Summary
Interactions between adjacent epithelial and mesenchymal tissues represent a highly conserved mechanism in embryonic organogenesis. In particular, the ability of the mesenchyme to instruct cellular differentiation of the epithelium is a fundamental requirement for the morphogenesis of tubular structures such as those found in the kidneys, lungs, and the developing male reproductive system. Once the tubular structure has formed, it receives signals from the mesenchyme, which can control proliferation, patterning, and differentiation of the epithelium inside the tube. However, the epithelium is not a “silent partner” in this process, and epithelium-derived factors are often required for proper maintenance of the mesenchymal compartment. Although much emphasis has been placed on the characterization of mesenchymally-derived signals required for epithelial differentiation, it is important to note that epithelial-mesenchymal interactions are a two-way street wherein each compartment requires the presence of the other for proper tubule morphogenesis and function. In this review, we discuss epithelial-mesenchymal interactions in the processes of Wolffian duct and fetal testis cord development using the mouse as a model organism and propose inhibin beta A as a conserved mesenchyme-derived regulator in these two male-specific tubular structures.
Keywords: epithelial-mesenchymal interaction, testis, Wolffian duct, activin, organogenesis
INTRODUCTION
Epithelial-mesenchymal interactions are a common motif in embryonic development, particularly in the formation of tubular structures throughout the body (reviewed by Lubarsky and Krasnow, 2003; Karihaloo et al., 2005). During organogenesis, it is typically the mesenchyme that provides instructive cues to promote the differentiation of the tubular epithelium. In kidney and ureter development, for example, sprouting of the ureteric buds from the Wolffian ducts is not the result of an intrinsic program of the Wolffian duct epithelium but is instead dependent upon signals from the nearby mesenchyme (reviewed by Airik and Kispert, 2007). Similarly, branching morphogenesis of the embryonic lung epithelium is also controlled by its adjacent mesenchyme (reviewed by Shannon and Hyatt, 2004). Mesenchymal contribution to epithelial differentiation has been demonstrated by tissue recombination experiments in which mesenchyme of a specific origin is cultured in physical contact with epithelium of a different origin to determine whether the fate of the epithelium can be altered. For example, lung mesenchyme is able to induce branching morphogenesis in salivary gland epithelium, a non-branching tissue, when cultured together as a tissue recombinant (Lawson, 1974). Similar tissue recombinations have established the instructive nature of the ureteric bud mesenchyme in determining the fate of the epithelia, which form the kidneys and lower urinary tract (Baskin et al., 1996; Cunha et al., 1991; Lipschutz et al., 1996).
In the male reproductive system, differentiation of tubular structures such as the Wolffian ducts and the testis cords (known as the seminiferous tubules in adult testes) also requires interactions between epithelium and mesenchyme. The Wolffian ducts and gonads share a common origin, the urogenital ridges which arise from the intermediate mesoderm by embryonic day 9.5 (E9.5) in the mouse (for details regarding spatiotemporal development of the urogenital ridges, please see review by Staack et al., 2003). Each urogenital ridge is divided into three overlapping regions—the pronephros, mesonephros, and metanephros. The pronephros is vestigal in mammals and thus regresses early in embryonic life; however, the pronephric ductal derivatives continue to progress in a cranial-to-caudal manner and become the Wolffian (or mesonephric) ducts by E10. The gonads are of mesonephric origin and are found on the medial side of the urogenital ridges (Staack et al., 2003). The meta-nephroi, on the other hand, give rise to the embryonic kidneys. Formation of the Wolffian and Müllerian (para-mesonephric) ducts occurs in embryos of both sexes; however, by the time of birth only one reproductive tract is present. Although formation of the Wolffian ducts does not require gonadal hormones, maintenance of the ducts requires testicular androgens (Jost, 1947, 1953). Therefore, the Wolffian ducts are retained only in males and eventually give rise to the epididymides, vasa deferens, and seminal vesicles. In contrast, both the formation and maintenance of the Müllerian ducts is hormone-independent. In male embryos, the Sertoli cells of the fetal testes produce anti-Müllerian hormone (AMH) that causes regression of the Müllerian ducts (Jost, 1947, 1953). In female embryos, the lack of AMH production creates a permissive environment for persistence of the Müllerian ducts.
In contrast to the transient coexistence of both male and female ducts, the sexual identity of the fetal gonads is defined according to the sex chromosome composition of the embryo. The gonadal primordia in mammals form either testes or ovaries based upon the presence or absence of the Y chromosome. In nearly all mammals, XY individuals develop testes due to the presence of the Sry (sex-determining region of the Y chromosome) gene (Koopman et al., 1990). Expression of either Sry or its downstream target Sox9 (Sry-related high mobility group box gene 9) is both necessary and sufficient to establish the Sertoli cell lineage and thus secure testis fate (Barrionuevo et al., 2006; Bishop et al., 2000; Chaboissier et al., 2004; Sekido and Lovell-Badge, 2008; Vidal et al., 2001). Under the influence of Sry and Sox9, Sertoli cells organize the male gonads into an epithelial compartment (the testis cords) and an adjacent mesenchymal compartment (the interstitium). In the absence of Sry or Sox9 expression, as is the case in normal XX individuals, the gonadal primordia will develop into ovaries which fail to maintain any cord-like structures during fetal life.
In this review, we discuss the epithelial-mesenchymal crosstalk involved in the maintenance and patterning of the Wolffian ducts and fetal testes. We also propose that inhibin beta A (Inhba), a subunit of activins, is a mesenchyme-produced factor involved in the elongation and convolution of both the anterior Wolffian ducts and the fetal testis cords.
EPITHELIAL-MESENCHYMAL INTERACTIONS IN THE INITIAL PATTERNING OF THE WOLFFIAN DUCTS
In mammals, the Wolffian (mesonephric) ducts develop in a cranial-to-caudal fashion from the remnants of the rudimentary pronephros (for details on Wolffian duct formation, please see reviews by Capel, 2000; MacLaughlin et al., 2001; and Hannema and Hughes, 2007). Once the Wolffian ducts have formed, the next key stage of morphogenesis involves the stabilization and basic patterning of the ducts. Androgens produced by the fetal testes are essential for maintaining the Wolffian ducts and eliciting the expression of downstream growth factors. Exposure of the Wolffian ducts to these testicular androgens follows an anterior-to-posterior gradient (Veyssière et al., 1982). In early Wolffian duct development, androgen receptor (AR) is expressed specifically within the mesenchyme and acts directly on the mesenchyme to stabilize the ducts (Huhtaniemi, 1994). In mice, AR is not detected in the Wolffian duct epithelium until E15.5, a critical timepoint in ductal morphogenesis (Cooke et al., 1991). At E15.5, the anterior Wolffian duct epithelium begins the process of elongation and convolution which will eventually result in highly-coiled epididymal structures by birth. Although the actions of androgens on the Wolffian duct mesenchyme are direct, the effects of androgens on the epithelium appear to be indirect and most likely occur via the production of para-crine factors from the mesenchyme.
Grafting experiments involving tissue recombinations have provided tremendous insight into the relationship between the Wolffian duct epithelium and mesenchyme during this critical period of development. Specifically, when epithelium from the anterior portion of the Wolffian ducts, which will form the epididymides, was grafted onto mesenchyme from the posterior Wolffian ducts, which will form the seminal vesicles, the epithelium was transformed to a seminal vesicle-like phenotype (Higgins et al., 1989). Similar recombination experiments have been conducted wherein urogenital epithelium, derived from the seminal vesicles or urogenital sinus, was combined with either seminal vesicle or skin mesenchyme (Cunha, 1972). In tissue recombinants composed of urogenital epithelium and seminal vesicle mesenchyme, the epithelium took on the glandular phenotype characteristic of the seminal vesicle epithelium. In grafts consisting of urogenital epithelium and integumental mesenchyme, the epithelium exhibited a keratinized structure characteristic of skin epithelium. These experiments confirm that it is the origin of the mesenchyme, rather than the origin of the epithelium, which determines the fate of the epithelial compartment. Tissue recombination experiments have also proven helpful in elucidating the compartment-specific requirement for androgens during Wolffian duct morphogenesis. To determine the role of androgens, Wolffian duct epithelium from androgen-insensitive testicular feminization mouse (Tfm) embryos was grafted onto wild-type mesenchyme derived from the urogenital sinus region, which gives rise to the androgen-dependent prostate. In these recombinations, the Tfm epithelium, which lacks a functional AR, still underwent ductal and cellular differentiation resembling that of the prostate (Sugimura et al., 1986). These data confirm that androgen action upon the mesenchyme, not the epithelium, is essential for maintenance and differentiation of the Wolffian ducts. Additionally, regional specification of the epithelium depends upon the identity of the adjacent mesenchyme.
Although epithelial differentiation is regulated by mesenchymal signals, communication between these compartments in the Wolffian ducts is in fact a two-way street. The Wolffian duct epithelium produces growth factors and other signaling molecules which are essential for the growth of the mesenchyme. Specifically, grafts of Wolffian duct mesenchyme cultured without the epithelium fail to develop properly, emphasizing the importance of the epithelium in maintenance of the Wolffian duct mesenchyme (Cunha et al., 1991; Higgins et al., 1989). Not surprisingly, the production of growth factors by the Wolffian ducts is closely tied to androgen signaling. Epidermal growth factor (Egf) is a particularly interesting example because not only is its expression regulated by androgens, but EGF can in turn regulate androgen signaling via effects on AR. Initially, EGF is expressed in both the mesenchyme and epithelium of the Wolffian ducts, but it is localized almost exclusively to the epithelium by E18 in the mouse (Gupta, 1997). The expression of EGF in the Wolffian ducts follows the anterior-to-posterior gradient of testosterone exposure, and expression of both EGF and its receptor can be increased by androgen treatment (Gupta and Jaumotte, 1993; Gupta and Singh, 1996; Gupta, 1996). Further evidence that EGF is a critical modulator of androgen-induced effects in the Wolffian ducts comes from both in vitro and in vivo experiments wherein EGF maintained the Wolffian ducts in the absence of the testes and induced Wolffian duct morphogenesis in female embryos (Gupta et al., 1991). In addition to its role as a downstream effector of androgens, in vitro experiments using cultured mesenchymal cells demonstrate that EGF works together with androgens in an additive fashion to enhance AR-mediated transcriptional activity (Gupta, 1999). Interestingly, EGF does not increase AR mRNA transcription or protein translation, suggesting that the ability of EGF to replace androgens in the developing Wolffian ducts is primarily due to its effect on AR-mediated transcription (Gupta, 1999).
Several other factors including growth hormone (GH), insulin-like growth factor 1 (Igf1), transforming growth factor beta 2 (Tgfβ2), and fibroblast growth factors (Fgf) have also been shown to play a role in Wolffian duct development both in vivo and in vitro. For example, the requirement for GH in Wolffian duct morphogenesis was established by in vitro experiments wherein E13 mouse gonads with Wolffian and Müllerian ducts attached were cultured in the presence of an anti-GH antibody (Nguyen et al., 1996). This blockage of GH signaling resulted in degeneration of the Wolffian ducts, a phenotype which was rescued by GH replacement. The negative effect of the anti-GH antibody could also be rescued by addition of IGF1, a downstream mediator of GH activity. Interestingly, adult mice with a null mutation in the Igf1 gene display extreme underdevelopment of the posterior epididymides, seminal vesicles, and prostate (Baker et al., 1996). However, normal development of the lower Wolffian duct derivatives relies heavily upon androgens and as testosterone production is reduced in Igf1-null males it is unclear whether the hypomorphic phenotype is due to local IGF1-deficiency, testosterone deficiency, or a combination of both during embryonic development (Baker et al., 1996). In addition to GH and IGF1, members of the TGFβ and FGF families have also been implicated in the stabilization and patterning of the Wolffian ducts although the specific requirements for these factors are not fully understood (Chua et al., 2002; Sanford et al., 1997; Thomson and Marker, 2006; reviewed by Hannema and Hughes, 2007). Analysis of male Tgfβ2 knockout mice revealed myriad defects in urogenital tract development, but the specific role of TGFβ2 in normal Wolffian duct development could not be elucidated due to the relatively small number of animals analyzed and the phenotypic variance present in these mice (Sanford et al., 1997). As is the case with Tgfβ2, the function of Fgf3 in the developing Wolffian ducts is not fully known, but male transgenic mice overexpressing Fgf3 in the Wolffian ducts and prostate display a severe dysmorphic hyperplasia of these tissues (Chua et al., 2002). Another member of the FGF family, Fgf10, is expressed in the mesenchyme of the lower Wolffian ducts and regulates epithelial growth of the seminal vesicles and prostate. Interestingly, although testosterone treatment increased Fgf10 transcription in the seminal vesicles, anti-androgen treatment did not alter Fgf10 levels, thus, suggesting that Fgf10 expression is not a direct target of androgen action (Thomson and Cunha, 1999). These results highlight the fact that although both androgens and growth factors are essential for Wolffian duct formation, the relationships between these two signaling families are rather complicated.
In addition to androgens and growth factors, homeotic (Hox) genes are essential for proper patterning of the Wolffian ducts. Although the Wolffian ducts start out as simple tubular structures, they give rise to an array of complex accessory reproductive organs including the epididymides, vasa deferens, and seminal vesicles. Hox genes are critical regulators of cell differentiation, positional arrangement, and patterning during the development of numerous organs displaying regional specificity, including the Wolffian ducts. Hox genes are expressed by both the Wolffian duct mesenchyme and epithelium although expression is notably more abundant in the mesenchyme, particularly the area directly adjacent to the ductal epithelium (Podlasek et al., 1999). The significance of this differential expression pattern is not known, although it indicates that Hox genes could be involved in epithelial-mesenchymal interactions of the Wolffian ducts. It is well-established that mutation of a particular Hox gene results in a homeotic transformation in which the phenotype of the affected Wolffian duct segment becomes that of the immediately anterior region (for more on Hox genes in Wolffian duct development, please see reviews by Lindsey and Wilkinson, 1996; Podlasek et al., 2002). Despite all that is known about the importance of Hox genes in establishing the identity of regions within the Wolffian ducts, the exact molecular mechanism by which Hox genes aid in the determination of morphological boundaries has yet to be established.
EXPANSION AND COILING OF THE WOLFFIAN DUCTS
Hox genes play an essential role in the establishment of regional identities along the Wolffian ducts; however, they are not known to be directly involved in further patterning of the Wolffian ducts. Although visible patterning of the posterior regions of the Wolffian ducts occurs largely during postnatal life in the mouse, the anterior Wolffian ducts develop their highly convoluted epididymal structure prior to birth. Whether this coiling is an intrinsic property of the epididymides or requires specific signaling inputs has been the subject of speculation since few factors have been shown to be required for proper epididymal convolution. We have identified inhibin beta A (Inhba) as a novel factor required for epididymal coiling (see Fig. 1) (Tomaszewski et al., 2007). Inhba encodes the βA subunit of inhibins, formed by dimerization of inhibin α and inhibin β subunits, and activins, formed by dimerization of inhibin β subunits (for more on inhibins and activins, please see review by Ball and Risbridger, 2001). Activins are members of the TGFβ superfamily and are known to signal canonically via phosphorylation of their downstream intracellular targets SMAD2/3. In contrast, inhibins function to antagonize activin signal transduction. Expression of Inhba within the Wolffian duct mesenchyme, coupled with the presence of phosphorylated-SMAD2/3 in the Wolffian duct epithelium suggests that the Inhba protein product activin A, rather than inhibin A, is a key player in epithelial-mesenchymal interactions of the developing epididymides. Additionally, inhibin α knockout mice, which can produce activins but not inhibins, have not been reported to have defects in epididymis or testis morphogenesis during fetal life, indicating that inhibins may not be required for normal development of these structures (Matzuk et al., 1992). Although epididymal coiling becomes evident in wild-type male mice after E15.5, the epididymides of Inhba knockout mice do not undergo convolution and thus remain a simple, straight tube even by the time of birth (E19.5). In wild-type embryos, expression of Inhba mRNA initially occurs in a waning anterior-to-posterior gradient, which corresponds to both the testosterone gradient in the Wolffian ducts as well as the degree of coiling found in the epididymides (Tomaszewski et al., 2007). Initial expression of Inhba mRNA in the anterior Wolffian ducts does not depend on the presence of testosterone; however, testosterone is required to maintain Inhba expression. Despite this relationship between Inhba expression and androgen signaling, the epididymal phenotype in Inhba knockout embryos does not arise from changes in androgen function based on the fact that testosterone production and testis descent, a parameter for androgen action, are similar to that of controls. Instead, the defects in epididymal convolution in Inhba knockout mice appear to result from decreased epithelial cell proliferation accompanied by abnormal cellular differentiation of the ductal epithelium and surrounding mesenchyme. In wild-type embryos, epithelial proliferation in the anterior Wolffian ducts increased steadily between E15.5 and birth; specifically, the percentage of epithelial cells that were actively proliferating increased from 40% at E15.5 to 65% at E19.5. Although epithelial cell proliferation did not differ between Inhba knockout embryos and controls at E15.5, there was no upregulation of epithelial cell proliferation after E15.5 in the Wolffian ducts of Inhba knockout embryos (Tomaszewski et al., 2007). Interestingly, although AR expression is maintained in the Wolffian duct mesenchyme of Inhba knockout mice, AR expression is not upregulated in the epithelium. As mentioned previously, AR expression is not detected in the Wolffian duct epithelium until E15.5 and appears to be under the control of mesenchymal factors (Cooke et al., 1991; Sugimura et al., 1986). Based upon our results, Inhba appears to be a prime candidate for the mesenchyme-derived factor required for epithelial proliferation as well as upregulation of AR in the Wolffian duct epithelium (see Fig. 1).
FIG. 1.
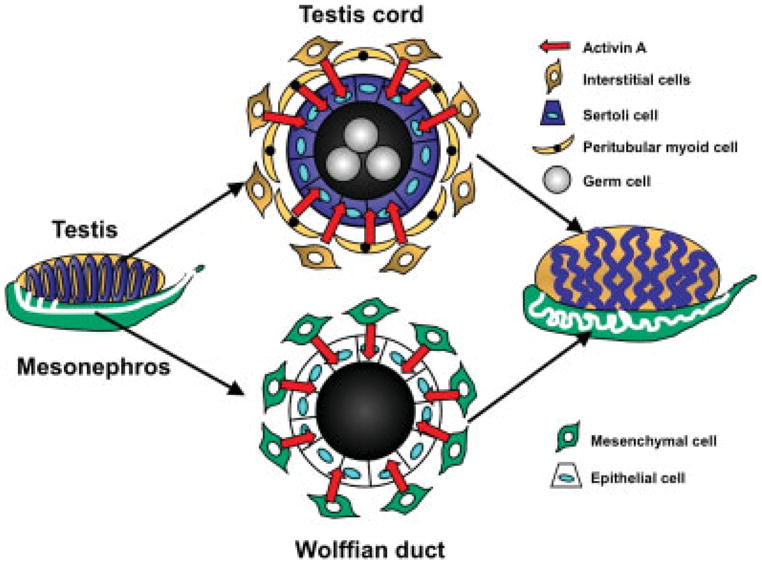
Model for the role of activin A in coiling of the embryonic testis cords and Wolffian ducts. A cross-section of the testis cord depicts germ cells within the lumen, surrounded by Sertoli cells. The peritubular myoid cells and other interstitial cells located outside the cords produce activin A, which signals to the Sertoli cell epithelium. A cross-section of the Wolffian ducts reveals an epithelial layer surrounded by a mesenchymal compartment. As is the case in the testis cords, the Wolffian duct mesenchyme also expresses activin A, which acts upon the ductal epithelium. In both the testis cords and the Wolffian ducts, mesenchyme-derived activin A stimulates proliferation of the neighboring epithelium, promoting elongation and convolution of the tubular structure.
ORGANIZATION OF THE FETAL TESTIS CORDS—ESTABLISHMENT OF EPITHELIUM AND MESENCHYME
Initially, the gonadal ridges are composed primarily of somatic components, namely the coelomic surface epithelium and its associated mesenchyme. Once the Sertoli cell lineage has been established within the fetal testes, it rapidly organizes the gonad into tubular “cords.” The testis cords consist of an epithelial layer (the Sertoli cells) which serves to separate the germ cells from the mesenchyme (the testis interstitium). These cords initially form as a series of adjacent, transverse circular loops through the gonad and are separated from one another by areas of interstitial cells. The exact cellular mechanisms involved in this complicated organization and positioning of the cords are largely unknown. Upon establishment of the testis cords, the Sertoli cells work in cooperation with the peritubular myoid cells, testis-specific squamous smooth muscle cells, to deposit the basal lamina, which serves as a structural barrier between the cords and the interstitium (Skinner et al., 1985). Initially it was thought that the peritubular myoid cells migrated into the XY gonads from the mesonephroi following the release of chemoattractants from the newly-differentiated Sertoli cells, a hypothesis supported by experiments which showed that testis cord formation can be impaired by blocking the migration of mesonephric cells into XY gonads (Martineau et al., 1997; Buehr et al., 1993; Tilmann and Capel, 1999). However, recent studies have indicated that these critical migrating mesonephric cells are most likely of endothelial origin, and that the peritubular myoid cells differentiate from precursor cells already present in the testis interstitium (Cool et al., 2008). As its name implies, the basal lamina is positioned between the basal surface of the Sertoli epithelium and the peritubular myoid cells which encircle the testis cords. The apical surface of the Sertoli epithelium is in contact with the germ cells located within the lumen of the cord. Proper formation of the testis cords does not rely upon the presence of the germ cells as agametic gonads undergo normal cord organization (Brennan and Capel, 2004). Thus far, organization of the testis cords has been shown to rely upon the coordinated efforts of the Sertoli cells and the peritubular myoid cells. Disruption of genes involved in the differentiation of or interaction between Sertoli cells and peri-tubular myoid cells can result in testis cord dysgenesis. For example, mice with a null mutation in the desert hedgehog (Dhh) gene have disorganized, poorly-formed testis cords (Clark et al., 2000). The testis cord malformations in Dhh-null mice are thought to result from improper development of the peritubular myoid cell population, which results in the laydown of a weak basal lamina, rather than from defects in Sertoli cell function (Clark et al., 2000; Pierucci-Alves et al., 2001).
Although numerous interactions between the testis cords and the interstitial mesenchyme have been identified in the adult testes, less is known about the role of compartmental communication in fetal testes (for more details on epithelial-mesenchymal interactions in adult testes, please see reviews by Skinner, 1990; Skinner et al., 1991) One of the best-characterized epithelial-mesenchymal interactions in the adult testes involves androgens, produced by the interstitial Leydig cells, and the maintenance of spermatogenesis by the Sertoli cell epithelium (reviewed by Sharpe, 1994; Skinner, 1990; and Walker and Cheng, 2005). However, this crosstalk is not present in embryonic testes as fetal Sertoli cells do not express AR, and the testes of newborn mice lacking Sertoli cell expression of AR do not differ from those of controls (Tan et al., 2005). In fetal testes, differentiation of the steroido-genic Leydig cell lineage within the interstitium occurs in response to signals from the Sertoli cells. After testis cord organization, the Sertoli epithelium produces DHH and platelet-derived growth factor (PDGF) proteins (Brennan et al., 2003; Yao et al., 2002). Both DHH and PDGF ligands are secreted by the Sertoli cells and receptors for these ligands are located on the Leydig cell precursors. These signals from the Sertoli epithelium to the pre-Leydig cells in the testicular mesenchyme initiate Leydig cell proliferation, differentiation, and subsequent androgen production in the fetal testes. Testosterone produced by the fetal Leydig cells is essential for maintenance of the Wolffian duct derivatives as discussed earlier.
EXPANSION AND COILING OF THE TESTIS CORDS
The fetal testes undergo a rapid increase in size following the organization of the testis cords at E12.5; however, the testis cords retain their original simple transverse loop structure until E15.5. Between E15.5 and birth (E19.5), the testis cords undergo a dramatic elongation and convolution process, the end result of which is the highly-coiled appearance of the seminiferous epithelium in newborn and adult testes. The expansion of the fetal testis cords before birth has been generally assumed to occur as a result of intrinsic programming of the Sertoli cells. The Sertoli cells are known to undergo rapid proliferation in the perinatal period, which is presumably essential for the testis cords to elongate; however, it is not known whether mesenchymal factors might stimulate this proliferation (Kluin et al., 1984; Steinberger and Steinberger, 1971). In an effort to identify mesenchymally-derived factors related to fetal testis cord expansion, we screened for genes expressed specifically within the testis interstitium which have the capability to signal to the epithelium. Interestingly, one such factor which followed this expression pattern was the aforementioned Inhba gene. Mirroring its expression in the Wolffian duct mesenchyme, Inhba mRNA is expressed by the fetal testis interstitium starting at E12.5 (Feijen et al., 1994; Tomaszewski et al., 2007). As is the case in the Wolffian ducts, the epithelium is the apparent target of this mesenchymal Inhba and its protein product activin A because the Sertoli cells are positive for phosphorylated-SMAD2/3, indicative of a functional activin pathway (unpublished data). Although inhibin beta B (Inhbb), a subunit of inhibin B, activin B and activin AB, is also expressed in the fetal testis, it is not present in the interstitium (Tomaszewski et al., 2007). The exclusive expression of Inhba mRNA in the interstitium indicates that activin A protein is the only active signaling product of this gene in the fetal testes. In examining histological sections of newborn Inhba knockout testes, we found dramatically fewer testis cord cross-sections compared to wild-type males. Transverse sections of wild-type and Inhba knockout testes were indistinguishable at E15.5; in both cases, the testis cords can be seen as a simple circular loop. After E15.5, a surge in Sertoli cell proliferation accompanies the elongation of the testis cords and is followed by a remarkable increase in coiling between E17.5 and E19.5 in wild-type testes. By the time of birth, the testis cords have been transformed into convoluted tubules that are seen as numerous small cross-sections of testis cord epithelium scattered throughout the testis, providing no evidence of its simple tubular beginnings.
In dramatic contrast, even at E19.5 the testis cords of Inhba knockout mice retain the same circular loop shape seen at E15.5. The failure in testis cord expansion in Inhba knockout mice appears to be the result of decreased Sertoli cell proliferation (Liu et al., 2006). In the absence of the upregulation of Sertoli cell proliferation after E15.5, the tubular structure of the Inhba knockout testis cords apparently do not elongate sufficiently to undergo convolution. Therefore, we propose Inhba as a novel interstitially-produced factor which is essential for the expansion of the testis cord epithelium prior to birth (see Fig. 1). Although a great deal of research has been conducted regarding factors required for the initial organization of the testis cords, activin A is the first signaling molecule shown to be dispensable for initial cord formation but required for later elongation and convolution. The identification of the Inhba protein product activin A as a factor coming from the interstitium which can alter Sertoli cell behavior late in fetal life suggests that perhaps other interstitial factors could be involved in the regulation of earlier Sertoli cell functions as well.
ACTIVIN A—CONSERVED REGULATOR OF TUBULAR MORPHOGENESIS
Similarities between the role of activin A in the Wolffian ducts and the fetal testes are numerous. In both tissue contexts, activin A expression begins in the mesenchyme at E12.5; yet, changes in epithelial morphogenesis are not observed until after E15.5. This delay in morphological alterations implies that either activin A elicits its effects indirectly through the activation of other downstream targets or a threshold level of activin A must be achieved before physiological response occurs. In the Wolffian ducts a clear anterior-to-posterior gradient of Inhba mRNA expression was evident along the length of the ducts (Tomaszewski et al., 2007). In both the Wolffian ducts and the testis cords, the most significant action of activin A is to elicit proliferation of the epithelial cells, which contributes to the coiling and convolution of the epithelial layer (Tomaszewski et al., 2007; unpublished data).
Although we have identified activin A as a critical factor for ductal coiling in the anterior Wolffian ducts and embryonic testes, the exact cellular and/or molecular mechanisms by which it produces this effect remain to be elucidated. Activin A is known to be involved in epithelial-mesenchymal interactions during the development of many tubular embryonic tissues, including the kidneys, salivary glands, lungs, pancreas, prostate, and dentition (summarized in Table 1) (Cancilla et al., 2001; Davies, 2001; Ferguson et al., 1998; Ritvos et al., 1995). During kidney, salivary gland, pancreas, and prostate development, the main function of activin A is to prevent branching of the primary tubular structure (Cancilla et al., 2001; Ritvos et al., 1995). In the embryonic lungs, activin A has been localized to the epithelial bronchiole tubes in the mouse (Roberts and Barth, 1994). A potential role for activin A in the prevention of epithelial branching in fetal mouse lungs is supported by data indicating that inhibition of SMAD 2/3, downstream targets of activin A, results in branching morphogenesis (Zhao et al., 1998). However, organ culture experiments conducted using embryonic rat lung explants indicate that in this tissue context it is TGFβ2 and not activin A which signals via SMAD2/3 to prevent ectopic branching of the lung epithelium (Liu et al., 2000). It remains to be determined whether epithelial expression of activin A prohibits bronchiole branching during embryonic development in the mouse (reviewed by Ball and Risbridger, 2001). During tooth development, the mesenchyme produces activin A that is required for epithelial cell proliferation and subsequent development of the incisors and mandibular molars beyond the tooth bud stage (Ferguson et al., 1998; Thesleff et al., 2007). Interestingly, whether activin is localized to the epithelium or the mesenchyme depends upon the specific tissue context, and in some tissues, such as the pancreas, activin is found to be expressed in both compartments (Ritvos et al., 1995; reviewed by Ball and Risbridger, 2001). For example, in contrast to our observations in the Wolffian ducts and fetal testes, the source of activin A in the embryonic kidneys and lungs is actually the epithelium which then signals to the adjacent mesenchyme (Maeshima et al., 2003; Roberts and Barth, 1994). Therefore, our observation of the highly compartment-specific expression of activin A and its downstream signaling targets (SMAD2/3) in the Wolffian ducts and testes may represent a role for activin that differs somehow from its actions during morphogenesis of the kidneys, salivary glands, dentition, or other tissues. Although activin A inhibits branching during the development of several organs, it is unlikely that it serves the same function in the Wolffian ducts and testes because Inhba knockout mice do not exhibit ectopic ductal branching in the absence of activin A. Similarly, the embryonic dentition, Wolffian ducts, and testes all share a mesenchymal expression pattern of activin A but the timing of action differs among these organs. In the developing teeth, the critical time for activin A expression is early, actually preceding development of the tooth buds (Ferguson et al., 1998). In the testes and Wolffian ducts, activin A appears to be important for late embryonic-stage patterning and expansion of the existing tubular structure.
Table 1.
Roles of Activin A in Tubular Morphogenesis
| Embryonic tissue | Epithelial expression | Mesenchymal expression | Function | Reference |
|---|---|---|---|---|
| Kidney | + | − | Inhibition of epithelial branching | Ritvos et al., 1995 |
| Lung | + | − | Possible inhibition of epithelial branching? | Roberts and Barth, 1994; Zhao et al., 1998 |
| Pancreas | ? | ? | Inhibition of epithelial branching | Ritvos et al., 1995 |
| Prostate | + | + | Inhibition of epithelial branching | Cancilla et al., 2001 |
| Salivary gland | − | + | Inhibition of epithelial branching | Ritvos et al., 1995 |
| Testis | − | + | Promotion of epithelial proliferation, elongation, and coiling | Feijen et al., 1994; unpublished data |
| Tooth bud | − | + | Promotion of epithelial proliferation | Ferguson et al., 1998; |
| Wolffian duct (epididymis) | − | + | Promotion of epithelial proliferation, elongation, and coiling | Tomaszewski et al., 2007 |
CONCLUSION
Epithelial-mesenchymal interactions during Wolffian duct and fetal testis morphogenesis are critical for the normal development of these organs. Particularly, in the testes, the study of epithelial-mesenchymal crosstalk has been limited by the fact that communication in the fetal testes has largely appeared to be one-way—namely, from the Sertoli cells to the interstitium. However, the identification of Inhba and its protein product activin A as an interstitially-derived factor which acts upon the Sertoli cells hints that crosstalk in the embryonic testes is in fact a two-way street. Despite all that has been discovered regarding epithelial-mesenchymal interactions during development of the male reproductive tract, numerous questions remain. For example, what controls Inhba expression in the mesenchyme? How does activin A, a protein that is not associated with coiling in any other system, elicit convolution of the testis cords and Wolffian ducts? Are other mesenchymal factors also required for expansion of the epididymides and testis cords? The answers to these questions will not only further our understanding of embryonic development of the male reproductive system but may also provide fresh perspectives regarding the crosstalk between mesenchyme and epithelium during the morphogenesis of other tubular organs.
Acknowledgments
Contract grant sponsor: March of Dimes Birth Defects Foundation (Basil O’Conner Starter Scholar Research Award), Contract grant number: #5-FY04-35, Contract grant sponsor: National Institute of Health; Contract grant numbers: HD46861, HD 052035; Contract grant sponsors: NIH (Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant Predoctoral Traineeships in Endocrine, Developmental, and Reproductive Toxicology), Contract grant sponsor: Howard Hughes Undergraduate Research Fellowship
The authors thank Dr. Martin Matzuk for providing the Inhba +/− mice. The authors also appreciate support from the members of the Yao laboratory.
Abbreviations
- Amh
anti-Müllerian hormone
- AR
androgen receptor
- Dhh
desert hedgehog
- E15.5
embryonic day 15.5
- Egf
epidermal growth factor
- Fgf
fibroblast growth factors
- GH
growth hormone
- Hox genes
homeotic genes
- Inhba
inhibin beta A
- Inhbb
inhibin beta B
- Igf1
insulin-like growth factor 1
- Pdgf
platelet-derived growth factor
- Sry
sex-determining region of the Y chromosome
- Sox9
Sry-related high mobility group box gene 9
- Tfm
testicular feminization mouse
- Tgfβ
transforming growth factor beta
LITERATURE CITED
- Airik R, Kispert A. Down the tube of obstructive nephropathies: The importance of tissue interactions during ureter development. Kidney Int. 2007;72:1459–1467. doi: 10.1038/sj.ki.5002589. [DOI] [PubMed] [Google Scholar]
- Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellvé AR, Efstratiadis A. Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol. 1996;10:903–918. doi: 10.1210/mend.10.7.8813730. [DOI] [PubMed] [Google Scholar]
- Ball EMA, Risbridger GP. Activins as regulators of branching morphogenesis. Dev Biol. 2001;238:1–12. doi: 10.1006/dbio.2001.0399. [DOI] [PubMed] [Google Scholar]
- Barrionuevo F, Bagheri-Fam S, Klattig J, Kist R, Taketo MM, Englert C, Scherer G. Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol Reprod. 2006;74:195–201. doi: 10.1095/biolreprod.105.045930. [DOI] [PubMed] [Google Scholar]
- Baskin LS, Hayward SW, Young P, Cunha GR. Role of mesenchymal-epithelial interactions in normal bladder development. J Urol. 1996;156:1820–1827. [PubMed] [Google Scholar]
- Bishop CE, Whitworth DJ, Qin Y, Agoulnik AI, Agoulnik IU, Harrison R, Behringer RR, Overbeek PA. A transgenic insertion upstream of Sox9 is associated with dominant XX sex reversal in the mouse. Nat Genet. 2000;26:490–494. doi: 10.1038/82652. [DOI] [PubMed] [Google Scholar]
- Brennan J, Capel B. One tissue, two fates: Molecular genetic events that underlie testis versus ovary development. Nat Rev Genet. 2004;5:509–521. doi: 10.1038/nrg1381. [DOI] [PubMed] [Google Scholar]
- Brennan J, Tilmann C, Capel B. Pdgfr-α mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes Dev. 2003;17:800–810. doi: 10.1101/gad.1052503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehr M, Gu S, McLaren A. Mesonephric contribution to testis differentiation in the fetal mouse. Development. 1993;117:273–281. doi: 10.1242/dev.117.1.273. [DOI] [PubMed] [Google Scholar]
- Cancilla B, Jarred RA, Wang H, Mellor SL, Cunha GR, Risbridger GP. Regulation of prostate branching morphogenesis by activin A and follistatin. Dev Biol. 2001;237:145–158. doi: 10.1006/dbio.2001.0364. [DOI] [PubMed] [Google Scholar]
- Capel B. The battle of the sexes. Mech Dev. 2000;92:89–103. doi: 10.1016/s0925-4773(99)00327-5. [DOI] [PubMed] [Google Scholar]
- Chaboissier M-C, Kobayashi A, Vidal VIP, Lützkendorf S, van de Kant HJG, Wegner M, De Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131:1891–1901. doi: 10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- Chua SS, Ma ZQ, Gong L, Lin SH, DeMayo FJ, Tsai SY. Ectopic expression of FGF-3 results in abnormal prostate and Wolffian duct development. Oncogene. 2002;21:1899–1908. doi: 10.1038/sj.onc.1205096. [DOI] [PubMed] [Google Scholar]
- Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult=type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod. 2000;63:1825–1838. doi: 10.1095/biolreprod63.6.1825. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Young P, Cunha GR. Androgen receptor expression in developing male reproductive organs. Endocrinology. 1991;128:2867–2873. doi: 10.1210/endo-128-6-2867. [DOI] [PubMed] [Google Scholar]
- Cool J, Carmona FD, Szucsik JC, Capel B. Peritubular myoid cells are not the migrating population required for testis cord formation in the XY gonad. Sex Dev. 2008;2:128–133. doi: 10.1159/000143430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR. Tissue interactions between epithelium and mesenchyme of urogenital and integumental origin. Anat Rec. 1972;172:529–541. doi: 10.1002/ar.1091720307. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Young P, Higgins SJ, Cooke PS. Neonatal seminal vesicle mesenchyme induces a new morphological and functional phenotype in the epithelia of adult ureter and ductus deferens. Development. 1991;111:145–158. doi: 10.1242/dev.111.1.145. [DOI] [PubMed] [Google Scholar]
- Davies J. Intracellular and extracellular regulation of ureteric bud morphogenesis. J Anat. 2001;198:257–264. doi: 10.1046/j.1469-7580.2000.19830257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijen A, Goumans MJ, van den Eijndenvan Raaij AJ. Expression of activin subunits, activin receptors and follistatin in postimplantation mouse embryos suggests specific developmental functions for different activins. Development. 1994;120:3621–3637. doi: 10.1242/dev.120.12.3621. [DOI] [PubMed] [Google Scholar]
- Ferguson CA, Tucker AS, Christensen L, Lau AL, Matzuk MM, Sharpe PT. Activin is an essential early mesenchymal signal in tooth development that is required for patterning of the murine dentition. Genes Dev. 1998;12:2636–2649. doi: 10.1101/gad.12.16.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta C. The role of epidermal growth factor receptor (EGFR) in male reproductive tract differentiation: Stimulation of EGFR expression and inhibition of Wolffian duct differentiation with anti-EGFR antibody. Endocrinology. 1996;137:905–910. doi: 10.1210/endo.137.3.8603602. [DOI] [PubMed] [Google Scholar]
- Gupta C. Cellular localization of immunoreactive epidermal growth factor during Wolffian duct differentiation of the fetal mouse. Urol Res. 1997;25:277–281. doi: 10.1007/BF00942098. [DOI] [PubMed] [Google Scholar]
- Gupta C. Modulation of androgen receptor (AR)-mediated transcriptional activity by EGF in the developing mouse reproductive tract primary cells. Mol Cell Endocrinol. 1999;25:169–178. doi: 10.1016/s0303-7207(99)00048-9. [DOI] [PubMed] [Google Scholar]
- Gupta C, Jaumotte J. Epidermal growth factor binding in the developing male reproductive duct and its regulation by testosterone. Endocrinology. 1993;133:1778–1782. doi: 10.1210/endo.133.4.8404620. [DOI] [PubMed] [Google Scholar]
- Gupta C, Siegel S, Ellis D. The role of EGF in testosterone-induced reproductive tract differentiation. Dev Biol. 1991;146:106–116. doi: 10.1016/0012-1606(91)90451-8. [DOI] [PubMed] [Google Scholar]
- Gupta C, Singh M. Stimulation of epidermal growth factor gene expression during the fetal mouse reproductive tract differentiation: Role of androgen and its receptor. Endocrinology. 1996;137:705–711. doi: 10.1210/endo.137.2.8593821. [DOI] [PubMed] [Google Scholar]
- Hannema SE, Hughes IA. Regulation of Wolffian duct development. Horm Res. 2007;67:142–151. doi: 10.1159/000096644. [DOI] [PubMed] [Google Scholar]
- Higgins SJ, Young P, Cunha GR. Induction of functional cytodifferentiation in the epithelium of tissue recombinants: II. Instructive induction of Wolffian duct epithelia by neonatal seminal vesicle mesenchyme. Development. 1989;106:235–250. doi: 10.1242/dev.106.2.235. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I. Fetal testis—a very special endocrine organ. Eur J Endocrinol. 1994;130:25–31. doi: 10.1530/eje.0.1300025. [DOI] [PubMed] [Google Scholar]
- Jost A. Recherches sur la differenciation sexuelle de l’embryon de lapin. Arch d’Anatomie Microscopique et de Morpholgie Exp. 1947;36:271–315. [Google Scholar]
- Jost A. Problems of fetal endocrinology: The gonadal and hypophyseal hormones. Recent Prog Horm Res. 1953;8:270–418. doi: 10.1016/b978-1-4831-9825-5.50017-8. [DOI] [PubMed] [Google Scholar]
- Karihaloo A, Nickel C, Cantley LG. Signals which build a tubule. Nephron Exp Nephrol. 2005;100:e40–e45. doi: 10.1159/000084111. [DOI] [PubMed] [Google Scholar]
- Kluin PM, Kramer MF, De Rooij DG. Proliferation of spermatogonia and Sertoli cells in maturing mice. Anat Embryol. 1984;169:73–78. doi: 10.1007/BF00300588. [DOI] [PubMed] [Google Scholar]
- Koopman P, Münsterberg A, Capel B, Vivian N, Lovell-Badge R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature. 1990;348:450–452. doi: 10.1038/348450a0. [DOI] [PubMed] [Google Scholar]
- Lawson KA. Mesenchyme specificity in rodent salivary gland development: The response of salivary epithelium to lung mesenchyme in vitro. J Embryol Exp Morphol. 1974;32:469–493. [PubMed] [Google Scholar]
- Lindsey S, Wilkinson MF. Homeobox genes and male reproductive development. J Assist Reprod Genet. 1996;13:182–192. doi: 10.1007/BF02072542. [DOI] [PubMed] [Google Scholar]
- Lipschutz JH, Young P, Taguchi O, Cunha GR. Urothelial transformation into functional glandular tissue in situ by instructive mesenchymal induction. Kidney Int. 1996;49:59–66. doi: 10.1038/ki.1996.8. [DOI] [PubMed] [Google Scholar]
- Liu C-F, Archambeault DR, Yao HHC. Testis cord morphogenesis: Determination, establishment, and maintenance. Anim Reprod. 2006;3:92–97. [Google Scholar]
- Liu J, Tseu I, Wang J, Tanswell K, Post M. Transforming growth factor β2, but not β1 and β3, is critical for early rat lung branching. Dev Dyn. 2000;217:343–360. doi: 10.1002/(SICI)1097-0177(200004)217:4<343::AID-DVDY2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Lubarsky B, Krasnow MA. Tube morphogenesis: Making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- MacLaughlin DT, Teixeira J, Donahoe PK. Perspective: Reproductive tract development—New discoveries and future directions. Endocrinology. 2001;142:2167–2172. doi: 10.1210/endo.142.6.8262. [DOI] [PubMed] [Google Scholar]
- Maeshima A, Yamashita S, Maeshima K, Kojima I, Nojima Y. Activin A produced by ureteric bud is a differentiation factor for meta-nephric mesenchyme. J Am Soc Nephrol. 2003;14:1523–1534. doi: 10.1097/01.asn.0000067419.86611.21. [DOI] [PubMed] [Google Scholar]
- Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B. Male-specific cell migration into the developing gonad. Curr Biol. 1997;7:958–968. doi: 10.1016/s0960-9822(06)00415-5. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Finegold MJ, Su JG-J, Hsueh AJW, Bradley A. Alpha-inhibin is a tumour suppressor gene with gonadal specificity in mice. Nature. 1992;360:313–319. doi: 10.1038/360313a0. [DOI] [PubMed] [Google Scholar]
- Nguyen AP, Chandorkar A, Gupta C. The role of growth hormone in fetal mouse reproductive tract differentiation. Endocrinology. 1996;137:3659–3666. doi: 10.1210/endo.137.9.8756530. [DOI] [PubMed] [Google Scholar]
- Pierucci-Alves F, Clark AM, Russell LD. A developmental study of the Desert hedgehog-null mouse testis. Biol Reprod. 2001;65:1392–1402. doi: 10.1095/biolreprod65.5.1392. [DOI] [PubMed] [Google Scholar]
- Podlasek CA, Seo RM, Clemens JQ, Ma L, Maas RL, Bushman W. Hoxa-10 deficient male mice exhibit abnormal development of the accessory sex organs. Dev Dyn. 1999;214:1–12. doi: 10.1002/(SICI)1097-0177(199901)214:1<1::AID-DVDY1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Podlasek CA, Houston J, McKenna KE, McVary KT. Posterior Hox gene expression in developing genitalia. Evol Dev. 2002;4:142–163. doi: 10.1046/j.1525-142x.2002.01068.x. [DOI] [PubMed] [Google Scholar]
- Ritvos O, Tuuri T, Erämaa M, Sainio K, Hildén K, Saxén L, Gilbert SF. Activin disrupts epithelial branching morphogenesis in the developing glandular organs of the mouse. Mech Dev. 1995;50:229–245. doi: 10.1016/0925-4773(94)00342-k. [DOI] [PubMed] [Google Scholar]
- Roberts VJ, Barth SL. Expression of messenger ribonucleic acids encoding the inhibin/activin system during mid- and late-gestation rat embryogenesis. Endocrinology. 1994;134:914–923. doi: 10.1210/endo.134.2.8299586. [DOI] [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFβ2 knockout mice have multiple developmental defects that are non-overlapping with other TGFβ knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–934. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol. 2004;66:625–645. doi: 10.1146/annurev.physiol.66.032102.135749. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neil JD, editors. The physiology of reproduction. New York: Raven Press; 1994. pp. 1363–1434. [Google Scholar]
- Skinner MK. Mesenchymal (stromal)-epithelial cell interactions in the testis and ovary which regulate gonadal function. Reprod Fertil Dev. 1990;2:237–243. doi: 10.1071/rd9900237. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Norton JN, Mullaney BP, Rosselli M, Whaley PD, Anthony CT. Cell-cell interactions and the regulation of testis function. Ann N Y Acad Sci. 1991;637:354–363. doi: 10.1111/j.1749-6632.1991.tb27322.x. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Tung PS, Fritz IB. Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol. 1985;100:1941–1947. doi: 10.1083/jcb.100.6.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staack A, Donjacour AA, Brody J, Cunha GR, Carroll P. Mouse urogenital development: A practical approach. Differentiation. 2003;71:402–413. doi: 10.1046/j.1432-0436.2003.7107004.x. [DOI] [PubMed] [Google Scholar]
- Steinberger A, Steinberger E. Replication pattern of Sertoli cells in maturing rat testis in vivo and in organ culture. Biol Reprod. 1971;4:84–87. doi: 10.1093/biolreprod/4.1.84. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Cunha GR, Bigsby RM. Androgenic induction of DNA synthesis in prostatic glands induced in the urothelium of testicular feminized (Tfm/Y) mice. Prostate. 1986;9:217–225. doi: 10.1002/pros.2990090302. [DOI] [PubMed] [Google Scholar]
- Tan KAL, De Gendt K, Atanassova N, Walker M, Sharpe RM, Saunders PTK, Denolet E, Verhoeven G. The role of androgens in Sertoli cell proliferation and functional maturation: Studies in mice with total or Sertoli cell-selective ablation of the androgen receptor. Endocrinology. 2005;146:2674–2683. doi: 10.1210/en.2004-1630. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Wang X-P, Suomalainen M. Regulation of epithelial stem cells in tooth regeneration. Comptes Rendus Biologies. 2007;330:561–564. doi: 10.1016/j.crvi.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Thomson AA, Cunha GR. Prostatic growth and development are regulated by FGF10. Development. 1999;126:3693–3701. doi: 10.1242/dev.126.16.3693. [DOI] [PubMed] [Google Scholar]
- Thomson AA, Marker PC. Branching morphogenesis in the prostate gland and seminal vesicles. Differentiation. 2006;74:382–392. doi: 10.1111/j.1432-0436.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- Tilmann C, Capel B. Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation in the mammalian gonad. Development. 1999;126:2883–2890. doi: 10.1242/dev.126.13.2883. [DOI] [PubMed] [Google Scholar]
- Tomaszewski J, Joseph A, Archambeault D, Yao HHC. Essential roles of inhibin beta A in mouse epididymal coiling. Proc Natl Acad Sci USA. 2007;104:11322–11327. doi: 10.1073/pnas.0703445104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyssière G, Berger M, Jean-Faucher C, de Turckheim M, Jean C. Testosterone and dihydrotestosterone in sexual ducts and genital tubercle of rabbit fetuses during sexual organogenesis: Effects of fetal decapitation. J Steroid Biochem. 1982;17:149–154. doi: 10.1016/0022-4731(82)90114-5. [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier M-C, De Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- Walker WH, Cheng J. FSH and testosterone signaling in Sertoli cells. Reproduction. 2005;130:15–28. doi: 10.1530/rep.1.00358. [DOI] [PubMed] [Google Scholar]
- Yao HHC, Whoriskey W, Capel B. Desert hedgehog/Patched1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 2002;16:1433–1440. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Lee M, Smith S, Warburton D. Abrogation of Smad3 and Smad2 or of Smad4 gene expression positively regulates murine embryonic lung branching morphogenesis in culture. Dev Biol. 1998;194:182–195. doi: 10.1006/dbio.1997.8825. [DOI] [PubMed] [Google Scholar]


