system of the female (Monsma et al. 1990), so it could also (or instead) act indirectly via neural or endocrine targets outside of the reproductive tract. Determining ovulin's site and nature of action is another important area of ongoing investigation.
Molecular studies of ovulin have uncovered another ballet. This ovulation-stimulating Acp is made in the male as a glycoprotein that is transferred intact to the female during mating (Monsma et al. 1990). Once inside the female, however, ovulin is cleaved in a sequential series of steps in a manner consistent with liberating bioactive peptides from a prohormonal precursor. Ectopic expression of ovulin's cleavage products in unmated females showed that each of the 2 final cleavage products can, on its own, stimulate ovulation (Heifetz et al. 2005). However, since full-length ovulin also can stimulate ovulation (Heifetz et al. 2005), at present we do not know whether the biological function of the cleavage is to liberate bioactive products from a precursor (as we believe, given the cleavage's site-specificity) or to control the stability of ovulin within the female's reproductive tract. In either case, the nature of the cleavage pathway is important, and thus we investigated it further.
In theory, a simple way to restrict ovulin cleavage to the mated female fly would be if the female's reproductive tract were the location for the entire proteolytic pathway that cleaves ovulin. If this were the case, then ectopic expression of ovulin within the reproductive tract of an unmated female should result in the protein's cleavage. However, it does not (Park and Wolfner 1995). Therefore, male contributions are also required for this cleavage, suggesting that there may again a crosstalk between males and females, this time at the molecular level, revolving around ovulin. Experiments in which ovulin cleavage was assessed in females that received normal or decreased levels of Acps showed that the male contribution was likely an Acp: cleavage was poor in females that received low levels of Acps (Heifetz et al. 2005). Therefore, to identify male contributions to ovulin cleavage, individual Acps were tested for roles in this process.
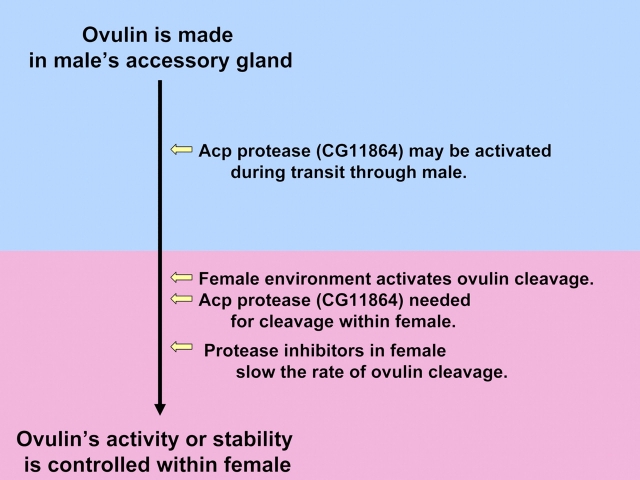
Approximately 25% of Acps are predicted proteolysis regulators (Mueller et al. 2004; Ravi Ram and Wolfner 2007a), and all those that have been tested are transferred to females during mating (Lung and Wolfner 1999; Ravi Ram et al. 2005; Findlay et al. 2008). Thus, these were candidates for the male contribution to ovulin cleavage. To test genetically whether any of the male-encoded proteolysis regulators were required for ovulin cleavage within mated females, males that had been knocked down (or knocked out) for a given proteolysis regulator were mated to females, and ovulin cleavage was assessed in those females. If ovulin cleavage required the presence of a particular Acp, the cleavage should be blocked or its rate should be changed in mates of males knocked down for that Acp. Of 12 proteolysis regulators tested, 2 affected ovulin cleavage: the predicted protease CG11864 was necessary for ovulin cleavage within females (Ravi Ram et al. 2006), and the trypsin inhibitor Acp62F controls the rate of ovulin proteolysis inside mated females (Mueller et al. 2008). CG11864 is a member of the astacin family of metalloproteases. Astacin family members are made as inactive zymogens (pro-proteases). They become active when a short pro-peptide sequence is cleaved from them (Bode et al. 1992). CG11864 contains a predicted pro-peptide. In the male accessory gland, where CG11864 and ovulin are both made, CG11864 appears to be in the pro-protein form. While in transit through the male reproductive tract, en route to the female, the molecular weight of CG11864 drops, consistent with removal of an inhibitory prosequence (Ravi Ram et al. 2006).
Despite the possible activation of CG11864 while in the mating male, no cleavage of ovulin is observed in the male. This suggests that some condition, cofactor, or contributing protease present in the female might be essential for cleavage of ovulin. To test if a female proteolysis regulator is necessary for, or modulates, ovulin cleavage, we generated females knocked down individually for proteases or protease inhibitors that are expressed in the female reproductive tract. To date, no female protease has been shown to contribute to ovulin cleavage, but at least one proteolysis regulator made by females controls the rate of ovulin cleavage within females (Sirot LK, Wong A, and Wolfner MF, unpublished data).
Thus, these studies have identified a proteolytic pathway that starts in one organism (the male) and ends in another (the female) and involves contributions by both organisms (Figure 4). Specifically, a target protein (i.e., ovulin) is made in the male's accessory gland, along with an inactive form of a protease needed for its cleavage (CG11864) and a protease inhibitor that controls the rate of cleavage (Acp62F). During transit to the females, CG11864 is cleaved, presumably to activate this astacin family protease; but cleavage of ovulin only occurs once CG11864 and ovulin have entered the female. The female environment (including potentially other proteases) enables ovulin cleavage, and a female proteolysis regulator (along with the male derived Acp62F) controls its rate. This joint pathway thus either controls the production of bioactive cleavage products of ovulin or regulates the stability and availability of ovulin within the female. Future research will fully flesh out the cleavage pathway, including the roles of female and male players (and targets) within it.
Figure 4.
The pathway for ovulin proteolysis begins in the male and ends in the female. This figure appears in color in the online version of Journal of Heredity.
Taken together, the studies described above indicate a nuanced ballet operating between female and male molecules and cells in Drosophila reproduction. For sperm storage, conformational changes in the female's reproductive tract are induced by male-derived Acps, likely by controlling release of preexisting female-derived neuromodulators. The subsequent retention, release, or viability of sperm requires female-derived spermathecal factors and several Acps. In the egg-production process, although the female makes the eggs, an Acp from the male is thought to raise her juvenile hormone levels, which would increase her oogenic rate. Ovulation of those eggs requires octopamine made by females and is stimulated by the male-derived Acp ovulin. Ovulin itself is cleaved in a pathway that requires at least one male-derived protease in the context of the female reproductive tract. The impression one is left with is that male and female components work together to facilitate the changes in the female that will ultimately promote reproduction by the mated pair.
The balletic interactions described above indicate that at the mechanistic level, male and female molecules and/or cells can work synergistically. And yet, that this occurs between 2 animals with differing “interests” at stake (despite the shared goal of reproduction) leads to the possibility of a conflict, or battle, at a different—evolutionary—level. Perhaps this can explain some otherwise perplexing findings that appeared as the mechanistic ballet has been dissected. For example, although the female is required for cleavage of ovulin, recent studies show that she contains at least one molecule that slows this cleavage rather than facilitating it. In another, hypothetical, example, the conformational changes that correlate with and may facilitate the storage of sperm might be sensitive to allelic forms of their triggering Acps, allowing choice by the female in terms of efficiency of storage of her mate's sperm. Indeed, as the evolutionary characteristics of Acps were examined (below), their results suggested that the view of a purely synergistic interaction between the sexes, although seen in several cases at the mechanistic level, is far too simple.
Battle
One might expect that molecules critical for reproductive success and that function in such highly tuned synergistic pathways would be constrained from change during evolution. Yet in contrast, many Acps show signs of having evolved rapidly, with positive selection having driven rapid change in regions of their amino acid sequence (reviewed in Civetta and Singh 1998; Swanson and Vacquier 2002; Clark et al. 2006; Panhuis et al. 2006; Ravi Ram and Wolfner 2007a). Rapidly evolving Acps include ones with known important functions, such as ovulin (one of the fastest-evolving genes in Drosophila [Aguadé et al. 1992; Tsaur and Wu 1997; Tsaur et al. 1998]) and CG9997 (Wong et al. 2008b), one of the 4 genes noted above that regulate sperm release from storage. Rapid evolution of the molecular was initially detected by amino acid comparisons between closely related species. Such studies between sibling species indicated that ∼20% of Acps have evolved rapidly (Swanson et al. 2001; Mueller et al. 2005), and the effect was evident also across the entire melanogaster subgroup (Haerty et al. 2007).
Such rapid amino acid change could conceivably change whole suites of Acps as species diverge. One can test this by taking advantage of the availability of complete genomic sequence for 12 species of Drosophila that span the genus (Clark et al. 2007). Using a reciprocal BLAST approach, we searched for orthologs of the known D. melanogaster Acps across the genus Drosophila. For D. melanogaster genes in aggregate (and even for genes expressed in their female reproductive tracts), it is easy to find orthologs in the other Drosophila species (∼90% of the D. melanogaster genes have detectable homologues in the other 11 species). In stark contrast, the number of melanogaster Acps for which a ortholog can be detected falls rapidly as one examines Drosophila species at increasing evolutionary distance from D. melanogaster. In comparison to Hawaiian or cactophilic Drosophila (40 million years of separation), only 21% of melanogaster Acps have detectable orthologs (Haerty et al. 2007). The simplest explanation is that each species or species group has a characteristic set of Acps, but the specific Acps differ among species or species groups. Support from this comes from studies of Acp genes in cactophilic Drosophila (Wagstaff and Begun 2005) and in the noncactophilic sibling species D. melanogaster and Drosophila simulans: even between close relatives that can mate and produce progeny (simulans and melanogaster), Acp genes specific to one species or the other have been found (Kern et al. 2004; Mueller et al. 2005).
Several hypotheses have been proposed to explain the rapid sequence evolution and apparent gene loss/gain seen for Drosophila Acps. These models are not mutually exclusive. First, the “interests” of female and male Drosophila may differ, especially in situations in which females mate with multiple males. Although the reproductive success of the mating pair benefits both of its members, the way in which that success is achieved may be more advantageous (or disadvantageous) to one sex than the other (Rice 1996; reviewed in Swanson and Vacquier 2002; Panhuis et al. 2006). For example, consider the stimulation by male-derived Acps of egg production in female Drosophila. Because egg production is energetically demanding, it would seem advantageous for females to have evolved to couple an increase egg production to mating, so that they only make large numbers of eggs when they contain stored sperm with which to fertilize those eggs. Viewed from this perspective, female flies could be seen to be using males as a hormone-delivery system, so that egg production only increases under the appropriate condition (i.e., after mating). Although it is also clearly to the benefit of males to increase females’ egg production above the basal premating level, one could imagine that it could be advantageous to males to increase their mates’ production beyond a level that was optimal for females, leading to more progeny in the short term, at a cost to female survival (causing females to expend too much of their resources on egg production, for example). The interests of females might be at odds with such high egg production that survival was impaired, since longer life would give them the opportunity to remate with a second male who might be better than the first male. In such a scenario, a male that produces a high-stimulating egg-inducing Acp might initially have higher than normal reproductive success, as would his male progeny that inherited this trait; but over time, selection could produce females that had become resistant to the male's excessive stimulation of egg production. At that point, the advantage of that original Acp mutation would be lost, but if a new Acp mutation arose that again stimulated increased egg production in females, that would be selected for as advantageous to males—until, again, females evolved resistance. This sort of conflict scenario could drive the rapid sequence evolution of Acps and theoretically the appearance of nonorthologous Acps that can accomplish the same function (or the presence of different Acps with redundant function). There is some evidence for such scenarios. For example, D. melanogaster females that have mated multiply die sooner than those that have not mated (Fowler and Partridge 1989), and a significant contributor to this cost-of-mating is Acps: females that do not receive Acps from their mates live longer than females mated to normal males (Chapman et al. 1995). The SP is one agent of this cost of mating (Wigby and Chapman 2005), although how it mediates this effect is unknown. SP and 3 other Acps have separately been shown to be toxic to Drosophila when expressed (ectopically) at high levels (Lung et al. 2002; Mueller et al. 2007), suggesting that they have a toxicity that could contribute to the premature death of the mated female. The existence of the cost of mating is suggestive of a “battle” between the sexes, in which Acps participate. Two other types of conflict that have also been suggested to drive rapid change in sequence or spectrum of Acps stem from the storage of sperm by mated Drosophila females and the fact that these females still do remate (even though Acps decrease the frequency of remating). That remated females can contain sperm/ejaculates from more than one male provides opportunities for sperm/ejaculates of different males to compete for fertilization opportunities (sperm competition [Parker 1970]) or for the female to exert preference (cryptic female choice [Eberhard 1996]) as to which sperm are being stored or utilized. Any of these types of conflict could result in rapid evolution of molecules, such as Acps, that are involved in these postmating responses.
Commonality, in the Face of Battle
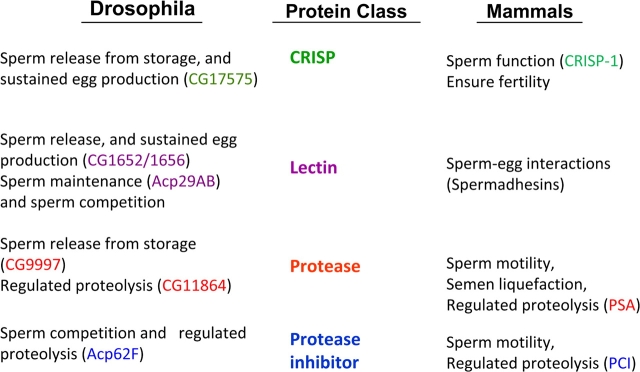
The presence of this rapid evolution, and the consequent suggestion of a battle, is intriguing in light of another feature of Acps. Despite the rapid sequence evolution and the apparent use of different suites of Acps, the molecular classes represented among Acps are conserved in seminal secretions across animals (Figure 5) (Mueller et al. 2004; reviewed in Poiani 2006). For example, proteolysis regulators are present in seminal fluid of mammals (e.g., He et al. 1999; Malm et al. 2000; Murer et al. 2001) as well as Drosophila and have been shown to play important roles in each. For example, in primates, the protease PSA is involved in liquefaction of semen (suggested to play a role in sperm storage or sperm competition, Kise et al. 1996; Peter et al. 1998; Robert and Gagnon 1999; Jensen-Seaman and Li 2003), and in Drosophila, the predicted serine protease CG9997 is one of the Acps that regulates release of stored sperm in mated females (Ravi Ram and Wolfner 2007b). Protective molecules in the same classes (antimicrobial, antiredox damage), lectins, and CRISPs are among other conserved classes. Thus, it appears that functions carried out by seminal proteins are needed across internally fertilizing animals, but different gene products have been co-opted to carry out those functions in different lineages. An intriguing future direction will be to determine how similar/dissimilar these molecules are in terms of their exact molecular and physiological functions and the evolutionary forces that maintained similar classes of molecules while selecting for different members of these classes in different lineages.
Figure 5.
Functions of Acps and mammalian seminal proteins that fall into common conserved classes. References: He et al. (1999); Malm et al. (2000); Mueller et al. (2004, 2008); O'Rand et al. (2006); Poiani (2006); Da Ros et al. (2007, 2008); Ravi Ram and Wolfner (2007a); Wong et al. (2008a). This figure appears in color in the online version of Journal of Heredity.
The conservation of overall classes, and of some particular members of those classes, suggests that despite the rapid evolution of some individual Acps or of suites of Acps, Drosophila Acps could nevertheless provide information for various practical applications. In mammals, including humans, for example, studies have shown that seminal proteins exert important enhancing effects on fertility (reviewed in Robertson et al. 2003). Physiological, biochemical, or behavioral functions dissected in Drosophila by virtue of its genetic tools can provide a framework and suggested pathways to test for action of seminal proteins in mammals and other animals. Flies could, in principle, be used as a sort of genetic test tube to determine some of the biochemical functions of the non-Drosophila seminal proteins by determining the effects of ectopic expression of those proteins in D. melanogaster. Another form of practical application is to use Drosophila Acps as a guide for the identity and function of important reproductive proteins in other insects. Knowledge of the identity and function of these proteins can be important in designing ways to promote the reproduction of beneficial insects and to control the reproduction of insect vectors of disease or of agricultural pests. Toward these ends, seminal proteins have been identified in several insects, including honeybees (which falls in to the beneficial class) (Collins et al. 2006), Anopheles gambiae, and Aedes aegypti mosquitoes (which transmit malaria and dengue and yellow fevers, respectively) (Dottorini et al. 2007; Sirot et al. 2008), medflies (an agricultural pest) (Davies and Chapman 2006), crickets (Andres et al. 2006; Braswell et al. 2006), and also in other arthropods, such as ticks (Weiss and Kaufman 2004). Again, Drosophila as a model, or assay system, can provide information on how Acps and other seminal proteins act and methods and paradigms useful for discovering the functions of the seminal proteins in these other important insects.
In conclusion, the class of Drosophila seminal proteins known as Acps has allowed discovery of a molecular interplay between male and female that continues even after the act of mating has completed. A dialogue of synergistic interactions occurs between male-derived seminal proteins, on the one hand, and molecules and tissues within the female who received them, on the other. This ballet is evident during sperm storage, in the induction of conformational changes by male molecules in a female reproductive tract that is poised to respond to those molecules. Retention/release of stored sperm also requires male and female contributions. Ballets occur in egg production as well, both in provision by the male of peptides and prohormones that stimulate oogenesis (likely through the female's neuro-endocrine system) and in the proteolytic cascade, initiated in males and completed only in the female environment, that cleaves an ovulation regulator. Future studies of these ballets will provide information about how molecules and mechanisms interact, including between individuals, to facilitate reproduction. These studies, in turn, are expected to provide information useful in consideration of the reproduction of insects of biomedical or agricultural importance. Yet, superimposed on this proximate ballet is a battle on an evolutionary timescale between the differing “interests” of males and of females and also occasioned by sperm competition and cryptic female choice that may have resulted in the very rapid change seen in the sequences and suites of seminal proteins even among closely related insects. Future studies can use Acp seminal proteins as a model to probe, on the one hand, the forces that can result in these rapid changes and, on the other, the mechanisms of gene loss/gain that result in conservation of seminal protein classes in the background of this rapid sequence evolution.
Funding
Our work on Drosophila Acps is supported by National Institutes of Health (grant HD038921), and was initiated with support from the National Science Foundation.
Acknowledgments
I am very grateful to the American Genetic Association and its current President Trudy Mackay for the honor to present the Wilhelmine Key Lecture that forms the basis for this article. It is impossible to fully express my gratitude to the members of my laboratory, past and present, for their elucidation of the identity, mechanism, and evolution of Drosophila Acps, described in this paper, and for the fun and fascination of doing those studies together. I also greatly appreciate their intellectual input into the studies (and figures) in this paper. Similar thanks extend to our numerous collaborators. I thank A. Clark for the title phrase “Battle and Ballet.” I am very grateful to L. Sirot, A. Wong, and K. Ravi Ram for their helpful advice in the development of this article, to them and C. Chow, B. LaFlamme, F. Avila, and J. Sitnik for comments on the final manuscript, and to Dr Ravi Ram for Figure 5.
References
- Adams EM, Wolfner MF. Seminal proteins but not sperm induce morphological changes in the Drosophila melanogaster female reproductive tract during sperm storage. J Insect Physiol. 2007;53:319–331. doi: 10.1016/j.jinsphys.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguadé M, Miyashita N, Langley C. Polymorphism and divergence in the Mst26A male accessory gland gene region in Drosophila. Genetics. 1992;132:755–770. doi: 10.1093/genetics/132.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigaki T, Fleischmann I, Chen PS, Kubli E. Ectopic expression of sex peptide alters reproductive behavior of female D. melanogaster. Neuron. 1991;7:557–563. doi: 10.1016/0896-6273(91)90368-a. [DOI] [PubMed] [Google Scholar]
- Allen AK, Spradling AC. The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development. 2008;135:311–321. doi: 10.1242/dev.015156. [DOI] [PubMed] [Google Scholar]
- Anderson RC. A study of the factors affecting fertility of lozenge females of Drosophila melanogaster. Genetics. 1945;30:280–296. doi: 10.1093/genetics/30.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres JA, Maroja LS, Bogdanowicz SM, Swanson WJ, Harrison RG. Molecular evolution of seminal proteins in field crickets. Mol Biol Evol. 2006;23:1574–1584. doi: 10.1093/molbev/msl020. [DOI] [PubMed] [Google Scholar]
- Baer B, Boomsma JJ. Mating biology of the leaf-cutting ants Atta colombica and A. cephalotes. J Morphol. 2006;267:1165–1171. doi: 10.1002/jmor.10467. [DOI] [PubMed] [Google Scholar]
- Bloch Qazi MC, Heifetz Y, Wolfner MF. The developments between gametogenesis and fertilization: ovulation and female sperm storage in Drosophila melanogaster. Dev Biol. 2003;256:195–211. doi: 10.1016/s0012-1606(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Bode W, Gomis-Ruth FX, Huber R, Zwilling R, Stocker W. Structure of astacin and implications for activation of astacins and zinc-ligation of collagenases. Nature. 1992;358:164–167. doi: 10.1038/358164a0. [DOI] [PubMed] [Google Scholar]
- Braswell WE, Andres JA, Maroja LS, Harrison RG, Howard DJ, Swanson WJ. Identification and comparative analysis of accessory gland proteins in Orthoptera. Genome. 2006;49:1069–1080. doi: 10.1139/g06-061. [DOI] [PubMed] [Google Scholar]
- Busso D, Cohen DJ, Da Ros V, Fissore R, Cuasnicu PS. Studies on the participation of epididymal sperm protein DE/CRISP-1 in egg activation. Cell Mol Biol. 2003;49:407–412. [PubMed] [Google Scholar]
- Carney GE, Taylor BJ. logjam encodes a predicted EMP24/GP25 protein that is required for Drosophila oviposition behavior. Genetics. 2003;164:173–186. doi: 10.1093/genetics/164.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho GB, Kapahi P, Anderson DJ, Benzer S. Allocrine modulation of appetite by the sex peptide of Drosophila. Curr Biol. 2006;16:692–696. doi: 10.1016/j.cub.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T. Seminal fluid-mediated fitness traits in Drosophila. Heredity. 2001;87:511–521. doi: 10.1046/j.1365-2540.2001.00961.x. [DOI] [PubMed] [Google Scholar]
- Chapman T, Bangham J, Vinti G, Seifried B, Lung O, Wolfner MF, Smith HK, Partridge L. The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc Natl Acad Sci USA. 2003;100:9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- Chen BX, Yuen ZX, Pan GW. Semen-induced ovulation in the bactrian camel (Camelus bactrianus) J Reprod Fertil. 1985;74:335–339. doi: 10.1530/jrf.0.0740335. [DOI] [PubMed] [Google Scholar]
- Chen PS, Stumm-Zollinger E, Aigaki T, Balmer J, Bienz M, Bohlen P. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell. 1988;54:291–298. doi: 10.1016/0092-8674(88)90192-4. [DOI] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- Civetta A, Singh RS. Sex-related genes, directional sexual selection, and speciation. Mol Biol Evol. 1998;15:901–909. doi: 10.1093/oxfordjournals.molbev.a025994. [DOI] [PubMed] [Google Scholar]
- Clark AG, Aguadé M, Prout T, Harshman LG, Langley CH. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics. 1995;139:189–201. doi: 10.1093/genetics/139.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AG, et al. Evolution of genes and genomes on the Drosophila phylogeny. Nature. 2007;450:203–218. doi: 10.1038/nature06341. [DOI] [PubMed] [Google Scholar]
- Clark NL, Aagaard JE, Swanson WJ. Evolution of reproductive proteins from animals and plants. Reproduction. 2006;131:11–22. doi: 10.1530/rep.1.00357. [DOI] [PubMed] [Google Scholar]
- Cohen DJ, Busso D, Da Ros V, Ellerman DA, Maldera JA, Goldweic N, Cuasnicu PS. Participation of cysteine-rich secretory proteins (CRISP) in mammalian sperm-egg interaction. Int J Dev Biol. 2008;52:737–742. doi: 10.1387/ijdb.072538dc. [DOI] [PubMed] [Google Scholar]
- Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. J Biol Chem. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- Collins AM, Caperna TJ, Williams V, Garrett WM, Evans JD. Proteomic analyses of male contributions to honey bee sperm storage and mating. Insect Mol Biol. 2006;15:541–549. doi: 10.1111/j.1365-2583.2006.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Ros V, Busso D, Cohen DJ, Maldera J, Goldweic N, Cuasnicu PS. Molecular mechanisms involved in gamete interaction: evidence for the participation of cysteine-rich secretory proteins (CRISP) in sperm-egg fusion. Soc Reprod Fertil Suppl. 2007;65:353–356. [PubMed] [Google Scholar]
- Da Ros VG, Maldera JA, Willis WD, Cohen DJ, Goulding EH, Gelman DM, Rubinstein M, Eddy EM, Cuasnicu PS. Impaired sperm fertilizing ability in mice lacking cysteine-rich secretory protein 1 (CRISP1) Dev Biol. 2008;320:12–18. doi: 10.1016/j.ydbio.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SJ, Chapman T. Identification of genes expressed in the accessory glands of male Mediterranean fruit flies (Ceratitis capitata) Insect Biochem Mol Biol. 2006;36:846–856. doi: 10.1016/j.ibmb.2006.08.009. [DOI] [PubMed] [Google Scholar]
- den Boer SPA, Boomsma JJ, Baer B. Seminal fluid enhances sperm viability in the leafcutter ant Atta colombica. Behav Ecol Sociobiol. 2008;62:1843–1849. [Google Scholar]
- DiBenedetto AJ, Lakich DM, Kruger WD, Belote JM, Baker BS, Wolfner MF. Sequences expressed sex-specifically in Drosophila melanogaster adults. Dev Biol. 1987;119:242–251. doi: 10.1016/0012-1606(87)90225-9. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Dottorini T, Nicolaides L, Ranson H, Rogers DW, Crisanti A, Catteruccia F. A genome-wide analysis in Anopheles gambiae mosquitoes reveals 46 male accessory gland genes, possible modulators of female behavior. Proc Natl Acad Sci USA. 2007;104:16215–16220. doi: 10.1073/pnas.0703904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard WG. Female control: sexual selection by cryptic female choice. Princeton (NJ): Princeton University Press; 1996. [Google Scholar]
- Fedorka KM, Linder JE, Winterhalter W, Promislow D. Post-mating disparity between potential and realized immune response in Drosophila melanogaster. Proc Biol Sci. 2007;274:1211–1217. doi: 10.1098/rspb.2006.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay GD, Yi X, Maccoss MJ, Swanson WJ. Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS Biol. 2008;6:e178. doi: 10.1371/journal.pbio.0060178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumera AC, Dumont BL, Clark AG. Sperm competitive ability in Drosophila melanogaster associated with variation in male reproductive proteins. Genetics. 2005;169:243–257. doi: 10.1534/genetics.104.032870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiumera AC, Dumont BL, Clark AG. Associations between sperm competition and natural variation in male reproductive genes on the third chromosome of Drosophila melanogaster. Genetics. 2007;176:1245–1260. doi: 10.1534/genetics.106.064915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler K, Partridge L. A cost of mating in female fruitflies. Nature. 1989;338:760–761. [Google Scholar]
- Gilbert DG. Ejaculate esterase and initial sperm use by female Drosophila melanogaster. J Insect Physiol. 1981;27:641–650. [Google Scholar]
- Gwathmey TM, Ignotz GG, Mueller JL, Manjunath P, Suarez SS. Bovine seminal plasma proteins PDC-109, BSP-A3, and BSP-30-kDa share functional roles in storing sperm in the oviduct. Biol Reprod. 2006;75:501–507. doi: 10.1095/biolreprod.106.053306. [DOI] [PubMed] [Google Scholar]
- Haerty W, Jagadeeshan S, Kulathinal RJ, Wong A, Ravi Ram K, Sirot LK, Levesque L, Artieri CG, Wolfner MF, Civetta A, et al. Evolution in the fast lane: rapidly evolving sex-related genes in Drosophila. Genetics. 2007;177:1321–1335. doi: 10.1534/genetics.107.078865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JC. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- Han KA, Millar NS, Davis RL. A novel octopamine receptor with preferential expression in Drosophila mushroom bodies. J Neurosci. 1998;18:3650–3658. doi: 10.1523/JNEUROSCI.18-10-03650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Lin YL, Liu YX. Functionally inactive protein C inhibitor in seminal plasma may be associated with infertility. Mol Hum Reprod. 1999;5:513–519. doi: 10.1093/molehr/5.6.513. [DOI] [PubMed] [Google Scholar]
- Heifetz Y, Lung O, Frongillo EA, Wolfner MF. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr Biol. 2000;10:99–102. doi: 10.1016/s0960-9822(00)00288-8. [DOI] [PubMed] [Google Scholar]
- Heifetz Y, Vandenberg LN, Cohn HI, Wolfner MF. Two cleavage products of the Drosophila accessory gland protein ovulin can independently induce ovulation. Proc Natl Acad Sci USA. 2005;102:743–748. doi: 10.1073/pnas.0407692102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz Y, Wolfner MF. Seminal fluid and mating mediate changes in nerve termini innervating the Drosophila reproductive tract. Proc Natl Acad Sci USA. 2004;101:6261–6266. doi: 10.1073/pnas.0401337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon LA, Wolfner MF. A Drosophila seminal fluid protein, Acp26Aa, stimulates egg-laying in females for one day following mating. Proc Natl Acad Sci USA. 1995;92:10114–10118. doi: 10.1073/pnas.92.22.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignotz GG, Lo MC, Perez CL, Gwathmey TM, Suarez SS. Characterization of a fucose-binding protein from bull sperm and seminal plasma that may be responsible for formation of the oviductal sperm reservoir. Biol Reprod. 2001;64:1806–1811. doi: 10.1095/biolreprod64.6.1806. [DOI] [PubMed] [Google Scholar]
- Jensen-Seaman MI, Li WH. Evolution of the hominoid semenogelin genes, the major proteins of ejaculated semen. J Mol Evol. 2003;57:261–270. doi: 10.1007/s00239-003-2474-x. [DOI] [PubMed] [Google Scholar]
- Kaufmann BP, Demerec M. Utilization of sperm by the female Drosophila melanogaster. Am Nat. 1942;76:445–469. [Google Scholar]
- Kern AD, Jones CD, Begun DJ. Molecular population genetics of male accessory gland proteins in the Drosophila simulans complex. Genetics. 2004;167:725–735. doi: 10.1534/genetics.103.020883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kise H, Nishioka J, Kawamura J, Suzuki K. Characterization of semenogelin II and its molecular interaction with prostate-specific antigen and protein C inhibitor. Eur J Biochem. 1996;238:88–96. doi: 10.1111/j.1432-1033.1996.0088q.x. [DOI] [PubMed] [Google Scholar]
- Kubli E. Sex-peptides: seminal peptides of the Drosophila male. Cell Mol Life Sci. 2003;60:1689–1704. doi: 10.1007/s00018-003-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langendijk P, Soede NM, Kemp B. Uterine activity, sperm transport, and the role of boar stimuli around insemination in sows. Theriogenology. 2005;63:500–513. doi: 10.1016/j.theriogenology.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Lawniczak MK, Begun DJ. A genome-wide analysis of courting and mating responses in Drosophila melanogaster females. Genome. 2004;47:900–910. doi: 10.1139/g04-050. [DOI] [PubMed] [Google Scholar]
- Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci USA. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung O, Kuo L, Wolfner MF. Drosophila males transfer antibacterial proteins from their accessory gland and ejaculatory duct to their mates. J Insect Physiol. 2001;47:617–622. doi: 10.1016/s0022-1910(00)00151-7. [DOI] [PubMed] [Google Scholar]
- Lung O, Tram U, Finnerty C, Eipper-Mains M, Kalb JM, Wolfner MF. The Drosophila melanogaster seminal fluid protein Acp62F is a protease inhibitor that is toxic upon ectopic expression. Genetics. 2002;160:211–224. doi: 10.1093/genetics/160.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung O, Wolfner MF. Drosophila seminal fluid proteins enter the circulatory system through the walls of the posterior vagina. Insect Biochem Mol Biol. 1999;29:1043–1052. doi: 10.1016/s0965-1748(99)00078-8. [DOI] [PubMed] [Google Scholar]
- Mack PD, Kapelnikov A, Heifetz Y, Bender M. Mating-responsive genes in reproductive tissues of female Drosophila melanogaster. Proc Natl Acad Sci USA. 2006;103:10358–10363. doi: 10.1073/pnas.0604046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson WE. Production of an embryo by an Acrochordus javanicus isolated for 7 years. Copeia. 1979;4:744–745. [Google Scholar]
- Malm J, Hellman J, Hogg P, Lilja H. Enzymatic action of prostate-specific antigen (PSA or hK3): substrate specificity and regulation by Zn(2+), a tight-binding inhibitor. Prostate. 2000;45:132–139. doi: 10.1002/1097-0045(20001001)45:2<132::aid-pros7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Manning A. The control of sexual receptivity in female Drosophila. Anim Behav. 1967;15:239–250. doi: 10.1016/0003-3472(67)90006-1. [DOI] [PubMed] [Google Scholar]
- McGraw LA, Gibson G, Clark AG, Wolfner MF. Genes regulated by mating, sperm or seminal proteins in mated female Drosophila melanogaster. Curr Biol. 2004;14:1509–1514. doi: 10.1016/j.cub.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Monastirioti M. Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster. Dev Biol. 2003;264:38–49. doi: 10.1016/j.ydbio.2003.07.019. [DOI] [PubMed] [Google Scholar]
- Monsma SA, Harada HA, Wolfner MF. Synthesis of two Drosophila male accessory gland proteins and their fate after transfer to the female during mating. Dev Biol. 1990;142:465–475. doi: 10.1016/0012-1606(90)90368-s. [DOI] [PubMed] [Google Scholar]
- Monsma SA, Wolfner MF. Structure and expression of a Drosophila male accessory gland gene whose product resembles a peptide pheromone precursor. Genes Dev. 1988;2:1063–1073. doi: 10.1101/gad.2.9.1063. [DOI] [PubMed] [Google Scholar]
- Moshitzky P, Fleischmann I, Chaimov N, Saudan P, Klauser S, Kubli E, Applebaum S. Sex-peptide activates juvenile hormone biosynthesis in the Drosophila melanogaster corpus allatum. Arch Insect Biochem Physiol. 1996;32:363–374. doi: 10.1002/(SICI)1520-6327(1996)32:3/4<363::AID-ARCH9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Mueller JL, Linklater JR, Ravi Ram K, Chapman T, Wolfner MF. Targeted gene deletion and phenotypic analysis of the Drosophila melanogaster seminal fluid protease inhibitor Acp62F. Genetics. 2008;178:1605–1614. doi: 10.1534/genetics.107.083766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller JL, Page JL, Wolfner MF. An ectopic expression screen reveals the protective and toxic effects of Drosophila seminal fluid proteins. Genetics. 2007;175:777–783. doi: 10.1534/genetics.106.065318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller JL, Ravi Ram K, McGraw LA, Bloch Qazi MC, Siggia ED, Clark AG, Aquadro CF, Wolfner MF. Cross-species comparison of Drosophila male accessory gland protein genes. Genetics. 2005;171:131–143. doi: 10.1534/genetics.105.043844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller JL, Ripoll DR, Aquadro CF, Wolfner MF. Comparative structural modeling and inference of conserved protein classes in Drosophila seminal fluid. Proc Natl Acad Sci USA. 2004;101:13542–13547. doi: 10.1073/pnas.0405579101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer V, Spetz JF, Hengst U, Altrogge LM, deAgostini A, Monard D. Male fertility defects in mice lacking the serine protease inhibitor protease nexin-1. Proc Natl Acad Sci USA. 2001;98:3029–3033. doi: 10.1073/pnas.051630698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubaum DM, Wolfner MF. Drosophila females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics. 1999a;153:845–857. doi: 10.1093/genetics/153.2.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubaum DM, Wolfner MF. Schatten G., Pederson R., editors. Wise, winsome or wierd: mechanisms of sperm storage in female animals. Curr Top Dev Biol. 1999b;41:67–97. doi: 10.1016/s0070-2153(08)60270-7. [DOI] [PubMed] [Google Scholar]
- O'Rand MG, Widgren EE, Wang Z, Richardson RT. Eppin: an effective target for male contraception. Mol Cell Endocrinol. 2006;250:157–162. doi: 10.1016/j.mce.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Ottiger M, Soller M, Stocker RF, Kubli E. Binding sites of Drosophila melanogaster sex peptide pheromones. J Neurobiol. 2000;44:57–71. [PubMed] [Google Scholar]
- Panhuis TM, Clark NL, Swanson WJ. Rapid evolution of reproductive proteins in abalone and Drosophila. Philos Trans R Soc Lond B Biol Sci. 2006;361:261–268. doi: 10.1098/rstb.2005.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Wolfner MF. Male and female cooperate in the processing of a Drosophila melanogaster seminal fluid protein. Dev Biol. 1995;171:694–702. doi: 10.1006/dbio.1995.1315. [DOI] [PubMed] [Google Scholar]
- Parker GA. Sperm competition and its evolutionary consequences in the insects. Biol Revs. 1970;45:525–567. [Google Scholar]
- Peng J, Chen S, Busser S, Liu H, Honegger T, Kubli E. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr Biol. 2005a;15:207–213. doi: 10.1016/j.cub.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Peng J, Zipperlen P, Kubli E. Drosophila sex-peptide stimulates female innate immune system after mating via the Toll and Imd pathways. Curr Biol. 2005b;15:1690–1694. doi: 10.1016/j.cub.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Peter A, Lilja H, Lundwall A, Malm J. Semenogelin I and semenogelin II, the major gel-forming proteins in human semen, are substrates for transglutaminase. Eur J Biochem. 1998;252:216–221. doi: 10.1046/j.1432-1327.1998.2520216.x. [DOI] [PubMed] [Google Scholar]
- Pilpel N, Nezer I, Applebaum SW, Heifetz Y. Mating-increases trypsin in female Drosophila hemolymph. Insect Biochem Mol Biol. 2008;38:320–330. doi: 10.1016/j.ibmb.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Pitnick S, Spicer GS, Markow TA. How long is a giant sperm? Nature. 1995;375:109. doi: 10.1038/375109a0. [DOI] [PubMed] [Google Scholar]
- Poiani A. Complexity of seminal fluid: a review. Behav Ecol Sociobiol. 2006;60:289–310. [Google Scholar]
- Prokupek A, Hoffmann F, Eyun SI, Moriyama E, Zhou M, Harshman L. An evolutionary expressed sequence tag analysis of Drosophila spermatheca genes. Evolution. 2008;62:2936–2947. doi: 10.1111/j.1558-5646.2008.00493.x. [DOI] [PubMed] [Google Scholar]
- Raina AK, Kingan TG, Giebultowicz JM. Mating-induced loss of sex pheromone and sexual receptivity in insects with emphasis on Helicoverpa zea and Lymantria dispar. Arch Insect Biochem Physiol. 1994;25:317–327. [Google Scholar]
- Ravi Ram K, Ji S, Wolfner MF. Fates and targets of male accessory gland proteins in mated female Drosophila melanogaster. Insect Biochem Mol Biol. 2005;35:1059–1071. doi: 10.1016/j.ibmb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Ravi Ram K, Sirot LK, Wolfner MF. A predicted seminal astacin-like protease is required for the processing of reproductive proteins in Drosophila melanogaster. Proc Natl Acad Sci USA. 2006;103:18674–18679. doi: 10.1073/pnas.0606228103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi Ram K, Wolfner MF. Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integr Comp Biol. 2007a;47:427–445. doi: 10.1093/icb/icm046. [DOI] [PubMed] [Google Scholar]
- Ravi Ram K, Wolfner MF. Sustained post-mating response in Drosophila melanogaster requires multiple seminal fluid proteins. PLoS Genet. 2007b;3:e238. doi: 10.1371/journal.pgen.0030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice WR. Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature. 1996;381:232–234. doi: 10.1038/381232a0. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. Hormones and Drosophila development. In: Bate M, Martinez AA, editors. The development of Drosophila melanogaster. Vol. 2. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory; 1993. pp. 899–939. [Google Scholar]
- Robert M, Gagnon C. Semenogelin I: a coagulum forming, multifunctional seminal vesicle protein. Cell Mol Life Sci. 1999;55:944–960. doi: 10.1007/s000180050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SA, Bromfield JJ, Tremellen KP. Seminal ‘priming’ for protection from pre-eclampsia—a unifying hypothesis. J Reprod Immunol. 2003;59:253–265. doi: 10.1016/s0165-0378(03)00052-4. [DOI] [PubMed] [Google Scholar]
- Schäfer U. Genes for male-specific transcripts in Drosophila melanogaster. Mol Gen Genet. 1986;202:219–225. [Google Scholar]
- Sirot LK, Poulson RL, Caitlin McKenna M, Girnary H, Wolfner MF, Harrington LC. Identity and transfer of male reproductive gland proteins of the dengue vector mosquito, Aedes aegypti: potential tools for control of female feeding and reproduction. Insect Biochem Mol Biol. 2008;38:176–189. doi: 10.1016/j.ibmb.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soller M, Bownes M, Kubli E. Mating and sex peptide stimulate the accumulation of yolk in oocytes of Drosophila melanogaster. Eur J Biochem. 1997;243:732–738. doi: 10.1111/j.1432-1033.1997.00732.x. [DOI] [PubMed] [Google Scholar]
- Soller M, Bownes M, Kubli E. Control of oocyte maturation in sexually mature Drosophila females. Dev Biol. 1999;208:337–351. doi: 10.1006/dbio.1999.9210. [DOI] [PubMed] [Google Scholar]
- Suarez SS. Regulation of sperm storage and movement in the mammalian oviduct. Int J Dev Biol. 2008;52:455–462. doi: 10.1387/ijdb.072527ss. [DOI] [PubMed] [Google Scholar]
- Swanson WJ, Clark AG, Waldrip-Dail HM, Wolfner MF, Aquadro CF. Evolutionary EST analysis identifies rapidly evolving male reproductive proteins in Drosophila. Proc Natl Acad Sci USA. 2001;98:7375–7379. doi: 10.1073/pnas.131568198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson WJ, Vacquier VD. The rapid evolution of reproductive proteins. Nat Rev Genet. 2002;3:137–144. doi: 10.1038/nrg733. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. On aims and methods of ethology. Z Tierphysiol. 1963;20:410–433. [Google Scholar]
- Tsaur SC, Ting CT, Wu C-I. Positive selection driving the evolution of a gene of male reproduction, Acp26Aa, of Drosophila: II. Divergence versus polymorphism. Mol Biol Evol. 1998;15:1040–1046. doi: 10.1093/oxfordjournals.molbev.a026002. [DOI] [PubMed] [Google Scholar]
- Tsaur SC, Wu C-I. Positive selection and the molecular evolution of a gene of male reproduction, Acp26Aa of Drosophila. Mol Biol Evol. 1997;14:544–549. doi: 10.1093/oxfordjournals.molbev.a025791. [DOI] [PubMed] [Google Scholar]
- Wagstaff BJ, Begun DJ. Molecular population genetics of accessory gland protein genes and testis-expressed genes in Drosophila mojavensis and D. arizonae. Genetics. 2005;171:1083–1101. doi: 10.1534/genetics.105.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MJ, Rylett CM, Keen JN, Audsley N, Sajid M, Shirras AD, Isaac RE. Proteomic identification of Drosophila melanogaster male accessory gland proteins, including a pro-cathepsin and a soluble gamma-glutamyl transpeptidase. Proteome Sci. 2006;4:9. doi: 10.1186/1477-5956-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss BL, Kaufman WR. Two feeding-induced proteins from the male gonad trigger engorgement of the female tick Amblyomma hebraeum. Proc Natl Acad Sci USA. 2004;101:5874–5879. doi: 10.1073/pnas.0307529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigby S, Chapman T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr Biol. 2005;15:316–321. doi: 10.1016/j.cub.2005.01.051. [DOI] [PubMed] [Google Scholar]
- Wigby S, Domanitskaya EV, Choffat Y, Kubli E, Chapman T. The effect of mating on immunity can be masked by experimental piercing in female Drosophila melanogaster. J Insect Physiol. 2008;54:414–420. doi: 10.1016/j.jinsphys.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfner MF. Tokens of love: functions and regulation of Drosophila male accessory gland products. Insect Biochem Mol Biol. 1997;27:179–192. doi: 10.1016/s0965-1748(96)00084-7. [DOI] [PubMed] [Google Scholar]
- Wolfner MF, Heifetz Y, Applebaum SW. Gonadal glands and their gene products. In: Gilbert LI, Iatrou K, Gil SS, editors. Comprehensive molecular insect science. Vol. 1. London: Elsevier; 2005. pp. 179–212. [Google Scholar]
- Wong A, Albright SN, Giebel JD, Ram KR, Ji S, Fiumera AC, Wolfner MF. A role for Acp29AB, a predicted seminal fluid lectin, in female sperm storage in Drosophila melanogaster. Genetics. 2008a;180:921–931. doi: 10.1534/genetics.108.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A, Turchin MC, Wolfner MF, Aquadro CF. Evidence for positive selection on Drosophila melanogaster seminal fluid protease homologs. Mol Biol Evol. 2008b;25:497–506. doi: 10.1093/molbev/msm270. [DOI] [PubMed] [Google Scholar]
- Yang CH, Belawat P, Hafen E, Jan LY, Jan YN. Drosophila egg-laying site selection as a system to study simple decision-making processes. Science. 2008;319:1679–1683. doi: 10.1126/science.1151842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapici N, Kim YJ, Ribeiro C, Dickson BJ. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451:33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]