Many genetic and genomic studies are conducted using a phenotype-driven approach: Cases and controls are identified based on the presence or absence of a particular condition and analyses are undertaken to identify gene variants associated with that condition. The inverse—a genotype-driven approach—is receiving increasing attention as another powerful research tool. In this setting, investigators use existing study populations for which genotype or complete sequence data are available to identify cases and controls based on the presence or absence of a particular gene variant. Participants are then recontacted for recruitment into follow-up studies involving in-depth phenotyping to understand the relationship between observable traits and the gene variant of interest. One driver for this framework is the genetics community's increasing focus on rarer gene variants that exert a large effect on risk for common diseases.
Enabling such a “bottom-up” approach to identifying and recruiting participants for follow-up studies could significantly advance the pace of genomic research on the functional significance of human genetic variation (McGuire and McGuire 2008). Genotype-driven recruitment, however, presents considerable ethical challenges. It is inextricably linked to the complex and much-debated issue of disclosing individual research results to participants: When individuals are recontacted, what if anything should they be told about the genotype that led to their being recontacted? There is a fundamental tension between avoiding the disclosure of potentially unwanted and uncertain information, and avoiding deception when explaining to prospective participants the purpose of the research and why they have been identified as eligible to participate.
To resolve this tension, McGuire and McGuire (2008) suggested that, when recontacted, participants should be told that the follow-up study is genotype-driven and what that means, what the genotype and biological pathway of interest is, that half of the participants are controls without the targeted gene variants, and that an invitation to participate is not contingent on the presence of any known phenotype. Here, we report our experiences and participants' reactions when we implemented a similar approach.
Case presentation
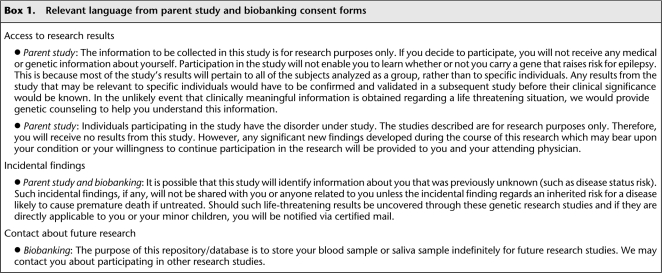
In 2005, researchers at Duke University began a study to identify gene variants associated with epilepsy and with response to antiepileptic drugs (Cavalleri et al. 2007). Participants were recruited from among patients attending adult and pediatric epilepsy clinics at Duke; subjects in another study on the genetics of memory served as controls. The consent form for the epilepsy study stated that participants would not receive individual research results (Box 1). Participants were also asked to sign a separate “biobanking” consent form to permit the long-term storage of biological specimens for possible use in future research. This second consent form alerted participants to the possibility that researchers might contact them about taking part in other studies (Box 1). Both consent forms included a standard statement recommended by the Duke University Health System Institutional Review Board (IRB) that incidental findings would be shared only if they concerned an inherited risk for a disease likely to cause premature death if untreated (Box 1).
Box 1.
Relevant language from parent study and biobanking consent forms
In late 2008, the research team observed that some patients with epilepsy have large heterozygous deletions (>2 Mb in size). Genomic deletions of this magnitude had been reported in patients with schizophrenia (Need et al. 2009); however, they had not been observed in neuropsychiatrically normal subjects and had seldom been seen in the general population (Hansson et al. 2007; Roos et al. 2008; Itsara et al. 2009). Collectively, these findings suggested that such deletions may confer a sizeable risk for neuropsychiatric disease, but additional research was needed to confirm their phenotypic consequences. However, the rarity of these deletions—the most common was observed in 23 out of 3812 patients (Heinzen et al. 2010)—made the task of identifying additional patients with this genotype daunting. One approach to confirming causality was to explore cosegregation patterns in the families of patients with a deletion. In addition, because a subset of these deletions had been shown to confer risk of other neuropsychiatric diseases (Hannes et al. 2008; Mefford et al. 2008; Stefansson et al. 2008; Need et al. 2009), collecting information about comorbidities in patients and family members was important for characterizing phenotypic consequences. Finally, the research team wanted to study the effects of these deletions on gene expression, which required collection of an additional biological sample. Thus, contact with patients already identified as having a deletion was a critical component to validating, understanding, and advancing the genetic association.
When the study coordinator began contacting participants who were eligible for the follow-up study (i.e., patients with a deletion), she started the conversation by reminding them of their participation in the epilepsy study and then stated that the researchers wanted to collect another blood sample and were also wondering whether their family members might be interested in participating in the research. This request was often met with immediate concern about “Why? Did you find something wrong with me?” The coordinator then faced the significant quandary of explaining the purpose of the contact without divulging individual research results.
The research team was initially split about how to address this difficulty. Some felt that participants should be given their results from the parent study, along with an explanation about their uncertainty, in order to avoid dissembling about the reason for recontact. Others felt that disclosing the results would provide no benefit and could cause undue anxiety and guilt among family members. The team sought research ethics consultation and eventually decided to send a letter to all participants in the parent study explaining the preliminary findings in aggregate—with emphasis on what was not known about their clinical validity and utility—and notifying them that they might be contacted about a follow-up study. With regard to access to individual results, the team's proposed letter included the following:
“So that we can learn more, we will be contacting a subset of participants about taking part in a follow-up study. Taking part in this follow-up study requires (a) that you learn your individual results from the original study, and (b) that you pass along information about the follow-up study to your family members so that they can call us if they want to take part. If you DO NOT want to be contacted about this follow-up study, please call [study coordinator] at [number] by [date] to let us know.”
This approach was intended to preserve participants' “right not to know” (Husted 1997) their genetic information, yet also allow the study coordinator to discuss openly the purpose of the study and the selection criteria during recruitment conversations.
When consulted about this plan, however, the IRB preferred that participants not be offered individual results, in part due to statements made to that effect in the original consent documents. More important were the basic ethical principles underlying the consent language, i.e., concern that harm (such as anxiety, guilt, unnecessary and possibly detrimental medical interventions, and unwarranted health and lifestyle decisions) could result from disclosure of unsubstantiated findings (National Bioethics Advisory Commision 1999; Bookman et al. 2006). Thus, the final letter (Appendix) included the statement:
“If we contact you about this follow up study, you should not assume it means that you have the deletion in question. Because we do not know what this deletion means and it will not affect your care right now, we will not be able to confirm whether or not you have the deletion we are studying.”
The letter was sent to all study participants (n = 975) in March 2009. To capitalize on this natural experiment, we created a form to track the responses of those who called as instructed in the letter. No letters were returned as undeliverable, but nine (0.9%) participants were found to have died. Of the 966 remaining, the study coordinator received a response from 51 (5.3%). Of these:
37 (72.5%) opted out of any further contact about the follow-up study
12 (23.5%) called to volunteer for the follow-up study
Two (3.9%) withdrew from the parent study
Although callers were not asked to state a reason for opting out, some noted personal circumstances, such as “no time.” Three said they did not want their family members contacted, one asked not to be contacted about any future studies, and one mother of a child participant said she was opting out because individual results would not be provided and thus the study would not help her son.
The letter did not ask for volunteers for the follow-up study, but those who called to opt in appeared to be happy to have been contacted and enthusiastic about participating in more research. One initially called to say that she was not a participant in the parent study, but, upon confirmation that she was, said she would like to take part in the new study. Another respondent was angry to have received the letter, claiming that she did not have epilepsy and demanding that any such references be removed from her medical records. After lengthy discussion of the broad case definition used (“two unprovoked seizures”) in the parent study, she then expressed interest in participating in the follow-up study.
Another respondent who was similarly angry, and had his attorney send a letter demanding that reference to epilepsy be expunged from medical records, withdrew from the parent study.
In addition to those who called the study coordinator as directed in the letter, the physician who sees a majority of these patients in clinic reported that ∼50 brought the letter up for discussion at their next visit. According to him, the most common reactions were (1) confusion (did not understand the letter); (2) questions about what the study findings meant for them; and (3) disappointment at being “left out” because, despite receiving the letter, they had not been contacted about the follow-up study.
None of those who responded to the letter were in fact eligible for the follow-up study. Among eligible participants contacted after the letter (n = 6), five agreed to participate and one was lost to follow-up. According to the study coordinator's experience, it appears that the letter may have facilitated her communication with participants in some instances by providing a basic foundation for discussion.
Discussion
Concerns about the use and disclosure of genetic information—more commonly associated with participation in genetic research—are shifted to the recruitment process when genetic information that is generated as the result of prior research participation is used as the basis for identifying and recontacting participants about further research. Specifically, harm may ensue if previously unknown and perhaps unwanted information is disclosed as part of the offer to participate in research, prior to actual consent. Concerns are exacerbated by the uncertain nature of most genetic research results: Further research is needed precisely because more must be learned to understand their meaning in terms of risk, inheritance, diagnosis, prognosis, and treatment.
Thus, in genotype-driven recontact and recruitment, a central tension exists between (1) avoiding the prospect of introducing unwanted information, as well as the foreseeable harms associated with conveying unsubstantiated and possibly misleading results; and (2) avoiding deception when explaining to prospective participants the purposes of the research and why they are eligible to participate. A growing number of researchers will likely face this dilemma given the recent and striking realization that, although common gene variants appear to play only a limited role in predisposition to common diseases, rare variants can have a very high impact (Goldstein 2010). Genotype-driven recruitment will become an increasingly important tool as scientists seek to recruit individuals already known to have such rare variants for in-depth phenotyping and further study.
Initial suggestions for how to approach this type of recruitment do not fully address the challenges. Although our letter constituted “pre-recontact” (prior notice that participants might be recontacted about additional research), it is likely that any recontact will spark disavowals from some participants that they are in the parent study or that they have the condition under study. Further, telling prospective participants that half of those being recruited are controls with no targeted gene variants is unlikely to mitigate fears or waylay assumptions that researchers found something in their blood. As stated by a participant in one study (Beskow and Dean 2008), “I can't imagine they would contact me unless they saw something in my blood that interested them and they needed more, needed to know more.”
Ethical issues arising in genotype-driven recruitment must be addressed throughout the research process, including during recruitment and consent for the original study, the recontact process itself, recruitment and consent for the follow-up study, and the conduct and dissemination phases of the follow-up study. There is a critical lack of data on the experiences and opinions of stakeholders, including research participants, researchers, study coordinators, IRB leaders, and physicians, to support the development of evidence-based guidelines. In the interim, we offer the following practical observations based on our experience:
First, genotype-driven studies—particularly those involving rare variants—will often involve identifying and recruiting family members, which itself raises ethical challenges (Beskow et al. 2004). One of the most complicated issues we faced was that many families of patients with epilepsy have spent years searching for the reason their family member is affected. Following a deletion through a family raised the specter of someone in the family assuming responsibility for the proband's epilepsy. This prospect weighed heavily in our decision not to offer results and also highlights the importance for certain kinds of studies of involving a treating physician who is familiar with the patient's condition and family situation.
Second, we sent our letter to all patients in the parent study (as opposed to only those eligible for the follow-up study) to preclude them from making inferences about their genetic results based on receipt of the letter itself. This approach had the unanticipated consequence of some patients being disappointed when they were not recruited for the follow-up study, and also led to the treating physician spending time during clinic visits answering questions about the letter and the study for patients who were not eligible in any event. To reduce the potential burden on those involved, an alternate approach for case-only studies (those that seek to enroll only people with the gene variant of interest) may be to send letters to eligible patients plus only a randomly selected subset rather than the entire parent study population. Even when controls will not be enrolled, being able truthfully to caution recipients of the letter against making assumptions about their genetic status may help preserve their right not to know.
Third, as noted, one mother opted out of further contact because she felt her affected son would not benefit if individual results were not provided. Although our letter explained in detail all that was not known about the results from the first study (see Appendix), perhaps it would have been helpful for the research team to contact her to discuss further that knowing the results would not alter her son's care. More generally, however, her response suggests that it may be important for researchers to consider carefully and convey clearly the circumstances under which results would be shared. In other words, hearing “we will not give you your individual results right now” may be easier to accept if accompanied by “we will give you your results if we learn anything that could be useful to you.” Researchers who are conducting genotype-driven studies may be in a better position than those conducting other, less hypothesis-driven genomic research to anticipate the kinds of findings they might uncover and to devise a plan for managing ethically appropriate disclosure, advice, and referral.
Fourth, defining what kinds of results might be “useful” to patients is a prominent aspect of the continuing and vigorous debate over the general issue of whether or not individual research results should be disclosed to participants (see, e.g., Shalowitz and Miller 2005; Clayton and Ross 2006; Meltzer 2006; Parker 2006; Sharp and Foster 2006; Fernandez 2008; Miller et al. 2008). Clinical utility—the usefulness of the results for informing risk reduction, treatment, or surveillance strategies—has been the most frequently recommended standard (National Bioethics Advisory Commission 1999; Bookman et al. 2006). For results concerning rare, high-impact gene variants, a threshold for disclosure that recognizes the possibility of personal utility, such as life planning or reproductive decision making (Ravitsky and Wilfond 2006), may be cautiously considered if there is strong evidence of analytic and clinical validity (Beskow 2006). Even when individual results will not be disclosed, researchers should communicate about their overall study findings with full awareness that, in some cases, participants who so choose may be able to pursue their results through the growing market for direct-to-consumer genetic testing (American Society of Human Genetics 2007).
Finally, the dissemination of the results of genotype-driven research requires particular attention. For example, when a case-only study is published in the scientific literature, participants may recognize the study and thereby learn their genotype even if researchers had not directly disclosed it to them. For case-control studies, where participation itself does not indicate genotype, participants may still be able to identify themselves in the scientific literature based on pedigree descriptions. Thus, even when privacy is protected in the sense that published information is “anonymous” to others, the risk of harm remains if participants could inadvertently discover unwanted genetic information.
Fundamental arguments for and against disclosure of individual research results to participants are based on the ethical considerations of respect for persons, beneficence, paternalism, reciprocity, and the boundaries between research and clinical practice (Haga and Beskow 2008). To this complex mixture, the emerging approach of genotype-driven recontact adds significant concerns about the prospect of familial disruption and about equivocation during the recruitment process. Further, we described our experiences when investigators went back to their participants for follow-up research on the same medical condition. In another application of genotype-driven recruitment, researchers could identify individuals with particular genotypes across multiple data sets stored in centralized databases, such as dbGaP, and potentially contact them about additional phenotypic studies (McGuire and McGuire 2008). This approach could maximize the utility of the massive amounts of data generated in GWAS, only a tiny fraction of which is related to the disease or condition originally under study—but will raise a host of additional issues. There is an urgent need for guidelines on ethical approaches to genotype-driven recontact and recruitment that provide appropriate protections for research participants, yet avoid overly restrictive policies that have a chilling effect on beneficial research and limit opportunities for those who would like to participate.
Acknowledgments
This report was supported in part by award number RC1HG005787 from the National Human Genome Research Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Human Genome Research Institute or the National Institutes of Health. We thank John Falletta and Gail Henderson for their insights, and Nicole Walley for her valuable assistance with data compilation.
Appendix
Letter to Research Participants
Thank you for your participation in the study entitled “The Genetics and Pharmacogenetics of Epilepsy” at Duke University Medical Center with Dr. Radtke, Dr. Husain, Dr. Gallentine, and Dr. Goldstein. There have been some advances in our research and we wanted to share what we have learned so far with you.
We have discovered that among patients with many different types of epilepsy, some are missing large sections of their DNA. DNA is the genetic code which is unique in each person and plays a role in many of the differences between people, including whether or not some people get diseases when others do not. Even people without a disease sometimes are missing sections of their DNA (a deletion). However, we have discovered that some patients with epilepsy have larger sections missing and we think it is possible that this might contribute to why they have seizures. At this point, there are many things we do not know. We do not know how such a deletion affects epilepsy clinically. We do not know how it affects the risk for getting epilepsy in an individual patient. We do not know what it means for inheriting epilepsy or if it even has any impact on inheritance. These preliminary findings will in no way change the way Dr. Radtke, Dr. Husain or Dr. Gallentine treat your epilepsy. We hope in the future that we will learn more about these deletions and therefore be able to advance the treatment of epilepsy but we are not there at this point.
In order to learn more about our findings we would like to contact some of you to obtain additional blood samples. We may also want to contact your family members, but will not do so without permission from you and from them. If we contact you about this follow up study, you should not assume it means that you have the deletion in question. Because we do not know what this deletion means and it will not affect your care right now, we will not be able to confirm whether or not you have the deletion we are studying. If you DO NOT want to be contacted for follow up please call [study coordinator] at [number]. If we do not hear from you by [date] we will assume you would like to hear more about helping us with this next exciting step.
Finally, we will be submitting our findings for publication. All are of course welcomed and encouraged to read the article; please contact us if you would like a copy. We caution you not to assume that information about you is included in the article, as we have studied thousands of patients and families. The study team will not be able to confirm whether or not information about specific individuals was included in the published results.
We look forward to an exciting year of discovery in epilepsy research and thank you for your continued assistance with this very important research.
Sincerely,
David B. Goldstein, PhD
Rodney A. Radtke, MD
Footnotes
Article published online before print. Article and publication date are at http://www.genome.org/cgi/doi/10.1101/gr.104455.109.
References
- American Society of Human Genetics (ASHG) 2007. Statement on direct-to-consumer genetic testing in the United States. Am J Hum Genet 81: 635–637 [Google Scholar]
- Beskow LM, Botkin JR, Daly M, Juengst ET, Lehmann LS, Merz JF, Pentz R, Press NA, Ross LF, Sugarman J, et al. 2004. Ethical issues in identifying and recruiting participants for familial genetic research. Am J Med Genet 130A: 424–431 [DOI] [PubMed] [Google Scholar]
- Beskow LM 2006. Considering the nature of individual research results. Am J Bioeth 6: 38–40 [DOI] [PubMed] [Google Scholar]
- Beskow LM, Dean E 2008. Informed consent for biorepositories: Assessing prospective participants' understanding and opinions. Cancer Epidemiol Biomarkers Prev 17: 1440–1451 [DOI] [PubMed] [Google Scholar]
- Bookman EB, Langehorne AA, Eckfeldt JH, Glass KC, Jarvik GP, Klag M, Koski G, Motulsky A, Wilfond B, Manolio TA, et al. 2006. Reporting genetic results in research studies: Summary and recommendations of an NHLBI working group. Am J Med Genet 140A: 1033–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalleri GL, Weale ME, Shianna KV, Singh R, Lynch JM, Grinton B, Szoeke C, Murphy K, Kinirons P, O'Rourke D, et al. 2007. Multicentre search for genetic susceptibility loci in sporadic epilepsy syndrome and seizure types: A case-control study. Lancet Neurol 6: 970–980 [DOI] [PubMed] [Google Scholar]
- Clayton EW, Ross LF 2006. Implications of disclosing individual results of clinical research. JAMA 295: 37. [DOI] [PubMed] [Google Scholar]
- Fernandez C 2008. Public expectations for return of results—time to stop being paternalistic? Am J Bioeth 8: 46–48 [DOI] [PubMed] [Google Scholar]
- Goldstein DB 2010. 2020 Visions: Personalised medicine and genomics. Nature (in press) [Google Scholar]
- Haga SB, Beskow LM 2008. Ethical, legal, and social implications of biobanks for genetics research. Adv Genet 60: 505–544 [DOI] [PubMed] [Google Scholar]
- Hannes FD, Sharp AJ, Mefford HC, de Ravel T, Ruivenkamp CA, Breuning MH, Fryns JP, Devriendt K, Van Buggenhout G, Vogels A, et al. 2008. Recurrent reciprocal deletions and duplications of 16p13.11: The deletion is a risk factor for MR/MCA while the duplication may be a rare benign variant. J Med Genet 46: 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson K, Szuhai K, Knijnenburg J, van Haeringen A, de Pater J 2007. Interstitial deletion of 6q without phenotypic effect. Am J Med Genet A 143A: 1354–1357 [DOI] [PubMed] [Google Scholar]
- Heinzen EL, Radtke RA, Urban TJ, Cavalleri GL, Depondt C, Need AC, Walley NM, Nicoletti P, Ge D, Catarino CB, et al. 2010. Rare deletions at 16p13.11 predispose to a diverse spectrum of sporadic epilepsy syndromes. Am J Hum Genet doi: 10.1016/j.ajhg.2010.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husted J 1997. Autonomy and a right not to know. In The right to know and the right not to know, (ed. Chadwick R et al. ), pp. 55–68 Avebury, Aldershot, England [Google Scholar]
- Itsara A, Cooper GM, Baker C, Girirajan S, Li J, Absher D, Krauss RM, Myers RM, Ridker PM, Chasman DI, et al. 2009. Population analysis of large copy number variants and hotspots of human genetic disease. Am J Hum Genet 84: 148–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, McGuire AL 2008. Don't throw the baby out with the bathwater: Enabling a bottom-up approach in genome-wide association studies. Genome Res 18: 1683–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mefford HC, Sharp AJ, Baker C, Itsara A, Jiang Z, Buysse K, Huang S, Maloney VK, Crolla JA, Baralle D, et al. 2008. Recurrent rearrangements of chromosome 1q21.1 and variable pediatric phenotypes. N Engl J Med 359: 1685–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer LA 2006. Undesirable implications of disclosing individual genetic results to research participants. Am J Bioeth 6: 28–30 [DOI] [PubMed] [Google Scholar]
- Miller FA, Christensen R, Giacomini M, Robert JA 2008. Duty to disclose what? Querying the putative obligation to return research results to participants. J Med Ethics 34: 210–213 [DOI] [PubMed] [Google Scholar]
- National Bioethics Advisory Commission (NBAC) 1999. Research involving human biological materials: Ethical issues and policy guidance, Vol. 1 NBAC, Rockville, Maryland [Google Scholar]
- Need AC, Ge D, Weale ME, Maia J, Feng S, Heinzen EL, Shianna KV, Yoon W, Kasperaviciūte D, Gennarelli M, et al. 2009. A genome-wide investigation of SNPs and CNVs in schizophrenia. PLoS Genet 5: e1000373 doi: 10.1371/journal.pgen.1000373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LS 2006. Rethinking respect for persons enrolled in research. Am Soc Bioethics & Humanit Exch 9: 6–7 [Google Scholar]
- Ravitsky V, Wilfond BS 2006. Disclosing individual genetic results to research participants. Am J Bioeth 6: 8–17 [DOI] [PubMed] [Google Scholar]
- Roos A, Elbracht M, Baudis M, Senderek J, Schonherr N, Eggermann T, Schuler HM 2008. A 10.7 Mb interstitial deletion of 13q21 without phenotypic effect defines a further non-pathogenic euchromatic variant. Am J Med Genet A 146A: 2417–2420 [DOI] [PubMed] [Google Scholar]
- Shalowitz DI, Miller FG 2005. Disclosing individual results of clinical research: Implications of respect for participants. JAMA 294: 737–740 [DOI] [PubMed] [Google Scholar]
- Sharp RR, Foster MW 2006. Clinical utility and full disclosure of genetic results to research participants. Am J Bioeth 6: 42–44 [DOI] [PubMed] [Google Scholar]
- Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S, Fossdal R, Sigurdsson E, Sigmundsson T, Buizer-Voskamp JE, et al. 2008. Large recurrent microdeletions associated with schizophrenia. Nature 455: 232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]