Abstract
Cyclic AMP Response Element Binding protein (CREB)-binding protein (CBP) is an acetyltransferase important for modifying histones and chromatin-associated proteins and thus affecting transcription and other DNA metabolic processes. We found that the Drosophila CBP (dCBP) is associated with the NAD+-dependent deacetylase, SIR2, which was originally identified as a silencing information regulator in yeast that models silenced and repeated sequence chromatin such as centric heterochromatin, telomeres, and the repeated rDNA sequences. As in yeast, Drosophila sir2 (dsir2) affects the formation and/or function of centric heterochromatin. The fact that we found dCBP in immunecomplexes with dSIR2 in vivo and found that dCBP can interact with dSIR2 directly in vitro suggested that dCBP might affect the packaging of silencing heterochromatin as well. A careful study of the dCBP mutations provides evidence that dCBP does not affect the formation and/or function of centric heterochromatin and thus may affect other dSIR2 functions.
Keywords: dCBP, Drosophila, PEV, SIR2
CREB-binding protein (CBP)/p300 is an acetyltransferase that can acetylate histones and factors within transcription complexes to activate, and in some cases suppress, transcription. The acetyltransferase activity is not always required for CBP/p300 coactivator function, and CBP/p300 can also act as a scaffold protein to recruit and model active transcription complexes. In addition to its role in acetylating chromatin and allowing epigenetic and short-range enhancer–promoter activity (Taverna et al. 2007), CBP appears to be involved in the modulation of epigenetic gene regulation during development (Bantignies et al. 2000; Petruk et al. 2001). Recent studies have identified CBP/p300 within protein complexes that regulate DNA replication, repair, and the DNA replication checkpoint (Hasan, Hassa, et al. 2001; Hasan, Stucki, et al. 2001; Blander et al. 2002; Hasan et al. 2002; Tini et al. 2002; Naryzhny and Lee 2004; Smolik and Jones 2007; Stauffer et al. 2007). These results suggest that CBP/p300 activity is required for many DNA and chromatin metabolic processes beyond its role as a transcriptional coactivator.
In our early studies of Drosophila CBP, dCBP, we used yeast 2-hybrid strategies to identify proteins that might interact with various dCBP domains (Akimaru et al. 1997). Among the candidate proteins isolated in 2 separate screens using the CREB-binding domain, we identified the Drosophila sir2 (dsir2) homologue. Silencing information regulator SIR2 is a NAD+-dependent deacetylase (Imai et al. 2000; Landry et al. 2000; Smith et al. 2000; Tanner et al. 2000) that among other functions in yeast maintains the silenced, hypoacetylated chromatin state at the 2 silent mating-type loci (Braunstein et al. 1993). dsir2 Mutations are reported to be recessive suppressors of position effect variegation (PEV), which occurs when centric heterochromatin variably inactivates a transcriptionally active euchromatic gene placed nearby (Newman et al. 2002; Astrom et al. 2003). Although the levels of suppression vary in the 2 studies that describe the dsir2 PEV phenotype, the detection of dSIR2 in centric heterochromatin (Newman et al. 2002; Rosenberg and Parkhurst 2002) supports a role for dsir2 in heterochromatin formation and/or function. The finding that a deacetylase, dSIR2, could interact directly with an acetyltransferase, dCBP, suggested that dCBP might modulate the formation and/or function of heterochromatin as well.
In this report, we explore the yeast 2-hybrid interaction further and show that dCBP and dSIR2 can be found in complexes in vivo by coimmunoprecipitation assays and can interact directly in in vitro binding assays. We also determined whether mutations in dCBP could affect PEV. Surprisingly, 5 dCBP mutant chromosomes carry dominant Suppressors of variegation (Su(var)s). Using transgenes, we show that a diploid dCBP copy number does not rescue suppression of PEV seen with mutant dCBP chromosomes. We use standard recombination to generate dCBP mutant chromosomes that do not suppress PEV. Furthermore, we demonstrate that multiple copies of dCBP do not enhance or suppress PEV. These results demonstrate that dCBP does not have a dosage-sensitive affect on the formation and/or function of pericentric heterochromatin and suggest that its interaction with dSIR2 may be important to other dSIR2 functions.
Materials and Methods
GST Pull-down Assays and Immunoprecipitations
Glutathione S-transferase (GST) pull-down assays were performed as described (Bantignies et al. 2000). In brief, [35S]-labeled dSIR2 (aa 461–821) was synthesized using the TNT reticulocyte translation kit from Promega. The radiolabeled dSIR2 was mixed with either 4 μl of the purified GST or 1, 2, and 4 μl of the purified GST–dCBP (CREB-binding domain) fusion protein (Bantignies et al. 2000) and glutathione agarose beads (Pharmacia). The samples were incubated for 2 h and precipitated with centrifugation. The beads were washed, boiled in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) buffer, and then precipitated again. The supernatant fluid was loaded onto SDS–PAGE gels and electrophoresed. The gels were then dried and exposed to film. The GST–dCBP fusion protein has a predicted approximate molecular weight of 54 kD. Immunoprecipitation assays were performed as follows: 2 confluent 10-cm plates of Kc cells were used for each sample. Cells were scraped into 15-ml Falcon tubes and rinsed twice in 5 ml of ice-cold phosphate-buffered saline (PBS). The cell pellets were resuspended in 1 ml of the following lysis buffer: PBS, 0.1% NP40, and Roche “mini” ethylenediaminetetraacetic acid–free protease inhibitor tablets (1 tablet/10 ml buffer). The suspension was sonicated 4 times for 30 s at medium power and then transferred to microfuge tubes and centrifuged at maximum speed for 15 min at 4 °C. Fifty microliters of 50% slurry of Protein L Agarose (Pierce) were washed in the lysis buffer and mixed with the Kc cell supernatant. Anti-dSIR2 serum (10 μl) (preimmune or immune) was added to each sample, and the mixtures were incubated at 4 °C for an hour. The beads were washed 4 times in the lysis buffer (without protease inhibitors), precipitated, and then boiled in 50 μl 2× SDS–PAGE buffer. Samples were precipitated and then separated on 6% SDS–PAGE gels. Westerns were performed with either anti-dSIR2 or anti-dCBP antibodies as described (Bantignies et al. 2000; Newman et al. 2002).
Fly Strains
We described the original 3 dCBP alleles previously (Akimaru et al. 1997). The dCBP0.3 allele is an ethylmethane sulfonate (EMS) mutation generated in our laboratory. The McGinnis laboratory generated and described the dCBP Q7 (nejQ7) allele (Florence and McGinnis 1998). All the dCBP mutant chromosomes carry a white (w) mutation as well. We obtained the wm4 inversion chromosome and X chromosome balancer FM7c from the Bloomington Stock Center and generated the y wm4 chromosome using standard recombination techniques. Standard cytology analysis confirmed the inversion on this recombinant chromosome.
We generated dCBP transgenes by cloning 6 kb of sequence upstream from the dCBP start to the dCBP cDNA that was tagged with V5 or not tagged. We then cloned this into the Carnegie yellow transformation vector (kindly supplied by Dr Pam Gueyer, University of Iowa). We generated several y w transgenic fly lines with these transformation vectors, screening for complementation of the yellow phenotype. One, on the second chromosome, carries the wild-type, untagged dCBP transgene and is referred to as dCBPTr42. Another wild-type dCBP transgene, dCBPTr31, on the third chromosome is tagged with the V5 epitope (Invitrogen) at the C terminus and has been described (Smolik and Jones 2007). Both transgenes rescue the lethality of all dCBP deletion mutations and dCBP0.3. Interestingly, the dCBP transgenes do not rescue the lethality associated with the Q7 dCBP allele generated in the McGinnis laboratory (Florence and McGinnis 1998), even in multiple copies. Increasing dosages of dCBP should ameliorate the lethality of this allele, which was hypothesized to be an antimorphic dCBP allele. Thus, these results strongly suggest that the Q7 chromosome carries a second lethal mutation in addition to the dCBP mutation. The homeotic phenotypes attributed to this chromosome most likely arise from this second site mutations because none of the deletion mutations have any homeotic effects. Because this chromosome is carrying an additional lethal, we did not analyze it further.
We generated recombinant w dCBP0.3 and w dCBP3 chromosomes by crossing w dCBP0.3/FM7c and w dCBP3/FM7c females to y w males and mating the w dCBP*/y w female progeny to wm4 males. We scored the y+ females that were not suppressed for the wm4 eye phenotype (scoring as described below) and mated them to FM7c males to balance and save. Approximately 20 w dCBP0.3 and w dCBP3 recombinant chromosomes were tested for their ability to suppress PEV as described below. Between 10% and 20% of the stocks were free of dominant Su(var)s. We saved one of each recombinant mutant chromosome in stock (w dCBP0.3R1 and w dCBP3R1). As with the original mutant chromosomes, we could rescue the lethality associated with each recombinant mutant chromosome with the dCBPTr42 and dCBPTr31 transgenes.
Determination of the dCBP Effects on PEV
We tested the ability of the 3 original dCBP alleles (Akimaru et al. 1997), an EMS allele, dCBP0.3, generated in the laboratory, and the recombinant dCBP0.3R1 and dCBP3R1 alleles to suppress the PEV seen with the wm4 chromosome. We crossed w dCBP*/y wm4 females to wm4 males and sorted the female progeny into 5 categories based on eye color. Class 1 represented almost completely white eyes, class 2 were white or pale yellow with a sprinkling of red ommatidia and/or small red patches, class 3 eyes had a combination of single red ommatidia and larger patches of red ommatidia, class 4 eyes had large patches of red ommatidia that encompassed most of the eye with some white or yellow, and class 5 eyes were completely red with no white or yellow. This assay is at least as accurate as pigment determination protocols, which can exhibit variability due to differences in eye and animal size (Sass and Henikoff 1998). Because all the progeny were y+, this analysis is completely blind to the presence of the dCBP mutant chromosomes. To determine whether the flies were carrying the y wm4 or w dCBP* mutant chromosomes, we crossed them to y*w* and scored for the presence of the y wm4 chromosome. The wm4 inversion is a fairly good recombination suppressor, and the rare recombinants, easily identified as 50% white males, were omitted from the data.
To determine whether a wild-type dCBP transgene could rescue the modified PEV phenotype of the dCBP3 deletion mutation and the dCBP0.3 EMS allele, we crossed y wm4 males to w dCBP3 or w dCBP0.3/FM7c females and mated the w dCBP0.3/y wm4 or w dCBP3/y wm4 females to wm4; Sco/dCBPTr42 males. The female progeny were sorted first by eye phenotype into 1 of the 5 classes. They were then scored for the presence of Sco. As before, the assay was blind for the X chromosomes, and it was easy to sort the eye colors without reference to the Sco phenotype. As before, the y wm4 and dCBP mutant chromosomes were distinguished by outcrosses to y w males and scoring the progeny for the presence of y wm4. We performed the same experiment with the dCBPTr31 chromosome and obtained the same result (data not shown).
To ensure that dCBP did not affect PEV, we generated flies with 3 and 4 copies of wild-type dCBP and determined whether these could enhance the PEV effect of the wm4 chromosome. In this case, the eyes of y wm4 flies that had 3 and 4 copies of dCBP should be whiter than y wm4 flies that have the wild-type complement of 2 copies of dCBP. We crossed both y w; dCBPTr47/Sco; dCBPTr31/MKRS and y wm4; dCBPTr47/Sco; dCBPTr31/MKRS males to y wm4 females and sorted the female progeny first for eye color into the 5 classes and then for the dominant markers. There were no differences in the distributions of variegation between these 2 crosses.
To determine whether the class distributions between the different genotypes were different or the same, we used the nonparametric Kolmogorov–Smirnov 2-sample test. The χ2 value was determined for a degree of freedom of 2. All the crosses above were repeated at least twice.
Results
dCBP and dSIR2 Are Found Together in Complex In Vivo and Can Interact Directly In Vitro
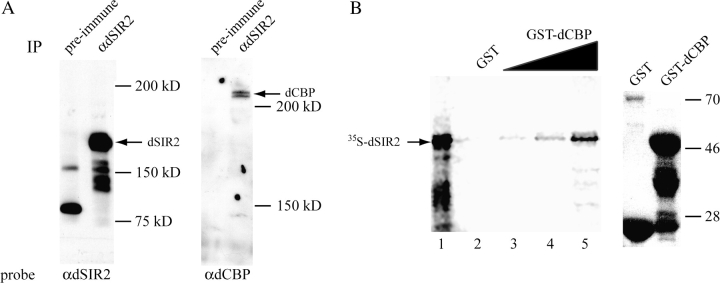
We originally identified dsir2 in yeast 2-hybrid screens designed to detect proteins that interacted with the CREB-binding domain in dCBP (Akimaru et al. 1997). To determine whether the dCBP–dSIR2 interaction occurred in vivo and might be direct, we analyzed immunoprecipitations of endogenous dSIR2 for the presence of dCBP and performed in vitro binding assays between dCBP and dSIR2. Figure 1 presents the results of these studies. We determined whether dCBP and dSIR2 could be found in the same protein complex in vivo using the anti-dSIR2 antibody to immunoprecipitate endogenous dSIR2 in Kc cells. Preimmune dSIR2 serum was used as a negative immunoprecipitation control. We separated the anti-dSIR2 and preimmune immunoprecipitates by PAGE and analyzed the proteins that coimmunoprecipitated with dSIR2 by western analysis. Figure 1A shows that the preimmune sera failed to precipitate dSIR2 as well as dCBP. However, the dSIR2 antibody immunoprecipitated both dSIR2 and dCBP. Thus, the 2 proteins are detected in protein complexes together in vivo. Our yeast 2-hybrid screens identified the C terminus of dSIR2 as an interaction domain with the dCBP CREB-binding domain. Thus, we expressed the CREB-binding domain as a GST fusion protein and added increasing amounts of it to the [35S]-methionine–labeled C terminus of dSIR2. Figure 1B shows that the in vitro–labeled dSIR2 protein does not bind to GST alone and binds specifically to increasing doses of the dCBP–GST fusion protein. Thus, these 2 proteins can interact directly through the protein domains originally identified in the yeast 2-hybrid screens.
Figure 1.
dCBP and dSIR2 are in complex in vivo and directly interact in vitro. (A) Antibody to dSIR2 immunoprecipitates dSIR2 (left panel) and dCBP (right panel) from Kc cell extracts. The preimmune sera were unable to immunoprecipitate either protein (negative control). (B) The left panel shows that the dSIR2 C terminus labeled in vitro with [35S]-methionine interacts with a GST–dCBP CREB-binding domain fusion protein in a dose-dependent manner (lane 1 is the 35S dSIR2 input, lanes 3, 4, and 5 are 1, 2, and 4 μl of the dCBP–GST purified fusion protein preparation added to the 35S dSIR2). The dSIR2 protein domain does not interact with GST alone (lane 2 is 4 μl of GST protein). The right panel is a western analysis of 4 μl of the GST and GST–dCBP protein preparations used in the pull-down assays. The GST–dCBP fusion protein has a predicted molecular weight of approximately 54 kD. The lower bands are breakdown products.
The Effects of dCBP on PEV
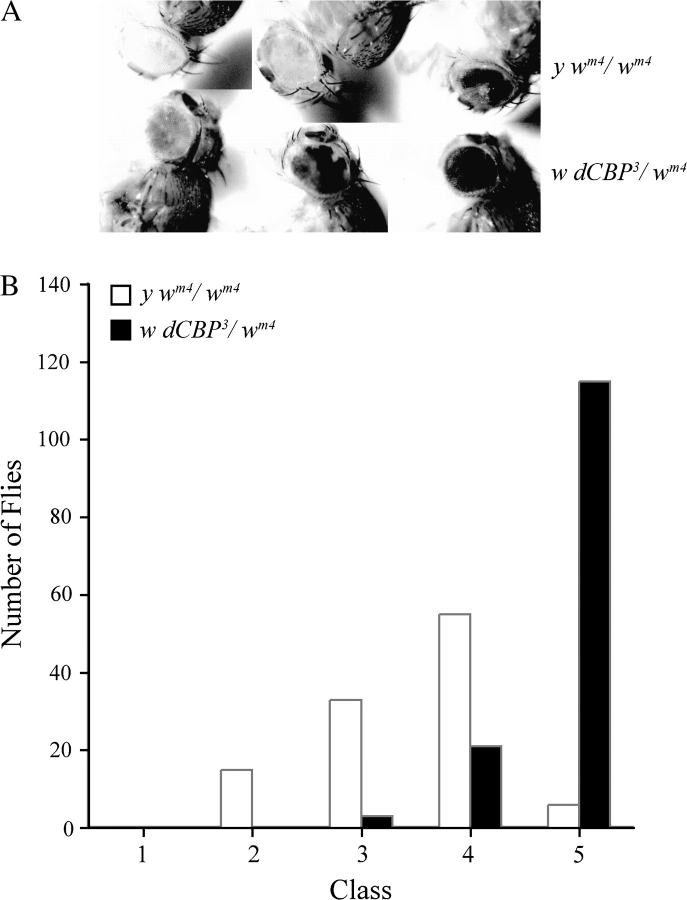
Like the yeast SIR2, dsir2 mutations suppress PEV and thus may affect heterochromatin formation and/or function (Newman et al. 2002; Astrom et al. 2003). The interaction between dSIR2 and dCBP suggested to us that mutations in the chromatin modification protein dCBP might also affect PEV. During our preliminary study of dCBP mutations, we found that the chromosomes carrying the 3 original dCBP alleles suppressed the variegated expression of the w gene on the X chromosome inversion chromosome, wm4. The wm4 inversion brings the w gene close to the centric heterochromatin, which can package and suppress the expression of w in some cells but does not in other cells, resulting in eyes that have a variable number of red and white ommatidia. We crossed the different w dCBP* heterozygous females to males carrying a y wm4 chromosome. We then mated the w dCBP*/y wm4 female progeny to wm4 males and separated the female progeny from this cross into 5 classes according to the degree of eye color mottling (Figure 2A). Because both the w dCBP*/wm4 and y wm4/wm4 females are y+, the assay is blind with respect to the dCBP mutant chromosomes. We determined the presence of the dCBP mutation by crossing the females to y w males and looking for the presence of the y wm4 chromosome in the progeny. The results from the dCBP3 studies are presented in Figure 2B and are identical to the results seen with the other dCBP mutant chromosomes. The distribution of variegation classes in the y wm4/wm4 is significantly different from the distribution of variegation seen in the w dCBP3/wm4 (P ≪ 0.001) and might suggest that dCBP affects the formation and/or function of heterochromatin. However, dominant Su(var) mutations are quite common, and it might be that the dCBP mutant chromosomes have secondary Su(var) mutations on them. To rule this possibility out, we used wild-type dCBP transgenes to complement the Su(var) phenotype. As shown in Figure 3A, additional copies of the wild-type dCBP gene were unable to rescue the variegation seen in the w dCBP0.3/wm4 eyes. The dCBPTr42 duplication chromosome did not have a Su(var) mutation or a PEV enhancer mutation (E(var)) on it as it did not affect the wm4 phenotype of the wm4/y wm4 heterozygotes. The dCBPTr42 chromosome does show a shift toward a Su(var) phenotype but is still not significantly different from wm4 homozygotes (0.05 < P < 0.02).
Figure 2.
The original dCBP mutant chromosomes have dominant suppressors of PEV. (A) The top row of flies shows the range of variegation seen in y wm4/wm4 or y w/wm4 eyes. The bottom row of flies shows the range of variegation seen in the w dCBP3/wm4 flies. All the mutant chromosomes tested were the same (not shown). (B) The red–white variegation was sorted by class and the distributions graphed. The differences in the distributions are significant (P≪0.001).
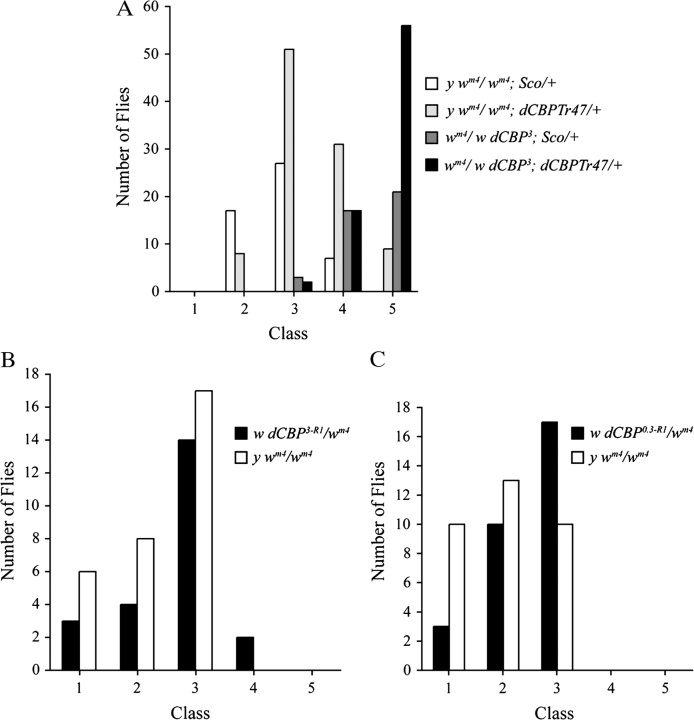
Figure 3.
dCBP mutations are not dominant suppressors of PEV (Su(var)s). (A) The addition of dCBP transgenes to the dCBP0.3/wm4 transheterozygotes restores the diploid copy number for dCBP but does not rescue the dominant Su(var) associated with the dCBP mutant chromosome. The dCBPTr42 chromosome does not have a Su(var) as there is no significant difference between the distribution of eye variegation in the wm4/y wm4 flies and the wm4/y wm4; dCBPTr42/+ flies (0.02 < P < 0.05). Furthermore, the flies that carry the dCBP0.3 mutant chromosome and the dCBPTr42 chromosome have the same distribution of variegation as the flies carrying the dCBP3 mutant chromosome alone (0.05 < P < 0.10). However, in all pairwise combinations, the flies carrying the dCBP0.3 mutant chromosome have a distribution of variegation significantly different from flies that do not carry this mutant chromosome (P≪0.001). (B and C) Recombinant dCBP0.3 and dCBP3 chromosomes do not carry dominant Su(var)s. The distribution of variegation in the eyes of the y wm4/wm4 flies is no different from the distribution of variegation in the dCBP3/wm4 and dCBP0.3/wm4 eyes (0.5 < P < 0.7 in both cases).
These results show that the dCBP mutant chromosomes carry secondary Su(var)s, but they do not rule out the possibility that dCBP mutations have a dosage-sensitive enhancer or suppressor effect on PEV. Furthermore, these data do not rule out variegation differences between y wm4 homozygotes and y wm4 heterozygotes. To rule out these possibilities, we generated a number of dCBP3 and dCBP0.3 recombinant chromosomes and determined whether we could separate the dCBP mutations from the dominant Su(var) phenotype. As shown in Figure 3B,C, we were able to do just that. The distribution of variegation in the wm4/y wm4 flies is not significantly different from the distribution of variegation seen in the w dCBP3/wm4 or w dCBP0.3/wm4 flies (0.5 < P < 0.7 in both comparisons). Thus, dCBP mutations are not dominant Su(var)s or E(var)s, and the variegation seen in wm4 homozygous flies is not different from the variegation seen in structurally heterozygous wm4/w flies. The 2 recombinant chromosomes still carry the dCBP3 and dCBP0.3 mutations because the dCBPTr42 and dCBPTr31 transgenes can rescue the lethality associated with them.
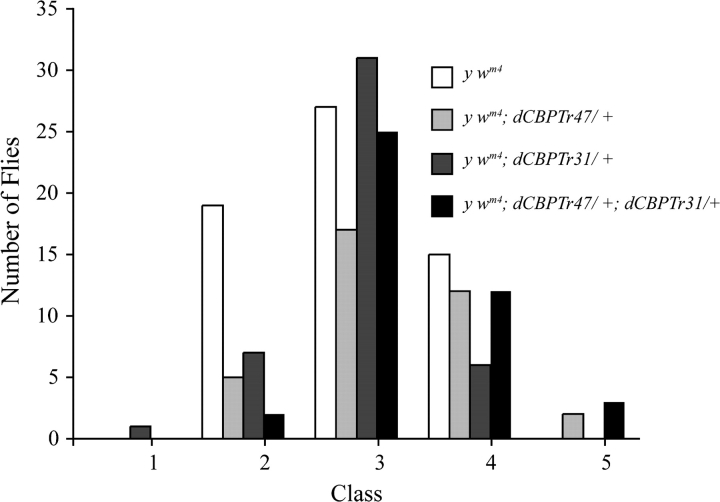
Although dCBP mutations are not dominant Su(var)s or E(var)s, it may be that dCBP modifies heterochromatin and affects PEV when present in increased dosages. Using the dCBPTr42 and dCBPTr31 transgenes, we generated y wm4 or y wm4/y w flies with 2, 3, and 4 copies of dCBP and determined whether the extra doses of dCBP could suppress or enhance wm4 PEV. As shown in Figure 4, multiple copies of dCBP did not enhance or suppress wm4 PEV. Although the presence of 4 copies of dCBP appears to suppress PEV, the distributions are not significantly different from eyes with 2 (0.02 < P < 0.05) or 3 copies (0.1 < P < 0.2 with dCBPTr42 alone and 0.7 < P < 0.8 with dCBPTr31 alone).
Figure 4.
Multiple copies of dCBP do not enhance or suppress PEV. Neither the dCBPTr42 nor the dCBPTr31 chromosome enhance or suppress PEV, and there is no significant difference between the distribution of variegation seen in the y wm4 flies and the y wm4; dCBPTr42/+ flies (0.2 < P < 0.3) and the y wm4; dCBPTr31/+ flies (0.3 < P < 0.5). Although the flies carrying 4 copies of dCBP appear to suppress PEV, the distribution of variegation in these flies is not different from that seen in the y wm4 flies (0.05 < P < 0.02). An experiment in y wm4/y w flies had similar results (not shown).
These results show that dCBP does not affect heterochromatin formation or function in a dosage-sensitive manner. They are supported by previous data showing that our antibodies to dCBP did neither detect dCBP in any quantity in centric heterochromatin (Newman et al. 2002) nor in heterochromatic nucleoli (Bantignies et al. 2002).
Discussion
In yeast, SIR2 is a NAD+-dependent deacetylase required for heterochromatic packaging and silencing at the mating-type loci, telomeres, and the rDNA repeat cluster (Rine and Herskowitz 1987; Gottschling et al. 1990; Bryk et al. 1997; Fritze et al. 1997; Smith and Boeke 1997). Yeast SIR2 also affects other aspects of DNA metabolism such as DNA repair through interactions with Ku70 and recombination through nonhomologous end joining (Tsukamoto et al. 1997). In yeast, SIR2 extends replicative life span (Kaeberlein et al. 1999) through caloric restriction (Lin et al. 2000), and in Caenorhabditis elegans, overexpression of sir2 can increase life span (Tissenbaum and Guarente 2001). In Drosophila, dSIR2 is also a NAD+-dependent deacetylase (Newman et al. 2002). The dsir2 mutations are recessive suppressors of PEV, although the extent of the suppression varies in the 2 studies that reported this result (Newman et al. 2002; Astrom et al. 2003). These differences are likely due to differences in genetic background and measurement protocols. The recessive dsir2 modification of PEV suggests that like SIR2 in yeast, dSIR2 represents a structural component of heterochromatin. The fact that antibodies to dSIR2 detect substantial amounts of dSIR2 in centric heterochromatin (Newman et al. 2002; Rosenberg and Parkhurst 2002) supports this conclusion. The effects of dsir2 mutations on longevity are subtle in Drosophila. The 2 studies that assessed life span in dsir2 mutant flies found that loss of dsir2 function did not decrease the maximal life span (Newman et al. 2002; Astrom et al. 2003). However, one report did find a small but significant decrease in the median life span of dsir2 mutant flies (Newman et al. 2002). A number of variables such as parental age, population density, temperature, nutrition, and genetic background affect life span in Drosophila, and the difference in the experimental parameters of the 2 studies may account for the differences in the median life span. Nevertheless, subsequent studies have provided convincing evidence that dsir2 plays a role in the dietary restriction–mediated pathways that extend life span (Rogina et al. 2002; Rogina and Helfand 2004). Thus, when we observed that dCBP, a known protein acetyltransferase, interacted with dSIR2, we hypothesized that dCBP might be involved in regulating dSIR2 functions as well. The idea that dCBP might be involved in heterochromatin formation and heterochromatic gene silencing was reasonable because dCBP appears to regulate epigenetic gene expression (Taverna et al. 2007). Furthermore, dCBP interacts with and affects the function of Absent, Small, or Homeotic discs 1, a trithorax group gene that is involved in epigenetic gene regulation during development (Bantignies et al. 2000), and dCBP is detected in complex with trithorax (Petruk et al. 2001). Genomic stability requires dCBP function; complete loss of dCBP function causes the degradation of chromosomes and shredding of chromatin and affects the DNA replication checkpoint. Furthermore, dCBP is detected in complex with the Drosophila ataxia telangiectasia mutated-related homologue MEI-41 (Smolik and Jones 2007).
We asked whether dCBP, like dSIR2, could modulate the formation and/or function of centric heterochromatin by determining whether dCBP mutations were suppressors or enhancers of PEV. All the original dCBP deletion mutations and an EMS mutation generated in the laboratory had a dominant Su(var) phenotype associated with them, suggesting that dCBP did affect centric heterochromatin formation and/or function. However, because Su(var) mutations are common in the fly genome, we undertook a careful study of the dCBP mutant chromosomes to determine whether the Su(var) phenotype was due to the dCBP mutations. We found that dCBP transgenes could not rescue the Su(var) phenotype associated with the dCBP mutant chromosomes, demonstrating the presence of second site Su(var)s that could mask any effects that the dCBP mutations might have on PEV. Thus, we generated recombinant dCBP mutant chromosomes to determine whether we could separate the Su(var) phenotype from the lethal dCBP phenotype. We found that we could generate recombinant dCBP mutant chromosomes that did not affect PEV in any way, demonstrating that the dCBP mutations themselves were not dominant Su(var)s or E(var)s. However, it could still be that overexpression of dCBP could affect PEV. To test this possibility, we generated flies with 2, 3, and 4 copies of dCBP and found that the extra doses of dCBP did not suppress or enhance PEV. These results demonstrate that dCBP does not have a dosage-sensitive effect on PEV and is unlikely to interact with dSIR2 to affect the formation and/or function of heterochromatin. This conclusion is supported by the observation that our dCBP antibody could not detect dCBP in centric heterochromatin (Newman et al. 2002) or in the repeated sequences packaged into nucleoli (Bantignies et al. 2002). However, these results do not rule out the possibility that a complete loss of dCBP activity may affect the expression of structural heterochromatin proteins or heterochromatin packaging genes.
What, then, does the dCBP–dSIR2 interaction affect? Because compromising the formation and integrity of centric heterochromatin in flies is not required to ensure a normal life span (Frankel and Rogina 2005), dCBP and dSIR2 might coregulate genes involved in mediating the lengthening of life span seen under dietary restriction or mediate other Sir2 functions such as recombination and DNA repair. For example, in mammals, CBP/p300 and SIRT1, a mammalian gene homologous to Sir2, cooperate to regulate androgen receptor-responsive genes (Saunders and Verdin 2007; Whittle et al. 2007). In addition, SIRT1 regulates the acetylation and function of forkhead transcription factor (FOXO) factors; it can be found in complex with p300 and can deacetylate p300 in vitro, suggesting that SIRT1 may inhibit the p300-mediated activation of FOXO transcriptional activity (Brunet et al. 2004; Motta et al. 2004). In flies, overexpression of FOXO in the cerebral fat body can extend life span and increase insulin-responsive gene expression (Hwangbo et al. 2004). In addition, overexpression of dsir2 affects FOXO-mediated apoptosis (Griswold et al. 2008). It may well be that dCBP and dSIR2 coregulate FOXO processes. Genetic studies have shown that dsir2 affects the ability of flies to deal with stress (Clancy et al. 2001; Wang et al. 2003; Hwangbo et al. 2004; Rogina and Helfand 2004; Broughton et al. 2005), and this is reflected in the reduction of stress response gene expression in dsir2 mutant flies (Girardot et al. 2006). It may be that the dCBP–dSIR2 interaction regulates the expression of these genes under different environmental conditions. Of course, the dCBP–dSIR2 interaction may affect processes we have yet to identify. Future genetic studies of dCBP and dsir2 will certainly elucidate the functional significance of the interaction between these 2 important genome regulatory proteins.
Funding
National Institutes of Health (RO1GM61837).
Note Added in Proof
While this manuscript was under review, an online prepublication by Zhao et al. (2008) shows dSIR2 in heterochromatin with their antibody. They demonstrate that they too can detect dCBP and dSIR2 in complexes together and furthermore that 2 lysines critical for dSIR2 function can be acetylated by CBP in vitro. Overexpression experiments suggest a dosage-dependent interaction between dSIR2 and dCBP in the thorax. These data further support the idea that dCBP and dSIR2 are interacting partners in the regulation of Drosophila gene expression.
Acknowledgments
I thank Brenda L. Newman for performing the molecular analysis and for her helpful discussions.
References
- Akimaru H, Chen Y, Dai P, Hou DX, Nonaka M, Smolik S, Armstrong S, Goodman R, Ishii S. Drosophila CBP is a co-activator of cubitus interruptus in hedgehog signalling. Nature. 1997;386:735–738. doi: 10.1038/386735a0. [DOI] [PubMed] [Google Scholar]
- Astrom SU, Cline TW, Rine J. The Drosophila melanogaster sir2+ gene is nonessential and has only minor effects on position-effect variegation. Genetics. 2003;163:931–937. doi: 10.1093/genetics/163.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantignies F, Goodman RH, Smolik SM. Functional interaction between the coactivator Drosophila CREB-binding protein and ASH1, a member of the trithorax group of chromatin modifiers. Mol Cell Biol. 2000;20:9317–9330. doi: 10.1128/mcb.20.24.9317-9330.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantignies F, Goodman RH, Smolik SM. The interaction between the coactivator dCBP and Modulo, a chromatin-associated factor, affects segmentation and melanotic tumor formation in Drosophila. Proc Natl Acad Sci USA. 2002;99:2895–2900. doi: 10.1073/pnas.052509799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander G, Zalle N, Daniely Y, Taplick J, Gray MD, Oren M. DNA damage-induced translocation of the Werner helicase is regulated by acetylation. J Biol Chem. 2002;277:50934–50940. doi: 10.1074/jbc.M210479200. [DOI] [PubMed] [Google Scholar]
- Braunstein M, Rose A, Holmes S, Allis CD, Broach J. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 1993;7:592–604. doi: 10.1101/gad.7.4.592. [DOI] [PubMed] [Google Scholar]
- Broughton SJ, Piper MD, Ikeya T, Bass TM, Jacobson J, Driege Y, Martinez P, Hafen E, Withers DJ, Leevers SJ, et al. Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci USA. 2005;102:3105–3110. doi: 10.1073/pnas.0405775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Bryk M, Banerjee M, Murphy M, Knudsen KE, Garfinkel DJ, Curcio MJ. Transcriptional silencing of Ty1 elements in the RDN1 locus of yeast. Genes Dev. 1997;11:255–269. doi: 10.1101/gad.11.2.255. [DOI] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Florence B, McGinnis W. A genetic screen of the Drosophila X chromosome for mutations that modify Deformed function. Genetics. 1998;150:1497–1511. doi: 10.1093/genetics/150.4.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel S, Rogina B. Drosophila longevity is not affected by heterochromatin-mediated gene silencing. Aging Cell. 2005;4:53–56. doi: 10.1111/j.1474-9726.2005.00143.x. [DOI] [PubMed] [Google Scholar]
- Fritze CE, Verschueren K, Strich R, Easton Esposito R. Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. EMBO J. 1997;16:6495–6509. doi: 10.1093/emboj/16.21.6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardot F, Lasbleiz C, Monnier V, Tricoire H. Specific age-related signatures in Drosophila body parts transcriptome. BMC Genomics. 2006;7:69. doi: 10.1186/1471-2164-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschling DE, Aparicio OM, Billington BL, Zakian VA. Position effect at S. cerevisiae telomeres: reversible repression of Pol II transcription. Cell. 1990;63:751–762. doi: 10.1016/0092-8674(90)90141-z. [DOI] [PubMed] [Google Scholar]
- Griswold AJ, Chang KT, Runko AP, Knight MA, Min KT. Sir2 mediates apoptosis through JNK-dependent pathways in Drosophila. Proc Natl Acad Sci USA. 2008;105:8673–8678. doi: 10.1073/pnas.0803837105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S, El-Andaloussi N, Hardeland U, Hassa PO, Burki C, Imhof R, Schar P, Hottiger MO. Acetylation regulates the DNA end-trimming activity of DNA polymerase beta. Mol Cell. 2002;10:1213–1222. doi: 10.1016/s1097-2765(02)00745-1. [DOI] [PubMed] [Google Scholar]
- Hasan S, Hassa PO, Imhof R, Hottiger MO. Transcription coactivator p300 binds PCNA and may have a role in DNA repair synthesis. Nature. 2001;410:387–391. doi: 10.1038/35066610. [DOI] [PubMed] [Google Scholar]
- Hasan S, Stucki M, Hassa PO, Imhof R, Gehrig P, Hunziker P, Hubscher U, Hottiger MO. Regulation of human flap endonuclease-1 activity by acetylation through the transcriptional coactivator p300. Mol Cell. 2001;7:1221–1231. doi: 10.1016/s1097-2765(01)00272-6. [DOI] [PubMed] [Google Scholar]
- Hwangbo DS, Gershman B, Tu MP, Palmer M, Tatar M. Drosophila dFOXO controls lifespan and regulates insulin signalling in brain and fat body. Nature. 2004;429:562–566. doi: 10.1038/nature02549. [DOI] [PubMed] [Google Scholar]
- Imai S-I, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–799. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, Sternglanz R. The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci USA. 2000;97:5807–5811. doi: 10.1073/pnas.110148297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Naryzhny SN, Lee H. The post-translational modifications of proliferating cell nuclear antigen: acetylation, not phosphorylation, plays an important role in the regulation of its function. J Biol Chem. 2004;279:20194–20199. doi: 10.1074/jbc.M312850200. [DOI] [PubMed] [Google Scholar]
- Newman BL, Lundblad JR, Chen Y, Smolik SM. A Drosophila homologue of Sir2 modifies position-effect variegation but does not affect life span. Genetics. 2002;162:1675–1685. doi: 10.1093/genetics/162.4.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruk S, Sedkov Y, Smith S, Tillib S, Kraevski V, Nakamura T, Canaani E, Croce CM, Mazo A. Trithorax and dCBP acting in a complex to maintain expression of a homeotic gene. Science. 2001;294:1331–1334. doi: 10.1126/science.1065683. [DOI] [PubMed] [Google Scholar]
- Rine J, Herskowitz I. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics. 1987;116:9–22. doi: 10.1093/genetics/116.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogina B, Helfand SL, Frankel S. Longevity regulation by Drosophila Rpd3 deacetylase and caloric restriction. Science. 2002;298:1745. doi: 10.1126/science.1078986. [DOI] [PubMed] [Google Scholar]
- Rosenberg MI, Parkhurst SM. Drosophila Sir2 is required for heterochromatic silencing and by euchromatic Hairy/E(Spl) bHLH repressors in segmentation and sex determination. Cell. 2002;109:447–458. doi: 10.1016/s0092-8674(02)00732-8. [DOI] [PubMed] [Google Scholar]
- Sass GL, Henikoff S. Comparative analysis of position-effect variegation mutations in Drosophila melanogaster delineates the targets of modifiers. Genetics. 1998;148:733–741. doi: 10.1093/genetics/148.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders LR, Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene. 2007;26:5489–5504. doi: 10.1038/sj.onc.1210616. [DOI] [PubMed] [Google Scholar]
- Smith JS, Boeke JD. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev. 1997;11:241–254. doi: 10.1101/gad.11.2.241. [DOI] [PubMed] [Google Scholar]
- Smith JS, Brachmann CB, Celic I, Kenna MA, Muhammad S, Starai VJ, Avalos JL, Escalante-Semerena JC, Grubmeyer C, Wolberger C, et al. A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc Natl Acad Sci USA. 2000;97:6658–6663. doi: 10.1073/pnas.97.12.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolik S, Jones K. Drosophila dCBP is involved in establishing the DNA replication checkpoint. Mol Cell Biol. 2007;27:135–146. doi: 10.1128/MCB.01283-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer D, Chang B, Huang J, Dunn A, Thayer M. p300/CREB-binding protein interacts with ATR and is required for the DNA replication checkpoint. J Biol Chem. 2007;282:9678–9687. doi: 10.1074/jbc.M609261200. [DOI] [PubMed] [Google Scholar]
- Tanner KG, Landry J, Sternglanz R, Denu JM. Silent information regulator 2 family of NAD-dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose. Proc Natl Acad Sci USA. 2000;97:14178–14182. doi: 10.1073/pnas.250422697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna SD, Li H, Ruthenburg AJ, Allis CD, Patel DJ. How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers. Nat Struct Mol Biol. 2007;14:1025–1040. doi: 10.1038/nsmb1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tini M, Benecke A, Um SJ, Torchia J, Evans RM, Chambon P. Association of CBP/p300 acetylase and thymine DNA glycosylase links DNA repair and transcription. Mol Cell. 2002;9:265–277. doi: 10.1016/s1097-2765(02)00453-7. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410:227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Kato J, Ikeda H. Silencing factors participate in DNA repair and recombination in Saccharomyces cerevisiae. Nature. 1997;388:900–903. doi: 10.1038/42288. [DOI] [PubMed] [Google Scholar]
- Wang MC, Bohmann D, Jasper H. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev Cell. 2003;5:811–816. doi: 10.1016/s1534-5807(03)00323-x. [DOI] [PubMed] [Google Scholar]
- Whittle JR, Powell MJ, Popov VM, Shirley LA, Wang C, Pestell RG. Sirtuins, nuclear hormone receptor acetylation and transcriptional regulation. Trends Endocrinol Metab. 2007;18:356–364. doi: 10.1016/j.tem.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Takeyama KI, Sawatsubashi S, Ito S, Suzuki E, Yamagata K, Tanabe M, Kimura S, Fujiyama S, Ueda T, et al. Co-repressive action of CBP on androgen receptor transactivation in pericentric heterochromatin in a Drosophila experimental model system. Mol Cell Biol. 2009;29:1017–1034. doi: 10.1128/MCB.02123-07. [DOI] [PMC free article] [PubMed] [Google Scholar]