Abstract
Hypoxia-inducible factor-1α (HIF-1α) is a transcription factor that regulates cellular stress responses. While the levels of HIF-1α protein are tightly regulated, recent studies suggest that it can be active under normoxic conditions. We hypothesized that HIF-1α is required for normal β cell function and reserve and that dysregulation may contribute to the pathogenesis of type 2 diabetes (T2D). Here we show that HIF-1α protein is present at low levels in mouse and human normoxic β cells and islets. Decreased levels of HIF-1α impaired glucose-stimulated ATP generation and β cell function. C57BL/6 mice with β cell–specific Hif1a disruption (referred to herein as β-Hif1a-null mice) exhibited glucose intolerance, β cell dysfunction, and developed severe glucose intolerance on a high-fat diet. Increasing HIF-1α levels by inhibiting its degradation through iron chelation markedly improved insulin secretion and glucose tolerance in control mice fed a high-fat diet but not in β-Hif1a-null mice. Increasing HIF-1α levels markedly increased expression of ARNT and other genes in human T2D islets and improved their function. Further analysis indicated that HIF-1α was bound to the Arnt promoter in a mouse β cell line, suggesting direct regulation. Taken together, these findings suggest an important role for HIF-1α in β cell reserve and regulation of ARNT expression and demonstrate that HIF-1α is a potential therapeutic target for the β cell dysfunction of T2D.
Introduction
The transcription factor HIF-1α is important for a range of functions, including cellular responses to hypoxia and other stressors, angiogenesis, and fetal development (1–6). It has strong antiapoptotic effects (7–11) and is implicated in the pathogenesis of cardiovascular diseases and some cancers (12–20).
HIF-1α is a member of the bHLH-PAS family (reviewed in refs. 2, 18, 21) and functions as an obligate dimer with other family members, including aryl hydrocarbon receptor (AhR) nuclear translocator (ARNT). We previously reported that ARNT was decreased in islets isolated from patients with type 2 diabetes (T2D) and that decreasing ARNT in Min6 cells or disrupting it in mouse β cells caused changes in gene expression and glucose-stimulated insulin secretion (GSIS) similar to those seen in islets isolated from humans with T2D (22). Recently, we reported a loss of ARNT expression in the livers of people with T2D, affecting dysregulation of gluconeogenesis (23). Though the specific ARNT partner which is important for its actions in β cells (or liver) is not known, candidates include AhR, HIF-1α, HIF-2α, HIF-3α, and circadian rhythm molecules, e.g., BMAL.
Because of its role in the regulation of glycolysis and other biological processes in other tissues (24, 25), we hypothesized that (a) HIF-1α might be the important partner for ARNT in β cells, (b) that decreasing HIF-1α would impair β cell reserve and thus lead to diabetes under conditions of β cell stress, and (c) that increasing HIF-1α in a nontoxic way would improve β cell function.
Consistent with its role in regulating a number of important biological processes, HIF-1α protein is tightly regulated (reviewed in refs. 2, 17, 19, 21, 25, 26). In the basal state, it is hydroxylated on proline residues and becomes competent to associate with von Hippel-Lindau (VHL) protein, leading to ubiquitination and rapid proteolysis, giving a half-life of minutes (19, 27, 28). Oxygen, iron, and 2-oxoglutarate are required for hydroxylation (29–32). Thus, hypoxia inhibits degradation, leading to a rapid increase. In addition, HIF-1α protein can be increased by genetic inactivation of VHL or the hydroxylases, treatment with heavy metals such as cobalt chloride, or iron chelation with deferoxamine (DFO) or deferasirox (DFS) (20, 29). An additional layer of regulation is added by asparaginyl-hydroxylation, which inhibits association with transcriptional cofactors, including p300 (21).
Until recently, it was thought that HIF-1α did not function under normoxic conditions. However, the presence of HIF-1α protein in brain, kidney, liver, embryonic stem cells, trophoblastic cells, and others (5, 6, 33) is now recognized. It is stabilized by inflammation, TGF, PDGF, EGF, and IL-1β (20, 34, 35) and by increased levels of ROS (36–38). Of potential relevance to β cells, insulin increases HIF-1α activity in liver, muscle, breast carcinoma, prostate carcinoma, and retinal epithelial-derived cells (39–42). PI3K-Akt pathway activation is required for the insulin-induced increase (43).
The role of HIF-1α in islets is not fully understood. Pancreatic islets are normally exposed to relatively low oxygen tension (20–37 mmHg) (44, 45) and to locally secreted insulin. These factors suggest a possible role for HIF-1α in islets and the possibility for decreased HIF-1α in the setting of insulin resistance.
This study found that targeted disruption of HIF-1α in β cells of C57BL/6 mice (referred to herein as β-Hif1a-null mice) led to glucose intolerance with impaired ATP generation and GSIS in isolated islets. Conversely, increasing HIF-1α using iron chelation with DFO or DFS caused significant changes in gene expression, which differed from severe hypoxia or VHL deletion (46–48). DFS significantly improved glucose tolerance in mice receiving a high-fat diet (HFD) but had no effect in β-Hif1a-null mice, demonstrating that β cell HIF-1α was required for its effect. Importantly, DFO treatment of T2D islets normalized expression of ARNT and downstream genes and improved GSIS. HIF-1α bound to the ARNT promoter, as revealed by ChIP, and increasing HIF-1α levels increased ARNT expression. Taken together, these findings suggest that decreased HIF-1α levels impair β cell reserve and that iron chelation, which increases HIF-1α activity in β cells, may be a therapeutic strategy for the treatment of human T2D.
Results
HIF-1α was present at low levels in islets and was decreased in humans with T2D.
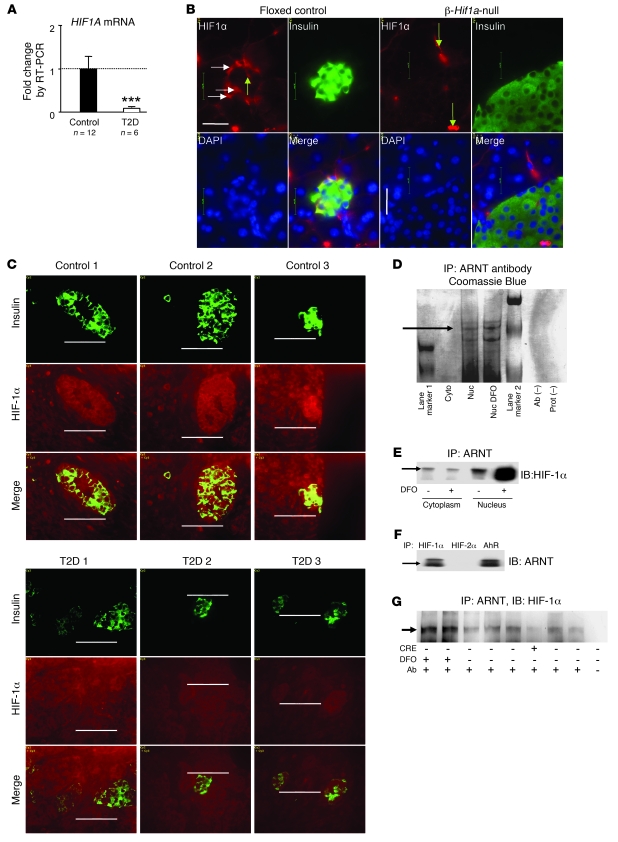
HIF1α levels were assessed using real-time PCR in isolated human T2D and control islets and using immunohistochemistry of pancreatic sections collected during partial pancreatectomy. HIF1A mRNA was decreased by 90% in T2D islets (P < 0.0001; Figure 1A). Immunohistochemistry revealed HIF-1α protein in some normal murine β cells (Figure 1B, horizontal arrows), and staining was present in blood vessels (Figure 1B, vertical arrows). In β-Hif1a-null islets, staining was present in vascular cells (Figure 1B, vertical arrows) but not β cells, demonstrating antibody specificity. In human partial pancreatectomy tissue, there was mild, diffuse HIF-1α staining, consistent with the operative procedure in which vessels are ligated prior to tissue removal, thus inducing hypoxia. Subjects with normal glucose tolerance showed more intense HIF-1α staining in islets than in the acinar pancreas (Figure 1C). In contrast, in T2D islets, HIF-1α staining was not higher than that in the acinar pancreas (Figure 1C).
Figure 1. HIF-1α is present in normoxic β cells, associates with ARNT, and is decreased in T2D.
(A) HIF1A mRNA was decreased in islets of people with T2D (n = 6) compared with people with normal glucose tolerance (n = 12). ***P < 0.001. (B) HIF-1α protein was present in β cells in floxed control mice (horizontal arrows) but was decreased in β-Hif1a-null mice. In both genotypes, HIF-1α was present in blood vessels (vertical arrows). Scale bar: 20 μm. (C) HIF-1α protein was higher than background in people with normal glucose tolerance (top panels) but was decreased in T2D pancreata (bottom panels). Scale bar: 50 μm. (D) HIF-1α protein (arrow) was associated with ARNT by affinity purification. Cyto, cytoplasm; Nuc, nucleus; Prot, protein. (E) HIF-1α protein associated with ARNT in the basal state in Min6 cells, and nuclear HIF-1α increased with DFO. (F) ARNT protein associated with HIF-1α by coimmunoprecipitation. (G) HIF-1α protein was increased by DFO treatment of isolated mouse islets and was decreased in islets from a β-Hif1a-null mouse (Cre+).
HIF-1α associated with ARNT.
To determine whether the HIF-1α in β cells and islets was associated with ARNT and therefore potentially transcriptionally active in the basal (nonhypoxic) state, we used ARNT affinity purification and tandem MALDI-TOF mass spectrometry. The Coomassie-stained gel showed a band at the appropriate size for HIF-1α in the untreated nuclear fraction and in the DFO-treated positive control (Figure 1D, black arrow). Mass spectrometry revealed 4 peptide sequences derived from HIF-1α. HIF-2α was also associated with ARNT but only in DFO-treated cells. AhR protein was not detected.
To confirm that ARNT was bound to HIF-1α, we performed coimmunoprecipitation studies in Min6 cells. Immunoprecipitation with ARNT antibodies revealed low levels of associated HIF-1α in the cytoplasm and nucleus, under normoxic conditions (Figure 1E). As expected, DFO treatment increased nuclear HIF-1α. The converse was also true; immunoprecipitation with HIF-1α antibodies purified ARNT, under normoxic conditions (Figure 1F, left lane). AhR antibodies were able to “pull-down” ARNT from whole-cell lysates (Figure 1F, right lane). However, without exogenous ligand, AhR antibodies did not purify detectable amounts of ARNT from nuclear extracts, suggesting that it was not functionally active (data not shown). As shown in Figure 1G, ARNT immunoprecipitation and HIF-1α immunoblotting was performed in whole-cell lysates from primary mouse islets. Lanes 1 and 2 are islets from mice treated with DFO and then cultured in DFO. Lanes 3–8 were islets cultured in normal media. The Cre+ lane shows islets from a β-Hif1a-null mouse in which there was decreased HIF-1α.
β cell–specific Hif1α-null mice have impaired β cell function.
Using the Cre-lox system, with Cre under control of the rat insulin promoter (RIP-Cre), and mice with a floxed Hif1a gene (floxed controls) (49), we generated β-Hif1a-null mice. HIF-1α immunostaining is shown in Figure 1B.
Importantly, in our colony, RIP-Cre mice have normal glucose tolerance (Figure 2A). β-Hif1a-null mice were fertile and did not differ in size or weight (data not shown). Fasting glucose did not differ among groups (Figure 2A); however, levels after glucose loading were significantly higher in β-Hif1a-null mice than in floxed control mice or in RIP-Cre mice. Disruption of HIF-1α in β cells did not alter fasting insulin. However, as shown in Figure 2B, β-Hif1a-null mice had significantly impaired first-phase GSIS. Consistent with these in vivo effects, islets isolated from β-Hif1a-null mice had impaired GSIS (>80% reduction at 3.3 and 11 mmol/l glucose; Figure 2C). The difference was not statistically significant at 22 mmol/l.
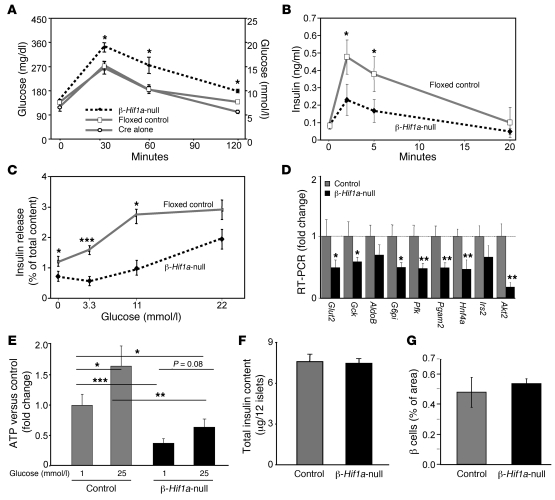
Figure 2. β cell deletion of Hif1a in mice causes glucose intolerance, impaired gene expression, ATP generation, and insulin secretion.
(A) β-Hif1a-null mice were glucose intolerant compared with either floxed controls or RIP-Cre alone mice. n = 15, 15, and 12, respectively. (B) GSIS was decreased in β-Hif1a-null mice. (C) GSIS was decreased in isolated β-Hif1a-null islets. (D) Expression of several genes was decreased in β-Hif1a-null islets. (E) ATP concentrations were significantly decreased in β-Hif1a-null islets at both basal and high glucose levels. (F) Insulin content did not differ between floxed control and β-Hif1a-null islets. (G) β cell mass did not differ between groups. *P < 0.05, **P < 0.01, and ***P < 0.001.
Gene expression was assessed in isolated islets using real-time PCR. In β-Hif1a-null islets, there was a more than 40% decrease in Glut2, glucokinase (Gck), glucose-6-phosphoisomerase (G6pi), phosphofructokinase (Pfk), hepatocyte nuclear factor 4α (Hnf4a), and others (Figure 2D). As expected, control islets had higher ATP content after exposure to high glucose (60% increase; Figure 2E). Islets from β-Hif1a-null mice had significantly lower basal ATP levels (60% decrease) and the increase in ATP levels with 25 mmol/l glucose was severely blunted. This was despite similar islet insulin content (Figure 2F) and nonsignificant, higher β cell content in β-Hif1a-null mice (Figure 2G). No differences were observed for CD31 staining, indicating similar islet vascularity (data not shown).
Hif1a knockdown in Min6 cells impaired β cell function.
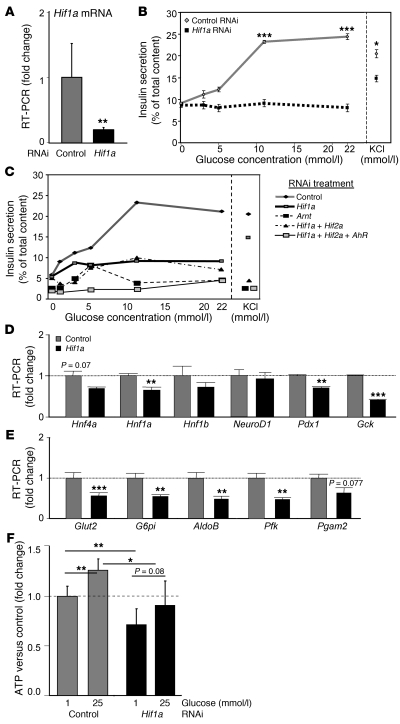
To confirm the β-Hif1a-null mice results in a β cell line, we used RNAi in Min6 cells. RNAi achieved approximately 70% knockdown of Hif1a mRNA (Figure 3A). This was accompanied by markedly impaired GSIS (Figure 3B). There was a milder (approximately 25%) impairment in KCl-stimulated insulin release (Figure 3B, right). In separate experiments examining combined RNAi treatments, slightly more severe impairment occurred with Arnt RNAi and with Hif1a plus Hif2a plus AhR knockdown, suggesting small additional roles for Hif-2α and AhR (Figure 3C). KCl-stimulated insulin secretion was again impaired by approximately 25% (Figure 3C, right), suggesting a partially glucose-specific effect.
Figure 3. Decreasing Hif1a by RNAi-impaired β cell function, gene expression, and ATP generation.
(A) RNAi decreased Hif1a mRNA. (B) Hif1a RNAi decreased GSIS in Min6 cells and caused a small decrease in KCl-stimulated insulin release. (C) Combination RNAi treatment caused slightly more severe impairment in insulin release. (D) Hif1a RNAi decreased expression of genes from the MODY family and (E) glucose-uptake and glycolysis genes. (F) Hif1a RNAi decreased basal and glucose-stimulated ATP concentrations. *P < 0.05, **P < 0.01, and ***P < 0.001.
Decreased expression of glucose transporter and glycolytic mRNAs was found, including Gck, Glut2, G6pi, Aldolase (AldoB), and Pfk (40%–60%; Figure 3, D and E). These changes were similar to those for β-Hif1a-null islets (Figure 2E). As shown in Figure 3F, Hif1a knockdown impaired ATP generation basally (25% decrease) and following glucose stimulation (only 90% of basal control).
Increasing HIF-1α improved glucose tolerance in HFD-fed C57BL/6 mice.
As noted above, β-Hif1a-null mice exhibited mild glucose intolerance, with preserved fasting glucose (Figure 2A). In a separate cohort of mice (Figure 4A), we performed glucose tolerance testing (GTT) and replicated the finding of impaired glucose tolerance, with preserved fasting levels (P = 0.007, ANOVA for repeated measures). For β-Hif1a-null mice, weight was 24.7 ± 0.6 g, and for controls, weight was 25.2 ± 0.6 g.
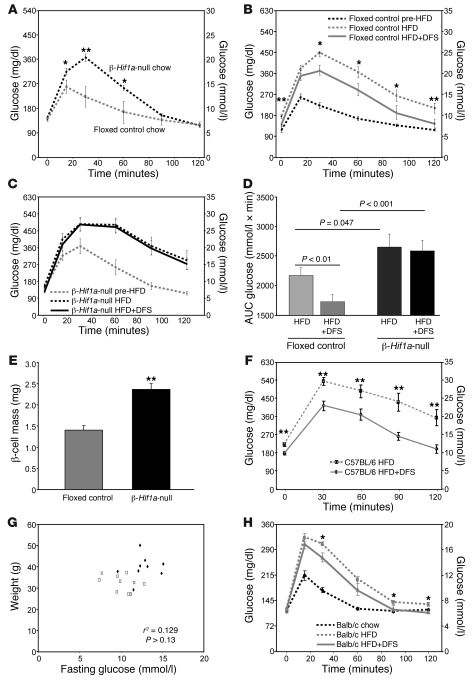
Figure 4. Lack of β cell HIF-1α leads to severe deterioration in glucose tolerance on a HFD and increasing HIF-1α levels with DFS improves glucose tolerance on a HFD.
(A) β-Hif1a-null mice (n = 10) had worse glucose tolerance than floxed control littermates (n = 17). (B) On HFD, glucose tolerance deteriorated in floxed controls and improved following DFS. (C) On HFD, glucose tolerance deteriorated markedly in β-Hif1a-null mice, and there was no improvement with DFS. (D) Glucose tolerance AUC for mice at completion of the HFD and HFD plus DFS stages. (E) β cell mass was increased in β-Hif1a-null mice at study completion. (F) Glucose tolerance was significantly better in C57BL/6 mice receiving HFD plus DFS versus mice receiving HFD alone (n = 10 per group). (G) Weight and fasting glucose were not significantly correlated in the mice. Rectangles indicate mice receiving HFD plus DFS, and triangles indicate mice receiving HFD alone. (H) Balb/c mice had deterioration in glucose tolerance on HFD (dotted line) compared with chow (dashed line). Their glucose tolerance improved significantly on HFD plus DFS (n = 12). *P < 0.05 and **P < 0.01.
To examine β cell compensation in the setting of insulin resistance, we placed mice on a HFD (45% of calories from fat) for 3 weeks. Weight increased to 29.4 ± 0.9 g for β-Hif1a-null mice and to 29.0 ± 0.8 g for controls. GTTs deteriorated in floxed controls (Figure 4B, dotted line) but more severely in β-Hif1a-null mice (Figure 4C, dotted line). AUCs are shown in Figure 4D (P = 0.047 for floxed control HFD-fed versus β-Hif1a-null HFD-fed mice).
Following 3 weeks of HFD, all mice were changed to HFD admixed with DFS (HFD plus DFS) to increase HIF-1α. After 3 weeks, weight was 29.0 ± 0.8 g in β-Hif1a-null mice versus 29.0 ± 0.8 g in controls. As shown in Figure 4C, despite continuing HFD, floxed controls had highly significantly improved glucose tolerance. In contrast, there was no improvement in β-Hif1a-null mice, demonstrating that β cell HIF-1α was required for DFS to improve glucose tolerance. AUCs are shown in Figure 4D. Interestingly, β-Hif1a-null mice had 69% greater β cell mass than controls at the end of the study (Figure 4E), despite worse glucose tolerance, suggesting attempted and unsuccessful β cell compensation.
There was some 4-hydroxynonenal staining in HFD-fed mice, consistent with increased ROS, but it was without obvious differences between genotypes (data not shown). Similarly, β cell apoptosis did not differ, as assessed by cleaved caspase-3 staining. Apoptosis rates were less than 1% in both floxed control and β-Hif1a-null groups with this short-term treatment (data not shown).
We confirmed the effects of DFS on HFD-induced glucose intolerance in a separate cohort of normal (not genetically modified) C57BL/6 mice fed HFD or HFD plus DFS for 26 weeks. Glucose tolerance was significantly better in mice receiving HFD plus DFS (Figure 4F, solid line) compared with mice receiving HFD alone (Figure 4F, dotted line). In this 26 week experiment, weight was lower in the HFD plus DFS–fed group (HFD-fed, 38.8 ± 4.2 g versus HFD plus DFS–fed, 33.0 ± 3.2 g; P = 0.037), which may have contributed to the difference in GTTs. However, weight and fasting glucose were not significantly correlated (P > 0.13; Figure 4G) and neither were weight and AUC of GTTs (data not shown). Multivariate regression using weight and DFS as univariate predictors showed that only DFS independently predicted fasting glucose (P = 0.010 for DFS, P = 0.811 for weight) and AUC of GTTs (P = 0.039 for DFS, P = 0.433 for weight). Mice were not anemic (hemoglobin, 120 ± 7 mg/dl in DFS mice versus 124 ± 3 mg/dl in controls; P > 0.6). Insulin tolerance testing (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI35846DS1) showed that the HFD plus DFS–fed mice had similar percentages of decreases in blood glucose after insulin administration.
Increasing HIF-1α improved glucose tolerance in HFD-fed Balb/c mice.
Because the above experiments were all performed in C57BL/6 mice, we sought to confirm the effects in another mouse strain. Balb/c mice were weighed (20.8 ± 0.4 g), underwent GTT (Figure 4H, dashed line), and were placed on HFD. After 2 weeks, mice weighed 24.5 ± 0.5 g. Repeat GTT showed marked deterioration (dotted line). The diet was then changed to HFD plus DFS. After 2 weeks, mice weighed 24.7 ± 0.4 g. GTTs were repeated and showed significant improvement (2.1 mmol/l in peak glucose), confirming an effect of DFS in another mouse line.
Increasing HIF-1α in human islets improved gene expression.
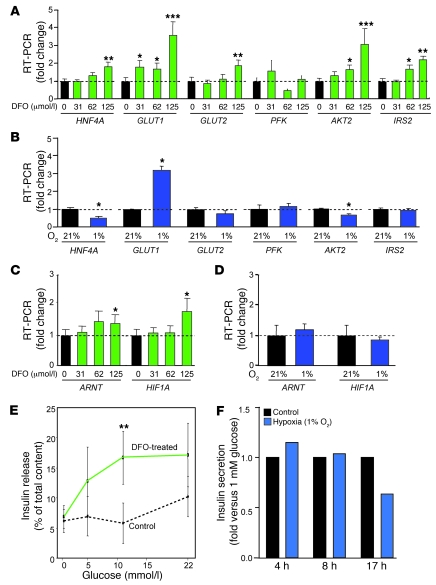
Given the deleterious effects of β cell HIF-1α disruption and the beneficial effects of DFS in vivo, we examined the effects of increasing HIF-1α with DFO upon cultured human islets and compared this with the effects of hypoxia. Islets were cultured with DFO at the doses shown (μmol/l) or at normoxia (21% oxygen) or hypoxia (1% oxygen). The human therapeutic dose is approximately 125 μmol/l. DFO caused dose-dependent increases in HNF4A, GLUT1, GLUT2, AKT2, and IRS2 mRNAs (Figure 5A). In contrast, hypoxia increased GLUT1 but not GLUT2, AKT2, or the other genes shown (Figure 5B). Interestingly, there was a small but significant increase in ARNT mRNA with DFO (Figure 5C), which was not seen with hypoxia (Figure 5D). There was a significant increase in GSIS at moderate hyperglycemia in DFO-cultured human islets compared with control-cultured islets from the same donors (Figure 5E). The approximately 60% increase at high glucose was usual for recently isolated human islets. As expected, GSIS was not improved in hypoxic human islets and declined with longer exposure (Figure 5F).
Figure 5. DFO improves gene expression and insulin secretion from human islets.
(A) DFO increased expression of several genes in isolated human islets. (B) Hypoxic culture increased GLUT1 expression but decreased that of HNF4A and AKT2. (C) DFO increased expression of ARNT and HIF1A. (D) Hypoxia did not alter ARNT or HIF1A expression. (E) DFO increased insulin secretion in isolated human islets. (F) GSIS was not improved in hypoxic islets and declined with longer exposure. *P < 0.05, **P < 0.01, and ***P < 0.001.
Hif1a regulated Arnt.
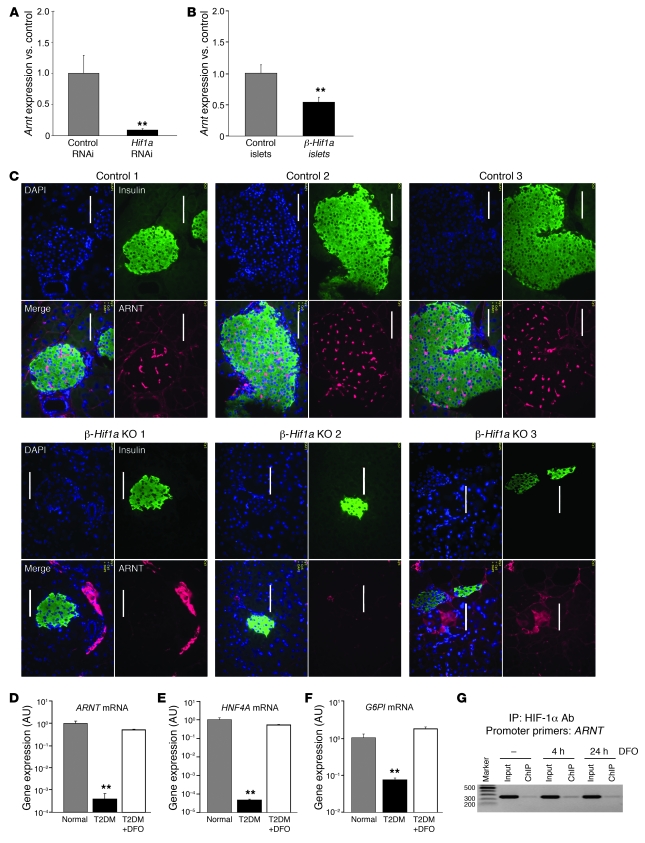
Having observed a small increase in ARNT expression with DFO treatment in normal human islets, we examined the effects of manipulating HIF-1α upon Arnt expression. RNAi-mediated knockdown of Hif1a in Min6 cells caused a more than 80% decrease in Arnt expression (Figure 6A; P < 0.01), and islets from β-Hif1a-null mice had a 50% decrease in Arnt expression (Figure 6B; P < 0.01), which was similar to the reduction in Hif1a itself. This was paralleled by a decrease in ARNT protein in immunostained pancreas of β-Hif1a-null versus floxed control mice (Figure 6C, compare bottom and top panels). Together these experiments show that decreasing HIF-1α in islets and β cells led to decreased ARNT.
Figure 6. HIF-1α regulates expression of ARNT and downstream genes.
(A) Hif1a RNAi in Min6 cells decreased Arnt expression. (B) β-Hif1a-null mice had decreased Arnt mRNA compared with floxed controls. (C) ARNT protein was decreased in β-Hif1a-null islets versus floxed controls. Scale bar: 50 μm. (D) ARNT mRNA was decreased in islets from people with T2D. DFO increased ARNT expression to levels that did not differ significantly from normal. (E) HNF4A mRNA was decreased in islets from people with T2D and was increased by DFO. (F) G6PI expression was decreased in islets from people with T2D and was increased by DFO. (G) HIF-1α associated with the proximal Arnt promoter by ChIP. **P < 0.01.
Increasing HIF-1α in human T2D islets increased ARNT, HNF4A, and G6PI expression.
Human islets isolated from a new cohort of 3 people with T2D had significantly decreased HIF1A and ARNT expression (Figure 1A and Figure 6D). We examined the effect of culturing T2D islets with DFO. In this new group of T2D donors, we also found a more than 80% decrease in ARNT expression (Figure 6D). HNF4A and G6PI were also decreased, consistent with our previous report (Figure 6, E and F). Culture of islets from the same T2D donors with DFO increased ARNT to near-normal levels (Figure 6D; P < 0.01). DFO increased HNF4A and G6PI expression to near-normal levels (Figure 6, E and F; P < 0.01 for both genes). Similar results were also seen for AKT2 (data not shown).
To determine whether HIF-1α bound directly to the Arnt promoter, we performed ChIP assays. In Min6 cells, HIF-1α antibodies pulled down the proximal Arnt promoter (Figure 6G). The amplified sequence (primers in Methods) contains a potential hypoxia-response element, GCGTG.
Differential effects with different methods of increasing HIF-1α.
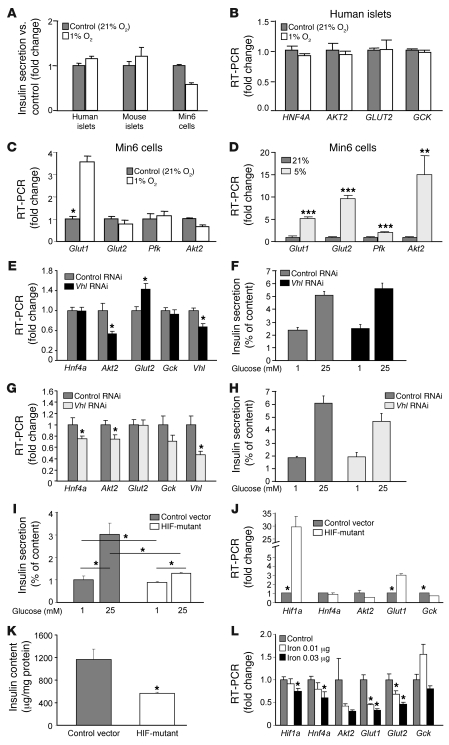
We examined the effects of hypoxia, Vhl knockdown, and DFO in isolated islets and Min6 cells with hypoxia in order to examine the mechanisms behind the different effects of DFO/DFS compared with hypoxia and to investigate the different results compared with those reported with Vhl knockouts (46–48). As shown in Figure 5F and Figure 7A, 1% oxygen did not promote GSIS. In human islets (Figure 7B) and Min6 cells (Figure 7C), 1% oxygen also did not increase expression of GLUT2 or AKT2. As expected, 1% oxygen increased Glut1 expression in Min6 cells. Interestingly, changes in gene expression differed between 5% oxygen and 1% oxygen. In cells exposed to 5% oxygen, Glut1, Glut2, and Akt2 increased (Figure 7D). RNAi-induced Vhl knockdown in Min6 cells decreased Vhl mRNA by 33%. This led to a small but significant increase in Glut2 (Figure 7E) and a nonsignificant increase in GSIS (Figure 7F). By doubling RNAi concentrations, approximately 55% Vhl knockdown was achieved. This led to decreased Hnf4a and Akt2 expression, and the increase in Glut2 was lost (Figure 7G). This was accompanied by a nonsignificant impairment in GSIS (Figure 7H).
Figure 7. The differing effects on gene expression and insulin secretion with increasing HIF-1α levels using hypoxia or VHL RNAi.
(A) Insulin secretion was not improved in human islets, mouse islets, or Min6 cells cultured under hypoxic conditions. (B) One percent oxygen did not increase GLUT2 expression in human islets. (C) One percent oxygen did not increase Glut2 expression in Min6 cells. (D) Moderate hypoxia (5% oxygen) increased Glut2 expression. (E) Modest Vhl knockdown (35%) increased expression of Glut2 and was associated with a nonsignificant increase in insulin release (F). (G) High-dose Vhl knockdown achieved a 55% decrease in Vhl and did not increase Glut2 expression. (H) High-dose Vhl RNAi lowered insulin secretion nonsignificantly. (I) Transfection with proline-to-alanine mutant HIF-1α significantly impaired insulin secretion. (J) Hif1a expression was increased more than 29-fold and was accompanied by increased Glut1 expression and decreased Gck. (K) Total insulin content was decreased in the proline mutant HIF–overexpressing cells. (L) Ferric citrate treatment significantly decreased Hif1a expression and was accompanied by decreased expression of Hnf4a, Akt2, Glut1, and Glut2. *P < 0.05, **P < 0.01, and ***P < 0.001.
Increasing HIF-1α levels by transfecting a proline-to-alanine mutant caused significant impairment in GSIS (Figure 7I). This was associated with the expected increases in Hif1a (>29-fold) and Glut1 (>3-fold). However, there was also a significant decrease in expression of Gck (Figure 7J). Interestingly, there was significantly decreased total insulin content in the mutant-HIF-1α–transfected cells (approximately 50% of vector transfected; Figure 7K).
Removing iron by chelation improved HIF-1α activity and β cell function. The effect of adding iron in the form of ferric citrate was studied. At high doses, there was obvious cell death. At lower doses, there were significant decreases in Hif1a, Hnf4a, Glut1, and Glut2 expression (Figure 7L). This identifies iron as a potential regulator of HIF1A in β cells. There was also significantly impaired GSIS, with only a nonsignificant 13% increase, following high glucose in iron-treated cells (P = 0.017 versus control high glucose).
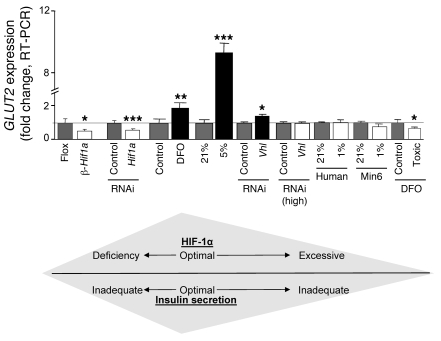
DFO treatment at 16-times the therapeutic dose (2,000 μmol/l) also decreased GLUT2 by 34% (Figure 8). Changes in GLUT2 expression with various treatments are compiled in Figure 8. Glut2 generally corresponded to GSIS, with lower Glut2 expression and lower insulin secretion in β-Hif1a-null islets after HIF-1α knockdown with ferric citrate, reported for genetic VHL deletion (46, 47), and with toxic doses of DFO.
Figure 8. Modest increases in HIF-1α improve insulin secretion.
Changes in HIF-1α, which were associated with decreased GLUT2, were associated with impaired insulin secretion. *P < 0.05, **P < 0.01, and ***P < 0.001.
Discussion
People with T2D characteristically have pronounced impairment of first-phase insulin secretion (50–52). This defect is intrinsic, as it persists in isolated islets (53, 54) and is relatively glucose specific (54, 55) in the earlier stages of the disease. In previous studies, we demonstrated that T2D islets had decreased ARNT expression and, using gene inactivation approaches, showed that this could contribute to altered β cell function. In the present study, we show that T2D islets had decreased HIF-1α, which we show is also important for islet function. Deletion of HIF-1α in C57BL/6 mice resulted in impaired ATP generation and impaired glucose tolerance, accompanied by altered gene expression. Similar results were found in Min6 cells using RNAi. The β cell defect was relatively glucose specific, with only approximately 25% impairment in KCl-stimulated insulin release.
Although HIF-1α protein is tightly regulated, several methods of increasing it exist. These include hypoxia, decreasing VHL protein, mutation or decreased expression of the prolyl hydroxylases, treatment with heavy metals such as cobalt chloride, and iron chelation. Severe hypoxia and cobalt chloride are toxic. Genetic modification is not usually a therapeutic option for humans, although the future possibility exists with antisense RNA strategies. Thus, we studied the effects of iron chelation with DFS or DFO.
Treating mice made diabetic by high-fat feeding with DFS improved glucose tolerance. DFS was also effective in C57BL/6 and Balb/c wild-type mice but was completely ineffective in mice lacking β cell HIF-1α, demonstrating that HIF-1α is required for the benefit. Our HFD had 45% of calories from fat, compared with 12% in normal chow. While this is high, the average fat intake in the American diet is more than 30%. The top 20% of the population consume 46% of calories from fat (56, 57). DFS was effective despite continuing the HFD, suggesting that it may be effective in people with T2D, in whom high fat-intake is common.
Surprisingly, DFO treatment normalized ARNT and other genes in T2D islets. HIF-1α is predominantly regulated at the protein level, and DFO treatment was apparently sufficient to normalize HIF-1α function, as assessed by expression of downstream genes. The magnitude of effect on ARNT, HNF4A, and G6PI was large in T2D islets (>10-fold), in which basal HIF-1α was low. This is the first time that a strong regulator of ARNT expression has been identified. In contrast, the change in ARNT expression in normal islets, in which HIF-1α was not low at baseline was modest. Hif1a itself was decreased by treatment with iron.
In other cell types, HIF-1α regulates PDK1 (10, 11), COX4.1, COX4.2, and LON protease changes associated with increased ATP (58). Consistent with these studies, we observed that decreasing HIF-1α decreased ATP. ATP generation is required in β cells for sensing of glucose, which in turn triggers insulin release. Furthermore, impaired ATP generation in our models was associated with impaired insulin release, consistent with HIF-1α being a regulator of β cell energy homeostasis and insulin release. Thus, decreased HIF-1α impaired glucose-stimulated ATP generation, providing the mechanism by which decreased availability of a transcription factor can cause β cell dysfunction.
Recently, 3 groups reported clear, adverse effects of homozygous deletion of VHL upon β cell function (46–48). In these models, there was a massive increase in HIF-1α protein. Disruption of VHL was accompanied by adverse gene expression changes, increased lactate, and severely impaired GSIS. These findings are interesting but were unexpected as heterozygous whole-body VHL knockout mice appeared grossly normal (59), with 5%–25% of mice developing abnormal vascular lesions in later life on some genetic backgrounds (60). People with VHL syndrome may develop endocrine pancreatic tumors, pancreatic cysts, and occasional insulinomas (16, 61–63). However, less than 3% of patients are reported to have abnormal glucose tolerance, despite frequently requiring steroids and/or pancreatic surgery (16, 61, 62, 64). This suggests that a heterozygous germline mutation, as occurs in VHL patients, may not cause an increased risk of diabetes. Mutations in subunits of the succinate dehydrogenase complex (65, 66) and the HIF-1α prolyl hydroxylases are also associated with increased HIF-1α protein, but there are no reported alterations in diabetes incidence.
Loss of VHL was associated with decreased Glut2 mRNA (46, 47). We also found decreased Glut2 with DFO at 16-times the therapeutic dose, exposure to 1% oxygen, high-dose VHL RNAi, and supplemental iron. In contrast, therapeutic levels of DFO and 5% oxygen treatment both caused different changes in gene expression, and in particular, Glut2 was increased. The different changes in gene expression with different methods of increasing HIF-1α were in accordance with the changes in β cell function (Figure 8).
Thus, there appears to be a dose-response curve for HIF-1α (Figure 8). Deletion is deleterious in C57BL/6 mice and Min6 cells. Mild increases are beneficial for β cell function and glucose tolerance but very high levels, such as those achieved with homozygous VHL deletion, severe hypoxia, or overexpression of a degradation resistant mutant, are clearly deleterious for β cell function.
Hydroxylation and proteolysis of HIF-1α requires iron, which is chelated by DFO and DFS. Iron overload due to transfusion dependency or hemochromatosis can cause β cell dysfunction and increases diabetes incidence (67, 68). It is perhaps less widely recognized that in the absence of transfusion-dependent iron overload or hemochromatosis, increases in serum ferritin or transferrin saturation are associated with increased risk of diabetes and the metabolic syndrome (69–74). High dietary iron intake is also associated with diabetes (71, 75). Conversely, venesection and blood donation can improve β cell function in people with diabetes (70, 76). Regular blood donation has been reported to protect against diabetes, as does a vegetarian diet (67, 70, 76). Disruption of the HIF-1α–partner ARNT in endothelial cells leads to pronounced iron accumulation in the liver (77), suggesting the intriguing potential for a vicious cycle of decreased HIF-1α, decreased ARNT, increased iron accumulation, and decreased HIF-1α. Based on our data and the absence of a DFS effect in mice lacking β cell HIF-1α, we postulate that a decrease in HIF-1α may be a mechanism contributing to the increased risk of diabetes with increased iron.
In summary, these studies demonstrate that β cell HIF-1α is important for β cell reserve, and increasing HIF-1α by iron chelation markedly improved glucose tolerance on a HFD. Increasing HIF-1α normalized gene expression in T2D islets. Therefore, we propose that increasing HIF-1α by iron chelation may be a valid therapeutic strategy for the treatment of human T2D.
Methods
Human islet studies were approved by St. Vincent’s Clinical School Human Research Ethics Committee. All participants gave informed consent. Animal studies were approved by the Garvan Institute Animal Ethics Committee. Human islets were purified using the modified Ricordi method as previously described (22). RNA was isolated using Qiagen RNeasy kits. Gene expression was measured by real-time PCR using the Invitrogen RT-for-PCR kit. The second step was performed in an ABI Prism 7700 Sequence Detection System (Applied Biosciences) with LightCycler-RNA Master SYBR Green I (Roche). Primers are in Supplemental Table 1. Every plate included a control gene (TATA-box binding protein/TBP) for every subject.
Immunohistochemistry and antibodies.
Slides were cut from paraffin-embedded pancreata. Antibodies were purchased from Novus Biologicals (HIF-1α, HIF2α/EPAS1), Orbigen (AhR), Cell Signaling Technology (insulin), or BD Biosciences (ARNT). Primary antibodies were applied overnight at 4°C. Secondary antibodies were Cy2, Cy3, or Cy5 conjugated and applied for 1-hour at room temperature. Slides were viewed on a Zeiss inverted microscope and images were taken with AxioVision software. For each figure, the images were taken in the same session with identical camera settings. For each antibody, species-matched nonimmune immunoglobulin and secondary antibody alone were tested as negative controls. HIF-1α antibody specificity was additionally supported by the lack of β cell HIF-1α staining in knockout mice.
Generation of β-Hif1a-null mice.
β-Hif1a-null mice were generated using the Cre-lox system. Mice, with floxed HIF-1α (49), were bred with mice expressing Cre-recombinase, under control of the rat insulin promoter (RIP-Cre mice). In our colony, RIP-Cre mice did not have abnormal glucose tolerance (Figure 2A). Recombination efficiency was estimated by semiquantitative PCR, using the genotyping primers (49) at 60%–80%. Anti-mouse and anti-rabbit secondary antibodies were from Santa Cruz Biotechnology Inc. ARNT affinity purification was done by binding ARNT antibody (12 μg) to 1 ml of packed protein A/G beads in 5 ml columns. Unbound antibody was removed by washing with 20 ml of PBST. Min6 cells (a gift from J. Miyazaki, Physiological Chemistry, Osaka University, Osaka, Japan; ref. 78) were grown to 80%–90% confluence, washed twice in PBS, and placed in serum-free high glucose DMEM for 4 hours with or without DFO. Cells were scraped into LID lysis buffer with protease inhibitors as previously described (79). Cytoplasmic extracts were collected after centrifugation. The nuclear-containing pellet was disrupted by sonication. Extracts were applied to columns, and the flow-through was reapplied twice to obtain maximal binding. After this, the columns were washed twice with 20 ml of LID buffer, followed by 2 washes with 20 ml of PBST. Bound proteins were eluted with reducing sample buffer and were size separated by 10% SDS-PAGE, followed by protein staining with Coomassie blue (Figure 1D).
For mass spectrometry, gel slices were digested with 5 ng/ml sequencing grade-modified trypsin (Promega) in 25 mM ammonium bicarbonate containing 0.01% n-octylglucoside for 18 hours at 37°C. Peptides were eluted from the gel slices with 80% acetonitrile and 1% formic acid. Tryptic digests were separated by capillary HPLC (C18, 75 mM i.d.; Picofrit column, New Objective), using a flow rate of 100 nl/min over a 3-hour reverse phase gradient, and analyzed using a LTQ linear Ion Trap LC/MSn system (Thermo Electron). Resultant MS/MS spectra were searched against the NCBI Refseq database ( http://www.ncbi.nlm.nih.gov/refseq/) (TurboSequest, BioWorks 3.1, Thermo Electron), with cross-correlation scores of greater than 1.5, 2.0, and 2.5 for charge states U′, u′, and Ʊ, respectively, more than 30% fragment ions, and a ranking of primary score (RsP) value of <3. Proteins were identified with more than 2 unique peptide matches.
Coimmunoprecipitation studies were performed using 2 μg of the indicated antibody and protein A/G beads and by incubating overnight with the indicated cell lysate, followed by washing, elution with reducing sample buffer, and separation by 10% SDS-PAGE. Proteins were detected with the indicated antibody, followed by the appropriate HRP-conjugated secondary antibody, and detection by enhanced chemiluminescence. For each antibody, species-matched nonimmune immunoglobulin and antibody-alone lanes were tested as negative controls to confirm antibody specificity.
Islet isolation from mice.
Islets were isolated from mice as previously described (22). All mice except for the Balb/c mice were inbred C57BL/6 for at least 12 generations.
DFO and hypoxia treatment.
DFO treatment was at 125 μM for 4 hours, unless otherwise specified. Hypoxia treatments were for 2 hours, unless otherwise indicated. Hypoxia was achieved with a hypoxic chamber and an oxygen sensor to confirm levels.
Alanine HIF-1α mutant.
Proline residues 402 and 577, in the murine HIF-1α cDNA, were mutated by site-directed mutagenesis to alanine and the construct was cloned into the pcDNA3 vector and sequenced. The construct and the vector were transfected into Min6 cells using Lipofectamine 2000, according to the manufacturer’s instructions, and selected using geneticin for 1 week. Total insulin content was measured and corrected for total protein, which was measured by DC Bradford assay.
In vivo testing.
GTTs, GSIS, in vitro GSIS, β cell mass, and mRNA expression in islets were assessed as previously described (22). AUC for the GTTs was calculated using the trapezoidal method. Insulin tolerance tests were performed by injecting insulin at 0.5 U/kg and measuring glucose at the times shown.
Measurement of intracellular ATP concentrations.
ATP concentrations were measured in islets and in Min6 cells following basal culture in 1 mM glucose for 1 hour, followed by washing and exposure to 1 mM or 25 mM glucose for 15 minutes. Cells were then placed on ice, washed twice in ice-cold PBS, and lysed. ATP was measured using the Roche Bioluminescence kit. Results were corrected for total protein.
RNAi treatment of Min6 cells and insulin release.
Using Min6 cells, HIF-1α, HIF-2α, AhR, and ARNT were decreased by treatment with smartpool RNAi (Dharmacon) and transfected using Lipofectamine 2000 (Invitrogen), according to the respective manufacturers’ protocols. Scrambled-sequence RNAi was used as a control in all experiments. Total RNAi concentrations were the same for the combination experiments (e.g., 3-times control versus HIF-1α plus AhR plus HIF-2α versus 2-times control plus HIF-1α). Cy3-labelled RNAi and FACS sorting were used to determine transfection efficiency, which was more than 75% (data not shown). Experiments were performed 48 hours after transfection. GSIS was assessed in triplicate wells in 3 separate experiments and corrected for total insulin content. In separate experiments, RNA was isolated for real-time PCR.
HFD studies.
Male floxed control (n = 17) or β-Hif1a-null mice (n = 10) had GTTs as described above. They were then placed on a HFD, based on Rodent Diet no. D12451 from Research Diets Incorporated, which contained 45% of calories from fat (lard). DFS was thoroughly mixed into the vitamin mix during diet formulation to achieve a 30 mg/kg/d dose. This was calculated by measuring food intake of separate C57BL/6 mice on HFD (HFD [g]/mouse weight [g]/d) and calculating accordingly.
T2D culture with DFO.
Islets were freshly isolated from 3 individuals with T2D. Islets were cultured overnight, in either control medium or control medium with 125 μM DFO, prior to RNA isolation.
ChIP.
ChIP was performed using the Active Motif kit (Carlsbad), according to the manufacturer’s instructions. The ARNT promoter primers were GCTTCCTAGCTCAGGCTTCC and AAGAGCCACTCCGCAGATTA, which produce a 250-bp band, which incorporates a GCGTG sequence.
Statistics.
Statistics were calculated in Excel or in SPSS version 14. Unless otherwise specified, Student’s t test with unequal variance was used to compare groups. For all figures, error bars indicate ± SEM. P values of less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
J.E. Gunton was funded by the National Health and Medical Research Council of Australia (NHMRC), Diabetes Australia Research Trust, Juvenile Diabetes Research Foundation (JDRF), the Royal Australasian College of Physicians Pfizer and Servier postdoctoral fellowships, and the L’Oreal Australian For Women in Science fellowship. W.J. Hawthorne, P.J. O’Connell, T. Loudovaris, and T.W. Kay were funded by JDRF and NHMRC. C.R. Kahn was supported by the Mary K. Iacocca Professorship and NIH grants RO1 DK33201 and DK60837-02. R.N. Kulkarni was supported by NIH grants K08, DK02885, and R01 DK67536. A.V. Biankin is supported by a Cancer Institute New South Wales fellowship. A.V. Biankin and J.G. Kench are supported by an NHMRC program grant. We would like to thank Andrew Dwyer, Sof Andrikopoulos, Cecile King, James Cantley, and Don Chisholm for helpful comments; Amber Johns for assistance with the human pancreatic slides; Alice Boulghourjian from the Garvan histology-core; Will Hughes from the Garvan microscope-core; Ed Feener from the Joslin Proteomics Core Facility DERC; staff at BTF for maintaining the mice; and Tina Patel and Lindy Williams from Westmead and Lina Mariana from St. Vincent’s Melbourne for human islet isolations.
Footnotes
Conflict of interest: Rohit N. Kulkarni declares that his laboratory received research funding from Novartis for an unrelated project.
Citation for this article: J Clin Invest. 2010;120(6):2171–2183. doi:10.1172/JCI35846.
References
- 1.Hofer T, Wenger H, Gassmann M. Oxygen sensing, HIF-1α stabilization and potential therapeutic strategies. Pflugers Arch. 2002;443(4):503–507. doi: 10.1007/s00424-001-0759-8. [DOI] [PubMed] [Google Scholar]
- 2.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 3.Kotch LE, Iyer NV, Laughner E, Semenza GL. Defective vascularization of HIF-1α-null embryos is not associated with VEGF deficiency but with mesenchymal cell death. Dev Biol. 1999;209(2):254–267. doi: 10.1006/dbio.1999.9253. [DOI] [PubMed] [Google Scholar]
- 4.Iyer NV, et al. Cellular and developmental control of O2 homeostasis by hypoxia inducible factor 1α. . Genes Dev. 1998;12(2):149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowden Dahl KD, et al. Hypoxia-inducible factors 1{alpha} and 2{alpha} regulate trophoblast differentiation. Mol Cell Biol. 2005;25(23):10479–10491. doi: 10.1128/MCB.25.23.10479-10491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan HE, Lo J, Johnson RS. HIF-1[alpha] is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17(11):3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown LM, et al. Reversing hypoxic cell chemoresistance in vitro using genetic and small molecule approaches targeting hypoxia inducible factor-1. Mol Pharmacol. 2006;69(2):411–418. doi: 10.1124/mol.105.015743. [DOI] [PubMed] [Google Scholar]
- 8.Buchler P, et al. Hypoxia-inducible factor 1 regulates vascular endothelial growth factor expression in human pancreatic cancer. Pancreas. 2003;26(1):56–64. doi: 10.1097/00006676-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Walmsley SR, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201(1):105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3(3):187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3(3):177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Hopfl G, et al. Rescue of hypoxia-inducible factor-1α-deficient tumor growth by wild-type cells is independent of vascular endothelial growth factor. Cancer Research. 2002;62(10):2962–2970. [PubMed] [Google Scholar]
- 13.Maxwell PH, Pugh CW, Ratcliffe PJ. Activation of the HIF pathway in cancer. Curr Opin Genet Dev. 2001;11(3):293–299. doi: 10.1016/s0959-437x(00)00193-3. [DOI] [PubMed] [Google Scholar]
- 14.Ryan HE, et al. Hypoxia-inducible factor-1α is a positive factor in solid tumor growth. Cancer Research. 2000;60(15):4010–4015. [PubMed] [Google Scholar]
- 15.Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14(16):1983–1991. [PubMed] [Google Scholar]
- 16.Woodward ER, Maher ER. Von Hippel-Lindau disease and endocrine tumour susceptibility. Endocr Relat Cancer. 2006;13(2):415–425. doi: 10.1677/erc.1.00683. [DOI] [PubMed] [Google Scholar]
- 17.Guillemin K, Krasnow MA. The hypoxic response: Huffing and HIFing. Cell. 1997;89(1):9–12. doi: 10.1016/S0092-8674(00)80176-2. [DOI] [PubMed] [Google Scholar]
- 18.Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol. 2004;36(2):189–204. doi: 10.1016/S1357-2725(03)00211-5. [DOI] [PubMed] [Google Scholar]
- 19.Bunn HF, Poyton RO. Oxygen sensing and molecular adoption to hypoxia. Physiological Reviews. 1996;76(3):839–885. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 20.Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1alpha): its protein stability and biological functions. Exp Mol Med. 2004;36(1):1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 21.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5(5):343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 22.Gunton JE, et al. Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;122(3):337–349. doi: 10.1016/j.cell.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 23.Wang XL, et al. Ablation of ARNT/HIF1β in liver alters gluconeogenesis, lipogenic gene expression, and serum ketones. Cell Metabolism. 2009;9(5):428–439. doi: 10.1016/j.cmet.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lum JJ, et al. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21(9):1037–1049. doi: 10.1101/gad.1529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ratcliffe PJ. HIF-1 and HIF-2: working alone or together in hypoxia. J Clin Invest. 2007;117(4):862–865. doi: 10.1172/JCI31750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruas JL, Poellinger L. Hypoxia–dependent activation of HIF into a transcriptional regulator. Semin Cell Dev Biol. 2005;16(4–5):514–522. doi: 10.1016/j.semcdb.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95(14):7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salceda S, Caro J. Hypoxia-inducible factor 1α (HIF-1α) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox induced changes. J Biol Chem. 1997;272(36):22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 29.Triantafyllou A, Liakos P, Tsakalof A, Georgatsou E, Simos G, Bonanou S. Cobalt induces hypoxia-inducible factor-1alpha (HIF-1alpha) in HeLa cells by an iron-independent, but ROS-, PI-3K- and MAPK-dependent mechanism. Free Radic Res. 2006;40(8):847–856. doi: 10.1080/10715760600730810. [DOI] [PubMed] [Google Scholar]
- 30.Maxwell PH, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 31.Epstein ACR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107(1):43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 32.Jaakkola P, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 33.Stroka DM, et al. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001;15(13):2445–2453. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- 34.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9(6):677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 35.Cramer T, et al. HIF-1α is essential for myeloid cell–mediated inflammation. Cell. 2003;112(5):645–657. doi: 10.1016/S0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonello S, et al. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol. 2007;27(4):755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- 37.Biswas S, Gupta MK, Chattopadhyay D, Mukhopadhyay CK. Insulin-induced activation of hypoxia-inducible factor-1 requires generation of reactive oxygen species by NADPH oxidase. Am J Physiol Heart Circ Physiol. 2007;292(2):H758–H766. doi: 10.1152/ajpheart.00718.2006. [DOI] [PubMed] [Google Scholar]
- 38.Kaelin WG., Jr ROS: really involved in oxygen sensing. Cell Metabolism. 2005;1(6):357–358. doi: 10.1016/j.cmet.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B. Insulin induces transcription of target genes through the hypoxia inducible factor HIF-1alpha/ARNT. EMBO J. 1998;17(17):5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK. Phosphatidylinositol 3-kinase signalling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 2001;12(7):363–369. [PubMed] [Google Scholar]
- 41.Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signalling pathway. J Biol Chem. 2002;277(31):27975–27981. doi: 10.1074/jbc.M204152200. [DOI] [PubMed] [Google Scholar]
- 42.Doronzo G, Russo I, Mattiello L, Riganti C, Anfossi G, Trovati M. Insulin activates hypoxia-inducible factor-1alpha in human and rat vascular smooth muscle cells via phosphatidylinositol-3 kinase and mitogen-activated protein kinase pathways: impairment in insulin resistance owing to defects in insulin signalling. Diabetologia. 2006;49(5):1049–1063. doi: 10.1007/s00125-006-0156-0. [DOI] [PubMed] [Google Scholar]
- 43.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1α (HIF-1α) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21(12):3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carlsson PO, Liss P, Andersson A, Jansson L. Measurements of oxygen tension in native and transplanted rat pancreatic islets. Diabetes. 1998;47(7):1027–1032. doi: 10.2337/diabetes.47.7.1027. [DOI] [PubMed] [Google Scholar]
- 45.Carlsson PO, Palm F. Oxygen tension in isolated transplanted rat islets and in islets of rat whole-pancreas transplants. Transpl Int. 2002;15(11):581–585. doi: 10.1111/j.1432-2277.2002.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 46.Cantley J, et al. Deletion of the von Hippel-Lindau gene in pancreatic beta cells impairs glucose homeostasis in mice. J Clin Invest. 2009;119(1):125–135. doi: 10.1172/JCI26934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puri S, Cano DA, Hebrok M. A role for von Hippel-Lindau protein in pancreatic beta-cell function. Diabetes. 2009;58(2):433–441. doi: 10.2337/db08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zehetner J, et al. pVHL is a regulator of glucose metabolism and insulin secretion in pancreatic beta-cells. Genes Dev. 2008;22(22):3135–3146. doi: 10.1101/gad.496908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomita S, et al. Defective brain development in mice lacking the Hif-1alpha gene in neural cells. Mol Cell Biol. 2003;23(19):6739–6749. doi: 10.1128/MCB.23.19.6739-6749.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerich JE. The genetic basis of type 2 diabetes mellitus: impaired insulin secretion versus impaired insulin sensitivity. Endocr Rev. 1998;19(4):491–503. doi: 10.1210/er.19.4.491. [DOI] [PubMed] [Google Scholar]
- 51.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 52.Bagdade JD, Bierman EL, Porte D., Jr The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest. 1967;46(10):1549–1557. doi: 10.1172/JCI105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marchetti P, et al. Pancreatic islets from type 2 diabetic patients have functional defects and increased apoptosis that are ameliorated by metformin. J Clin Endocrinol Metab. 2004;89(11):5535–5541. doi: 10.1210/jc.2004-0150. [DOI] [PubMed] [Google Scholar]
- 54.Del Guerra S, et al. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes. 2005;54(3):727–735. doi: 10.2337/diabetes.54.3.727. [DOI] [PubMed] [Google Scholar]
- 55.Deng S, et al. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes. 2004;53(3):624–632. doi: 10.2337/diabetes.53.3.624. [DOI] [PubMed] [Google Scholar]
- 56.Hu FB, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337(21):1491–1499. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]
- 57.Miller WC, Niederpruem MG, Wallace JP, Lindeman AK. Dietary fat, sugar, and fiber predict body fat content. J Am Diet Assoc. 1994;94(6):612–615. doi: 10.1016/0002-8223(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 58.Fukuda R, Zhang H, Kim J, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129(1):111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 59.Gnarra JR, et al. Defective placental vasculogenesis causes embryonic lethality in VHL-deficient mice. Proc Natl Acad Sci U S A. 1997;94(17):9102–9107. doi: 10.1073/pnas.94.17.9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kleymenova E, Everitt JI, Pluta L, Portis M, Gnarra JR, Walker CL. Susceptibility to vascular neoplasms but no increased susceptibility to renal carcinogenesis in Vhl knockout mice. Carcinogenesis. 2004;25(3):309. doi: 10.1093/carcin/bgh017. [DOI] [PubMed] [Google Scholar]
- 61.Chetty R, Kennedy M, Ezzat S, Asa SL. Pancreatic endocrine pathology in von Hippel-Lindau disease: an expanding spectrum of lesions. Endocr Pathol. 2004;15(2):141–148. doi: 10.1385/EP:15:2:141. [DOI] [PubMed] [Google Scholar]
- 62.Perigny M, et al. Pancreatic endocrine microadenomatosis in patients with von Hippel-Lindau disease: characterization by VHL/HIF pathway proteins expression. Am J Surg Pathol. 2009;33(5):739–748. doi: 10.1097/PAS.0b013e3181967992. [DOI] [PubMed] [Google Scholar]
- 63.Lott ST, et al. High frequency loss of heterozygosity in von Hippel-Lindau (VHL)-associated and sporadic pancreatic islet cell tumors evidence for a stepwise mechanism for malignant conversion in VHL Tumorigenesis 1. Cancer Res. 2002;62(7):1952–1955. [PubMed] [Google Scholar]
- 64.Hammel PR, et al. Pancreatic involvement in von Hippel-Lindau disease. Gastroenterology. 2000;119(4):1087–1095. doi: 10.1053/gast.2000.18143. [DOI] [PubMed] [Google Scholar]
- 65.Pollard PJ, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1{alpha} in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14(15):2231–2239. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 66.Ciara MM, Diana EB, Deborah JM, Bruce GR, Margaret RZ. K40E: a novel succinate dehydrogenase (SDH)B mutation causing familial phaeochromocytoma and paraganglioma. Clin Endocrinol (Oxf). 2004;61(4):510–514. doi: 10.1111/j.1365-2265.2004.02122.x. [DOI] [PubMed] [Google Scholar]
- 67.Ascherio A, Rimm EB, Giovannucci E, Willett WC, Stampfer MJ. Blood donations and risk of coronary heart disease in men. Circulation. 2001;103(1):52–57. doi: 10.1161/01.cir.103.1.52. [DOI] [PubMed] [Google Scholar]
- 68.De Sanctis, V, Zurlo MG, Senesi E, Boffa C, Cavallo L, Di Gregorio F. Insulin dependent diabetes in thalassaemia. Arch Dis Child. 1988;63(1):58–62. doi: 10.1136/adc.63.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ford ES, Cogswell ME. Diabetes and serum ferritin concentration among US adults. Diabetes Care. 1999;22(12):1978–1983. doi: 10.2337/diacare.22.12.1978. [DOI] [PubMed] [Google Scholar]
- 70.Fernandez-Real JM, Lopez-Bermejo A, Ricart W. Iron stores, blood donation, and insulin sensitivity and secretion. Clin Chem. 2005;51(7):1201–1205. doi: 10.1373/clinchem.2004.046847. [DOI] [PubMed] [Google Scholar]
- 71.Jiang R, Manson JE, Meigs JB, Ma J, Rifai N, Hu FB. Body iron stores in relation to risk of type 2 diabetes in apparently healthy women. JAMA. 2004;291(6):711–717. doi: 10.1001/jama.291.6.711. [DOI] [PubMed] [Google Scholar]
- 72.Wrede CE, Buettner R, Bollheimer LC, Scholmerich J, Palitzsch KD, Hellerbrand C. Association between serum ferritin and the insulin resistance syndrome in a representative population. Eur J Endocrinol. 2006;154(2):333–340. doi: 10.1530/eje.1.02083. [DOI] [PubMed] [Google Scholar]
- 73.Salonen JT, Tuomainen TP, Nyyssonen K, Lakka HM, Punnonen K. Relation between iron stores and non–insulin–dependent diabetes in men: case–control study. BMJ. 1999;317(7160):727. doi: 10.1136/bmj.317.7160.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fernández-Real JM, et al. Serum ferritin as a component of the insulin resistance syndrome. Diabetes Care. 1998;21(1):62–68. doi: 10.2337/diacare.21.1.62. [DOI] [PubMed] [Google Scholar]
- 75.Lee DH, Folsom AR, Jacobs DRJ. Dietary iron intake and type 2 diabetes incidence in postmenopausal women: the Iowa Women’s Health Study. Diabetologia. 2004;47(2):185–194. doi: 10.1007/s00125-003-1307-1. [DOI] [PubMed] [Google Scholar]
- 76.Fernandez-Real JM, Penarroja G, Castro A, Garcia-Bragado F, Hernandez-Aguado I, Ricart W. Blood letting in high-ferritin type 2 diabetes: effects on insulin sensitivity and beta-cell function. Diabetes. 2002;51(4):1000–1004. doi: 10.2337/diabetes.51.4.1000. [DOI] [PubMed] [Google Scholar]
- 77.Yim SH, et al. Disruption of the Arnt gene in endothelial cells causes hepatic vascular defects and partial embryonic lethality in mice. Hepatology. 2006;44(3):550–560. doi: 10.1002/hep.21284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Miyazaki J, et al. Establishment of a pancreatic beta cell line that retains glucose inducible insulin secretion: Special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127(1):126–132. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- 79.Gunton JE, Delhanty PJ, Takahashi S, Baxter RC. Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2. J Clin Endocrinol Metab. 2003;88(3):1323–1332. doi: 10.1210/jc.2002-021394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.