Abstract
Background
Renal cell carcinoma (RCC) is recognized as a neoplasm resistant to chemotherapy. In vitro experiments demonstrated that suramin, at noncytotoxic doses, enhanced the activity of chemotherapy including 5-fluorouracil (5-FU) in xenograft models.
Patients and Methods
A phase I/II trial of noncytotoxic suramin in combination with weekly 5-FU in patients with metastatic RCC was conducted. The treatment consisted of intravenous (I.V.) suramin followed by a 500 mg/m2 I.V. bolus of 5-FU given 4.5 hours after starting suramin. In the phase I portion, a cohort of 6 patients received a suramin dose calculated to achieve a plasma level of 10–50 μmol/L. Therapy was administered once weekly for 6 doses, followed by 2 weeks off. This was followed by a phase II portion in which the primary goal was to determine the objective response rate.
Results
Twenty-three patients were enrolled in the study: 6 in the phase I portion and 17 in phase II. Seventy-eight percent of patients were men, the mean age was 58.8 years, 96% had previous nephrectomy, and 70% had received previous systemic therapy. Histologic subtype was clear cell in 91%. Dose-limiting toxicity was observed in 1 of 6 patients (grade 3 hypersensitivity related to suramin infusion). The suramin dosing nomogram used in phase I and II portions of the trial yielded the desired plasma level of 10–50 μmol/L from 4.5 hours to 48 hours after infusion in 94 of 115 treatments. No objective responses were noted, and the median time to treatment failure was 2.5 months. The major toxicities (all grades) were fatigue (83%), nausea/vomiting (78%), diarrhea (61%), and chills (61%).
Conclusion
Suramin levels expected to reverse fibroblast growth factor–induced resistance can be achieved with the dosing regimen used in this study. The toxicity observed with suramin and 5-FU was acceptable. The combination does not have clinical activity in patients with metastatic RCC.
Keywords: Dose-limiting toxicity, Fibroblast growth factor, Pharmacokinetics, Plasma concentration
Introduction
Renal cell carcinoma (RCC) is the most common malignancy of the kidney and accounts for approximately 3% of tumors in adults. Estimates of the annual incidence of RCC indicate steady increases, with over a third of newly diagnosed patients presenting with advanced or metastatic disease.1–4 Cyto-reductive nephrectomy and/or metastasectomy remain important treatment options in patients regardless of stage of disease at presentation.5–7
Recently, systemic therapy for advanced RCC has changed significantly. Cytokines such as interleukin (IL)-2 and interferon (IFN)-α have been used in the past, with an objective response rate (ORR) of approximately 15% and limited or no survival advantage.8–10 Since 2006, 3 new drugs have been approved for patients with advanced RCC, namely sorafenib, sunitinib, and temsirolimus.11–15 These agents are kinase inhibitors that prolong progression-free survival (PFS),11 produce significant tumor regression,13,14 or improve survival in patients with RCC with poor prognostic features.15 Past studies have clearly demonstrated RCC is a chemotherapy-resistant tumor with response rates of ≤ 5%.1,16
Of the earlier agents used before the era of kinase inhibitors, 5-fluorouracil (5-FU) might be one of the most effective.16 Au et al have demonstrated that acidic and basic fibroblast growth factors (aFGF and bFGF) produced by the tumor cells induce drug resistance to agents with diverse structures and mechanisms of action, including 5-FU.17,20 These investigators also reported that suramin at noncytotoxic levels (ie, 10–50 μmol/L) could reverse the FGF-induced resistance and enhance the activity of chemotherapy in animals bearing prostate tumors. Additional data also indicated that high levels of aFGF and bFGF are produced by RCC tumors obtained from patients, and low levels of suramin enhanced the antitumor activity of 5-FU in histocultures of tumors.17,18 An earlier phase II clinical trial using cytotoxic doses of suramin (about 10 times the noncytotoxic doses) in advanced RCC had demonstrated its tolerability.19
With this as the background, a phase I/II trial of suramin in combination with weekly 5-FU in patients with metastatic RCC was initiated. The objectives of this clinical trial were to (1) determine the dose of weekly suramin that would result in plasma concentrations of suramin of 10–50 μmol/L in 4.5–48 hours, (2) assess the ORR in patients with RCC to the combination of 5-FU and suramin, and (3) define the pharmacokinetics of low-dose suramin in patients with RCC when treated with 5-FU.
Patients and Methods
Patients
Eligibility was similar in the phase I and phase II portions of the trial and included histologically documented advanced/metastatic RCC, Eastern Cooperative Oncology Group performance status of ≤ 2, and measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST). Also required for enrollment was adequate organ function defined by an absolute neutrophil count ≥ 1500 cells/mm3, platelet count ≥ 100,000 cells/mm3, hemoglobin level ≥ 9 g/dL, serum creatinine ≤ 1.8 mg/dL, aspartate aminotransferase ≤ 2.5 times the institutional upper limit normal (ULN), alkaline phosphatase ≤ 5 × ULN, total bilirubin ≤ 1.5 mg/dL, and serum calcium less than or equal to ULN.
Exclusion criteria included untreated hypercalcemia; pregnancy or lactation; active autoimmune disease; active infection; untreated or progressive metastases to the central nervous system; requirement of concurrent administration of nonphysiologic doses of corticosteroids; and/or coexisting malignancies other than localized prostate cancer, basal cell carcinoma, and/or cervical carcinoma in situ. Patients treated with another investigational drug in the past 30 days, having presence of any unresolved grade ≥ 2 National Cancer Institute Common Toxicity Criteria (NCI-CTC) adverse event, having uncontrolled intercurrent infection, and having hypersensitivity to suramin were also excluded. No restriction was imposed based on the number of previous therapies for enrollment in this study. This study was approved by the Case Comprehensive Cancer Center and the Cleveland Clinic Institutional Review Boards, and informed consent was obtained from all patients in accordance with institutional and US FDA guidelines.
Design of the Phase I Trial
The goal of the phase I portion of the trial was to identify the dose of weekly suramin that would produce a plasma level of 10–50 μmol/L from 4.5 hours to 48 hours after infusion. The 4.5-hour time point corresponded to initiation of 5-FU (500 mg/m2) administration. Based on the published terminal half-life of < 10 minutes for 5-FU,20 it was calculated that > 99% of the area under plasma concentration curve of 5-FU occurs in < 48 hours. Cohorts of 3 patients were entered to assess whether the dose of suramin administered achieved a plasma level of 10–50 μmol/L. A cohort would be expanded if 1 of 3 patients encountered dose-limiting toxicity (DLT).
Drug Administration
Suramin was supplied as 600-mg vials by the Division of Cancer Treatment and Diagnosis of the NCI and required reconstitution in 6 mL of distilled water. The desired dose was further diluted in 0.9% sodium chloride or 5% dextrose solution water and administered in-travenously (I.V.) over 30 minutes. Commercially available 5-FU was used (500 mg per 10-mL single-use vials). 5-Fluorouracil was administered as an I.V. bolus weekly for 6 weeks of an 8-week cycle.
Suramin Dose
The protocol specified the following: (1) suramin dose escalation or de-escalation in the event that ≥ 2 of the first 3 patients experience suramin levels out of the target range (with patients already on study to be treated with the adjusted dose), and (2) repeating treatment cycles until a suramin dose that produced the target plasma concentrations were achieved in 5 of 6 patients who received the same suramin dose in all treatments. As shown in the Results section herein, there was no need to adjust the suramin dose. The suramin dose was calculated using a dosing nomogram developed based on population pharmacokinetic analysis using the data in patients with non–small-cell lung cancer (NSCLC).21 The dosing nomogram provided the value of FACTOR that was used in the following equation for dose calculation (also used in the subsequent phase II trial):
It was subsequently determined that the suramin dose calculated using the above equation yielded the target plasma levels of 10–50 μmol/L at 4.5–48 hours after starting the suramin infusion, with only 1 DLT among the 6 patients. This equation was therefore used in the phase II portion of the study.
Dose Modifications
Dose modifications were required per the protocol for any grade ≥ 3 toxicity lasting > 3 days (NCI-CTC for Adverse Events [CTCAE], version 3.0), with the exception of grade 3 nausea and vomiting that was controllable with antiemetic therapy.
For DLTs that occurred with target plasma suramin concentration of ≤ 50 μmol/L, toxicities were attributed to suramin or 5-FU. For DLTs attributable to suramin, patients were removed from the study. For DLTs attributable to 5-FU, the dose was reduced by 25% with no change in suramin dose. If the plasma suramin level was > 50 μmol/L and the DLTs were attributable to 5-FU, the dose of suramin was reduced per the nomogram. However, if the DLTs were attributable to suramin at a plasma suramin level of > 50 μmol/L, treatment was discontinued. If DLTs were attributed to 5-FU, dose reductions of 25% for hematologic (except hemoglobin) and nonhematologic toxicities were required. If a patient developed > 2 episodes of a DLT during the dose reduction or any infection in the presence of grade ≥ 3 febrile neutropenia, the patient was removed from study.
Definition of Dose-Limiting Toxicity
Dose-limiting toxicity was defined as grade ≥ 3 toxicity observed in the study that required the doses to be held. Dose-limiting toxicities were attributed to suramin or 5-FU based on the plasma suramin level and also the type of toxicity. If 2–3 of the 3 patients in the cohort had suramin levels within the target range and 1 of 3 showed DLTs, the cohort would be expanded to 6 patients. If any DLT required holding the therapy for ≥ 14 days, the patients would come off the study. Also, if any patient developed grade ≥ 3 toxicity suspected secondary to disease progression, this warranted an interim evaluation for tumor status irrespective of the scheduled study visits.
Definition of Response
Response Evaluation Criteria in Solid Tumors was used for objective response assessment.22 Serial computed topography scans of the chest, abdomen, and pelvis were performed at the end of every cycle (every 8 weeks) for assessment. Patients with progressive disease were removed from the study.
Pharmacokinetic Studies
The pharmacokinetics of 5-FU are well established and were therefore not investigated because the data are well described in the literature and because no drug-drug interactions were anticipated because the 2 drugs used different routes of elimination (suramin is excreted unchanged, and 5-FU is metabolized before excretion).23
The pharmacokinetics of suramin was studied in all 23 patients, including 6 and 17 patients in the phase I and phase II portions, respectively. Patients in the phase I study provided multiple samples during and after suramin administration (pre-dose, 10, 20, and 30 minutes and 1, 1.5, 2, 3, 4.5, 5.5, 9, 24, 48, and 72 hours). Limited samples were obtained from patients in the phase II study (pre-dose, 30 minutes and 3, 4.5, 5.5, and 6 hours) in order to determine whether the target concentrations were achieved while at the same time minimized the inconvenience to patients. A 2-compartment, open pharmacokinetic model was fit to the plasma concentration time data for individual patients to obtain the concentrations at 48 hours of each treatment for the phase II patients.
Heparinized peripheral blood samples were obtained at specified intervals. Plasma samples for suramin determinations were collected and centrifuged. The plasma fraction containing suramin was then extracted and analyzed using high-performance liquid chromatography as described previously.24 Briefly, a plasma sample was mixed with tetrabutylammonium bromide and extracted with acetonitrile. The organic layer was refrigerated, filtered, and analyzed. The detection limit was 0.5 μg/mL. The plasma concentration profiles of suramin were analyzed using standard methods.24,25
Biostatistical Design
The primary endpoint of the phase I portion was to determine the ideal dose of suramin that would result in a plasma level of 10–50 μmol/L at 4.5–48 hours after infusion. It was estimated that this would require no more than 2 cohorts of 3 patients each (expanded cohort with 3 + 3 = 6 patients). The primary endpoint of the phase II portion was objective response. A 2-stage accrual design was used to allow the trial to be stopped early if preliminary evidence indicated a relative lack of efficacy. The initial accrual objective was 17 patients with RCC, of whom 15 would be eligible and evaluable. If ≥ 2 responses were observed, an additional 11 patients with RCC, of whom 10 would be eligible and evaluable, would be recruited. At least 3 objective responses among the 25 patients were used as the cutoff for determining the efficacy of suramin plus 5-FU in this population. This would have corresponded to an observed response rate of ≥ 12%. If < 3 responses were observed, the combination of 5-FU and suramin would not be considered for further testing in this population. The probability that the trial would be declared to have minimal antitumor activity after the first accrual stage was ≥ 83% if the underlying response rate was ≥ 5%. In contrast, the probability of stopping early was ≥ 4% if the underlying response rate was ≥ 30%. The overall probability of rejecting the combination of chemotherapy and suramin, if it had minimal activity, was ≥ 91%, while there was at most a 4% chance of rejecting if it was active.
Response and toxicity were summarized using frequency counts and percentages. Time to failure was estimated using the Kaplan-Meier method. Toxicity and time to failure (defined as the interval from the start of treatment to documented progression, discontinuation of therapy for other reasons such as toxicity, or death, whichever came first) were secondary endpoints. Based on the results of previous trials in RCC, this neoplasm is resistant to chemotherapy, with a response rate of < 10% to a wide range of chemotherapeutic agents.8,9 Consequently, an ORR (complete plus partial response) of approximately 30% would be deemed sufficiently important to warrant further study, whereas an underlying response rate of ≤ 5% would indicate a relative lack of efficacy.
Results
Patient Demographics
The trial was carried out at 2 sites, the Cleveland Clinic Taussig Cancer Institute and the University Hospitals Case Medical Center, between March 2004 and March 2005. Twenty-three patients (6 in the phase I portion and 17 in phase II) were enrolled. All 23 patients were considered eligible and evaluable. The characteristics of these patients were similar to age and sex ratios in the typical RCC population (Table 1). Seventy-eight percent of patients were men, mean age was 58.8 years, 96% of patients had undergone previous nephrectomy, and histologic subtype was clear cell in 91%. Previous systemic therapy was not required; however, 70% of patients had a history of previous treatment, primarily IL-2 and/or IFN-α and/or antiangiogenic agents such as thalidomide and lenalidomide. Fifty-two percent of patients (12 of 23) had received ≥ 2 previous regimens, and 26% received ≥ 3 regimens (range, 1–5 previous regimens). Overall, patients received a median of 1 cycle of treatment (range, 1–7 cycles). Twenty of 23 patients (87%) discontinued therapy because of disease progression, 1 patient stopped because of adverse events, and 1 patient was taken off the study per physician’s discretion.
Table 1.
Patient Demographics
| Characteristic | N (%) |
|---|---|
| Dose Level | |
| Phase I | 6 |
| Phase II | 17 |
| Sex | |
| Male | 18 (78) |
| Female | 5 (22) |
| Age (Years) | |
| Mean ± SD | 58.8 ± 8.7 |
| Median (Range) | 57 (42–74) |
| Performance Status | |
| 0 | 14 (61) |
| 1 | 9 (39) |
| Previous Nephrectomy | 22 (96) |
| Previous Radiation Therapy | 10 (43) |
| Previous Systemic Treatment* | |
| None | 7 (30) |
| Antiangiogenic only† | 1 (4) |
| IL-2 and/or IFN-α only | 3 (13) |
| IL-2 and/or IFN-α based | 12 (52) |
| Chemotherapy | 2 (9) |
| Antiangiogenic therapy† | 7 (30) |
| Other | 3 (13) |
| Histology | |
| Clear cell | 21 (91) |
| Papillary | 1 (4) |
| Other | 1 (4) |
| Diagnosis to On-Study (Months) | |
| Median (Range) | 27.5 (5.1 Months–19.3 Years) |
Some patients were on > 1 type of anticancer therapy before the study.
Primarily thalidomide and lenalidomide.
Abbreviations: IFN = interferon; IL = interleukin; SD = standard deviation
Phase I Study
One patient in the first cohort of 3 patients experienced a DLT (grade 3 hypersensitivity to suramin). Per protocol, the cohort was expanded by entering 3 more patients. The suramin dose was calculated using the dosing nomogram established based on the pharmacokinetic data in patients with NSCLC.21 The previous nomogram was developed for the every-3-week treatment schedule. Because of the different dosing schedule used in the current study, it was necessary to confirm the validity of the dosing nomogram. Hence, the pharmacokinetics of suramin was studied; the results confirmed that the dosing nomogram correctly predicted the dose needed to deliver the desired target drug concentrations (see below). Table 2 shows the values of FACTOR as a function of the time elapsed since the previous treatment2; the time-based adjustment was necessary because of the unusually long elimination half-life of suramin (eg, > 10 days).
Table 2.
Suramin Dosing Nomogram
| Cycle 1* | FACTOR = 125 |
|---|---|
| Days from Administration of Previous Dose | FACTOR |
| 7 | 39 |
| 8 | 43 |
| 9 | 47 |
| 10 | 51 |
| 11 | 55 |
| 12 | 58 |
| 13 | 61 |
| 14 | 64 |
| 15 | 67 |
| 16 | 69 |
| 17 | 72 |
| 18 | 74 |
| 19 | 76 |
| 20 | 78 |
| 21 | 80 |
| 22 | 82 |
| 23 | 84 |
| 24 | 86 |
| 25 | 87 |
| 26 | 88 |
| 27 | 90 |
| 28 | 91 |
Subsequent cycles: values of FACTOR depend on the elapsed time (in days) since the administration of the previous dose as provided.
The suramin dose was calculated using the following equation: Dose (mg) = FACTOR × (absolute value of body-surface area without unit). The same FACTOR was used for men and women. Starting dose calculation used a FACTOR of 125, and the FACTOR for subsequent dose calculations were derived from this nomogram.
The Pharmacokinetics of Suramin
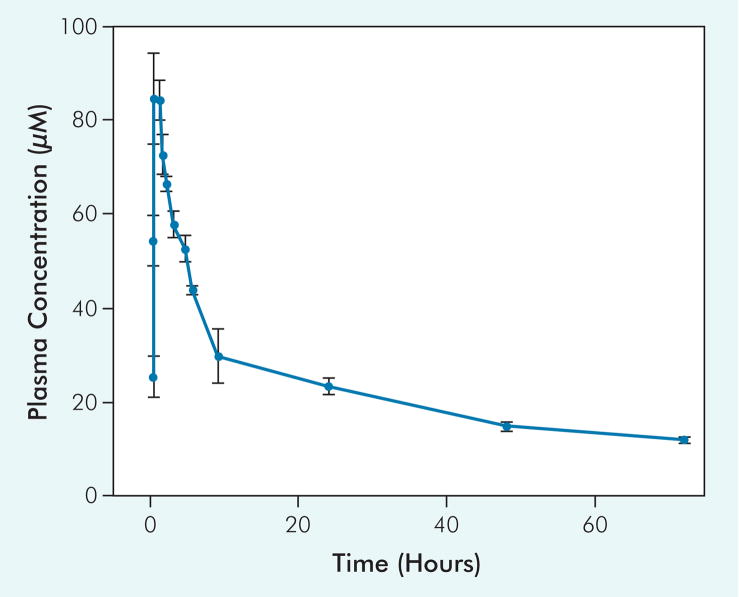
Figure 1 illustrates the mean plasma concentration time profiles of suramin in the 6 patients entered in the phase I portion of the trial. Table 3 shows the pharmacokinetic parameters. The maximum plasma concentrations attained after drug administration was about 90 μmol/L, declining to about 50 μmol/L at 4.5 hours or when 5-FU infusion was initiated and subsequently to about 15 μmol/L at 48 hours. The pharmacokinetic study, but with a more limited sampling schedule, was extended to the 17 patients enrolled in the phase II portion of the trial. In total, the pharmacokinetics of suramin was studied in 115 treatment cycles (a total of 138 treatments were given). The suramin concentrations were < 50 μmol/L in 84.3% of treatments (97 of 115) at 4.5 hours and > 10 μmol/L at 48 hours in 97.3% of treatments (112 of 115), indicating that the target concentrations of 10–50 μmol/L from 4.5 hours to 48 hours were attained in 81.7% of the treatments (Table 3).
Figure 1.
Plasma Concentration Time Profiles of Suramin in Phase I Patients
Six patients were treated with noncytotoxic suramin and 5-fluorouracil as described in the text. These data were used to establish the pharmacokinetic parameters of suramin (Table 3).
Table 3.
Pharmacokinetic Parameters of Suramin
| Patient Group | Cmax* (μmol/L) | C4.5 hours (μmol/L) | C48 hours (μmol/L) | AUC0-72 hours (μmol/L per Hour) | Terminal Half-Life (Days) |
|---|---|---|---|---|---|
| Phase I (n = 6) | 93.3 ± 14.8 | 52.7 ± 7.5 | 14.8 ± 2.6 | 1672 ± 223 | 11.4 ± 5.75 |
| Phase II (n = 17) | 84.4 ± 17.8 | 54.2 ± 9.8 | 21.6 ± 11.5 | NA | NA |
Cmax is the maximum concentration. C4.5 hours and C48 hours are the concentrations at 4.5 hours and 48 hours. AUC0–72 hours is area under the concentration time profile from 0 to 72 hours and was calculated using the trapezoid rule. The terminal half-life was calculated as 0.69315/λ, where λ (terminal rate constant) was calculated by applying linear regression to the linear part of the profile using noncompartmental analysis and the WinNolin program. In the phase I patients, Cmax was attained immediately after the 30-minute infusion, with the exception of 1 patient who, for unknown reasons, showed Cmax at 60 minutes. In the phase II patients, the concentrations at 4.5 hours were actually determined, whereas the concentrations at 48 hours were computer simulated as described in the Patients and Methods section.
Values are means ± standard deviations.
Abbreviations: AUC = area under the curve; NA = not applicable or not available
Phase II Study
In the phase II portion, all of the 17 patients with RCC screened in the first stage were eligible and evaluable for the study. Because no objective responses were observed, the study was terminated after the first stage of accrual.
Toxicity
The safety of the treatment was evaluated by using the NCI-CTCAE, version 3.0. Table 4 illustrates the commonly reported adverse events in all the 23 patients (phase I and phase II). Seven patients (30%) experienced a total of 12 serious adverse events. The most common toxicities observed were (all grades) fatigue (83%), nausea/vomiting (78%), and diarrhea (61%). The toxicity pattern observed is characteristic for patients receiving bolus fluoropy-rimidine therapy, and except for a high incidence of chills (61%), no differences were discernible. The most common higher-grade toxicities included diarrhea (grade 3, n = 4) and thromboembolism (grade 4, n = 2).
Table 4.
Adverse Reactions Observed in All 23 Patients
| Adverse Event | Number of Events | |||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Hypersensitivity/Allergic Reaction | 0 | 0 | 1 | 0 |
| Anemia | 2 | 0 | 1 | 0 |
| Leukopenia | 2 | 0 | 0 | 0 |
| Thrombocytopenia | 3 | 0 | 0 | 0 |
| Hypotension | 0 | 1 | 0 | 0 |
| Thromboembolism | 0 | 0 | 0 | 2 |
| Chills | 14 | 0 | 0 | 0 |
| Fatigue | 11 | 8 | 0 | 0 |
| Hyperpigmentation | 5 | 0 | 0 | 0 |
| Rash/Desquamation | 4 | 1 | 0 | 0 |
| Anorexia | 11 | 6 | 0 | 0 |
| Constipation | 6 | 0 | 0 | 0 |
| Diarrhea | 5 | 5 | 4 | 0 |
| Nausea/Vomiting | 12 | 5 | 1 | 0 |
| Stomatitis | 5 | 2 | 0 | 0 |
| Taste Changes | 7 | 0 | 0 | 0 |
| Elevated Aspartate Aminotransferase | 1 | 0 | 0 | 0 |
| Hyperglycemia | 0 | 1 | 1 | 0 |
| Hypokalemia | 0 | 0 | 1 | 0 |
| Dizziness | 5 | 1 | 0 | 0 |
| Neuropathy | 4 | 0 | 0 | 0 |
| Tearing | 7 | 0 | 0 | 0 |
| Dyspnea | 2 | 5 | 0 | 0 |
Efficacy (Antitumor Activity)
This study included previously treated (n = 15) and untreated (n = 8) patients; therefore, the 2 different Memorial Sloan-Kettering Cancer Center risk stratifications were used.26,27 Nine (60%) of the previously treated patients were at favorable risk, 5 patients (33%) were at intermediate risk, and 1 patient (7%) was at unfavorable risk. For the untreated patients, 5 (62%) were at favorable risk, 2 (25%) were at intermediate risk, and 1 (13%) was at unfavorable risk.
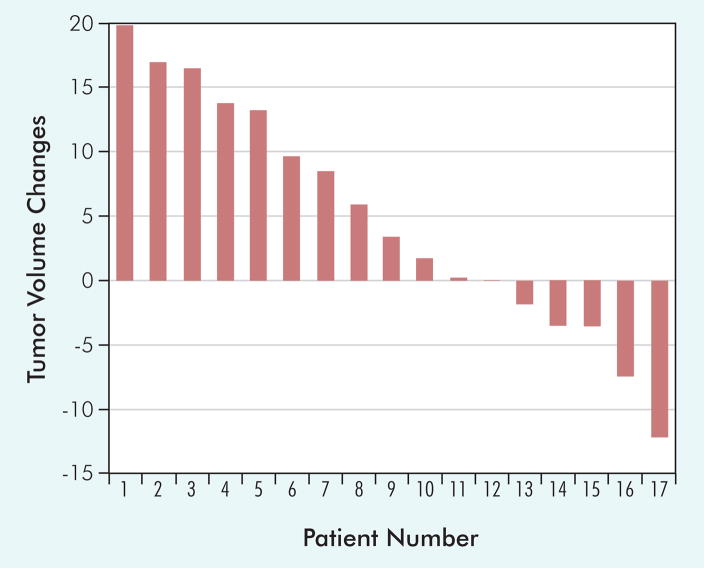
There were no objective responses to the combination of noncytotoxic suramin and 5-FU. The best response in the evaluable patients was 17 with stable disease and 6 with progressive disease per RECIST. Median time to failure was 2.5 months. Overall median survival is estimated to be 19.1 months, with 10 of 23 patients (44%) remaining alive. The possibility of antitumor activity of the regimen was also examined using a waterfall plot (Figure 2). In 17 patients with stable disease, 5 had tumor shrinkage not qualifying as a partial response. All of these individuals were heavily pretreated with cytokines, including IL-2.
Figure 2.
Tumor Volume Changes Corresponding to Best Response in the 17 Patients in the Phase II Portion
Discussion
Results of pharmacokinetic studies in the phase I and II portions of the trial show that the suramin plasma levels associated with reversal of chemotherapy resistance in preclinical models (10–50 μmol/L at 4.5–48 hours after starting the infusion) could be readily achieved. These findings indicate the dosing nomogram previously defined using data obtained in patients with NSCLC using an every-3-week schedule successfully identified the suramin dose using the weekly schedule in patients with RCC.
The data further show that the suramin and 5-FU combination produced acceptable toxicity. Overall, the toxicity observed with suramin and 5-FU was significant, with 12 serious adverse events (10 grade 3 and 2 grade 4) observed in 7 patients as well as 2 patients with grade 4 toxicities. Although in vitro studies suggested the combination of 5-FU and suramin increase the activity of 5-FU in histocultures of tumors obtained from patients with RCC (unpublished data), no significant improvement was observed in patients with metastatic RCC. Although patients in this trial were generally heavily pretreated, no objective responses were observed, and failure-free survival was fairly short (median, 2.5 months).
Toxicity related to the use of suramin alone or in combination with other chemotherapy agents is dose related and is observed at high suramin concentrations (> 200 μg/mL) that were maintained for more than a few weeks.28 The noncytotoxic suramin doses/concentrations used in the current trial are about 10 times lower than the high-dose suramin used earlier as a cytotoxic agent and were not associated with DLTs. In the phase I portion of this study, 1 patient developed hypersensitivity reaction soon after receiving the suramin infusion. The reaction included urticarial rash, shortness of breath, and chest tightness, all of which subsided in a few hours with symptomatic management. This type of reaction has not been reported with low doses of suramin.
Conclusion
After this study was initiated, the treatment of patients with advanced RCC has changed, and the use of cytokines has been replaced by a variety of kinase and vascular endothelial growth factor inhibitors. These agents have significant activity with robust tumor shrinking and prolongation of PFS and overall survival.11,14,15 Nonetheless, continued investigation of the mechanisms responsible for the drug resistance of RCC remains necessary and might provide additional insights for future treatment approaches.
Acknowledgments
This work was supported in part by a research grant(R01CA93871) awarded to Jessie J. L. Au, PhD, by the NCI Department of Health and Human Services.
References
- 1.Motzer RJ, Russo P. Systemic therapy for renal cell carcinoma. J Urol. 2000;163:408–17. [PubMed] [Google Scholar]
- 2.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335:865–75. doi: 10.1056/NEJM199609193351207. [DOI] [PubMed] [Google Scholar]
- 3.Chow WH, Devesa SS, Warren JL, et al. Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–31. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 4.Tsui KH, Shvarts O, Smith RB, et al. Renal cell carcinoma: prognostic significance of incidentally detected tumors. J Urol. 2000;163:426–30. doi: 10.1016/s0022-5347(05)67892-5. [DOI] [PubMed] [Google Scholar]
- 5.Staehler G, Brkovic D. The role of radical surgery for renal cell carcinoma with extension into the vena cava. J Urol. 2000;163:1671–5. [PubMed] [Google Scholar]
- 6.Van Poppel H, Bamelis B, Oyen R, et al. Partial nephrectomy for renal cell carcinoma can achieve long-term tumor control. J Urol. 1998;160:674–8. doi: 10.1016/S0022-5347(01)62751-4. [DOI] [PubMed] [Google Scholar]
- 7.Flanigan R, Blumenstein B, Salmon S, et al. Cytoreduction nephrec-tomy in metastatic renal cancer: the results of Southwest Oncology Group trial 8949. Proc Am Soc Clin Oncol. 2000;19:2a. (Abstract 3) [PubMed] [Google Scholar]
- 8.Bukowski R. Natural history and therapy of metastatic renal cell carcinoma: role of interleukin-2. Cancer. 1997;80:1198–220. doi: 10.1002/(sici)1097-0142(19971001)80:7<1198::aid-cncr3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 9.Parkinson DR, Sznol M. High dose interleukin-2 in the therapy of metastatic renal cell carcinoma. Semin Oncol. 1995;22:61–6. [PubMed] [Google Scholar]
- 10.Stadler WM, Vogelzang NJ. Low-dose interleukin-2 in the treatment of metastatic renal-cell carcinoma. Semin Oncol. 1995;22:67–73. [PubMed] [Google Scholar]
- 11.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 12.Escudier B, Szczylik C, Demkow T, et al. Randomized phase II trial of the multi-kinase inhibitor sorafenib versus interferon (IFN) in treatment-naïve patients with metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2006;24(18 suppl):217s . doi: 10.1200/JCO.2008.19.3342. (Abstract 4501) [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 15.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 16.Yagoda A, Abi-Rached B, Petrylak D. Chemotherapy for advanced renal-cell carcinoma: 1983–1993. Semin Oncol. 1995;22:42–60. [PubMed] [Google Scholar]
- 17.Song S, Wientjes MG, Gan Y, et al. Fibroblast growth factors: an epigenetic mechanism of broad spectrum resistance to anticancer drugs. Proc Natl Acad Sci U S A. 2000:8658–63. doi: 10.1073/pnas.140210697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein CA, LaRocca RV, Thomas R, et al. Suramin: an anticancer drug with a unique mechanism of action. J Clin Oncol. 1989;7:499–508. doi: 10.1200/JCO.1989.7.4.499. [DOI] [PubMed] [Google Scholar]
- 19.Dreicer R, Smith DC, Williams RD, et al. Phase II trial of suramin in patients with metastatic renal cell carcinoma. Invest New Drugs. 1999;17:183–6. doi: 10.1023/a:1006331518952. [DOI] [PubMed] [Google Scholar]
- 20.Au JL, Rustum YM, Ledesma EJ, et al. Clinical pharmacological studies of concurrent infusion of 5-fluorouracil and thymidine in treatment of colorectal carcinomas. Cancer Res. 1982;42:2930–7. [PubMed] [Google Scholar]
- 21.Chen D, Song SH, Wientjes MG, et al. Nontoxic suramin as a chemosensitizer in patients: dosing nomogram development. Pharm Res. 2006;23:1265–74. doi: 10.1007/s11095-006-0165-1. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P. Evaluation of response: new and standard criteria. Ann Oncol. 2002;13(suppl 4):127–9. doi: 10.1093/annonc/mdf649. [DOI] [PubMed] [Google Scholar]
- 23.Falcone A, Pfanner E, Brunetti I, et al. Suramin in combination with 5-fluorouracil (5-FU) and leucovorin (LV) in metastatic colorectal cancer patients resistant to 5-FU+LV-based chemotherapy. Tumori. 1998;84:666–8. doi: 10.1177/030089169808400610. [DOI] [PubMed] [Google Scholar]
- 24.Kassack M, Nickel P. Rapid, highly sensitive gradient narrow-bore high-performance liquid chromatographic determination of suramin and its analogues. J Chromatogr B Biomed Appl. 1996;686:275–84. doi: 10.1016/s0378-4347(96)00214-9. [DOI] [PubMed] [Google Scholar]
- 25.Villalona-Calero MA, Wientjes MG, Otterson GA, et al. Phase I study of low-dose suramin as a chemosensitizer in patients with advanced non-small cell lung cancer. Clin Cancer Res. 2003;9:3303–11. [PubMed] [Google Scholar]
- 26.Motzer RJ, Bacik J, Schwartz LH, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–63. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 27.Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–40. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 28.Bitton RJ, Figg WD, Venzon DJ, et al. Pharmacologic variables associated with the development of neurologic toxicity in patients treated with suramin. J Clin Oncol. 1995;13:2223–9. doi: 10.1200/JCO.1995.13.9.2223. [DOI] [PubMed] [Google Scholar]