Abstract
Reduced production of type I procollagen is a prominent feature of chronologically-aged human skin. Connective tissue growth factor (CTGF/CCN2), a downstream target of the transforming growth factor-β (TGF-β)/Smad pathway, is highly expressed in numerous fibrotic disorders, where it is believed to stimulate excessive collagen production. CTGF is constitutively expressed in normal human dermis in vivo, suggesting that CTGF is a physiological regulator of collagen expression. We report here that the TGF-β/Smad/CTGF axis is significantly reduced in dermal fibroblasts, the major collagen-producing cells, in aged (80+ years) human skin in vivo. In primary human skin fibroblasts, neutralization of endogenous TGF-β or knockdown of CTGF substantially reduced expression of type I procollagen mRNA, protein, and promoter activity. In contrast, overexpression of CTGF stimulated type I procollagen expression, and increased promoter activity. Inhibition of TGF-β receptor kinase, knockdown of Smad4, or overexpression of inhibitory Smad7 abolished CTGF stimulation of type I procollagen expression. However, CTGF did not stimulate Smad3 phosphorylation or Smad3-dependent transcriptional activity. These data indicate that in human skin fibroblasts, type I procollagen expression is dependent on endogenous production of both TGF-β and CTGF, which act through interdependent yet distinct mechanisms. Down regulation of the TGF-β/Smad/CTGF axis likely mediates reduced type I procollagen expression, in aged human skin in vivo.
Keywords: Connective tissue growth factor, TGF-β, Type I procollagen, Human skin
INTRODUCTION
Connective tissue growth factor (CTGF/CCN2) is a member of the CCN (CCN1-6) family of cystein-rich, matricellular secreted proteins. CTGF exhibits diverse biological activities in vitro, such as cell proliferation, adhesion, migration, and extracellular matrix (ECM) production (Babic et al., 1999; Brigstock, 1999; Chen et al., 2001a; Duncan et al., 1999; Grotendorst, 1997; Gupta et al., 2000). CTGF is primarily induced by transforming growth factor-β (TGF-β) in human skin fibroblasts (Quan et al., 2002b) and appears to function as a downstream mediator of TGF-β’s ability to stimulate extracellular matrix synthesis. CTGF stimulates collagen synthesis when injected into mouse skin or added to cultured renal fibroblasts (Duncan et al., 1999). Several lines of evidence indicate that CTGF is markedly elevated in numerous fibrotic disorders, involving skin, lungs, and kidneys, where it is believed to stimulate excessive deposition of collagen. Recently, Ivkovic et al (Ivkovic et al., 2003) developed CTGF-null mice, which die shortly after birth, primarily due to respiratory failure caused by skeletal defects, indicating that CTGF plays a crucial role in regulation of cartilage ECM production during development.
Although the role of CTGF in fibrotic disorders has received considerable attention, the role of CTGF in physiological regulation of type I procollagen expression in normal human skin has received less attention (Shi-Wen et al., 2008). CTGF was shown to be expressed in fibrotic skin diseases such as scleroderma (Abraham et al., 2000; Bradham et al., 1991; Frazier et al., 1996; Grotendorst, 1997; Grotendorst et al., 1996). CTGF is also constitutively expressed, and readily detectable in normal adult human skin in vivo and normal human skin fibroblasts (Quan et al., 2002b).
In contrast to fibrotic disease, chronologically-aged human skin displays reduced production of type I procollagen, the major structural protein in human skin connective tissue. This loss of dermal collagen significantly contributes to increased skin fragility and impaired wound healing, in aged human skin. Underlying mechanisms responsible for reduced collagen biosynthesis in chronologically-aged human skin are largely unknown.
We report here that TGF-β1, CTGF and type I procollagen are significantly down regulated in fibroblasts in aged human skin, compared to young human skin in vivo. In primary human skin fibroblasts, TGF-β and CTGF act in concert, through interdependent yet distinct mechanisms, to regulate type I procollagen production. These data support the conclusion that TGF-β/Smad/CTGF forms an axis that regulates collagen production in human skin fibroblasts, and that attenuation of this axis contributes to reduced collagen content observed in aged human skin.
RESULTS
TGF-β1, CTGF, and type I procollagen expression in dermal fibroblasts is reduced in aged human skin in vivo
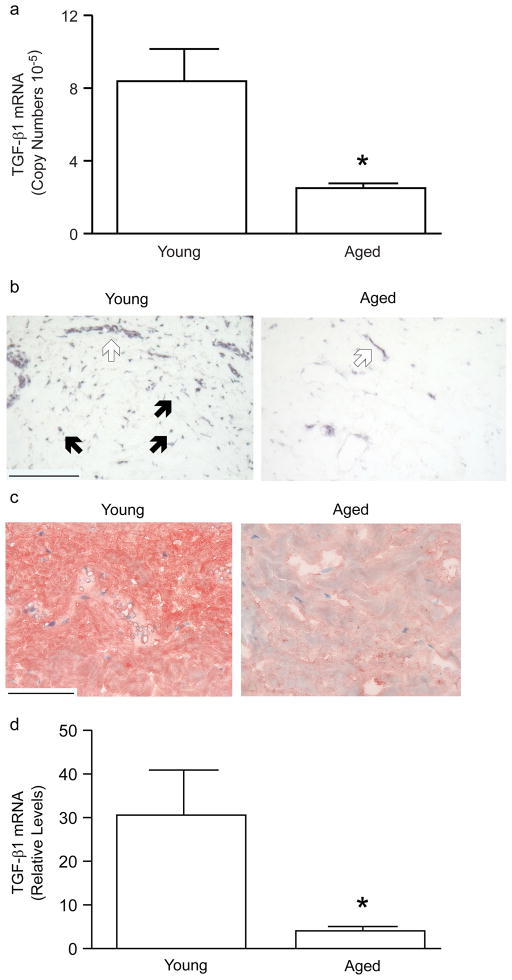
Adult human dermis expresses transcripts for TGF-β1, β2, and β3 in a ratio of 1:0.6:0.4. Levels of TGF-β2 or TGF-β3 transcripts, in the dermis of young (21–30 years of age) compared to aged (80 or greater years of age) individuals, did not significantly differ (n=6, data not shown). In contrast TGF-β1 mRNA levels were significantly reduced (70%) in aged, compared to young individuals (Figure 1A). Localization of TGF-β1 mRNA and protein, in the dermis of young and aged individuals, was determined by in situ hybridization and immunohistology, respectively. TGF-β1 mRNA (Figure 1B) and protein (Figure 1C) were expressed primarily in vascular cells and interstitial fibroblasts in the upper dermis, in young skin. TGF-β1 protein was primarily localized in the ECM, consistent with its known secretion and binding to ECM components. In aged skin, TGF-β1 mRNA (Figure 1B) and protein (Figure 1C) expression, in the dermis, were markedly reduced, compared to young skin. Laser capture microdissection was used to obtain fibroblasts from young and aged skin. TGF-β1 mRNA in captured fibroblasts was quantified by real-time RT-PCR. TGF-β1 gene expression in fibroblasts in aged skin was reduced 87%, compared to fibroblasts in young skin (Figure 1D).
Figure 1.
TGF-β1 mRNA and protein expression are reduced in the dermis of aged human skin in vivo. (A) Total RNA was prepared from dermis, obtained by dissection of full thickness young (21–30 years) or aged (80 or greater years of age) human skin. TGF-β1 and 36B4 (internal reference for normalization) mRNA were quantified in the dermis of young (21–30 years of age) and aged (80 and greater years of age) human skin, by real time RT-PCR. Data are means+SEM, N=6 young and 6 aged subjects, *p<0.05. (B) Localization of TGF-β1 mRNA in the dermis of young and aged human skin was determined by antisense riboprobe in situ hybridization. Open arrows indicate vascular cell. Solid arrows indicate fibroblasts. Results are representative five young and five aged individuals. Bar=300 m. (C) Localization of TGF-β1 protein, in the dermis of young and aged human skin, was determined by immunohistology. Results are representative of five young and five aged individuals. Bar=300 m. (D) Dermal fibroblasts were obtained by laser capture microdissection from frozen sections of young (21–30 years) and aged (80+ years) human skin. TGF-β1 and 36B4 (internal reference) mRNA levels were quantified by real-time RT-PCR. Data are means SEM, N=6 young and 6 aged subjects, *p<0.05.
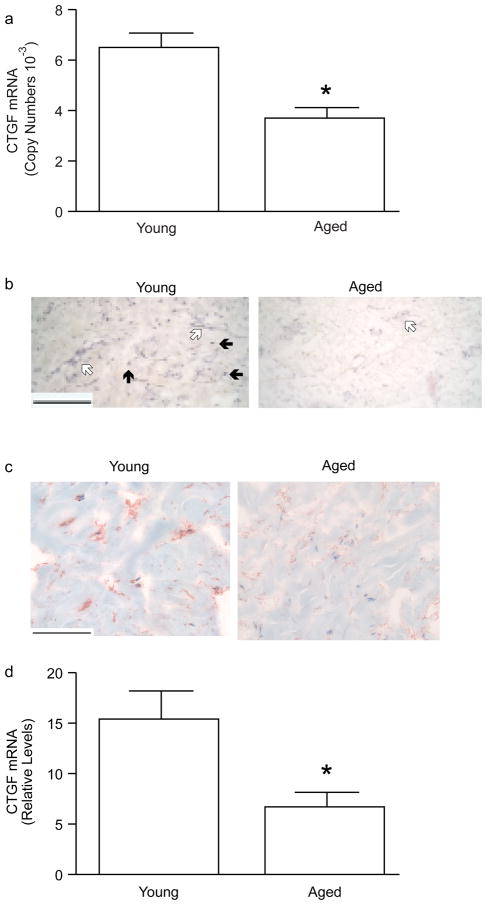
CTGF gene expression is directly regulated by TGF-β-activated Smad proteins, and TGF-β and CTGF are often overexpressed in fibrotic diseases. Therefore, we examined CTGF expression in young and aged human skin dermis. We found that basal expression of CTGF mRNA (Figure 2A) was approximately 200-times higher than that of TGF-β (Figure 1A), based on relative copy number. CTGF mRNA was substantially reduced (50%) in aged dermis, compared to young dermis (Figure 2A). CTGF mRNA (Figure 2B) and protein (Figure 2C), similar to TGF-β1, were expressed in vascular cells and interstitial fibroblasts in young skin dermis. In aged dermis, expression of CTGF mRNA (Figure 2B) and protein (Figure 2C) were substantially reduced, compared to young dermis. CTGF immunostaining was primarily localized in cells. Secreted CTGF, which binds to the ECM, was not detected with the antibody that was used. Fibroblasts from young and aged skin were obtained by laser capture microdissection. CTGF mRNA levels were approximately 57% lower in fibroblasts in aged dermis compared to fibroblasts in young dermis (Figure 2D).
Figure 2.
CTGF mRNA and protein expression are reduced in the dermis of aged human skin in vivo. (A) Total RNA was prepared from the dermis, obtained by dissection of full thickness young (21–30 years) and aged (80 or greater years of age) human skin. CTGF and 36B4 (internal reference for normalization) mRNA levels were quantified by real-time RT-PCR. Data are means + SEM, N=5 young and 5 aged subjects, *p<0.05. (B) Localization of CTGF mRNA in the dermis of young and aged human skin was determined by antisense riboprobe in situ hybridization. Results are representative five young and five aged individuals. Bar=300 μm. Open arrows indicate vascular cell. Solid arrows indicate fibroblasts. (C) Localization of CTGF protein, in the dermis of young and aged human skin, was determined by immunohistology. Results are representative of five young and five aged individuals. Bar=300 μm. (D) Dermal fibroblasts were obtained by laser capture microdissection from frozen sections of young (21–30 years) and aged (80+ years) human skin. CTGF and 36B4 (internal reference for normalization) mRNA levels were quantified by real-time RT-PCR. Data are means + SEM, N=8 young and 8 aged subjects, *p<0.05.
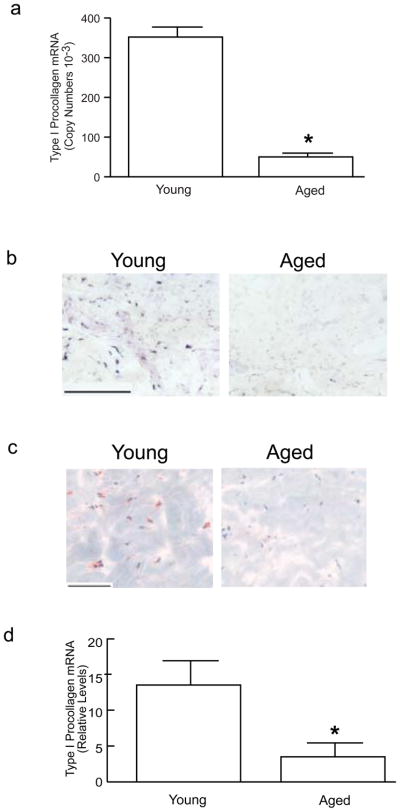
Given that TGF-β and CTGF are important mediators of fibrosis, their reduced expression in aged dermis could lower levels of collagen production. To examine this possibility, mRNA and protein expression of type I procollagen, the major structural component of the dermal ECM, were determined in young and aged human skin dermis. Type I procollagen mRNA levels in aged dermis were significantly lower than in young dermis (Figure 3A). Type I procollagen mRNA (Figure 3B) and protein (Figure 3C) were readily detected in fibroblasts in young skin dermis. Fibroblast expression of both type I procollagen mRNA (Figure 3B) and protein (Figure 3C) were strikingly reduced in aged dermis. Type I procollagen gene expression was reduced approximately 75% in fibroblasts, obtained by laser capture microdissection, in aged dermis, compared to fibroblasts in young dermis (Figure 3D).
Figure 3.
Type I procollagen mRNA and protein expression are reduced in the dermis of aged human skin in vivo. (A) Total RNA was prepared from the dermis, obtained by dissection of full thickness young (21–30 years) and aged (80 or greater years of age) human skin. Type I procollagen and 36B4 (internal reference) mRNA levels were quantified by real-time RT-PCR. Data are means + SEM, N=6 young and 6 aged subjects, *p<0.05. (B) Localization of type I procollagen mRNA, in the dermis of young and aged human skin, was determined by antisense riboprobe in situ hybridization. Results are representative five young and five aged individuals. Bar=300 μm. (C) Localization of type I procollagen protein, in the dermis of young and aged human skin, was determined by immunohistology. Results are representative of five young and five aged individuals. Bar=300 μm. (D) Dermal fibroblasts were obtained by laser capture microdissection from frozen sections of young (21–30 years) and aged (80+ years) human skin. Type I procollagen and 36B4 (internal reference for normalization) mRNA levels were quantified by real-time RT-PCR. Data are means + SEM, N=13 young and 13 aged subjects, *p<0.05.
Autocrine TGF-β stimulates CTGF and type I procollagen expression in human dermal fibroblasts
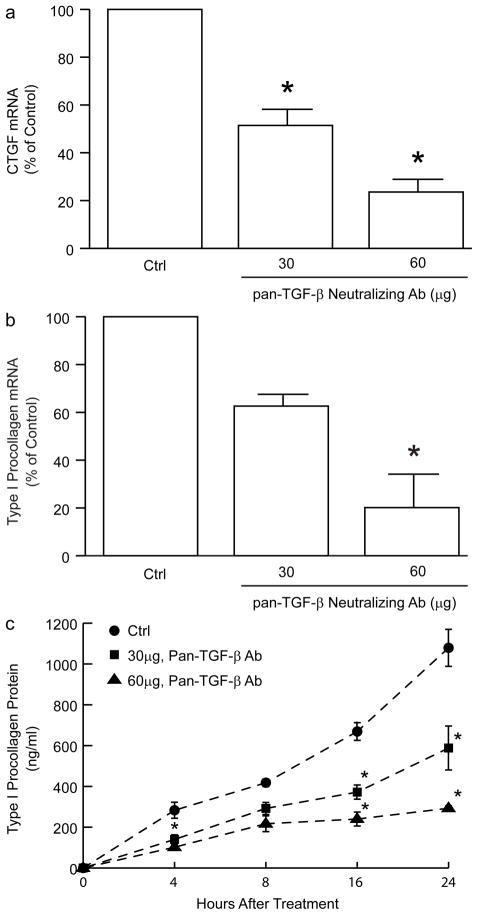
Given the compelling evidence that increased TGF-β and CTGF contribute to overproduction of collagen in fibrotic diseases, the above data suggest that reduced TGF-β and CTGF may contribute to physiological, age-related reduction of type I collagen production in human skin. To examine this possibility, we utilized primary human skin dermal fibroblasts to investigate functional relationships among TGF-β, CTGF, and type I procollagen expression. Human dermal fibroblasts express all three isoforms of TGF-β: TGF-β1, TGF-β2, and TGF-β3 (data not shown). Treatment of fibroblast cultures with pan-TGF-β neutralizing antibody significantly reduced expression of CTGF and type I procollagen. CTGF mRNA expression was reduced approximately 75% (Figure 4A); type I procollagen mRNA (Figure 4B) and protein (Figure 4C) were reduced approximately 80%.
Figure 4.
Neutralization of endogenous TGF-β reduces expression of CTGF and type I procollagen in primary adult human dermal fibroblasts. Fibroblasts were cultured in the presence of the indicated amounts of pan TGF-β neutralizing antibody for 24 hours. (A) CTGF and (B) type I procollagen mRNA were quantified by real-time RT-PCR. 36B4 mRNA levels were used as internal reference. Data are means + SEM, N=3, *p<0.05. (C) Fibroblasts were cultured in the presence of the indicated amounts of pan TGF-β neutralizing antibody for the indicated times. Type I procollagen protein secreted into the culture media was quantified by ELISA. Data are means + SEM, N=3, *p<0.05.
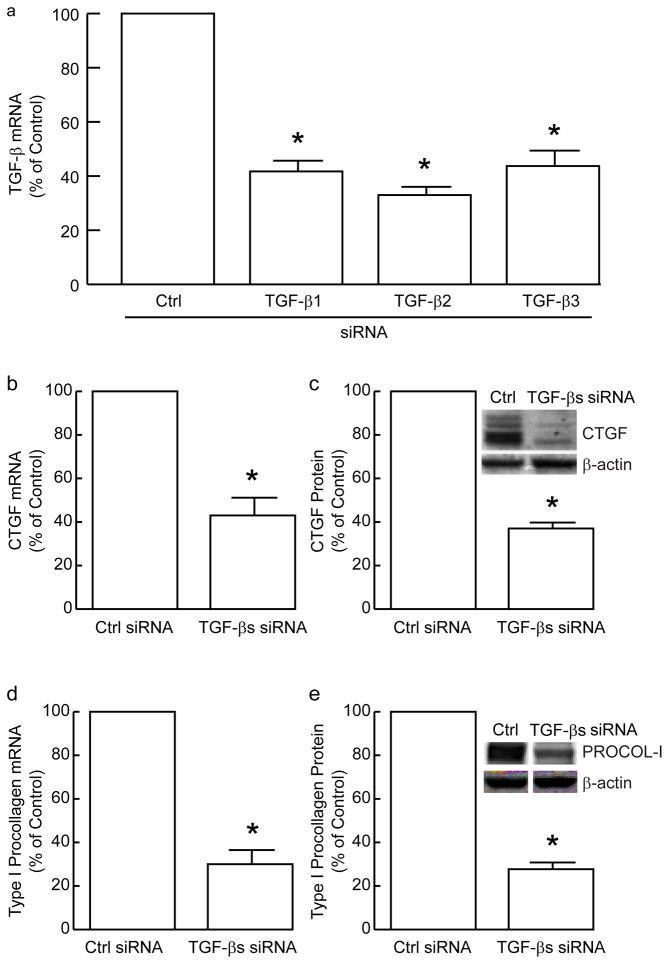
To confirm the importance of autocrine TGF-β in the regulation of CTGF and type I procollagen, we reduced endogenous expression of TGF-β1, TGF-β2, and TGF-β3 with siRNA. Each TGF-β isoform was reduced approximately 60% (Figure 5A). Knockdown of each TGF-β isoform separately reduced CTGF and type I procollagen 20–30% (data not shown). In combination, knockdown of TGF-β1, TGF-β2, and TGF-β3 yielded approximately 60% reduction of CTGF mRNA (Figure 5B) and protein (Figure 5C), confirming that basal expression of CTGF is substantially dependent on fibroblast production of TGF-β. Human dermal fibroblasts expressed primarily 38kDa and 35kDa forms of CTGF, although other small CTGF fragments were also detected. TGF-β1/2/3 knockdown reduced type I procollagen mRNA (Figure 5D) and protein (Figure 5E) 70%.
Figure 5.
Knockdown of endogenous TGF-β reduces expression of CTGF and type I procollagen in primary adult human dermal fibroblasts. (A) Fibroblasts were transfected with the indicated non-specific (Ctrl) or TGF-β isoform-specific siRNA. TGF-β1, TGF-β2, and TGF-β3 mRNA levels were determined by real-time RT-PCR, 48 hours after transfection. 36B4 mRNA levels were used as internal reference. Data are means + SEM, N=3, *p<0.05. (B–E) Fibroblasts were transfected with pooled TGF-β1, TGF-β2, and TGF-β3 siRNA. Cells were harvested 48 hours after transfection and analyzed for CTGF or type I procollagen mRNA or protein levels. Transcript levels were determined by real-time RT-PCR (36B4 mRNA was used as internal reference), and protein levels were determined by Western analyses (β-actin was used as internal control). (B) CTGF mRNA levels. Data are means + SEM, N=3, *p<0.05. (C) CTGF protein levels. Insets shows representative Western blots. Data are means + SEM, N=3, *p<0.05. (D) Type I procollagen mRNA levels. Data are means + SEM, N=3, *p<0.05. (E) Type I procollagen protein levels. Insets shows representative Western blots. Data are means + SEM, N=3, *p<0.05.
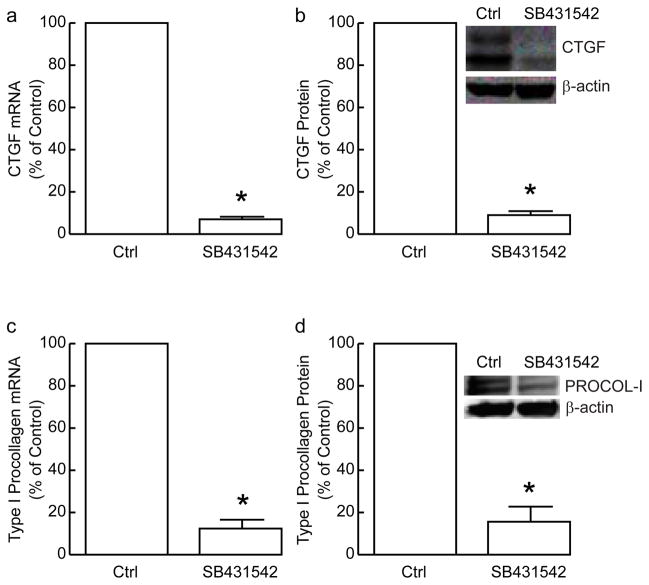
TGF-β acts primarily through its cell surface receptor complex composed of type I (ALK5), type II and type III (betaglycan) receptors. We utilized a specific TGF-β type I receptor (TβRI) kinase inhibitor SB431542 (Halder et al., 2005; Laping et al., 2002), which we have confirmed effectively blocks TGF- β-induced Smad2 phosphorylation and TGF- β-induced Smad-dependent reporter gene activity in human skin fibroblasts (data not shown), to investigate TGF-β receptor-dependence of CTGF and type I procollagen expression by aurocrine TGF-β. Treatment of fibroblasts with SB431542 reduced CTGF mRNA (Figure 6A) and protein (Figure 6B) levels approximately 90%. Type I procollagen mRNA (Figure 6C) and protein (Figure 6D) expression were also decreased approximately 90% in fibroblasts treated with SB431542.
Figure 6.
Inhibition of type I TGF-β receptor reduces expression of CTGF and type I procollagen in primary adult human dermal fibroblasts. Fibroblasts were treated with DMSO vehicle (Ctrl) or type I TGF-β receptor inhibitor SB431542 (10μM) for 24 hours. CTGF and type I procollagen mRNA and protein levels were determined by real-time RT-PCR and Western analyses, respectively. (A) CTGF mRNA levels were normalized to 36B4 mRNA levels, used as internal reference. Data are means+SEM, N=3, *p<0.05. (B) CTGF protein levels were normalized to s-actin used as internal control. Insets shows representative Western blots. Data are means+SEM, N=3, *p<0.05. (C) Type I procollagen mRNA levels were normalized to 36B4 mRNA levels, used as internal reference. Data are means+SEM, N=3, *p<0.05. (D) Type I procollagen protein levels were normalized to s-actin used as internal control. Insets shows representative Western blots. Data are means+SEM, N=3, *p<0.05.
Knockdown of CTGF reduces expression of type I procollagen in human skin fibroblasts
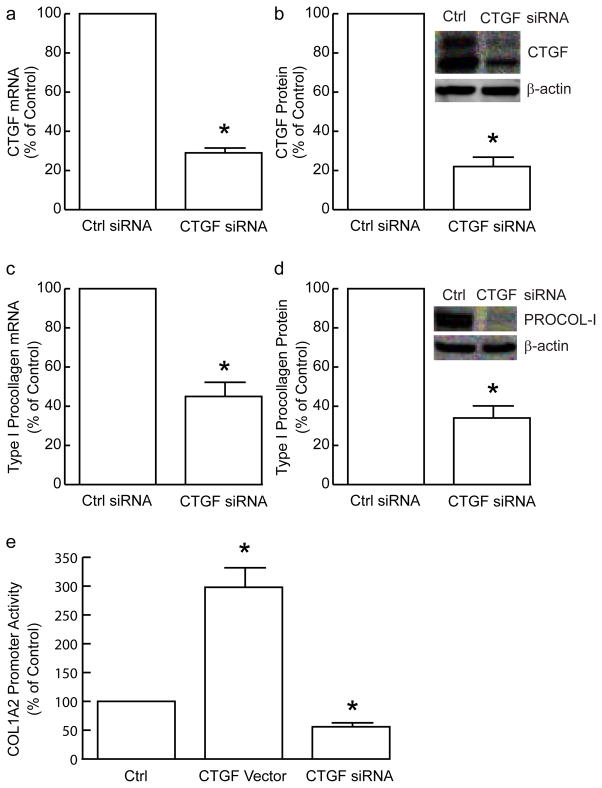
Taken together, the above data demonstrate that autocrine TGF-β is the major mediator of CTGF and type I procollagen production in primary human dermal fibroblasts. To explore the extent to which CTGF is involved in autocrine TGF-β stimulation of type I procollagen production, we utilized siRNA-mediated knockdown. CTGF mRNA (Figure 7A) and protein (Figure 7B) levels were reduced 71% and 78%, respectively, three days following transfection of CTGF siRNA, and remained at this minimum level for at least five days (data not shown). Knockdown of CTGF had no effect on the mRNA levels of any of the other five CCN family members (data not shown), indicating the effect of the CTGF siRNA was specific (and the levels of other CCN family members are not dependent on CTGF). Additionally, CTGF knockdown did not alter expression of TGF-β1/2/3 (data not shown). Three days after transfection of CTGF siRNA, type I procollagen mRNA (Figure 7C) and protein (Figure 7D) were reduced 55% and 65%, respectively. These data indicate that CTGF mediates, at least in part, autocrine TGF-β-dependent type I procollagen production in human dermal fibroblasts.
Figure 7.
Knockdown of CTGF reduces expression of type I procollagen in primary adult human dermal fibro-blasts. Fibroblasts were transfected with non-specific siRNA (Ctrl) or CTGF siRNA. Total RNA or protein was prepared 48 hours after transfection. (A) CTGF and 36B4 (internal reference for normalization) mRNA levels were quantified by real-time RT-PCR. Data are means + SEM, N=3, *p<0.05. (B) CTGF and s-actin (internal reference for normalization) protein levels were quantified by Western analyses. Insets show representative Western blots. Data are means + SEM, N=3, *p<0.05. (C) Type I procollagen and 36B4 (internal reference for normalization) mRNA levels were quantified by real-time RT-PCR. Data are means + SEM, N=3, *p<0.05. (D) Type I procollagen and s-actin (internal reference) protein levels were quantified by Western analyses. Insets show representative Western blots. Data are means + SEM, N=3, *p<0.05. (E) Fibroblasts were transfected with type I procollagen α2 promoter (COL1A2) CAT reporter and Lac Z reporter (internal control for normalization), with empty vector (pCDNA3.1) and non-specific siRNA (Ctrl), or CTGF expression vector, or CTGF siRNA. CAT and β-galactosidase activities were determined 48 hours after transfection. Data are means + SEM, N=3, *p<0.05.
Type I (α2) procollagen (COL-1A2) promoter activity is regulated by CTGF in human skin fibroblasts
Since knockdown of CTGF reduces type I procollagen mRNA level, we next investigated whether CTGF is able to regulate type I procollagen gene promoter activity. COL-1A2 promoter/reporter construct was co-transfected with CTGF siRNA or CTGF expression vector. As shown in Figure 7E, knockdown of CTGF significantly attenuated COL-1A2 promoter activity. In contrast, overexpression of CTGF significantly potentiated COL-1A2 promoter activity.
CTGF regulation of type I procollagen expression is dependent on TGF-β/Smad signaling pathway in human skin fibroblasts
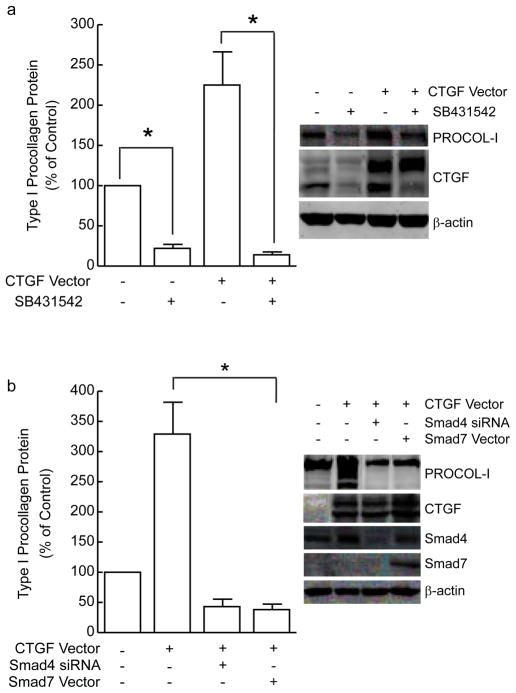
The above data indicate that CTGF functions as endogenous regulator of autocrine TGF-β-dependent type I procollagen production in human skin fibroblasts. However, molecular mechanism(s) by which CTGF regulates type I procollagen are largely unknown. We first determined whether CTGF regulation of type I procollagen expression is dependent on TGF-β signaling pathway. We employed three approaches to address this question. First, control and CTGF over-expressing fibroblasts were treated with specific TβRI kinase inhibitor SB431542 or vehicle. As shown in Figure 8A, CTGF overexpression increased type I procollagen expression, and this increase was completely abolished by SB431542. CTGF over-expression did not alter gene expression levels of TGF-β1, 2, or 3 (data not shown). These data indicate that TGF-β receptor activity is required for CTGF-mediated stimulation of type I procollagen in human skin fibroblasts. To further substantiate this conclusion, we simultaneously over-expressed CTGF and knocked down Smad4, or over-expressed inhibitory Smad7. Induction of type I procollagen by CTGF over-expression was completely blocked by either knockdown of Smad4, or over-expression of Smad7 (Figure 8B). These data further demonstrate that the ability of CTGF to augment TGF-β induction of type I procollagen expression is dependent on intact TGF-β signaling pathway.
Figure 8.
CTGF regulation of type I procollagen is dependent on TGF-β/Smad signaling pathway in primary adult human dermal fibroblasts. (A) Fibroblasts were transfected with empty vector or CTGF expression vector. Thirty-two hours after transfection, cells were treated for 16 hours with vehicle or type I TGF-β receptor inhibitor SB431542 (10μM). Type I procollagen (PROCOL-I), CTGF, and s-actin (internal reference for normalization) protein levels were quantified by Western analyses. Insets show representative Western blots. Data are means + SEM, N=3, *p<0.05. (B) Fibroblasts were transfected with empty vector (−) (pCDNA3.1) and non-specific siRNA (Ctrl siRNA), or CTGF expression vector with Smad4 siRNA, or Smad7 expression vector. Whole cell protein extracts were prepared 48 hours after transfection. Type I procollagen, CTGF, Smad4, Smad7, and s-actin (internal reference) protein levels were quantified by Western analyses. Insets show representative Western blots. Data are means + SEM, N=3, *p<0.05.
CTGF regulation of type I procollagen expression is not mediated through direct activation of TGF-β/Smad signaling pathway in human skin fibroblasts
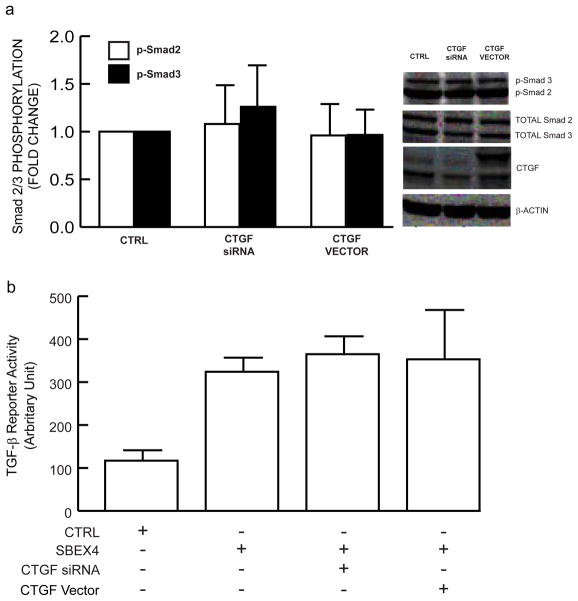
It has recently been reported that CTGF potentiates binding of TGF-β to its receptor complex, thereby stimulating Smad2 phosphorylation and Smad-dependent transcriptional activity, in mink lung epithelial cells (Abreu et al., 2002). Therefore, we examined whether similar mechanisms operate in human dermal fibroblasts, under conditions we determined in which CTGF knockdown or overexpression regulated type I procollagen expression. Interestingly, CTGF knockdown did not alter Smad2 or Smad3 phosphorylation (Figure 9A). Similarly, CTGF overexpression did not alter either Smad2 or Smad3 phosphorylation (Figure 9A). Consistent with these results, CTGF knockdown or overexpression had no effect on transcription of TGF-β/Smad3-dependent reporter gene (Figure 9B). These data argue against CTGF acting to regulate type I procollagen expression through direct augmentation of the TGF-β/Smad signaling pathway.
Figure 9.
CTGF does not modulate TGF-β/Smad signaling pathway in primary adult human dermal fibro-blasts. (A) Fibroblasts were transfected with CTGF siRNA or CTGF expression vector or their appropriate controls (CTRL), non-specific siRNA, orempty vector pCDNA3.1, respectively. Whole cell lysates were prepared 48 hours after transfection. Phosphorylated Smad2 (p-Smad2), phosphorylated Smad3 (p-Smad3), total Smad2, total Smad3, CTGF, and s-actin (internal reference for normalization) protein levels were determined by Western analyses. Inset shows representative Western blots. N=3. (B) Fibroblasts were co-transfected with Smad3 luciferse reporter construct (SBEX4) and LacZ reporter (internal control for normalization), or CTGF siRNA, or CTGF expression vector, or their appropriate controls (CTRL), empty luciferase reporter plasmid (pGL3), or non-specific siRNA, or empty expression vector, respectively. Whole cell extracts were prepared 48 hours after transfection, and assayed for luciferase and β-galactosidase activities. Data are means + SEM, N=3.
DISCUSSION
Type I collagen is the major structural protein in human skin. Age-dependent collagen loss causes elderly skin to become thin and fragile. In the present study, we investigated the role of TGF-β/Smad pathway and CTGF in age-dependent reduction of collagen production in human skin. Our results demonstrate significant decrease of TGF-β1 and CTGF in fibroblasts in aged human skin in vivo, and type I procollagen production by human dermal fibroblasts is dependent on both TGF-β and CTGF.
We find that expression levels of TGF-β1, CTGF or type I procollagen do not significantly differ between dermal fibroblasts cultured from aged skin or young skin (unpublished data). These data highlight the importance of factors within the tissue environment, rather than inherent genetic or epigenetic alterations, as key determinants of down regulation of the TGF-β/Smad/CTGF/procollagen axis in aged human skin. We have previously described increased fragmentation of the collagenous extracellular matrix and attendant reduction of fibroblast stretch in aged human skin (Fisher et al., 2002; Fisher et al., 2009; Varani et al., 2006). Decreased mechanical tension is known to reduce collagen production by skin fibroblasts (Fisher et al., 2008; Fligiel et al., 2003; Varani et al., 2006). Thus, age-dependent decline of the TGF-β/Smad/CTGF/procollagen axis in human skin may originate from altered structural and mechanical properties of the dermal extracellular matrix. Molecular mechanisms that sense mechanical tension and couple it to cellular processes, such as collagen production are not well understood. Interestingly, we have observed that reduced mechanical tension impairs the TGF-β/Smad pathway in human skin fibroblasts (Quan, TH and Fisher, GJ, unpublished observation). These data suggest that TGF-β signaling may be regulated in part by mechano-sensing mechanisms.
CTGF was originally isolated from cultured endothelial cells and later reported to be highly expressed in fibrotic disorders (Abraham et al., 2000; Bradham et al., 1991; Frazier et al., 1996; Grotendorst, 1997; Grotendorst et al., 1996; Leask and Abraham, 2003, 2004). Here we provide evidence that CTGF functions as an intrinsic, physiological mediator of type I procollagen expression, and that reduced expression of CTGF contributes to age-dependent reduction of type I procollagen production observed in human skin. We find that reduced expression of CTGF in dermal fibroblasts in aged human skin in vivo mirrors reduced expression of type I procollagen. These data are in agreement with our previous report that CTGF is constitutively expressed in normal human skin in vivo (Quan et al., 2002b). CTGF knockdown or overexpression results in reduced or increased expression, respectively, of type I procollagen in human dermal fibroblasts. This regulation of type I procollagen production by CTGF is mediated, at least in part, by transcriptional mechanisms.
It is well documented that CTGF is rapidly and potently induced by TGF-β in a variety of cells including primary human skin fibroblasts (Quan et al., 2002b). Synergy between CTGF and TGF-β has been reported in a mouse fibrosis model (Mori et al., 1999). Injection of TGF-β or CTGF alone into mouse skin caused transient fibrotic tissue formation. However, simultaneous injection of TGF-β plus CTGF produced long-term, persistent fibrotic tissue formation. Consistent with these data, recently Chujo et al reported that serial subcutaneous injection of CTGF after TGF-β resulted in a significant increase of COL1A2 promoter activity and mRNA expression, compared with TGF-β treatment alone (Chujo et al., 2005). CTGF has also been shown to induce COL1A2 promter activity in promimal tubular epithelial cells (Gore-Hyer et al., 2002). Furthermore, Yang et al (Yang et al., 2004) demonstrated that CTGF augments TGF-β-induced myofibroblast differentiation in normal rat kidney fibroblasts in vitro.
CTGF has been reported to directly bind TGF-β1, and increase interaction of TGF-β with its receptors in non-fibroblast cell lines (Abreu et al., 2002). This enhancement of TGF-β-binding led to increased Smad phosphorylation and Smad-dependent transcriptional activity. These effects of exogenous CTGF were observed only at sub-nanomolar TGF-β concentrations (10pM or less). In our studies, under conditions in which overexpression or knock-down of CTGF substantially altered type I procollagen expression, there were no significant effects of either overexpression or knockdown of CTGF on TGF-β-dependent Smad2/3 phosphorylation or Smad3 transcriptional activity. These data indicate that the ability of endogenous CTGF to regulate type I procollagen expression is not dependent on direct potentiation of Smad activation in human dermal fibroblasts. Shi-Wen et al have reported that CTGF action is Smad independent (Shi-Wen et al., 2006).
We found that the ability of CTGF to upregulate type I procollagen is dependent on intact TGF-β signaling. Recently Qi et al reported that the profibrotic effect of CTGF was completely abrogated in the presence of pan-specific TGF-β and TGF-β type II receptor neutralizing antibodies in renal cortical fibroblasts (Qi et al., 2005), indicating that CTGF requires intact TGF-β signaling to exert its effect on ECM production. CTGF is potently induced by TGF-β and this increased CTGF stimulates type I procollagen expression. Thus, CTGF is both regulated by TGF-β signaling, and is required for downstream action of TGF-β.
The mechanism by which CTGF exerts is regulatory effects on TGF-β-dependent type I collagen production is not known. Emerging evidence indicates that the actions of CTGF, at least in part, are mediated through its interactions with integrins, which bind to ECM proteins. CTGF has been shown to bind to a variety of integrins including α5β1 (Nishida et al., 2007), αvβ3 (Babic et al., 1999), α 6β1 (Heng et al., 2006); (Chen et al., 2001b), α4β1 (Chen et al., 2004), α Mβ2 (Schober et al., 2002) and α IIbβ3 (Jedsadayanmata et al., 1999), in a cell type specific manner. This binding can activate integrin signaling pathways leading to activation of focal adhesion kinase and MAP kinases. CTGF has also been reported to bind to ECM heparin sulfate proteoglycans perlecan (Nishida et al., 2003) and syndecan 4 (Chen et al., 2004). These data support the concept that CTGF cooperates with extracellular matrix proteins to specify functional interactions with their cell surface receptors (Yeger and Perbal, 2007).
TGF-β is the most potent stimulator of CTGF gene expression (Holmes et al., 2001; Leask et al., 2003). Therefore, reduced expression of TGF-β 1 in aged human skin is likely a major contributing factor to the observed reduction of CTGF. We have previously reported that UV irradiation down-regulates type II TGF-β receptor and thereby substantially reduces cellular responsiveness to TGF-β (Quan et al., 2001). Interestingly, type II TGF-β receptor expression is reduced in dermal fibroblasts in aged human skin in vivo (Quan et al., 2006). These data indicate that down regulation of type II TGF-β receptor may also contribute to reduced expression of CTGF in aged human skin. Additionally, it has been shown that JNK/c-Jun/MAPK pathway antagonizes TGF-β induction of CTGF transcription (Leask et al., 2003; Leivonen et al., 2001). We have previously reported that JNK/c-Jun/MAPK pathway is increased in aged compared to young human skin in vivo (Chung et al., 2000), suggesting that alterations of JNK/c-Jun/MAPK pathway may contribute to reduced expression of CTGF in aged human skin. c-Jun directly interacts with activated Smad proteins in the nucleus to prevent their binding to target genes (Verrecchia et al., 2000). c-Jun also competes with Smad proteins for the common transcription co-activator p300. Both mechanisms can simultaneously contribute to the c-Jun-mediated inhibition of TGF-β/Smad signaling pathway. Therefore, elevated c-Jun may impair TGF-β/Smad signaling, which may in turn contribute to reduced expression of CTGF observed in aged human skin.
In summary, data presented above demonstrate that TGF-β1 and CTGF are significantly reduced in aged human skin in vivo. Given the pivotal, synergistic role of endogenous TGF-β and CTGF in regulating type I procollagen synthesis by dermal adult human fibroblasts, it is likely that reduced expression of these two pro-fibrotic cytokines is key to reduced production of type I procollagen, which is a prominent feature of aged human skin.
METHODS AND MATERIALS
Materials
Dulbecco’s Modified Eagle’s Media (DMEM), fetal bovine serum, trypsin solution, penicillin, and streptomycin were purchased from Invitrogen Life Technology (Carlsbad, CA). CTGF antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA); type I procollagen antibody used for Western blot was purchased from RDI Research Diagnostics (Flanders, NJ); rat type I procollagen antibody (Millipore Corp., Burlington, MA) was used for immunohistology; phospho-Smad2 and phospho-Smad3 antibodies was purchased from Cell Signaling Technology (Beverly, MA); total Smad2 and Smad3 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA); human recombinant TGF-β1 was purchased from R&D Systems (Minneapolis, MN). Unless otherwise stated, all other reagents were purchased from Sigma Chemical Company (St. Louis, MO).
Procurement of human tissue samples
Human skin samples were obtained from adult volunteers, as previously described (Fisher et al., 1991a; Fisher et al., 1998; Fisher et al., 1997). Subjects were grouped according to age: 21–30 years for young group and 80+ years for aged group. Full-thickness skin punch biopsies (4 mm) were obtained from sun-protected buttock skin. All procedures involving human subjects were approved by the University of Michigan Institutional Review Board, and all subjects provided written informed consent.
Cell culture
Primary human skin fibroblasts were cultured from punch biopsies of adult normal buttock skin (aged 21–55 years), as described previously (Fisher et al., 1991b). Cells were cultivated in DMEM supplemented with 10% Fetal Bovine Serum (Invitrogen). Cells were plated at 70–80% confluence, and used one day later. Cells were utilized between passages 3 and 7. Independent replicates of studies, indicated by N number in figure legends, were performed with cells from different individuals.
RNA isolation, laser capture microdissection, and quantitative real-time RT-PCR
Dermis was separated from epidermis by dissection and total RNA was extracted from human skin dermis using commercial kit (RNeasy Midi Kit, Qiagen, Chatsworth, CA) as previously described (Fisher et al., 2009; Quan et al., 2002b) and described in Supplemental Material. Fibroblasts (approximately 200 cells) from frozen skin sections, from six young (21–30 years) and six aged (80+ years) individuals were obtained by laser capture microdissection using a Leica AS LMD, as previously described (Quan et al., 2002b, 2004; Quan et al., 2006). Quantitative real-time RT-PCR and PCR primer and probe sequences have been previously described (Quan et al., 2002b, 2004; Quan et al., 2006) or are described in Supplemental Material Table 1).
In situ hybridization
RNA probes were prepared from cDNA of TGF-β1 (Quan et al., 2002a), type I(α1) procollagen (Fisher et al., 2000), and CTGF (generously provided by Dr. Grotendorst, Department of Cell Biology and Anatomy, University of Miami School of Medicine, Miami, FL). Digoxigenin-containing sense and antisense riboprobes were synthesized using T7 and SP6 ribonucleic polymerase, respectively. Frozen skin sections (5 μm) were mounted, fixed, treated, and hybridized as previously described (Fisher et al., 1997; Quan et al., 2002b). Hybridization signals were detected immunohistochemically by alkaline phosphatase-conjugated anti-digoxigenin antibody. The sense riboprobe yielded minimal background signal (data not shown).
Immunohistology
Detection of type I procollagen, TGF-β1 and CTGF in young and aged human skin by immunohistology was performed as previously described (Quan et al., 2002b; Quan et al., 2006; Quan et al., 2001; Quan et al., 2005) and described in Supplemental Material.
Western analysis and type I procollagen ELISA
Western analyses and type I procollagen ELISA were performed as previously described (Quan et al., 2002b, 2004; Quan et al., 2005) and described in Supplemental Material.
Transfection
Oligonucletide siRNA sequences targeting CTGF, TGF-β1, β 2, and β3 were designed from human mRNA open reading frames according to OligoEngine (Seattle, WA) website instructions and submitted to a BLAST search against human genome database to confirm specificity (see Supplemental Material for sequences, Table II). siRNA oligonucleotides were synthesized by Qiagen (Chatsworth, CA). CTGF expression vector (CTGF-V5 TOPO) (Wahab et al., 2001) was generously provided by Dr. Wahab (Cell and Molecular Biology Section, Imperial College School of Medicine, London). TGFβ/Smad reporter construct (P3T-Lux) was generously provided by Dr. Joan Massague (Sloan-Kettering Institute, NY). Human skin fibroblasts were transiently transfected by electroporation, using Amaxa Nucleofector (Koeln, Germany). After transfection (48 hours), total RNA and cellular protein were extracted and mRNA and protein levels were determined by real-time RT-PCR and Western analysis, respectively, as described above. Luciferase activity was measured using an enhanced luciferase assay kit (PharMingen International, San Diego, CA) according to the manufacturer’s protocol. COL1a2 CAT reporter gene plasmid (−772ti +58) was provided by Dr Trojanowski (Ihn et al., 1997). CAT assays were carried out as previously described (Quan and Fisher, 1999; Quan et al., 2004). Aliquots containing identical β-galactosidase activity were used for each luciferase assay.
Statistical analysis
Comparisons were made with the paired t-test (two groups) or the repeated measures of ANOVA (more than two groups). Multiple pair-wise comparisons were made with the Tukey Studentized Range test. All p values are two-tailed, and considered significant when <0.05.
Supplementary Material
Acknowledgments
We are indebted to Suzan Rehbine for the procurement of tissue specimens. Kenne Currie, Trupta Purohit, and Zhaoping Qin provided technical support. Laura VanGoor and Diane Fiolek assisted in preparation of graphic material and administrative support. This work was supported by a grant from the NIH (AG019364 and AG025186 to GJF).
Abbreviations
- CTGF
connective tissue growth factor
- TGF-β
transforming growth factor-β
- COL1A2
type I (α2) procollagen 1A2
- ECM
extracellular matrix
- MAPK
mitogen-activated protein kinase
- JNK
c-Jun NH2-terminal kinase
References
- Abraham D, Shiwen X, Black C, Sa S, Xu Y, Leask A. Tumor necrosis factor β suppresses the induction of connective tissue growth factor by transforming growth factor-β in normal and scleroderma fibroblasts. J Biol Chem. 2000;275:15220–15225. doi: 10.1074/jbc.275.20.15220. [DOI] [PubMed] [Google Scholar]
- Abreu J, Ketpura N, Reversade B, De Roberts E. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-β. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic A, Chen C, Lau F. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin αvβ3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol. 1999;19:2958–2966. doi: 10.1128/mcb.19.4.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradham D, Igarashi A, Potter R, Grotendorst G. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–1294. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigstock D. The connective tissue growth factor/Cysteine-rich 61/nephroblastoma overexpressed (CCN) family. Endocrine Rev. 1999;20:189–206. doi: 10.1210/edrv.20.2.0360. [DOI] [PubMed] [Google Scholar]
- Chen C-C, Chen N, Lau L. The angiogenic factors Cyr61 and connective tissue growth factor induce adhesive signaling in primary human skin fibroblasts. J Biol Chem. 2001a;276:10443–10452. doi: 10.1074/jbc.M008087200. [DOI] [PubMed] [Google Scholar]
- Chen C-C, Mo F-E, Lau L. The angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblasts. J Biol Chem. 2001b;276:47329–47337. doi: 10.1074/jbc.M107666200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Abraham D, Shi-Wen X, Pearson J, Black C, Lyons K, et al. CCN2 (connective tissue growth factor) promotes fibroblast adhesion to fibronectin. Mol Biol Cell. 2004;15:5635–5646. doi: 10.1091/mbc.E04-06-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chujo S, Shirasaki F, Kawara S, Inagaki Y, Kinbara T, Inaoki M, et al. Connective tissue growth factor causes persistent proalpha2(I) collagen gene expression induced by transforming growth factor-beta in a mouse fibrosis model. J Cell Physiol. 2005;203:447–456. doi: 10.1002/jcp.20251. [DOI] [PubMed] [Google Scholar]
- Chung J, Kang S, Varani J, Lin J, Fisher G, Voorhees J. Decreased extracellular-signal-regulated kinase and increased stress-activated MAP kinase activities in aged human skin in vivo. J Invest Dermatol. 2000;114:177–182. doi: 10.1046/j.1523-1747.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- Duncan M, Frazier K, Abramson S, Williams S, Klapper H, Huang X, et al. Connective tissue growth factor mediates transforming growth factor β-induced collagen synthesis: down-regulation by cAMP. FASEB J. 1999;13:1774–1786. [PubMed] [Google Scholar]
- Fisher G, Datta S, Wang Z, Li X, Quan T, Chung J, et al. c-Jun dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoid acid. J Clin Invest. 2000;106:661–668. doi: 10.1172/JCI9362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher G, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138:1462–1470. doi: 10.1001/archderm.138.11.1462. [DOI] [PubMed] [Google Scholar]
- Fisher G, Quan T, Purohit T, Shao Y, Cho M, He T, et al. Collagen fragmentation promotes oxidates stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Path. 2009;174:101–114. doi: 10.2353/ajpath.2009.080599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher G, Varani J, Voorhees J. Looking Older: Fibroblast collapse and therapeutic implications. Arch Dermatol. 2008;144:666–672. doi: 10.1001/archderm.144.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Esmann J, Griffiths CEM, Voorhees JJ. Cellular, immunologic and biochemical characterization of topical retinoic acid-treated human skin. J Invest Dermatol. 1991a;96:699–707. doi: 10.1111/1523-1747.ep12470632. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Henderson PA, Voorhees JJ, Baldassare JJ. Epidermal growth factor-induced hydrolysis of phosphatidylcholine by phospholipase D and phospholipase C in human dermal fibroblasts. J Cell Physiol. 1991b;146:309–317. doi: 10.1002/jcp.1041460216. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Talwar HS, Lin JY, Lin PP, McPhillips F, Wang ZQ, et al. Retinoic acid inhibits induction of c-Jun protein by ultraviolet irradiation that occurs subsequent to activation of mitogen-activated protein kinase pathways in human skin in vivo. J Clin Invest. 1998;101:1432–1440. doi: 10.1172/JCI2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. New Eng J Med. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- Fligiel S, Varani J, Datta S, Kang S, Fisher G, Voorhees J. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J Invest Dermatol. 2003;120:842–848. doi: 10.1046/j.1523-1747.2003.12148.x. [DOI] [PubMed] [Google Scholar]
- Frazier K, Williams S, Kothapalli D, Klapper H, Grotendorst G. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Invest Dermatol. 1996;107:404–411. doi: 10.1111/1523-1747.ep12363389. [DOI] [PubMed] [Google Scholar]
- Gore-Hyer E, Shegogue D, Markiewicz M, Lo S, Hazen-Martin D, Greene E, et al. TGF-beta and CTGF have overlapping and distinct fibrogenic effects on human renal cells. Am J Physiol Renal Physiol. 2002;283:F707–716. doi: 10.1152/ajprenal.00007.2002. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. Connective tissue growth factor: a mediator of TGF-β action on fibroblasts. Cytokine Growth Factor Rev. 1997;8:171–179. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Grotendorst G, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996;7:469–480. [PubMed] [Google Scholar]
- Gupta S, Clarkson M, Duggan J, Brady H. Connective tissue growth factor: Potential role in glomerulosclerosis and tubulointerstitial fibrosis. Kidney Intl. 2000;58:1389–1399. doi: 10.1046/j.1523-1755.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- Halder S, Beauchamp R, Datta P. A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia. 2005;7:509–521. doi: 10.1593/neo.04640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng E, Huang Y, Black S, Jr, Trackman P. CCN2, connective tissue growth factor stimulates collagen deposition by gingival fibroblasts via module 3 and α6 and β1 integrins. J Cell Biochem. 2006;98:409–420. doi: 10.1002/jcb.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Sa S, Shiwen X, Black C, Abraham D, Leask A. CTGF and Smads: Maintenance of scleroderma phenotype is independent of Smad signalling. J Biol Chem. 2001;276:10594–10601. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]
- Ihn H, LeRoy E, Trojanowska M. Oncostatin M stimulates transcription of the human α2(I) collagen gene via the Sp1/Sp3-binding site. J Biol Chem. 1997;272:24666–24672. doi: 10.1074/jbc.272.39.24666. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Yoon B, Popoff S, Safadi F, Libuda D, Stephenson R, et al. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedsadayanmata A, Chen C-C, Kireeva M, Lau L, Lam SC-T. Activation-dependent adhesion of human platelets to Cyr61 and Fisp12/mouse connective tissue growth factor is mediated through integrin αIIb β3. Biol Chem. 1999;274:24321–24327. doi: 10.1074/jbc.274.34.24321. [DOI] [PubMed] [Google Scholar]
- Laping N, Grygielko G, Mathur A, Butter S, Bonmberger J, Tweed C, et al. Inhibition of transforming growth factor (TGF)-β1-induced extracellular matrix with a novel inhibitor of the TGF-β type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002;62:58–64. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham D. The role of connective tissue growth factor, a multifunctional matricellular protein, in fibroblast biology. Biochem Cell Biol. 2003;81:355–363. doi: 10.1139/o03-069. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham D. TGF-β signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- Leask A, Holmes A, Black C, Abraham D. Connective tissue growth factor gene regualtion. Requirements for its induction by transforming growth factor-beta 2 in fibroblasts. J Biol Chem. 2003;278:13008–13015. doi: 10.1074/jbc.M210366200. [DOI] [PubMed] [Google Scholar]
- Leivonen S, Hakkinen L, Lui D, Kahari V. Smad3 and extracellular signal-regulated kinase 1/2 coordinately mediate transforming growth factor-β-induced expression of connective tissue growth factor in human fibroblasts. J Invest Dermatol. 2001;124:1162–1169. doi: 10.1111/j.0022-202X.2005.23750.x. [DOI] [PubMed] [Google Scholar]
- Mori T, Kawara S, Shinozaki M, Hayaski N, Kakinuma T, Igarashi A, et al. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: A mouse fibrosis model. J Cell Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Nishida T, Kawaki H, Baxter R, Deyoung R, Takigawa M, Lyons K. CCN2 (Connective Tissue Growth Factor) is essential for extracellular matrix production and integrin signaling in chondrocytes. J Cell Commun Signal. 2007;1:45–58. doi: 10.1007/s12079-007-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T, Kubota S, Fukunaga T, Kondo S, Yosimichi G, Nakanishi T, et al. CTGF/Hcs24, hypertrophic chondrocyte-specific gene product, interacts with perlecan in regulating the proliferation and differentiation of chondrocytes. J Cell Physiol. 2003;196:265–275. doi: 10.1002/jcp.10277. [DOI] [PubMed] [Google Scholar]
- Qi W, Trigg S, Chen X, Polhill T, Poronnik P, Gilbert R, et al. Integrated actions of transforming growth factor-beta1 and connective tissue growth factor in renal fibrosis. Am J Physiol Renal Physiol. 2005;288:F800–F809. doi: 10.1152/ajprenal.00179.2004. [DOI] [PubMed] [Google Scholar]
- Quan T, Fisher GJ. Cloning and characterization of the human protein kinase c-η promoter. J Biol Chem. 1999;274:28566–28574. doi: 10.1074/jbc.274.40.28566. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees J, Fisher G. Ultraviolet irradiation alters transforming growth factor β/Smad pathway in human skin in vivo. J Invest Dermatol. 2002a;119:499–506. doi: 10.1046/j.1523-1747.2002.01834.x. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Connective tissue growth factor: expression in human skin in vivo and inhibition by ultraviolet irradiation. J Invest Dermatol. 2002b;118:402–408. doi: 10.1046/j.0022-202x.2001.01678.x. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am J Pathol. 2004;165:741–751. doi: 10.1016/s0002-9440(10)63337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, He T, Shao Y, Lin L, Kang S, Voorhees JJ, et al. Elevated cysteine-rich 61 mediates aberrant collagen homeostasis in chronologically aged and photoaged human skin. Am J Pathol. 2006;169:482–490. doi: 10.2353/ajpath.2006.060128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan T, He T, Voorhees J, Fisher G. Ultraviolet irradiation blocks cellular responses to transforming growth factor-β by down-regulating its type-II receptor and inducing Smad7. J Biol Chem. 2001;276:26349–26356. doi: 10.1074/jbc.M010835200. [DOI] [PubMed] [Google Scholar]
- Quan T, He T, Voorhees JJ, Fisher GJ. Ultraviolet irradiation induces Smad7 via induction of transcription factor AP-1 in human skin fibroblasts. J Biol Chem. 2005;280:8079–8085. doi: 10.1074/jbc.M409647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober J, Chen N, Grzeszkiewicz T, Jovanovic I, Emerson E, Ugarova T, et al. Identification of integrin alpha(M)beta(2) as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN-1) and connective tissue growth factor (CCN2): immediate-early gene products expressed in atherosclerotic lesions. Blood. 2002;99:4457–4465. doi: 10.1182/blood.v99.12.4457. [DOI] [PubMed] [Google Scholar]
- Shi-Wen X, Leask A, Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth Factor Rev. 2008;19:133–144. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Shi-Wen X, Stanton L, Kennedy L, Pala D, Chen Y, Howat S, et al. CCN2 is necessary for adhesive responses to transforming growth factor-beta1 in embryonic fibroblasts. J Biol Chem. 2006;281:10715–10726. doi: 10.1074/jbc.M511343200. [DOI] [PubMed] [Google Scholar]
- Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, Fisher GJ, et al. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol. 2006;168:1861–1868. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrecchia F, Pessah M, Atfi A, Mauviel A. Tumor necrosis factor-α inhibits transforming growth factor-β/Smad signaling in human dermal fibroblasts via AP-1 activation. J Biol Chem. 2000;275:30226–30231. doi: 10.1074/jbc.M005310200. [DOI] [PubMed] [Google Scholar]
- Wahab N, Yevdokimova N, Weston B, Roberts T, Li X, Brinkman H, et al. Role of connective tissue growth factor in the pathogenesis of diabetic nephropathy. Biochem J. 2001;359:77–87. doi: 10.1042/0264-6021:3590077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Huang H, Li J, Li D, Wang H. Tyrosine phosphorylation of the LDL receptor-related protein (LRP) and activation of the ERK pathyway are required for connective tissue growth factor to potentiate myofibroblast differentiation. FASEB J. 2004;18:1920–1921. doi: 10.1096/fj.04-2357fje. [DOI] [PubMed] [Google Scholar]
- Yeger H, Perbal B. The CCN family of genes: aperspective on CCN biology and therapeutic potential. J Cell Commun Signal. 2007;1:159–164. doi: 10.1007/s12079-008-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.